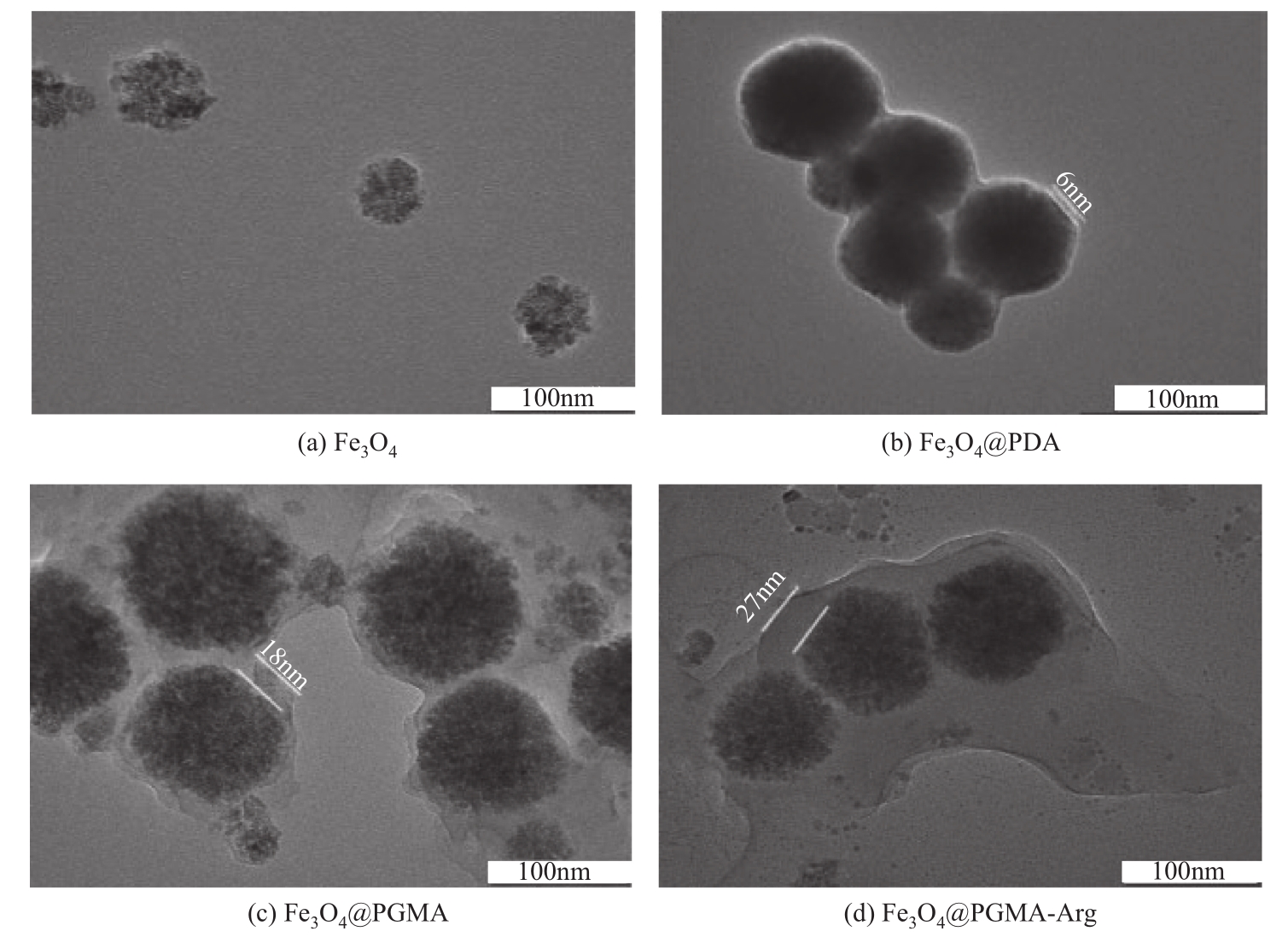
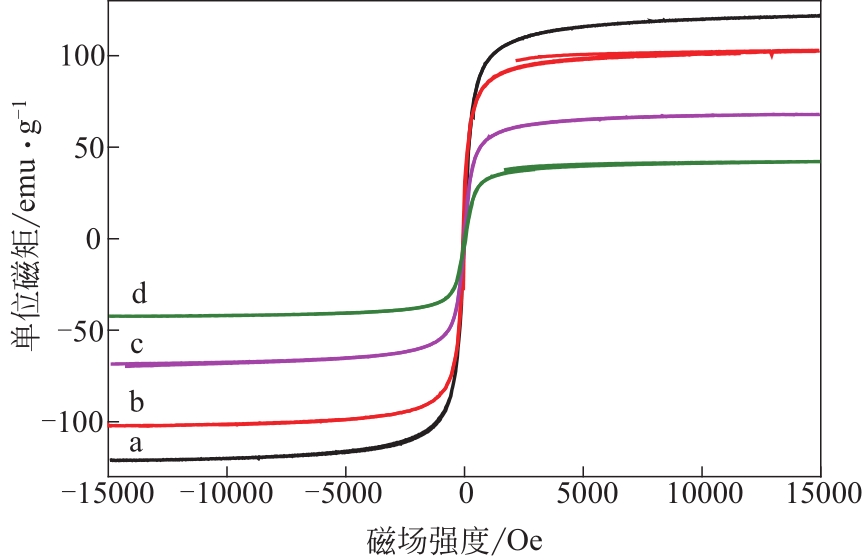
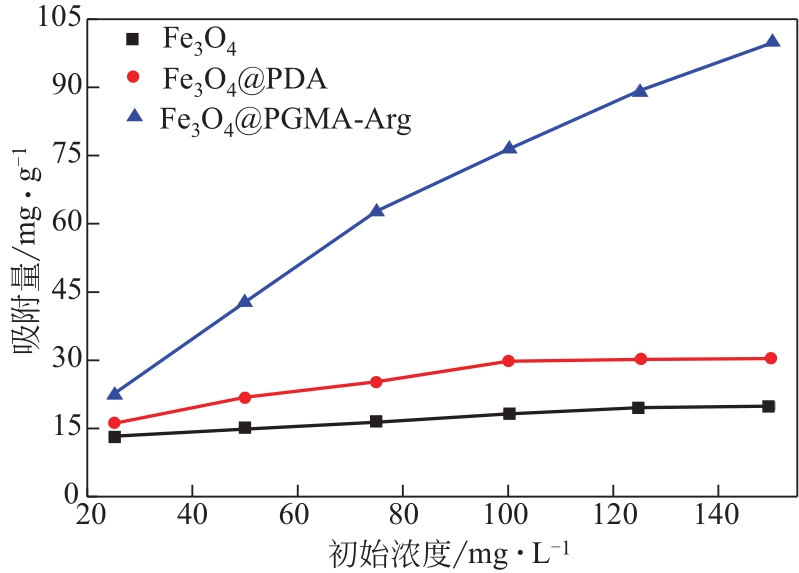
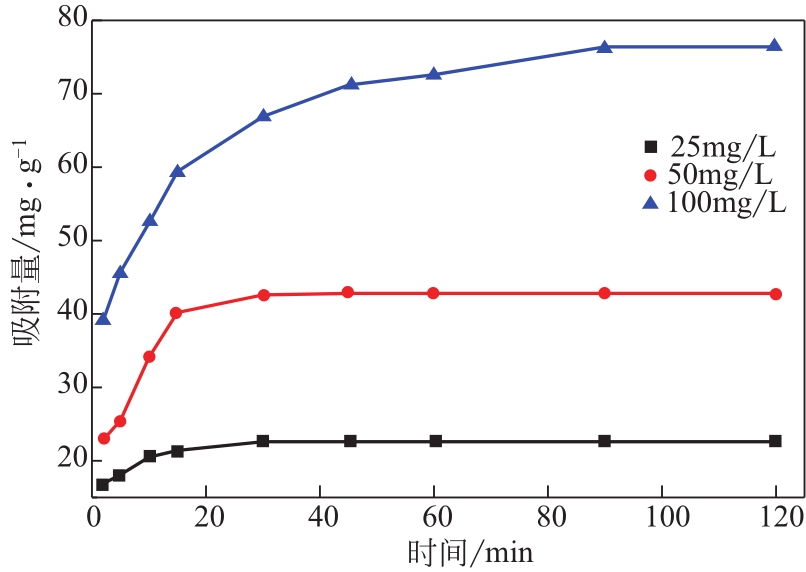
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (2): 881-891.DOI: 10.16085/j.issn.1000-6613.2021-0109
• Materials science and technology • Previous Articles Next Articles
Preparation of Fe3O4@PGMA-Arg magnetic adsorbents and its adsorption performance for ferrous ion
TIAN Zhongyu( ), YANG Jingyi(
), YANG Jingyi( ), WANG Yixing, XU Xinru
), WANG Yixing, XU Xinru
- International Joint Research Center of Green Energy Chemical Engineering, School of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China
-
Received:2021-01-16Revised:2021-04-07Online:2022-02-23Published:2022-02-05 -
Contact:YANG Jingyi
Fe3O4@PGMA-Arg磁性吸附剂的制备及对亚铁离子的吸附性能
- 华东理工大学化工学院, 绿色能源化工国际联合研究中心,上海 200237
-
通讯作者:杨敬一 -
作者简介:田忠宇(1992—),男,硕士研究生,研究方向为石油化学。E-mail:tianzysl@163.com 。
CLC Number:
Cite this article
TIAN Zhongyu, YANG Jingyi, WANG Yixing, XU Xinru. Preparation of Fe3O4@PGMA-Arg magnetic adsorbents and its adsorption performance for ferrous ion[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 881-891.
田忠宇, 杨敬一, 王怿兴, 徐心茹. Fe3O4@PGMA-Arg磁性吸附剂的制备及对亚铁离子的吸附性能[J]. 化工进展, 2022, 41(2): 881-891.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-0109
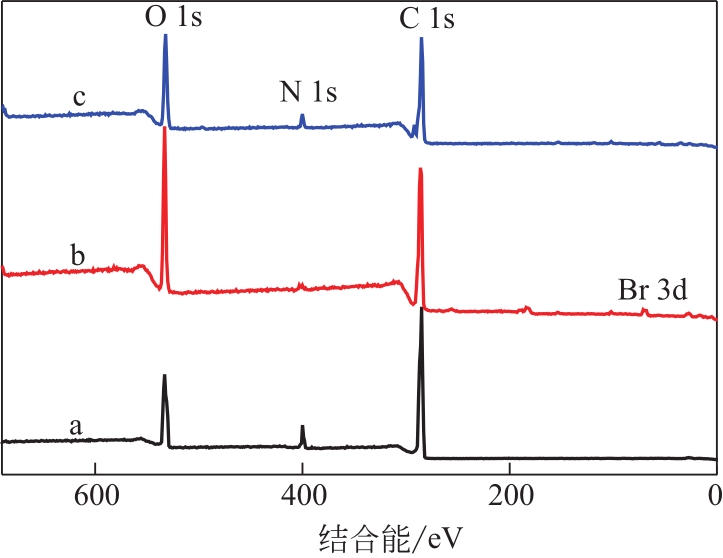
| 样品 | C 1s(摩尔分数)/% | N 1s(摩尔分数)/% | O 1s(摩尔分数)/% | Br 3d(摩尔分数)/% |
|---|---|---|---|---|
| Fe3O4@PDA | 73.97 | 7.73 | 18.66 | — |
| Fe3O4@PGMA | 72.04 | 0.42 | 24.48 | 1.06 |
| Fe3O4@PGMA-Arg | 70.99 | 4.10 | 24.73 | 0.18 |
| 样品 | C 1s(摩尔分数)/% | N 1s(摩尔分数)/% | O 1s(摩尔分数)/% | Br 3d(摩尔分数)/% |
|---|---|---|---|---|
| Fe3O4@PDA | 73.97 | 7.73 | 18.66 | — |
| Fe3O4@PGMA | 72.04 | 0.42 | 24.48 | 1.06 |
| Fe3O4@PGMA-Arg | 70.99 | 4.10 | 24.73 | 0.18 |
浓度 /mg·L-1 | 准一级动力学模型 | 准二级动力学模型 | |||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | Qe/mg·g-1 | R2 | k2/g·mg-1·min-1 | Qe/mg·g-1 | R2 | ||
| 25 | 0.7465 | 22.5 | 0.8833 | 0.05338 | 22.8 | 0.9999 | |
| 50 | 1.9614 | 42.8 | 0.8601 | 0.01167 | 43.8 | 0.9994 | |
| 100 | 1.8076 | 70.5 | 0.8520 | 0.00030 | 79.0 | 0.9990 | |
浓度 /mg·L-1 | 准一级动力学模型 | 准二级动力学模型 | |||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | Qe/mg·g-1 | R2 | k2/g·mg-1·min-1 | Qe/mg·g-1 | R2 | ||
| 25 | 0.7465 | 22.5 | 0.8833 | 0.05338 | 22.8 | 0.9999 | |
| 50 | 1.9614 | 42.8 | 0.8601 | 0.01167 | 43.8 | 0.9994 | |
| 100 | 1.8076 | 70.5 | 0.8520 | 0.00030 | 79.0 | 0.9990 | |
温度 /℃ | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
| KL/L·mg-1 | Qm/mg·g-1 | R2 | KF/mg1-1/n·L1/n·g-1 | n | R2 | |
| 20 | 0.08276 | 121.2 | 0.9931 | 16.05 | 2.05 | 0.9704 |
| 30 | 0.13181 | 123.3 | 0.9924 | 22.37 | 2.28 | 0.9803 |
| 40 | 0.39558 | 127.2 | 0.9921 | 42.24 | 3.04 | 0.9783 |
| 50 | 0.67623 | 136.8 | 0.9877 | 54.51 | 3.15 | 0.9325 |
温度 /℃ | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
| KL/L·mg-1 | Qm/mg·g-1 | R2 | KF/mg1-1/n·L1/n·g-1 | n | R2 | |
| 20 | 0.08276 | 121.2 | 0.9931 | 16.05 | 2.05 | 0.9704 |
| 30 | 0.13181 | 123.3 | 0.9924 | 22.37 | 2.28 | 0.9803 |
| 40 | 0.39558 | 127.2 | 0.9921 | 42.24 | 3.04 | 0.9783 |
| 50 | 0.67623 | 136.8 | 0.9877 | 54.51 | 3.15 | 0.9325 |
| 温度/℃ | ΔG?/kJ·mol-1 | ΔH?/kJ·mol-1 | ΔS?/J·mol-1·K-1 |
|---|---|---|---|
| 20 | -13.55 | 67.34 | 298.39 |
| 30 | -22.44 | ||
| 40 | -26.04 | ||
| 50 | -28.32 |
| 温度/℃ | ΔG?/kJ·mol-1 | ΔH?/kJ·mol-1 | ΔS?/J·mol-1·K-1 |
|---|---|---|---|
| 20 | -13.55 | 67.34 | 298.39 |
| 30 | -22.44 | ||
| 40 | -26.04 | ||
| 50 | -28.32 |
| 1 | XIONG Boya, MILLER Z, ROMAN-WHITE S, et al. Chemical degradation of polyacrylamide during hydraulic fracturing[J]. Environmental Science & Technology, 2018, 52(1): 327-336. |
| 2 | ZHANG Ping, ZHANG Zhang, LIU Yuan, et al. Investigation of the impact of ferrous species on the performance of common oilfield scale inhibitors for mineral scale control[J]. Journal of Petroleum Science and Engineering, 2019, 172: 288-296. |
| 3 | KAVEESHWAR A R, SANDERS M, PONNUSAMY S K, et al. Chitosan as a biosorbent for adsorption of iron (II) from fracking wastewater[J]. Polymers for Advanced Technologies, 2018, 29(2): 961-969. |
| 4 | 黄漫, 李美蓉, 田兰兰, 等. 亚铁离子对驱油聚合物溶液黏度的影响及其降黏机理[J]. 应用化学, 2013, 30(12): 1399-1403. |
| HUANG Man, LI Meirong, TIAN Lanlan, et al. Effect of ferrous ion on reducing the viscosity of oil flooding polymer solution and its mechanism[J]. Chinese Journal of Applied Chemistry, 2013, 30(12): 1399-1403. | |
| 5 | GREGORY K B, VIDIC R D, DZOMBAK D A. Water management challenges associated with the production of shale gas by hydraulic fracturing[J]. Elements, 2011, 7(3): 181-186. |
| 6 | 刘金燕, 刘立华, 薛建荣, 等. 重金属废水吸附处理的研究进展[J]. 环境化学, 2018, 37(9): 2016-2024. |
| LIU Jinyan, LIU Lihua, XUE Jianrong, et al. Research progress on treatment of heavy metal wastewater by adsorption[J]. Environmental Chemistry, 2018, 37(9): 2016-2024. | |
| 7 | 汪彩虹, 陈硕然, 叶常青, 等. 单分散磁性Fe3O4纳米粒子的研究进展[J]. 化工进展, 2016, 35(S1): 242-247. |
| WANG Caihong, CHEN Shuoran, YE Changqing, et al. Research progress on monodisperse Fe3O4 magnetic nanoparticles[J]. Chemical Industry and Engineering Progress, 2016, 35(S1): 242-247. | |
| 8 | MASOUMI A, GHAEMY M, BAKHT A N. Removal of metal ions from water using poly(MMA-co-MA)/modified-Fe3O4 magnetic nanocomposite: isotherm and kinetic study[J]. Industrial & Engineering Chemistry Research, 2014, 53(19): 8188-8197. |
| 9 | 刘明强, 王会才, 孙强. 端氨基超支聚合物功能化磁性复合材料对Cu2+和甲基橙的吸附研究[J]. 化工新型材料, 2017, 45(2): 91-94. |
| LIU Mingqiang, WANG Huicai, SUN Qiang. Study on adsorption of amino terminated hyperbranched polymer functional magnetic composite materials for Cu2+ and methyl orange[J]. New Chemical Materials, 2017, 45(2): 91-94. | |
| 10 | ZHANG Sai, ZHOU Yifeng, NIE Wangyan, et al. Preparation of Fe3O4/chitosan/poly(acrylic acid) composite particles and its application in adsorbing copper ion (II)[J]. Cellulose, 2012, 19(6): 2081-2091. |
| 11 | MA Zhiyuan, JIA Xin, HU Jiamei, et al. Mussel-inspired thermosensitive polydopamine-graft-poly(N-isopropylacrylamide) coating for controlled-release fertilizer[J]. Journal of Agricultural and Food Chemistry, 2013, 61(50): 12232-12237. |
| 12 | 陈思远, 董旭, 查刘生. 表面引发原子转移自由基聚合法合成无机/有机核壳复合纳米粒子[J]. 化学进展, 2015, 27(7): 831-840. |
| CHEN Siyuan, DONG Xu, ZHA Liusheng. Inorganic/organic core-shell composite nanoparticles by surface-initiated atom transfer radical polymerization[J]. Progress in Chemistry, 2015, 27(7): 831-840. | |
| 13 | BUAMAH R, PETRUSEVSKI B, SCHIPPERS J C. Oxidation of adsorbed ferrous iron: kinetics and influence of process conditions[J]. Water Science and Technology, 2009, 60(9): 2353-2363. |
| 14 | SRIVASTAVA V, WENG C H, SINGH V K, et al. Adsorption of nickel ions from aqueous solutions by nano alumina: kinetic, mass transfer, and equilibrium studies[J]. Journal of Chemical & Engineering Data, 2011, 56(4): 1414-1422. |
| 15 | NEELI S T, RAMSURN H, NG C Y, et al. Removal of Cr (Ⅵ), As (Ⅴ), Cu(Ⅱ), and Pb(Ⅱ) using cellulose biochar supported iron nanoparticles: a kinetic and mechanistic study[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 103886. |
| 16 | ÖZACAR M, ŞENGIL İ A. A kinetic study of metal complex dye sorption onto pine sawdust[J]. Process Biochemistry, 2005, 40(2): 565-572. |
| 17 | PHOLOSI A, NAIDOO E B, OFOMAJA A E. Intraparticle diffusion of Cr(Ⅵ) through biomass and magnetite coated biomass: a comparative kinetic and diffusion study[J]. South African Journal of Chemical Engineering, 2020, 32: 39-55. |
| 18 | TSENG R L, WU F C. Inferring the favorable adsorption level and the concurrent multi-stage process with the Freundlich constant[J]. Journal of Hazardous Materials, 2008, 155(1/2): 277-287. |
| 19 | GUO Xuan, WANG Jianlong. Comparison of linearization methods for modeling the Langmuir adsorption isotherm[J]. Journal of Molecular Liquids, 2019, 296: 111850. |
| [1] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [2] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [3] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [4] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [5] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [6] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [7] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [8] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [9] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [10] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| [11] | YU Jingwen, SONG Luna, LIU Yanchao, LYU Ruidong, WU Mengmeng, FENG Yu, LI Zhong, MI Jie. An indole-bearing hypercrosslinked polymer In-HCP for iodine adsorption from water [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3674-3683. |
| [12] | LI Yanling, ZHUO Zhen, CHI Liang, CHEN Xi, SUN Tanglei, LIU Peng, LEI Tingzhou. Research progress on preparation and application of nitrogen-doped biochar [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3720-3735. |
| [13] | BAI Yadi, DENG Shuai, ZHAO Ruikai, ZHAO Li, YANG Yingxia. Exploration on standardized test scheme and experimental performance of temperature swing adsorption carbon capture unit [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3834-3846. |
| [14] | ZHANG Xuewei, HUANG Yaji, XU Yueyang, CHENG Haoqiang, ZHU Zhicheng, LI Jinlei, DING Xueyu, WANG Sheng, ZHANG Rongchu. Adsorption characteristics of SO3 from coal flue gas by alkaline adsorbent [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3855-3864. |
| [15] | XIE Zhiwei, WU Zhangyong, ZHU Qichen, JIANG Jiajun, LIANG Tianxiang, LIU Zhenyang. Viscosity properties and magnetoviscous effects of Ni0.5Zn0.5Fe2O4 vegetable oil-based magnetic fluid [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3623-3633. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||