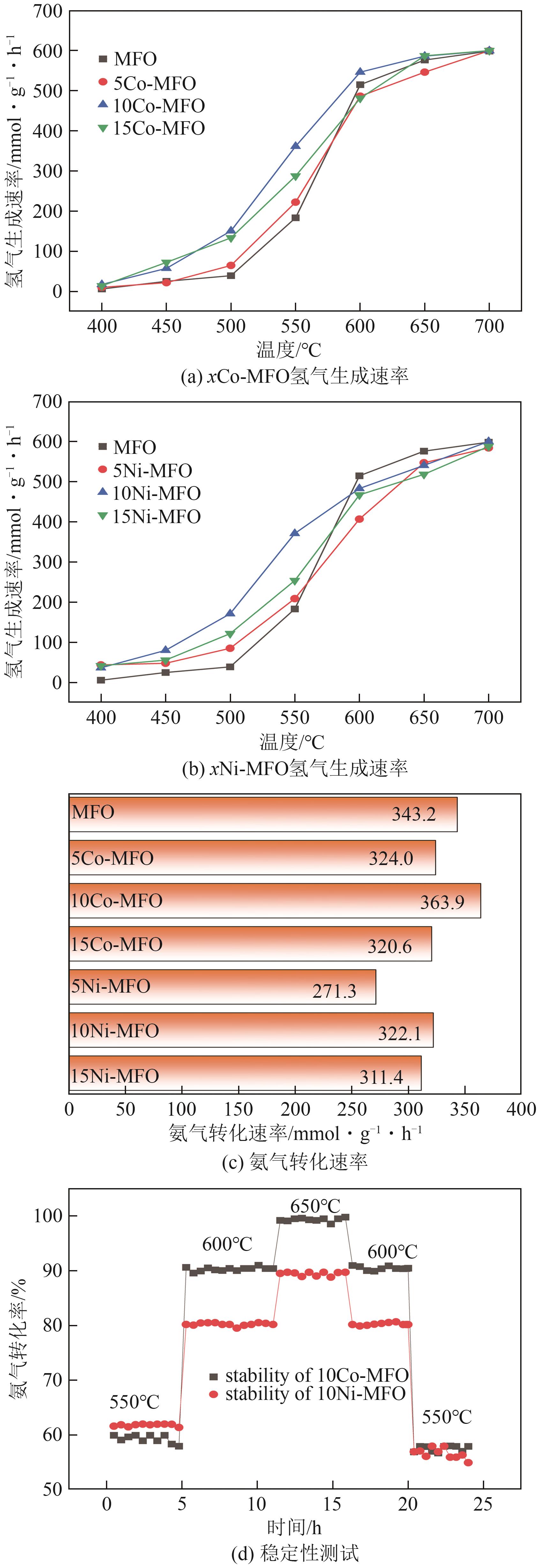
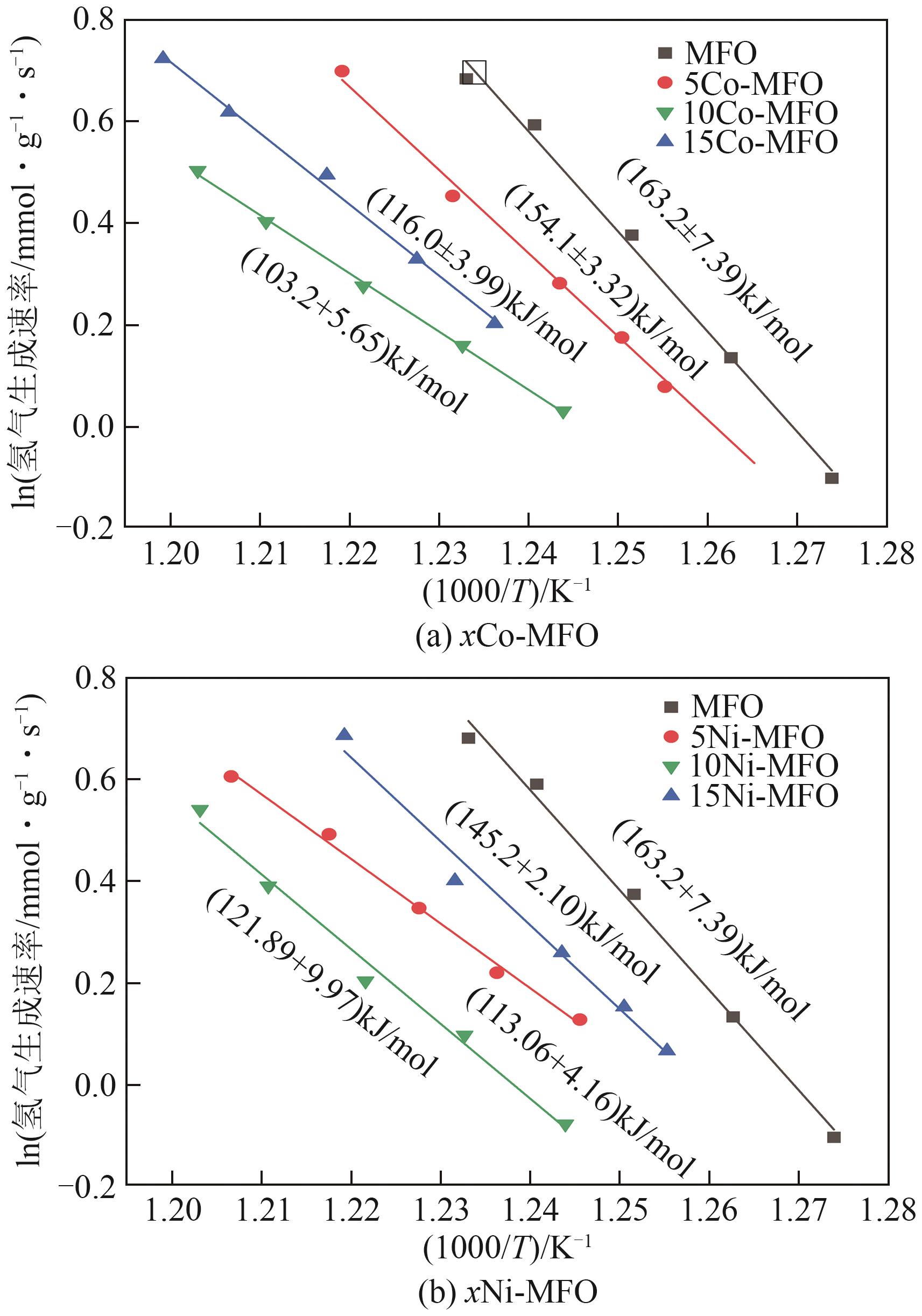
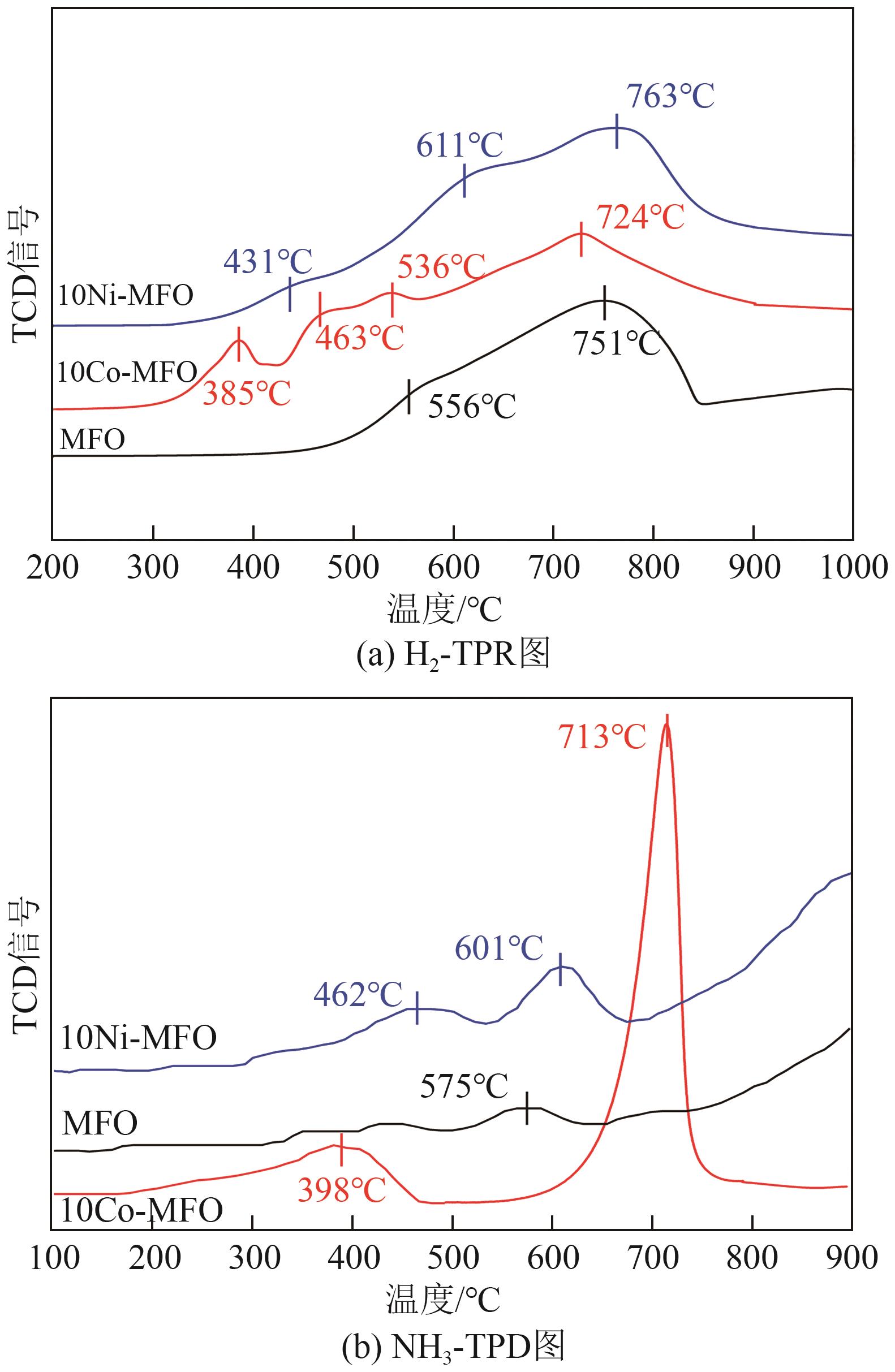
| [1] |
GANLEY J C, THOMAS F S, SEEBAUER E G, et al. A priori catalytic activity correlations: The difficult case of hydrogen production from ammonia[J]. Catalysis Letters, 2004, 96(3): 117-122.
|
| [2] |
TANG Shu, DING Jun, LI Wenyi, et al. Ultrafine Ru nanoparticles supported on mesoporous silica for hydrogen production via decomposition of ammonia[J]. ChemCatChem, 2024, 16(20): e202400241.
|
| [3] |
CHEN Biaohua, LIU Ning, LIU Xingyong, et al. Study on the direct decomposition of nitrous oxide over Fe-beta zeolites: From experiment to theory[J]. Catalysis Today, 2011, 175(1): 245-255.
|
| [4] |
HU Zhongpan, WENG Chenchen, CHEN Chong, et al. Two-dimensional mica nanosheets supported Fe nanoparticles for NH3 decomposition to hydrogen[J]. Molecular Catalysis, 2018, 448: 162-170.
|
| [5] |
JI Jian, DUAN Xuezhi, QIAN Gang, et al. Fe particles on the tops of carbon nanofibers immobilized on structured carbon microfibers for ammonia decomposition[J]. Catalysis Today, 2013, 216: 254-260.
|
| [6] |
MENG Shengyan, LI Shangkun, SUN Shuaiqi, et al. NH3 decomposition for H2 production by thermal and plasma catalysis using bimetallic catalysts[J]. Chemical Engineering Science, 2024, 283: 119449.
|
| [7] |
SU Qin, WANG Hehui, GU Lingli, et al. Fe-based catalyst derived from MgFe-LDH: Very efficient yet simply obtainable for hydrogen production via ammonia decomposition[J]. International Journal of Hydrogen Energy, 2021, 46(61): 31122-31132.
|
| [8] |
FEYEN Mathias, WEIDENTHALER Claudia, Robert GÜTTEL, et al. High-temperature stable, iron-based core-shell catalysts for ammonia decomposition[J]. Chemistry:A European Journal, 2011, 17(2): 598-605.
|
| [9] |
ZHANG Jian, XU Hengyong, LI Wenzhao. Kinetic study of NH3 decomposition over Ni nanoparticles: The role of La promoter, structure sensitivity and compensation effect[J]. Applied Catalysis A: General, 2005, 296(2): 257-267.
|
| [10] |
DONALD Jaclyn, XU Chunbao, HASHIMOTO Hiroyuki, et al. Novel carbon-based Ni/Fe catalysts derived from peat for hot gas ammonia decomposition in an inert helium atmosphere[J]. Applied Catalysis A: General, 2010, 375(1): 124-133.
|
| [11] |
LI Guoru, YU Xiaoting, LEI Zhiping, et al. Preparation of lanthanum hexaaluminate supported nickel catalysts for hydrogen production by ammonia decomposition[J]. Catalysis Letters, 2023, 153(10): 3148-3158.
|
| [12] |
WANG Chanchan, WANG Fen, SHI Jianjun. FeO x -modified ultrafine platinum particles supported on MgFe2O4 with high catalytic activity and promising stability toward low-temperature oxidation of CO[J]. Molecules, 2024, 29(5): 1027.
|
| [13] |
PODILA Seetharamulu, DRISS Hafedh, ZAMAN Sharif F, et al. Effect of preparation methods on the catalyst performance of Co/MgLa mixed oxide catalyst for CO x -free hydrogen production by ammonia decomposition[J]. International Journal of Hydrogen Energy, 2017, 42(38): 24213-24221.
|
| [14] |
ALAMOUDI Omar M, KHAN Wasim Ullah, HANTOKO Dwi, et al. Catalytic activity of Co/γ-Al2O3 catalysts for decomposition of ammonia to produce hydrogen[J]. Fuel, 2024, 372: 132230.
|
| [15] |
QIU Yu, FU Enkang, GONG Feng, et al. Catalyst support effect on ammonia decomposition over Ni/MgAl2O4 towards hydrogen production[J]. International Journal of Hydrogen Energy, 2022, 47(8): 5044-5052.
|
| [16] |
SIMA Dewen, WU Haojin, TIAN Koukou, et al. Enhanced low temperature catalytic activity of Ni/Al-Ce0.8Zr0.2O2 for hydrogen production from ammonia decomposition[J]. International Journal of Hydrogen Energy, 2020, 45(16): 9342-9352.
|
| [17] |
SU Qin, GU Lingli, YAO Yao, et al. Layered double hydroxides derived Ni x (Mg y Al z O n ) catalysts: Enhanced ammonia decomposition by hydrogen spillover effect[J]. Applied Catalysis B: Environmental, 2017, 201: 451-460.
|
| [18] |
CHELLAPPA AS, FISCHER CM, THOMSON WJ. Ammonia decomposition kinetics over Ni-Pt/Al2O3 for PEM fuel cell applications[J]. Applied Catalysis A: General, 2002, 227(1/2): 231-240.
|
| [19] |
CHEN Chongqi, WU Kai, REN Hongju, et al. Ru-based catalysts for ammonia decomposition: A mini-review[J]. Energy & Fuels, 2021, 35(15): 11693-11706.
|
| [20] |
WANG Fagen, ZHANG Linjia, DENG Jianquan, et al. Embedded Ni catalysts in Ni-O-Ce solid solution for stable hydrogen production from ethanol steam reforming reaction[J]. Fuel Processing Technology, 2019, 193: 94-101.
|
| [21] |
LI Xinshu, WANG Qing, WU Shuang, et al. Critical upstream technologies for hydrogen energy industry: Research progress on ammonia decomposition catalysts[J]. Sustainable Chemistry and Pharmacy, 2024, 38: 101492.
|
| [22] |
MAZZONE S, LEISHMAN C, ZHANG G, et al. A compact non-PGM catalytic hollow fibre converter for on-board hydrogen production[J]. Sustainable Energy & Fuels, 2022, 6(6): 1554-1567.
|
| [23] |
HE Haihua, CHEN Chonglai, BIAN Chaoqun, et al. Enhanced ammonia decomposition by tuning the support properties of Ni/Gd x Ce1- x O2- δ at 600 ℃[J]. Molecules, 2023, 28(6): 2750.
|
| [24] |
HU Lihua, CHEN Jingye, TONG Jinming, et al. Synthesis of Fe/Co/Ni based catalysts and NH3 cracking performance for H2 production[J]. Clean Coal Technology, 2024, 30(8): 1-10.
|
| [25] |
BENRABAA Rafik, RUBBENS Annick, Axel LÖFBERG, et al. Evidence of surface properties by isopropanol decomposition reaction and NH3-TPD over Ni-Fe spinel nanoparticles prepared via hydrothermal route[J]. ChemistrySelect, 2023, 8(4): e202204361.
|
| [26] |
LI Shigang, LIU Xiaohui, GUO Yong, et al. Highly active and stable Ni@SiO2 catalyst for ammonia decomposition[J]. Fuel, 2024, 368: 131543.
|
 ), LI Zhuo1(
), LI Zhuo1( ), CHEN Song2, LI Xuebing2, WANG Zhong2
), CHEN Song2, LI Xuebing2, WANG Zhong2