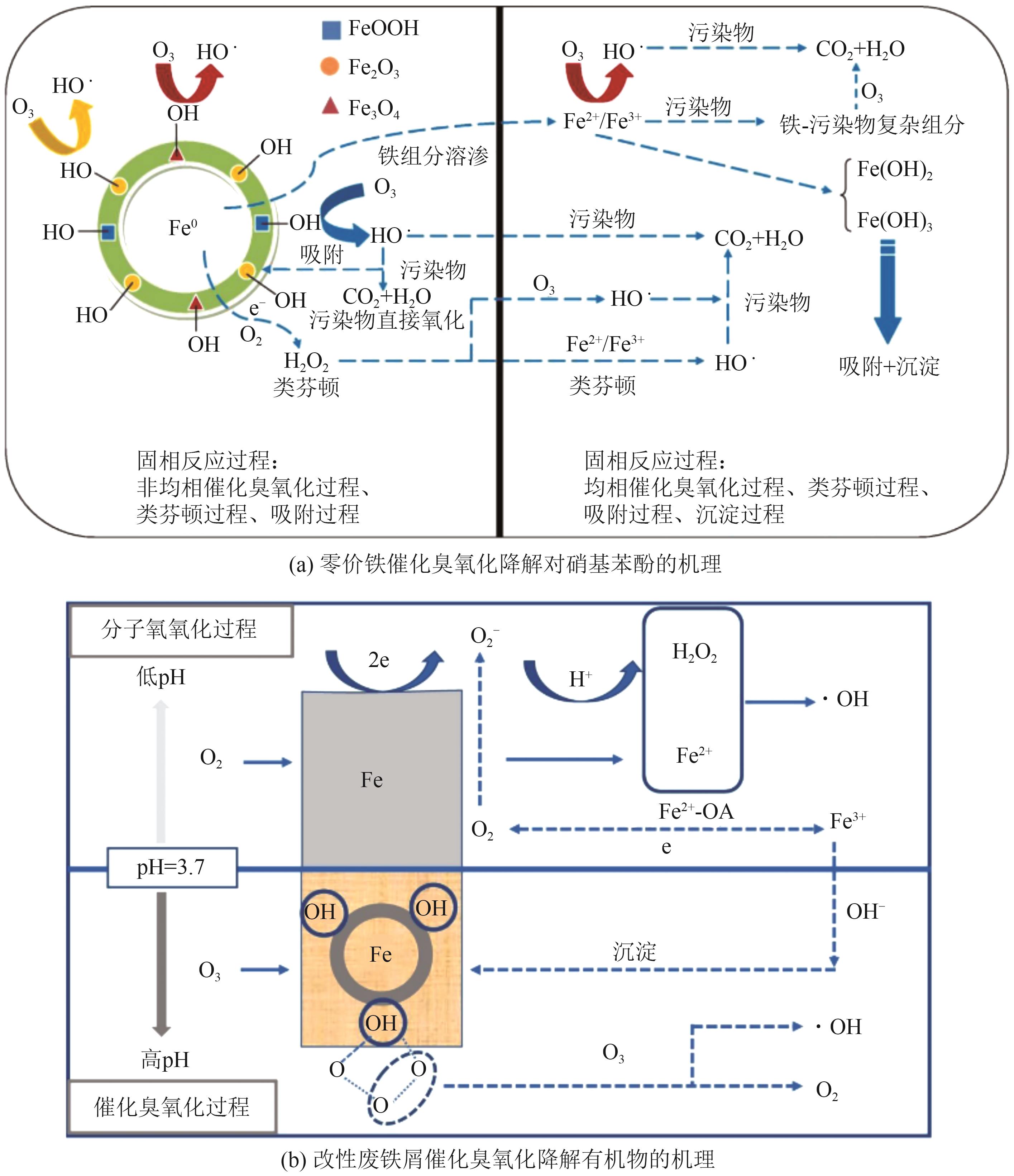
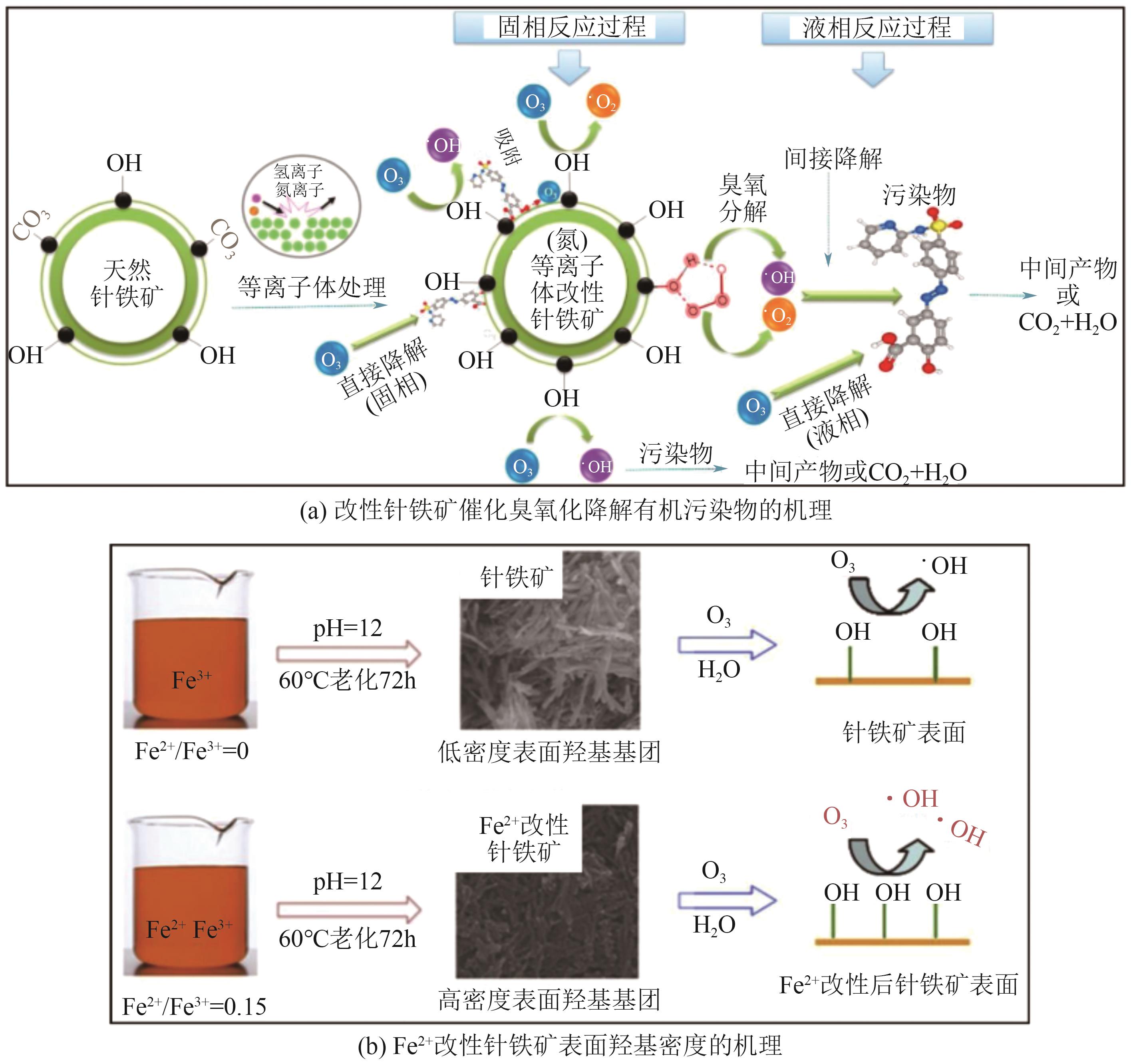
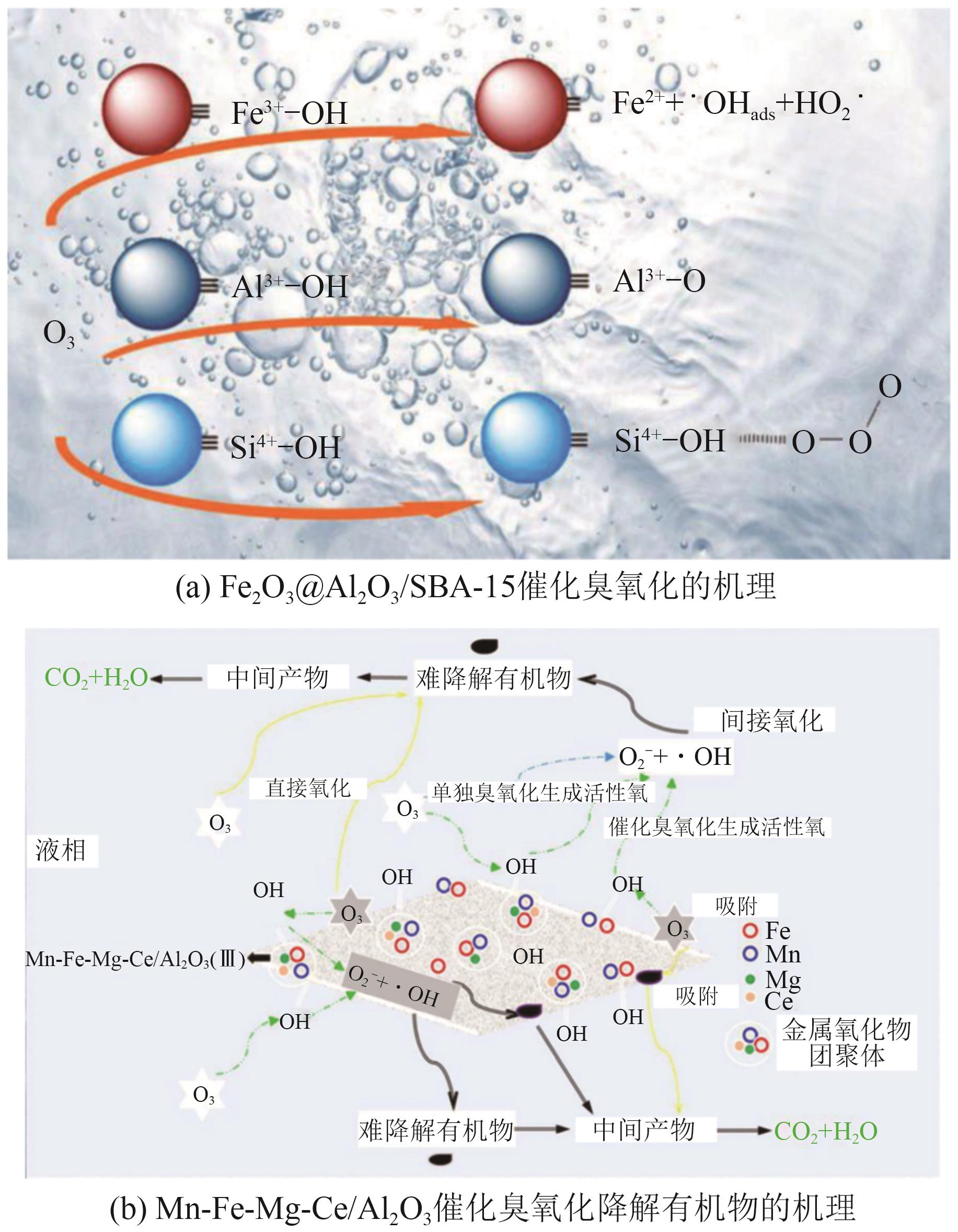
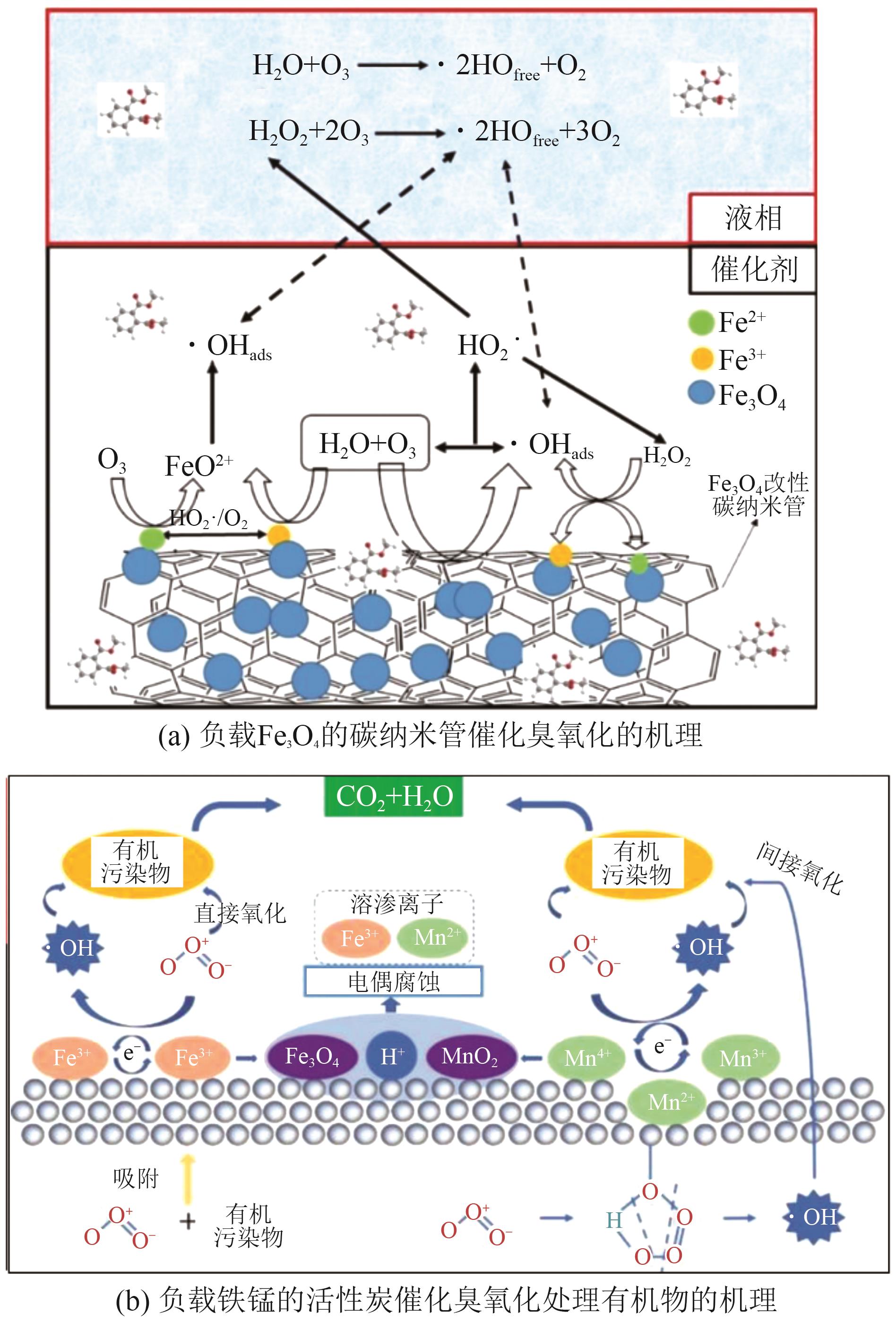
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (12): 6885-6905.DOI: 10.16085/j.issn.1000-6613.2024-1804
• Industrial catalysis • Previous Articles
Research progress of heterogeneous iron-based ozone catalysts for degradation of pollutants in water
YUAN Run1,2( ), QIN Yihe2, HE Xuwen3(
), QIN Yihe2, HE Xuwen3( )
)
- 1.School of Environment and Energy, South China University of Technology, Guangzhou 510006, Guangdong, China
2.School of Environmental and Civil Engineering, Dongguan University of Technology, Dongguan 523808, Guangdong, China
3.School of Chemical and Environmental Engineering, China University of Mining & Technology-Beijing, Beijing 100083, China
-
Received:2024-11-06Revised:2025-05-14Online:2026-01-06Published:2025-12-25 -
Contact:HE Xuwen
非均相铁基臭氧催化剂催化降解水中污染物的研究进展
- 1.华南理工大学环境与能源学院,广东 广州 510006
2.东莞理工学院生态环境与建筑工程学院,广东 东莞 523808
3.中国矿业大学(北京)化学与环境工程学院,北京 100083
-
通讯作者:何绪文 -
作者简介:员润(1996—),男,博士,研究方向为废水处理及资源化利用。E-mail:qyuanrun@163.com。 -
基金资助:国家水体污染控制与治理科技重大专项(2017ZX07402002-05-02)
CLC Number:
Cite this article
YUAN Run, QIN Yihe, HE Xuwen. Research progress of heterogeneous iron-based ozone catalysts for degradation of pollutants in water[J]. Chemical Industry and Engineering Progress, 2025, 44(12): 6885-6905.
员润, 秦燚鹤, 何绪文. 非均相铁基臭氧催化剂催化降解水中污染物的研究进展[J]. 化工进展, 2025, 44(12): 6885-6905.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1804
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| α-FeOOH | 磺胺嘧啶类抗生素(SMT) | 初始污染物浓度为10mg/L;反应pH为7;臭氧投加量为5mg/L;催化剂投加量为1.5g/L;气体流量为1L/h | SO:D[SMT]=61.44%CO:D[SMT]=96.05% | [ |
| Fe2+-α-FeOOH | 4-氯硝基苯(4-CNB) | 反应温度为25℃;反应pH为6;初始污染物浓度为50μg/L;臭氧投加量为0.6mg/L;催化剂投加量为50mg/L;反应时间为10min | SO:D[4-CNB]=34.2% CO:D[4-CNB]=57.2% | [ |
| 硅酸铁盐 | 对氯硝基苯(p-CNB) | 初始污染物浓度为100μg/L;臭氧投加量为0.6mg/L;催化剂投加量为100mg/L;反应pH为7;反应时间为15min;反应温度为25℃ | SO:D[p-CNB]=60% CO:D[p-CNB]=97% | [ |
| β-FeOOH | 对氯苯酚(4-CP) | 反应温度为20℃;反应pH为3.5;初始污染物浓度为2mmol/L;臭氧投加量为0.6mg/min | SO:D[4-CP]=67% CO:D[4-CP]=99% | [ |
| 硅酸铁盐 | 4-氯硝基苯(4-CNB) | 反应温度为25℃;初始污染物浓度为0.1mg/L;臭氧投加量为0.9mg/L;催化剂投加量为100mg/L | SO:D[4-CNB]=49.2%,[TOC]=27.2% CO:D[4-CNB]=82.2%,[TOC]=67.4% | [ |
| 改性磁铁矿 | 活性红120(RR-120) | 初始污染物浓度为100mg/L;催化剂投加量为2g/L;臭氧投加量为1mg/min;反应温度为25℃;反应pH为11 | SO:D[RR-120]=39% CO:D[RR-120]=83.4% | [ |
| α-FeOOH | 经农业污水和生活污水污染后的河水 | 气体流量为50mL/min;反应温度为20℃;催化剂投加量为1.5g/L;臭氧投加量为2.5g/L;初始TOC为9.21mg/L | SO:[TOC]=7.8% CO:[TOC]=43.54% | [ |
| α-FeOOH | 草酸(OA) | 气体流量为0.4L/min;臭氧投加量为0.45mg/min;反应pH为7;反应时间为30min;初始污染物浓度为0.01mmol/L;催化剂投加量为2g/L;反应温度为20℃ | SO:D[OA]=22% CO:D[OA]=54% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| α-FeOOH | 磺胺嘧啶类抗生素(SMT) | 初始污染物浓度为10mg/L;反应pH为7;臭氧投加量为5mg/L;催化剂投加量为1.5g/L;气体流量为1L/h | SO:D[SMT]=61.44%CO:D[SMT]=96.05% | [ |
| Fe2+-α-FeOOH | 4-氯硝基苯(4-CNB) | 反应温度为25℃;反应pH为6;初始污染物浓度为50μg/L;臭氧投加量为0.6mg/L;催化剂投加量为50mg/L;反应时间为10min | SO:D[4-CNB]=34.2% CO:D[4-CNB]=57.2% | [ |
| 硅酸铁盐 | 对氯硝基苯(p-CNB) | 初始污染物浓度为100μg/L;臭氧投加量为0.6mg/L;催化剂投加量为100mg/L;反应pH为7;反应时间为15min;反应温度为25℃ | SO:D[p-CNB]=60% CO:D[p-CNB]=97% | [ |
| β-FeOOH | 对氯苯酚(4-CP) | 反应温度为20℃;反应pH为3.5;初始污染物浓度为2mmol/L;臭氧投加量为0.6mg/min | SO:D[4-CP]=67% CO:D[4-CP]=99% | [ |
| 硅酸铁盐 | 4-氯硝基苯(4-CNB) | 反应温度为25℃;初始污染物浓度为0.1mg/L;臭氧投加量为0.9mg/L;催化剂投加量为100mg/L | SO:D[4-CNB]=49.2%,[TOC]=27.2% CO:D[4-CNB]=82.2%,[TOC]=67.4% | [ |
| 改性磁铁矿 | 活性红120(RR-120) | 初始污染物浓度为100mg/L;催化剂投加量为2g/L;臭氧投加量为1mg/min;反应温度为25℃;反应pH为11 | SO:D[RR-120]=39% CO:D[RR-120]=83.4% | [ |
| α-FeOOH | 经农业污水和生活污水污染后的河水 | 气体流量为50mL/min;反应温度为20℃;催化剂投加量为1.5g/L;臭氧投加量为2.5g/L;初始TOC为9.21mg/L | SO:[TOC]=7.8% CO:[TOC]=43.54% | [ |
| α-FeOOH | 草酸(OA) | 气体流量为0.4L/min;臭氧投加量为0.45mg/min;反应pH为7;反应时间为30min;初始污染物浓度为0.01mmol/L;催化剂投加量为2g/L;反应温度为20℃ | SO:D[OA]=22% CO:D[OA]=54% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| 泡沫铁 | 制药废水 | 臭氧投加量为9mg/L;催化剂投加量为80g/L;初始TOC为40~50mg/L;初始COD为120~150mg/L;反应pH为8.4;反应时间为120min | SO:[TOC]=32%,[COD]=73% CO:[TOC]=53%,[COD]=73% | [ |
| 泡沫铁镍 | 石化废水 | 臭氧投加量为12.2mg/L;催化剂投加量为110g/L;初始TOC为31~35mg/L;初始COD为116~143mg/L;反应pH为8.3;反应时间为120min | SO:[TOC]=11%,[COD]=65% CO:[TOC]=43%,[COD]=65% | [ |
| 泡沫铁 | 农药废水 | 臭氧投加量为5.3mg/L;催化剂投加量为20g/L;初始COD为900mg/L;反应pH为9;反应时间为30min | SO:[COD]=14.3% CO:[COD]=28.9% | [ |
| 纳米零价铁 | 对硝基苯酚 | 初始污染物浓度为500mg/L;初始COD为833mg/L;催化剂投加量为40mg/L;反应pH为5.3;气体流量为1.5L/min;臭氧投加量为7.6mg/L;反应时间为60min | SO:[COD]=29.4% CO:[COD]=89.5% | [ |
| 纳米零价铁 | 酚酞(PHL) | 臭氧投加量为5mg/L;初始污染物浓度为60mg/L;反应pH为3;反应时间为60min;催化剂投加量为1.8g/L | SO:[TOC]=49%,D[PHL]=84% CO:[TOC]=96%,D[PHL]=100% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| 泡沫铁 | 制药废水 | 臭氧投加量为9mg/L;催化剂投加量为80g/L;初始TOC为40~50mg/L;初始COD为120~150mg/L;反应pH为8.4;反应时间为120min | SO:[TOC]=32%,[COD]=73% CO:[TOC]=53%,[COD]=73% | [ |
| 泡沫铁镍 | 石化废水 | 臭氧投加量为12.2mg/L;催化剂投加量为110g/L;初始TOC为31~35mg/L;初始COD为116~143mg/L;反应pH为8.3;反应时间为120min | SO:[TOC]=11%,[COD]=65% CO:[TOC]=43%,[COD]=65% | [ |
| 泡沫铁 | 农药废水 | 臭氧投加量为5.3mg/L;催化剂投加量为20g/L;初始COD为900mg/L;反应pH为9;反应时间为30min | SO:[COD]=14.3% CO:[COD]=28.9% | [ |
| 纳米零价铁 | 对硝基苯酚 | 初始污染物浓度为500mg/L;初始COD为833mg/L;催化剂投加量为40mg/L;反应pH为5.3;气体流量为1.5L/min;臭氧投加量为7.6mg/L;反应时间为60min | SO:[COD]=29.4% CO:[COD]=89.5% | [ |
| 纳米零价铁 | 酚酞(PHL) | 臭氧投加量为5mg/L;初始污染物浓度为60mg/L;反应pH为3;反应时间为60min;催化剂投加量为1.8g/L | SO:[TOC]=49%,D[PHL]=84% CO:[TOC]=96%,D[PHL]=100% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| 铈改性针铁矿 | 邻苯二甲酸二甲酯(DMP) | 初始污染物浓度为10mg/L;反应pH为6.8;臭氧投加量为6mg/min;催化剂投加量为0.2g/L;反应时间为60min | SO:D[DMP]=80%,[TOC]=15%CO:D[DMP]=88%,[TOC]=40% | [ |
| 铁镍金属氧化物 | 邻苯二甲酸二甲酯(DMP) | 初始污染物浓度为440mg/L;臭氧(液相)投加量为0.5mg/L;催化剂投加量为0.1g/L;反应时间为10min | SO:D[DMP]=39.08% CO:D[DMP]=86.83% | [ |
| 铁钴金属氧化物 | 2,4,6-三溴苯酚(2,4,6-TBP) | 反应时间为20min;臭氧投加量为100mg/L;催化剂投加量为4g/L;初始污染物浓度为50mg/L | SO:D[2,4,6-TBP]=40%,[TOC]=11%CO:D[2,4,6-TBP]=100%,[TOC]=58% | [ |
| 镍铁层状氢氧化物 | 双酚A(BPA) | 催化剂投加量为0.3g/L;臭氧(液相)投加量为9mg/L;反应pH为8.2;初始污染物浓度为10mg/L | SO:[TOC]=30%,D[BPA]=100%CO:[TOC]=89%,D[BPA]=100% | [ |
| Ce0.1Fe0.9OOH | 磺胺二甲嘧啶 | 气体流量为0.4L/min;初始污染物浓度为0.2g/L;臭氧投加量为15mg/L;催化剂投加量为0.2g/L;反应时间为60min | SO:[TOC]=24.1% CO:[TOC]=42.1% | [ |
| Co-FeOOH | 阿替洛尔 | 初始污染物浓度为20mg/L;初始TOC浓度为14.48mg/L;臭氧投加量为2.04mg/L;催化剂投加量为0.2g/L;反应pH为7;反应时间为20min | SO:[TOC]≈16% CO:[TOC]=22% | [ |
| ZnFe2O4 | 苯酚 | 反应时间为30min;反应温度为20℃;臭氧投加量为14mg/L;反应pH为6.38;气体流量为1L/min;初始污染物浓度为300mg/L;催化剂投加量为1g/L | SO:D[苯酚]=63.4% CO:D[苯酚]=92.6% | [ |
| NiFe2O4 | 苯酚 | 臭氧投加量为0.75mg/min;初始污染物浓度为300mg/L;催化剂投加量为1g/L;反应pH为6.5;反应温度为20℃ | SO:D[苯酚]=38.9% CO:D[苯酚]=55.2% | [ |
| 硅酸铁掺杂羟基氧化铁 | 对氯硝基苯 | 初始污染物浓度为100μg/L;液相臭氧投加量为0.6mg/L;反应pH为7;催化剂投加量为0.5g/L | SO:[TOC]=28.1% CO:[TOC]=61.3% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| 铈改性针铁矿 | 邻苯二甲酸二甲酯(DMP) | 初始污染物浓度为10mg/L;反应pH为6.8;臭氧投加量为6mg/min;催化剂投加量为0.2g/L;反应时间为60min | SO:D[DMP]=80%,[TOC]=15%CO:D[DMP]=88%,[TOC]=40% | [ |
| 铁镍金属氧化物 | 邻苯二甲酸二甲酯(DMP) | 初始污染物浓度为440mg/L;臭氧(液相)投加量为0.5mg/L;催化剂投加量为0.1g/L;反应时间为10min | SO:D[DMP]=39.08% CO:D[DMP]=86.83% | [ |
| 铁钴金属氧化物 | 2,4,6-三溴苯酚(2,4,6-TBP) | 反应时间为20min;臭氧投加量为100mg/L;催化剂投加量为4g/L;初始污染物浓度为50mg/L | SO:D[2,4,6-TBP]=40%,[TOC]=11%CO:D[2,4,6-TBP]=100%,[TOC]=58% | [ |
| 镍铁层状氢氧化物 | 双酚A(BPA) | 催化剂投加量为0.3g/L;臭氧(液相)投加量为9mg/L;反应pH为8.2;初始污染物浓度为10mg/L | SO:[TOC]=30%,D[BPA]=100%CO:[TOC]=89%,D[BPA]=100% | [ |
| Ce0.1Fe0.9OOH | 磺胺二甲嘧啶 | 气体流量为0.4L/min;初始污染物浓度为0.2g/L;臭氧投加量为15mg/L;催化剂投加量为0.2g/L;反应时间为60min | SO:[TOC]=24.1% CO:[TOC]=42.1% | [ |
| Co-FeOOH | 阿替洛尔 | 初始污染物浓度为20mg/L;初始TOC浓度为14.48mg/L;臭氧投加量为2.04mg/L;催化剂投加量为0.2g/L;反应pH为7;反应时间为20min | SO:[TOC]≈16% CO:[TOC]=22% | [ |
| ZnFe2O4 | 苯酚 | 反应时间为30min;反应温度为20℃;臭氧投加量为14mg/L;反应pH为6.38;气体流量为1L/min;初始污染物浓度为300mg/L;催化剂投加量为1g/L | SO:D[苯酚]=63.4% CO:D[苯酚]=92.6% | [ |
| NiFe2O4 | 苯酚 | 臭氧投加量为0.75mg/min;初始污染物浓度为300mg/L;催化剂投加量为1g/L;反应pH为6.5;反应温度为20℃ | SO:D[苯酚]=38.9% CO:D[苯酚]=55.2% | [ |
| 硅酸铁掺杂羟基氧化铁 | 对氯硝基苯 | 初始污染物浓度为100μg/L;液相臭氧投加量为0.6mg/L;反应pH为7;催化剂投加量为0.5g/L | SO:[TOC]=28.1% CO:[TOC]=61.3% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| 废铁屑 | 印染废水 | 催化剂投加量为20g/L;反应pH为7.37;臭氧投加量为10.8g/L;气体流量为0.5L/min;反应时间为120min;初始COD浓度为142mg/L;初始TOC浓度为44mg/L;反应温度为20℃ | CO:[COD]=50%,[TOC]=50%SO:[COD]=26.8%,[TOC]=25% | [ |
| 铈改性磁硫铁矿烧渣 | 活性黑5 | 反应pH为5.5;初始污染物浓度为200mg/L;催化剂投加量为2.5g/L;臭氧投加量为5.6mg/L;反应时间为120min | SO:[TOC]≈40%CO:[TOC]=83.32% | [ |
| 铁屑 | 橄榄厂加工废水 | 反应温度为20℃;反应pH为3;反应时间为120min;臭氧投加量为20mg/L;催化剂投加量为1g/L | SO:[COD]≈30%CO:[COD]=65% | [ |
| 负载铁活性组分的稻壳灰 | 亚甲基蓝 | 初始污染物浓度为15mg/L;反应pH为3;催化剂投加量为1g/L;反应温度为30℃;臭氧投加量为0.5mg/min | SO:色度去除率为45% CO:色度去除率为98% | [ |
| 铁磁性污泥基活性炭 | 对氯苯甲酸(p-CBA) | 臭氧投加量为1mg/L;催化剂投加量为40mg/L;初始污染物浓度为20mg/L;反应pH为6;反应温度为20℃ | SO:D[p-CBA]=41% CO:D[p-CBA]=80% | [ |
| 铁泥 | 活性红24 | 气体流量为15mL/min;初始COD为100mg/L;臭氧投加量为3.04g/h;反应pH为11;催化剂投加量为1g/L;初始污染物浓度为100mg/L | SO:[COD]=58%CO:[COD]=67% | [ |
| 负载铁氧化物的粉煤灰 | 邻甲基苯酚(OMP) | 初始污染物浓度为0.2g/L;臭氧投加量为92.78mg/L;气体流量为4L/min;催化剂投加量为2g/L | SO:[COD]≈20%,D[OMP]≈60%CO:[COD]≈40%,D[OMP]≈90% | [ |
| 活性炭负载四氧化三铁的牛粪炭 | 煤气化废水 | 反应pH为7;催化剂投加量为3g/L;臭氧投加量为1.96mg/min;气体流量为0.4L/min;催化剂投加量为3g/L | SO:[COD]=55%CO:[COD]=74% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| 废铁屑 | 印染废水 | 催化剂投加量为20g/L;反应pH为7.37;臭氧投加量为10.8g/L;气体流量为0.5L/min;反应时间为120min;初始COD浓度为142mg/L;初始TOC浓度为44mg/L;反应温度为20℃ | CO:[COD]=50%,[TOC]=50%SO:[COD]=26.8%,[TOC]=25% | [ |
| 铈改性磁硫铁矿烧渣 | 活性黑5 | 反应pH为5.5;初始污染物浓度为200mg/L;催化剂投加量为2.5g/L;臭氧投加量为5.6mg/L;反应时间为120min | SO:[TOC]≈40%CO:[TOC]=83.32% | [ |
| 铁屑 | 橄榄厂加工废水 | 反应温度为20℃;反应pH为3;反应时间为120min;臭氧投加量为20mg/L;催化剂投加量为1g/L | SO:[COD]≈30%CO:[COD]=65% | [ |
| 负载铁活性组分的稻壳灰 | 亚甲基蓝 | 初始污染物浓度为15mg/L;反应pH为3;催化剂投加量为1g/L;反应温度为30℃;臭氧投加量为0.5mg/min | SO:色度去除率为45% CO:色度去除率为98% | [ |
| 铁磁性污泥基活性炭 | 对氯苯甲酸(p-CBA) | 臭氧投加量为1mg/L;催化剂投加量为40mg/L;初始污染物浓度为20mg/L;反应pH为6;反应温度为20℃ | SO:D[p-CBA]=41% CO:D[p-CBA]=80% | [ |
| 铁泥 | 活性红24 | 气体流量为15mL/min;初始COD为100mg/L;臭氧投加量为3.04g/h;反应pH为11;催化剂投加量为1g/L;初始污染物浓度为100mg/L | SO:[COD]=58%CO:[COD]=67% | [ |
| 负载铁氧化物的粉煤灰 | 邻甲基苯酚(OMP) | 初始污染物浓度为0.2g/L;臭氧投加量为92.78mg/L;气体流量为4L/min;催化剂投加量为2g/L | SO:[COD]≈20%,D[OMP]≈60%CO:[COD]≈40%,D[OMP]≈90% | [ |
| 活性炭负载四氧化三铁的牛粪炭 | 煤气化废水 | 反应pH为7;催化剂投加量为3g/L;臭氧投加量为1.96mg/min;气体流量为0.4L/min;催化剂投加量为3g/L | SO:[COD]=55%CO:[COD]=74% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| Fe/AC | 结晶紫 | 臭氧投加量为4.44mg/min;气体流量为0.3L/min;催化剂投加量为2.5g/L;反应pH为7;反应时间为90min;初始污染物浓度为400mg/L | SO:[COD]=41% CO:[COD]=57% | [ |
| Fe-Mn/AC | 苯酚 | 催化剂投加量为1g/L;反应pH为7;气体流量为3L/min;初始污染物浓度为0.2g/L;臭氧投加量为22mg/L | SO:[TOC]=31.8% CO:[TOC]=60.1% | [ |
| 负载四氧化三铁的碳纳米管 | 对羟基苯甲酸(HBA) | 初始污染物浓度为20mg/L;臭氧投加量为9mg/min;反应时间为10min;处理水量为1L | SO:D[HBA]≈80% CO:D[HBA]=100% | [ |
| 负载铁氧化物的活性炭 | 炼油废水 | 反应温度为20℃;反应pH为7.8;臭氧投加量为45L/h;催化剂投加量为5g/L;反应时间为30min;气体流量为5L/min | SO:[COD]=17% CO:[COD]=53.9% | [ |
| Fe2O3/AC | 草酸(OA) | 催化剂投加量为0.71g/L;初始污染物浓度为30mg/L;反应pH为3.3;臭氧投加量为50mg/h | SO:D[OA]=9.6% CO:D[OA]=86% | [ |
| Fe3O4/AC | 邻苯二甲酸二丁酯(DBP) | 反应pH为6;初始污染物浓度为2mg/L;臭氧投加量为0.15mg/L;反应时间为60min;催化剂投加量为10mg/L | SO:D[DBP]=38% CO:D[DBP]=63% | [ |
| Fe-Mn/AC | 甲基橙 | 初始污染物浓度为50mg/L;催化剂投加量为2.5g/L;反应pH为7;反应时间为25min;气体流量为2L/min;反应温度为20℃ | SO:[TOC]=17.6%[COD]=14% CO:[TOC]=39.6%[COD]=25.4% | [ |
| 负载四氧化三铁的碳纳米管 | 磺胺二甲基嘧啶类抗生素 | 初始污染物浓度为20mg/L;臭氧投加量为9mg/min;催化剂投加量为0.5g/L;反应pH为7 | SO:[TOC]=21.8% CO:[TOC]=27.2% | [ |
| Fe3O4/AC | 高盐石化废水 | 臭氧投加量为0.1g/h;反应时间为60min;催化剂投加量为0.3g/L;初始COD为362mg/L;反应温度为25℃ | SO:[COD]=15.6% CO:[COD]=45.1% | [ |
| Fe-Ni/AC | 2,4-二氯苯氧乙酸 | 反应时间为60min;臭氧投加量为50mg/h;催化剂投加量为0.5g/L;初始污染物浓度为10mg/L;反应温度为25℃;气体流量为1.2L/min | SO:[TOC]=34% CO:[TOC]=72% | [ |
| 负载四氧化三铁的碳纳米管 | 邻苯二甲酸二甲酯(DMP) | 气体流量为0.8L/min;初始污染物浓度为20mg/L;催化剂投加量为0.3mg/L;反应pH为6.8;臭氧投加量为4.8mg/min | SO:D[DMP]=70%[TOC]=15% CO:D[DMP]≈96%[TOC]=35% | [ |
| 负载零价铁的碳纳米管 | 亚甲基蓝 | 气体流量为5L/min;反应pH为9;臭氧投加量为5g/h;反应温度为20℃;初始污染物浓度为0.025mmol/L | SO:动力学常数=0.134min-1 CO:动力学常数=0.255min-1 | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| Fe/AC | 结晶紫 | 臭氧投加量为4.44mg/min;气体流量为0.3L/min;催化剂投加量为2.5g/L;反应pH为7;反应时间为90min;初始污染物浓度为400mg/L | SO:[COD]=41% CO:[COD]=57% | [ |
| Fe-Mn/AC | 苯酚 | 催化剂投加量为1g/L;反应pH为7;气体流量为3L/min;初始污染物浓度为0.2g/L;臭氧投加量为22mg/L | SO:[TOC]=31.8% CO:[TOC]=60.1% | [ |
| 负载四氧化三铁的碳纳米管 | 对羟基苯甲酸(HBA) | 初始污染物浓度为20mg/L;臭氧投加量为9mg/min;反应时间为10min;处理水量为1L | SO:D[HBA]≈80% CO:D[HBA]=100% | [ |
| 负载铁氧化物的活性炭 | 炼油废水 | 反应温度为20℃;反应pH为7.8;臭氧投加量为45L/h;催化剂投加量为5g/L;反应时间为30min;气体流量为5L/min | SO:[COD]=17% CO:[COD]=53.9% | [ |
| Fe2O3/AC | 草酸(OA) | 催化剂投加量为0.71g/L;初始污染物浓度为30mg/L;反应pH为3.3;臭氧投加量为50mg/h | SO:D[OA]=9.6% CO:D[OA]=86% | [ |
| Fe3O4/AC | 邻苯二甲酸二丁酯(DBP) | 反应pH为6;初始污染物浓度为2mg/L;臭氧投加量为0.15mg/L;反应时间为60min;催化剂投加量为10mg/L | SO:D[DBP]=38% CO:D[DBP]=63% | [ |
| Fe-Mn/AC | 甲基橙 | 初始污染物浓度为50mg/L;催化剂投加量为2.5g/L;反应pH为7;反应时间为25min;气体流量为2L/min;反应温度为20℃ | SO:[TOC]=17.6%[COD]=14% CO:[TOC]=39.6%[COD]=25.4% | [ |
| 负载四氧化三铁的碳纳米管 | 磺胺二甲基嘧啶类抗生素 | 初始污染物浓度为20mg/L;臭氧投加量为9mg/min;催化剂投加量为0.5g/L;反应pH为7 | SO:[TOC]=21.8% CO:[TOC]=27.2% | [ |
| Fe3O4/AC | 高盐石化废水 | 臭氧投加量为0.1g/h;反应时间为60min;催化剂投加量为0.3g/L;初始COD为362mg/L;反应温度为25℃ | SO:[COD]=15.6% CO:[COD]=45.1% | [ |
| Fe-Ni/AC | 2,4-二氯苯氧乙酸 | 反应时间为60min;臭氧投加量为50mg/h;催化剂投加量为0.5g/L;初始污染物浓度为10mg/L;反应温度为25℃;气体流量为1.2L/min | SO:[TOC]=34% CO:[TOC]=72% | [ |
| 负载四氧化三铁的碳纳米管 | 邻苯二甲酸二甲酯(DMP) | 气体流量为0.8L/min;初始污染物浓度为20mg/L;催化剂投加量为0.3mg/L;反应pH为6.8;臭氧投加量为4.8mg/min | SO:D[DMP]=70%[TOC]=15% CO:D[DMP]≈96%[TOC]=35% | [ |
| 负载零价铁的碳纳米管 | 亚甲基蓝 | 气体流量为5L/min;反应pH为9;臭氧投加量为5g/h;反应温度为20℃;初始污染物浓度为0.025mmol/L | SO:动力学常数=0.134min-1 CO:动力学常数=0.255min-1 | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| Fe2O3@Al2O3/SBA-15 | 布洛芬 | 初始污染物浓度为10mg/L;催化剂投加量为1.5g/L;臭氧投加量为30mg/L;反应温度为20℃;气体流量为0.2L/min;反应pH为7 | SO:[TOC]=26% CO:[TOC]=90% | [ |
| Fe-Cu/MCM-41 | 双氯芬酸 | 臭氧投加量为50mg/h;反应温度为20℃;反应pH为7;催化剂投加量为1g/L;初始污染物浓度为20mg/L | SO:[TOC]=42% CO:[TOC]=78% | [ |
| Fe/MCM-41 | 对氯苯甲酸 | 臭氧投加量为100mg/h;催化剂投加量为1g/L;初始污染物浓度为10mg/L;反应pH为4.3;反应温度为25℃;气体流量为1.2L/min | SO:[TOC]=62.2% CO:[TOC]=91.3% | [ |
| Fe/SBA-15 | 邻苯二甲酸二甲酯 | 臭氧投加量为50mg/h;反应温度为25℃;反应pH为5.7;催化剂投加量为0.28g/L;初始污染物浓度为10mg/L;气体流量为0.8L/min | SO:[TOC]=10.2% CO:[TOC]=34.8% | [ |
| 负载铁活性组分的沸石 | 腐殖酸 | 初始污染物浓度为30mg/L;反应pH为6.5;臭氧投加量为10mg/L;反应温度为24℃;反应时间为60min | SO:[TOC]≈20% CO:[TOC]=62% | [ |
| Fe/ZSM-5 | 环丙沙星 | 反应时间为15min;催化剂投加量为1g/L;初始污染物浓度为0.1g/L;臭氧投加量为30L/h;反应pH为3;反应温度为30℃ | SO:[TOC]=13% CO:[TOC]=42% | [ |
| 负载铁活性组分的4A型沸石 | 对氯苯甲酸(p-CBA)和草酸(OA) | 催化剂投加量为0.4g/L;初始p-CBA浓度为10mg/L;初始OA浓度为40mg/L;气体流量为3L/min;臭氧投加量为250mg/L;反应温度为25℃ | SO:D[OA]≈60% D[p-CBA]≈10% CO:D[OA]≈90% D[p-CBA]≈100% | [ |
| Fe/SBA-16 | 双氯芬酸 | 臭氧投加量为60mg/L;催化剂投加量为0.2g/L;初始污染物浓度为10mg/L;气体流量为1L/min;反应温度为25℃;处理水量为1L | SO:[TOC]=33.4% CO:[TOC]=79.3% | [ |
| Fe-Cu/MCM-41 | 草酸(OA) | 反应pH为6;处理水量为1.4L;初始污染物浓度为10mg/L;臭氧投加量为0.1g/h;气体流量为1L/min;反应温度为25℃ | SO:D[OA]=16.5% CO:D[OA]=95% | [ |
| Fe/MCM-48 | 双氯芬酸 | 臭氧投加量为0.1g/L;反应温度为25℃;催化剂投加量为0.15g/L;初始污染物浓度为15mg/L | SO:[TOC]≈25% CO:[TOC]=49.9% | [ |
| Fe/SBA-15 | 草酸 | 反应时间为60min;气体流量为1.2L/min;催化剂投加量为0.2g/L;臭氧投加量为0.1g/h;反应pH为3.7 | SO:动力学常数=10.9% CO:动力学常数=64.6% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| Fe2O3@Al2O3/SBA-15 | 布洛芬 | 初始污染物浓度为10mg/L;催化剂投加量为1.5g/L;臭氧投加量为30mg/L;反应温度为20℃;气体流量为0.2L/min;反应pH为7 | SO:[TOC]=26% CO:[TOC]=90% | [ |
| Fe-Cu/MCM-41 | 双氯芬酸 | 臭氧投加量为50mg/h;反应温度为20℃;反应pH为7;催化剂投加量为1g/L;初始污染物浓度为20mg/L | SO:[TOC]=42% CO:[TOC]=78% | [ |
| Fe/MCM-41 | 对氯苯甲酸 | 臭氧投加量为100mg/h;催化剂投加量为1g/L;初始污染物浓度为10mg/L;反应pH为4.3;反应温度为25℃;气体流量为1.2L/min | SO:[TOC]=62.2% CO:[TOC]=91.3% | [ |
| Fe/SBA-15 | 邻苯二甲酸二甲酯 | 臭氧投加量为50mg/h;反应温度为25℃;反应pH为5.7;催化剂投加量为0.28g/L;初始污染物浓度为10mg/L;气体流量为0.8L/min | SO:[TOC]=10.2% CO:[TOC]=34.8% | [ |
| 负载铁活性组分的沸石 | 腐殖酸 | 初始污染物浓度为30mg/L;反应pH为6.5;臭氧投加量为10mg/L;反应温度为24℃;反应时间为60min | SO:[TOC]≈20% CO:[TOC]=62% | [ |
| Fe/ZSM-5 | 环丙沙星 | 反应时间为15min;催化剂投加量为1g/L;初始污染物浓度为0.1g/L;臭氧投加量为30L/h;反应pH为3;反应温度为30℃ | SO:[TOC]=13% CO:[TOC]=42% | [ |
| 负载铁活性组分的4A型沸石 | 对氯苯甲酸(p-CBA)和草酸(OA) | 催化剂投加量为0.4g/L;初始p-CBA浓度为10mg/L;初始OA浓度为40mg/L;气体流量为3L/min;臭氧投加量为250mg/L;反应温度为25℃ | SO:D[OA]≈60% D[p-CBA]≈10% CO:D[OA]≈90% D[p-CBA]≈100% | [ |
| Fe/SBA-16 | 双氯芬酸 | 臭氧投加量为60mg/L;催化剂投加量为0.2g/L;初始污染物浓度为10mg/L;气体流量为1L/min;反应温度为25℃;处理水量为1L | SO:[TOC]=33.4% CO:[TOC]=79.3% | [ |
| Fe-Cu/MCM-41 | 草酸(OA) | 反应pH为6;处理水量为1.4L;初始污染物浓度为10mg/L;臭氧投加量为0.1g/h;气体流量为1L/min;反应温度为25℃ | SO:D[OA]=16.5% CO:D[OA]=95% | [ |
| Fe/MCM-48 | 双氯芬酸 | 臭氧投加量为0.1g/L;反应温度为25℃;催化剂投加量为0.15g/L;初始污染物浓度为15mg/L | SO:[TOC]≈25% CO:[TOC]=49.9% | [ |
| Fe/SBA-15 | 草酸 | 反应时间为60min;气体流量为1.2L/min;催化剂投加量为0.2g/L;臭氧投加量为0.1g/h;反应pH为3.7 | SO:动力学常数=10.9% CO:动力学常数=64.6% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| Mn-Fe-Mg-Ce/Al2O3 | 炼油废水 | 臭氧投加量为5mg/min;反应温度为30℃;反应时间为15min;初始TOC为35.24mg/L;气体流量为0.5L/min;催化剂投加量为5g/L | SO:[TOC]≈22% CO:[TOC]=45.6% | [ |
| Fe3O4–CoO/Al2O3 | 2-(2,4-二氯苯氧)丙酸(DP) | 初始污染物浓度为100mg/L;臭氧投加量为98.5mg/min;催化剂投加量为20g/L;反应温度为25℃;反应pH为3.3 | SO:[COD]=6.42%D[DP]=29.56% CO:[COD]=34.66% D[DP]=95.94% | [ |
| Fe/ZrO2 | 苯酚 | 臭氧投加量为1.2g/h;反应温度为25℃;催化剂投加量为0.5g/L;初始污染物浓度为100mg/L;气体流量为40L/h;反应时间为120min | SO:[TOC]≈27% CO:[TOC]≈62% | [ |
| Fe-Co/ZrO2 | 2-(2,4-二氯苯氧)丙酸 | 反应pH为7;催化剂投加量为2g/L;臭氧投加量为30mg/L;反应时间为20min;反应温度为20℃;初始污染物浓度为50mg/L | SO:[TOC]=19% CO:[TOC]=94% | [ |
| Fe3O4/Al2O3 | 2-(2,4-二氯苯氧)乙酸 | 初始污染物浓度为20mg/L;催化剂投加量为1g/L;臭氧投加量为30mg/L;反应时间为40min;反应pH为6 | SO:[TOC]=40% CO:[TOC]=95% | [ |
| 铁锰硅酸盐 | 磺胺甲𫫇唑 | 初始污染物浓度为25.3mg/L;催化剂投加量为1g/L;气体流量为0.4L/min;臭氧投加量为3.62mg/min;处理水量为0.5L | SO:[TOC]=27% CO:[TOC]=79.8% | [ |
| Mn-Fe-Cu/Al2O3 | 炼油废水 | 反应pH为8.2;臭氧投加量为2.19g/h;反应温度为60℃;反应时间为60min | SO:[COD]=34.4% CO:[COD]=67.1% | [ |
| FeO/Al2O3 | 对氯苯甲酸 (p-CBA) | 臭氧投加量为3mg/L;反应pH为2.5;初始污染物浓度为1.2mg/L;催化剂投加量为17.7g/L;反应时间为30min | SO:D[p-CBA]=10% CO:D[p-CBA]=100% | [ |
| 负载硅酸铁的浮石 | 双氯芬酸 | 臭氧投加量为5.52mg/L;反应温度为25℃;气体流量为1L/min;初始污染物浓度为29.6mg/L;催化剂投加量为0.8g/L | SO:[TOC]=32.3% CO:[TOC]=73.3% | [ |
| 负载铁活性组分的浮石 | 腐殖酸 | 初始污染物浓度为10mg/L;臭氧投加量为10mg/L;催化剂投加量为0.05g/L;反应温度为22℃;反应pH为6.72 | SO:[TOC]=24.87% CO:[TOC]=77.86% | [ |
| Fe/SiO2 | 磺胺二甲基嘧啶(SMT) | 初始污染物浓度为20mg/L;臭氧投加量为6mg/min;催化剂投加量为0.3g/L;反应pH为6.8;气体流量为0.4L/min;反应温度为25℃ | SO:D[SMT]=100%[TOC]=25% CO:D[SMT]=100%[TOC]=41% | [ |
| 铁锰组分改性的铝土矿 | 2,4,6-三氯苯酚(TCA) | 初始污染物浓度为28.2μg/L;臭氧投加量为0.62mg/L;催化剂投加量为0.5g/L;反应pH为6.5;反应时间为60min | SO:D[TCA]=55.8% CO:D[TCA]≈99% | [ |
| Fe-Cu/SiO2 | 水杨酸 | 反应时间为120min;反应温度为25℃;初始污染物浓度为20mg/L;气体流量为1mL/min;催化剂投加量为0.1g/L;臭氧投加量为4.6mg/L | SO:[TOC]=51% CO:[TOC]=88% | [ |
| β-FeOOH/NiO | 4-氯酚(4-CP) | 反应温度为20℃;初始污染物浓度为0.002mol/L;臭氧投加量为0.6mg/min;反应pH为7 | SO:D[4-CP]=47% CO:D[4-CP]=85% | [ |
| Cu x O-Fe3O4 | 邻苯二甲酸二甲酯 | 初始污染物浓度为50mg/L;反应pH为5.7;臭氧投加量为1.8mg/min;催化剂投加量为0.1g/L;反应时间为120min | SO:动力学常数=0.024min-1 CO:动力学常数=0.193min-1 | [ |
| β-FeOOH/Al2O3 | 医药活性物质 | 反应pH为7;催化剂投加量为1.5g/L;臭氧投加量为30mg/L | SO:[TOC]=20% CO:[TOC]=90% | [ |
| 催化剂 | 处理对象 | 反应条件 | 降解效率 | 参考文献 |
|---|---|---|---|---|
| Mn-Fe-Mg-Ce/Al2O3 | 炼油废水 | 臭氧投加量为5mg/min;反应温度为30℃;反应时间为15min;初始TOC为35.24mg/L;气体流量为0.5L/min;催化剂投加量为5g/L | SO:[TOC]≈22% CO:[TOC]=45.6% | [ |
| Fe3O4–CoO/Al2O3 | 2-(2,4-二氯苯氧)丙酸(DP) | 初始污染物浓度为100mg/L;臭氧投加量为98.5mg/min;催化剂投加量为20g/L;反应温度为25℃;反应pH为3.3 | SO:[COD]=6.42%D[DP]=29.56% CO:[COD]=34.66% D[DP]=95.94% | [ |
| Fe/ZrO2 | 苯酚 | 臭氧投加量为1.2g/h;反应温度为25℃;催化剂投加量为0.5g/L;初始污染物浓度为100mg/L;气体流量为40L/h;反应时间为120min | SO:[TOC]≈27% CO:[TOC]≈62% | [ |
| Fe-Co/ZrO2 | 2-(2,4-二氯苯氧)丙酸 | 反应pH为7;催化剂投加量为2g/L;臭氧投加量为30mg/L;反应时间为20min;反应温度为20℃;初始污染物浓度为50mg/L | SO:[TOC]=19% CO:[TOC]=94% | [ |
| Fe3O4/Al2O3 | 2-(2,4-二氯苯氧)乙酸 | 初始污染物浓度为20mg/L;催化剂投加量为1g/L;臭氧投加量为30mg/L;反应时间为40min;反应pH为6 | SO:[TOC]=40% CO:[TOC]=95% | [ |
| 铁锰硅酸盐 | 磺胺甲𫫇唑 | 初始污染物浓度为25.3mg/L;催化剂投加量为1g/L;气体流量为0.4L/min;臭氧投加量为3.62mg/min;处理水量为0.5L | SO:[TOC]=27% CO:[TOC]=79.8% | [ |
| Mn-Fe-Cu/Al2O3 | 炼油废水 | 反应pH为8.2;臭氧投加量为2.19g/h;反应温度为60℃;反应时间为60min | SO:[COD]=34.4% CO:[COD]=67.1% | [ |
| FeO/Al2O3 | 对氯苯甲酸 (p-CBA) | 臭氧投加量为3mg/L;反应pH为2.5;初始污染物浓度为1.2mg/L;催化剂投加量为17.7g/L;反应时间为30min | SO:D[p-CBA]=10% CO:D[p-CBA]=100% | [ |
| 负载硅酸铁的浮石 | 双氯芬酸 | 臭氧投加量为5.52mg/L;反应温度为25℃;气体流量为1L/min;初始污染物浓度为29.6mg/L;催化剂投加量为0.8g/L | SO:[TOC]=32.3% CO:[TOC]=73.3% | [ |
| 负载铁活性组分的浮石 | 腐殖酸 | 初始污染物浓度为10mg/L;臭氧投加量为10mg/L;催化剂投加量为0.05g/L;反应温度为22℃;反应pH为6.72 | SO:[TOC]=24.87% CO:[TOC]=77.86% | [ |
| Fe/SiO2 | 磺胺二甲基嘧啶(SMT) | 初始污染物浓度为20mg/L;臭氧投加量为6mg/min;催化剂投加量为0.3g/L;反应pH为6.8;气体流量为0.4L/min;反应温度为25℃ | SO:D[SMT]=100%[TOC]=25% CO:D[SMT]=100%[TOC]=41% | [ |
| 铁锰组分改性的铝土矿 | 2,4,6-三氯苯酚(TCA) | 初始污染物浓度为28.2μg/L;臭氧投加量为0.62mg/L;催化剂投加量为0.5g/L;反应pH为6.5;反应时间为60min | SO:D[TCA]=55.8% CO:D[TCA]≈99% | [ |
| Fe-Cu/SiO2 | 水杨酸 | 反应时间为120min;反应温度为25℃;初始污染物浓度为20mg/L;气体流量为1mL/min;催化剂投加量为0.1g/L;臭氧投加量为4.6mg/L | SO:[TOC]=51% CO:[TOC]=88% | [ |
| β-FeOOH/NiO | 4-氯酚(4-CP) | 反应温度为20℃;初始污染物浓度为0.002mol/L;臭氧投加量为0.6mg/min;反应pH为7 | SO:D[4-CP]=47% CO:D[4-CP]=85% | [ |
| Cu x O-Fe3O4 | 邻苯二甲酸二甲酯 | 初始污染物浓度为50mg/L;反应pH为5.7;臭氧投加量为1.8mg/min;催化剂投加量为0.1g/L;反应时间为120min | SO:动力学常数=0.024min-1 CO:动力学常数=0.193min-1 | [ |
| β-FeOOH/Al2O3 | 医药活性物质 | 反应pH为7;催化剂投加量为1.5g/L;臭氧投加量为30mg/L | SO:[TOC]=20% CO:[TOC]=90% | [ |
| 活性氧种类 | 淬灭剂 |
|---|---|
| 羟基自由基 | 叔丁醇 苯甲酸 对苯二甲酸 |
| 超氧自由基 | 超氧化物歧化酶 对硝基蓝四氯化唑 氯仿 |
| 单线氧 | 叠氮化钠(NaN3)、 4-氨基-2,2,6,6-四甲基丙烯(TEMP) |
| 过氧化氢 | 过氧化氢酶 对苯醌 |
| 活性氧种类 | 淬灭剂 |
|---|---|
| 羟基自由基 | 叔丁醇 苯甲酸 对苯二甲酸 |
| 超氧自由基 | 超氧化物歧化酶 对硝基蓝四氯化唑 氯仿 |
| 单线氧 | 叠氮化钠(NaN3)、 4-氨基-2,2,6,6-四甲基丙烯(TEMP) |
| 过氧化氢 | 过氧化氢酶 对苯醌 |
| 样品 | 表面羟基密度/mmol·g-1 | pH | 表面羟基密度/mmol·g-1 | |
|---|---|---|---|---|
| 20℃ | 100℃ | |||
| α-Fe2O3 | 57 | 7.2 | 0 | 0 |
| α-FeOOH | 330 | 8.6 | 795.4 | 106.7 |
| Fe3O4 | 116 | 6.8 | 307.6 | 0 |
| 样品 | 表面羟基密度/mmol·g-1 | pH | 表面羟基密度/mmol·g-1 | |
|---|---|---|---|---|
| 20℃ | 100℃ | |||
| α-Fe2O3 | 57 | 7.2 | 0 | 0 |
| α-FeOOH | 330 | 8.6 | 795.4 | 106.7 |
| Fe3O4 | 116 | 6.8 | 307.6 | 0 |
| 催化剂 | 比表面积/m2·g-1 | 平均孔径/nm | 孔容/cm3·g-1 | 表面羟基密度/mmol·g-1 | pH |
|---|---|---|---|---|---|
| Cl--FeOOH | 99.16 | 7.32 | 0.15 | 0.59 | 7.31 |
| SO42--FeOOH | 307.57 | 2.69 | 0.21 | 1.04 | 7.12 |
| NO3--FeOOH | 109.04 | 5.51 | 0.18 | 0.81 | 7.21 |
| 催化剂 | 比表面积/m2·g-1 | 平均孔径/nm | 孔容/cm3·g-1 | 表面羟基密度/mmol·g-1 | pH |
|---|---|---|---|---|---|
| Cl--FeOOH | 99.16 | 7.32 | 0.15 | 0.59 | 7.31 |
| SO42--FeOOH | 307.57 | 2.69 | 0.21 | 1.04 | 7.12 |
| NO3--FeOOH | 109.04 | 5.51 | 0.18 | 0.81 | 7.21 |
| 催化剂 | 表面羟基密度/mmol·g-1 | pH | 比表面积/m2·g-1 | 孔容/cm3·g-1 |
|---|---|---|---|---|
| α-FeOOH | 0.5 | 7.0 | 68.4 | 0.25 |
| β-FeOOH | 1.21 | 7.3 | 132.4 | 0.27 |
| γ-FeOOH | 0.74 | 6.6 | 100.3 | 0.63 |
| γ-AlOOH | 1.15 | 7.5 | 120.1 | 0.14 |
| 催化剂 | 表面羟基密度/mmol·g-1 | pH | 比表面积/m2·g-1 | 孔容/cm3·g-1 |
|---|---|---|---|---|
| α-FeOOH | 0.5 | 7.0 | 68.4 | 0.25 |
| β-FeOOH | 1.21 | 7.3 | 132.4 | 0.27 |
| γ-FeOOH | 0.74 | 6.6 | 100.3 | 0.63 |
| γ-AlOOH | 1.15 | 7.5 | 120.1 | 0.14 |
| 催化剂 | 总酸性位点/μmol·g-1 | 中性(强性)酸位点 /μmol·g-1 |
|---|---|---|
| Fe2O3/SBA-15 | 21.7 | 19.2 |
| Al2O3/SBA-15 | 246.2 | 184.3 |
| Fe2O3@Al2O3/SBA-15 | 264.3 | 193.1 |
| 催化剂 | 总酸性位点/μmol·g-1 | 中性(强性)酸位点 /μmol·g-1 |
|---|---|---|
| Fe2O3/SBA-15 | 21.7 | 19.2 |
| Al2O3/SBA-15 | 246.2 | 184.3 |
| Fe2O3@Al2O3/SBA-15 | 264.3 | 193.1 |
| [65] | AHMADI Mehdi, KAKAVANDI Babak, JAAFARZADEH Nemat, et al. Catalytic ozonation of high saline petrochemical wastewater using PAC@FeⅡFe2 ⅢO4: Optimization, mechanisms and biodegradability studies[J]. Separation and Purification Technology, 2017,177: 293-303. |
| [66] | Xianghong LYU, ZHANG Qiuyun, YANG Wenqing, et al. Catalytic ozonation of 2,4-dichlorophenoxyacetic acid over novel Fe-Ni/AC[J]. RSC Advances, 2015, 5(14): 10537-10545. |
| [67] | BAI Zhiyong, YANG Qi, WANG Jianlong. Catalytic ozonation of dimethyl phthalate using Fe3O4/multi-wall carbon nanotubes[J]. Environmental Technology, 2017, 38(16): 2048-2057. |
| [68] | ZHANG Shuo, WANG Dong, QUAN Xie, et al. Multi-walled carbon nanotubes immobilized on zero-valent iron plates (Fe0-CNTs) for catalytic ozonation of methylene blue as model compound in a bubbling reactor[J]. Separation and Purification Technology, 2013, 116: 351-359. |
| [69] | BING Jishuai, HU Chun, NIE Yulun, et al. Mechanism of catalytic ozonation in Fe₂O₃/Al₂O₃@SBA-15 aqueous suspension for destruction of ibuprofen [J]. Environmental Science & Technology, 2015, 49(3): 1690-1697. |
| [70] | CHEN Weirui, LI Xukai, TANG Yiming, et al. Mechanism insight of pollutant degradation and bromate inhibition by Fe-Cu-MCM-41 catalyzed ozonation[J]. Journal of Hazardous Materials, 2018, 346: 226-233. |
| [71] | LAN Bingyan, HUANG Ruihuan, LI Laisheng, et al. Catalytic ozonation of p-chlorobenzoic acid in aqueous solution using Fe-MCM-41 as catalyst[J]. Chemical Engineering Journal, 2013, 219: 346-354. |
| [72] | HUANG Ruihuan, YAN Huihua, LI Laisheng, et al. Catalytic activity of Fe/SBA-15 for ozonation of dimethyl phthalate in aqueous solution[J]. Applied Catalysis B: Environmental, 2011, 106(1/2): 264-271. |
| [73] | Dilek GÜMÜŞ, AKBAL Feryal. A comparative study of ozonation, iron coated zeolite catalyzed ozonation and granular activated carbon catalyzed ozonation of humic acid[J]. Chemosphere, 2017, 174: 218-231. |
| [74] | Katia GONZÁLEZ-LABRADA, RICHARD Romain, ANDRIANTSIFERANA Caroline, et al. Enhancement of ciprofloxacin degradation in aqueous system by heterogeneous catalytic ozonation[J]. Environmental Science and Pollution Research International, 2020, 27(2): 1246-1255. |
| [75] | LI Xue, WANG Dong, ZHANG Shuo. Evaluation of Fe-functionalized 4A zeolite as ozone catalyst for an enhancement of hydroxyl radical pathway in a multiphase reactor[J]. Ozone: Science & Engineering, 2019, 41(2): 156-165. |
| [76] | TONG Xinyuan, LI Zhidong, CHEN Weirui, et al. Efficient catalytic ozonation of diclofenac by three-dimensional iron (Fe)-doped SBA-16 mesoporous structures[J]. Journal of Colloid and Interface Science, 2020, 578: 461-470. |
| [77] | CHEN Weirui, LI Xukai, LIU Meiqi, et al. Effective catalytic ozonation for oxalic acid degradation with bimetallic Fe-Cu-MCM-41: Operation parameters and mechanism[J]. Journal of Chemical Technology & Biotechnology, 2017, 92(11): 2862-2869. |
| [78] | LI Xukai, CHEN Weirui, TANG Yiming, et al. Relationship between the structure of Fe-MCM-48 and its activity in catalytic ozonation for diclofenac mineralization[J]. Chemosphere, 2018, 206: 615-621. |
| [79] | YAN Huihua, CHEN Weirui, LIAO Gaozu, et al. Activity assessment of direct synthesized Fe-SBA-15 for catalytic ozonation of oxalic acid[J]. Separation and Purification Technology, 2016, 159: 1-6. |
| [80] | CHEN Chunmao, LI Yang, MA Wenfeng, et al. Mn-Fe-Mg-Ce loaded Al2O3 catalyzed ozonation for mineralization of refractory organic chemicals in petroleum refinery wastewater[J]. Separation and Purification Technology, 2017, 183: 1-10. |
| [81] | TONG Shao-Ping, SHI Rui, ZHANG Hua, et al. Kinetics of Fe3O4-CoO/Al2O3 catalytic ozonation of the herbicide 2-(2,4-dichlorophenoxy) propionic acid[J]. Journal of Hazardous Materials, 2011, 185(1): 162-167. |
| [82] | SABLE Shailesh S, SHAH Kinjal J, CHIANG Pen-Chi, et al. Catalytic oxidative degradation of phenol using iron oxide promoted sulfonated-ZrO2 by Advanced Oxidation Processes (AOPs)[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 91: 434-440. |
| [83] | NIE Yulun, XING Shengtao, HU Chun, et al. Efficient removal of toxic pollutants over Fe-Co/ZrO2 bimetallic catalyst with ozone[J]. Catalysis Letters, 2012, 142(8): 1026-1032. |
| [84] | YANG Zhendong, Aihua LYU, NIE Yulun, et al. Catalytic ozonation performance and surface property of supported Fe3O4 catalysts dispersions[J]. Frontiers of Environmental Science & Engineering, 2013, 7(3): 451-456. |
| [85] | GAO Guoying, KANG Jing, SHEN Jimin, et al. Heterogeneous catalytic ozonation of sulfamethoxazole in aqueous solution over composite iron-manganese silicate oxide[J]. Ozone: Science & Engineering, 2017, 39(1): 24-32. |
| [86] | CHEN Chunmao, YOZA Brandon A, WANG Yandan, et al. Catalytic ozonation of petroleum refinery wastewater utilizing Mn-Fe-Cu/Al2O3 catalyst[J]. Environmental Science and Pollution Research International, 2015,22(7): 5552-5562. |
| [87] | PARK Hosik, KIM Jun, JUNG Haeryong, et al. Iron oxide nanoparticle-impregnated alumina for catalytic ozonation of Para-chlorobenzoic acid in aqueous solution[J]. Water, Air, & Soil Pollution, 2014, 225(6): 1975. |
| [88] | GAO Guoying, CHU Wei, CHEN Zhonglin, et al. Catalytic ozonation of diclofenac with iron silicate-loaded pumice in aqueous solution[J]. Water Supply, 2017, 17(5): 1458-1467. |
| [1] | YANG Linyan, SHENG Mei, LI Yejin, et al. A hybrid process of Fe-based catalytic ozonation and biodegradation for the treatment of industrial wastewater reverse osmosis concentrate[J]. Chemosphere, 2020, 238: 124639. |
| [2] | WANG Fan, LUO Yuangfeng, RAN Gang, et al. Sequential coagulation and Fe0-O3/H2O2 process for removing recalcitrant organics from semi-aerobic aged refuse biofilter leachate: Treatment efficiency and degradation mechanism[J]. Science of the Total Environment, 2020, 699: 134371. |
| [3] | CHEN Keyong, ZHANG Xiaobing, LI Jun. Advanced treatment of oilfield production wastewater by an integration of coagulation/flotation, catalytic ozonation and biological processes[J]. Environmental Technology, 2016, 37(19): 2536-2544. |
| [4] | Qurat Ul AIN, RASHEED Usman, YASEEN Muhammad, et al. Superior dye degradation and adsorption capability of polydopamine modified Fe3O4-pillared bentonite composite[J]. Journal of Hazardous Materials, 2020, 397: 122758. |
| [5] | WAN Zhong, HU Jun, WANG Jianlong. Removal of sulfamethazine antibiotics using CeFe-graphene nanocomposite as catalyst by Fenton-like process[J]. Journal of Environmental Management, 2016,182: 284-291. |
| [6] | ZHU Hongyuan, GUO Zhenyu, YU Wei, et al. Illuminating for purity: Photocatalytic and photothermal membranes for sustainable oil-water separation[J]. Water Research, 2025, 272: 122919. |
| [7] | YANG Wenhua, DONG Hongyu, TIAN Yonglan, et al. Electrochemistry enhanced multi-stage biological contact oxidation treating sulfamethoxazole wastewater: Performance, degradation pathway and microbial community[J]. Separation and Purification Technology, 2025, 357: 130044. |
| [8] | ALORAINI Dalal A, ALMUQRIN Aljawhara H, SAYYED M I, et al. Analysis of potential waste materials of protection capacity against ionizing radiation using innovative methods[J]. Journal of Radiation Research and Applied Sciences, 2024,17(3): 101030. |
| [9] | YIN Baohe, LI Chenxi, TIAN Zhifu, et al. Effective catalytic ozonation of Orange G by supported Ce-Fe-Co oxides on Al2O3: Performance, mechanism and selective oxidation[J]. Journal of Water Process Engineering, 2025, 69: 106752. |
| [10] | TANG Min, WU Dong, NIE Yulun, et al. Efficiently catalytic ozonation of 2,4-dichlorophenoxyacetic acid by natural ferrihydrite: A pH dependent and surface-OH group involved reaction mechanism[J]. Environmental Research, 2025, 264: 120410. |
| [11] | ZHAN Shujuan, LI Yiqing, SUN Lianpeng, et al. Construction of solid frustrated-Lewis-pairs in porous nanoceria for boosting catalytic ozonation of refractory organic pollutants[J]. Separation and Purification Technology, 2025, 359: 130613. |
| [12] | CHENG Yizhen, CHEN Zhonglin, BI Jinhong, et al. Surface coordination species in heterogeneous catalytic ozonation for water purification[J]. Cell Reports Physical Science, 2024, 5(11): 102269. |
| [89] | ALVER Alper, Ahmet KıLıÇ. Catalytic ozonation by iron coated pumice for the degradation of natural organic matters[J]. Catalysts, 2018, 8(5): 219. |
| [90] | BAI Zhiyong, WANG Jianlong, YANG Qi. Iron doped fibrous-structured silica nanospheres as efficient catalyst for catalytic ozonation of sulfamethazine[J]. Environmental Science and Pollution Research International, 2018, 25(10): 10090-10101. |
| [91] | QI Fei, XU Bingbing, ZHAO Lun, et al. Comparison of the efficiency and mechanism of catalytic ozonation of 2,4,6-trichloroanisole by iron and manganese modified bauxite[J]. Applied Catalysis B: Environmental, 2012, 121: 171-181. |
| [92] | CHEN Weirui, BAO Yixiang, LI Xukai, et al. Mineralization of salicylic acid via catalytic ozonation with Fe-Cu@SiO2 core-shell catalyst: A two-stage first order reaction[J]. Chemosphere, 2019, 235: 470-480. |
| [93] | OPUTU Ogheneochuko, CHOWDHURY Mahabubur, NYAMAYARO Kudzanai, et al. A novel β-FeOOH/NiO composite material as a potential catalyst for catalytic ozonation degradation of 4-chlorophenol[J]. RSC Advances, 2015, 5(73): 59513-59521. |
| [94] | HE Hongping, LIU Ying, WU Deli, et al. Ozonation of dimethyl phthalate catalyzed by highly active Cu x O-Fe3O4 nanoparticles prepared with zero-valent iron as the innovative precursor[J]. Environmental Pollution, 2017, 227: 73-82. |
| [95] | YANG Li, HU Chun, NIE Yulun, et al. Surface acidity and reactivity of β-FeOOH/Al2O3 for pharmaceuticals degradation with ozone: In situ ATR-FTIR studies[J]. Applied Catalysis B: Environmental, 2010, 97(3/4): 340-346. |
| [96] | LIN Kun-Yi Andrew, LIN Tienyu, CHEN Yu-Chien, et al. Ferrocene as an efficient and recyclable heterogeneous catalyst for catalytic ozonation in water[J]. Catalysis Communications, 2017, 95: 40-45. |
| [97] | YU Deyou, WU Minghua, HU Qian, et al. Iron-based metal-organic frameworks as novel platforms for catalytic ozonation of organic pollutant: Efficiency and mechanism[J]. Journal of Hazardous Materials, 2019, 367: 456-464. |
| [98] | LI Ying, YEUNG King Lun. Polymeric catalytic membrane for ozone treatment of DEET in water[J]. Catalysis Today, 2019, 331: 53-59. |
| [99] | ZHU Hao, MA Wencheng, HAN Hongjun, et al. Degradation characteristics of two typical N-heterocycles in ozone process: Efficacy, kinetics, pathways, toxicity and its application to real biologically pretreated coal gasification wastewater[J]. Chemosphere, 2018, 209: 319-327. |
| [100] | LI Xufang, CHEN Weiyu, MA Luming, et al. Industrial wastewater advanced treatment via catalytic ozonation with an Fe-based catalyst[J]. Chemosphere, 2018, 195: 336-343. |
| [13] | MENG Hongying, CHANG Jing, WANG Shaopo, et al. Fabrication of MOF-carbonized materials as ozone catalysts for water purification: Exploration of catalytic mechanisms[J]. Journal of Environmental Chemical Engineering, 2024, 12(6): 114632. |
| [14] | WANG Jianlong, BAI Zhiyong. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater[J]. Chemical Engineering Journal, 2017, 312: 79-98. |
| [15] | SHARMA Virender K, ZBORIL Radek, VARMA Rajender S. Ferrates: Greener oxidants with multimodal action in water treatment technologies[J]. Accounts of Chemical Research, 2015, 48(2): 182-191. |
| [16] | JIA Ziyu, YANG Yuming, YANG Chunwei, et al. Magnetic γ-Fe2O3/ZnO@CNTs synthesized by a green precipitation method for the degradation of aniline through photocatalysis coupling catalytic ozonation[J]. Applied Surface Science, 2024, 659: 159866. |
| [17] | LIU Xinghao, ZHU Wenxiu, YANG Zhaoguang, et al. Efficient ozone catalysis by manganese iron oxides/activated carbon for sulfamerazine degradation[J]. Journal of Water Process Engineering, 2022, 49: 103050. |
| [18] | WANG Zhicheng, XU Tao, TANG Dingding, et al. Catalytic ozonation with γ-Al2O3 sphere supported highly dispersed iron species: Preparation, performance and catalytic mechanism[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 658: 130560. |
| [19] | SUN Yongjun, CHENG Yueqian, XIE Shuqian, et al. Catalytic ozonation of 2,4-dichlorophenoxyacetic acid wastewater by Fe-La@ZE catalyst[J]. Journal of Water Process Engineering, 2024, 61: 105362. |
| [20] | WANG Xiao, WU Shuhui, YAO Xue, et al. Catalytic ozone oxidation performance of Fe-Ce@γ-Al2O3 in reverse osmosis concentrate treatment[J]. Journal of Water Process Engineering, 2024, 68: 106449. |
| [21] | ZHANG Nianbo, ZHANG Baoyong, HE Ao, et al. Activation of ozone by CoFe-LDO-BC heterogeneous catalyst for efficient mineralization of methylene blue: The role of oxygen vacancies and acidic sites[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 110717. |
| [22] | REZVANI Bardia, NABAVI Seyed Reza, GHANI Milad. Magnetic nanohybrid derived from MIL-53(Fe) as an efficient catalyst for catalytic ozonation of cefixime and process optimization by optimal design[J]. Process Safety and Environmental Protection, 2023, 177: 1054-1071. |
| [23] | YAN Pengwei, SHEN Jimin, ZHOU Yanchi, et al. Interface mechanism of catalytic ozonation in an α-Fe0.9Mn0.1OOH aqueous suspension for the removal of iohexol[J]. Applied Catalysis B: Environmental, 2020, 277: 119055. |
| [24] | QIANG Dilong, JIN Hongyang, QING Qishun, et al. Iron-doped manganese oxide octahedral molecular sieve catalysts for ozone decomposition[J]. Journal of Environmental Chemical Engineering, 2024, 12(5): 113769. |
| [101] | GAO Guoying, KANG Jing, SHEN Jimin, et al. Investigation on the kinetics of heterogeneous catalytic ozone decomposition in aqueous solution over composite iron-manganese silicate oxide[J]. Ozone: Science & Engineering, 2016, 38(6): 434-442. |
| [102] | MALIK Sameena N, GHOSH Prakash C, VAIDYA Atul N, et al. Catalytic ozone pretreatment of complex textile effluent using Fe2+ and zero valent iron nanoparticles[J]. Journal of Hazardous Materials, 2018,357: 363-375. |
| [103] | MA Jieting, CHEN Yunlu, NIE Jianxin, et al. Pilot-scale study on catalytic ozonation of bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst[J]. Scientific Reports, 2018, 8(1): 7555. |
| [104] | YUAN Yuting, XING Guowei, GARG Shikha, et al. Mechanistic insights into the catalytic ozonation process using iron oxide-impregnated activated carbon[J]. Water Research, 2020,1 77: 115785. |
| [105] | WANG Da, XU Haodan, MA Jun, et al. Enhanced mineralization of atrazine by surface induced hydroxyl radicals over light-weight granular mixed-quartz sands with ozone[J]. Water Research, 2019, 149: 136-148. |
| [106] | ZHENG Tianlong, WANG Qunhui, ZHANG Tao, et al. Microbubble enhanced ozonation process for advanced treatment of wastewater produced in acrylic fiber manufacturing industry[J]. Journal of Hazardous Materials, 2015, 287: 412-420. |
| [107] | AZEVEDO A, OLIVEIRA H, RUBIO J. Bulk nanobubbles in the mineral and environmental areas: Updating research and applications[J]. Advances in Colloid and Interface Science, 2019, 271: 101992. |
| [108] | XIA Zhiran, HU Liming. Treatment of organics contaminated wastewater by ozone micro-nano-bubbles[J]. Water, 2019, 11(1): 55. |
| [109] | AZEVEDO A, ETCHEPARE R, CALGAROTO S, et al. Aqueous dispersions of nanobubbles: Generation, properties and features[J]. Minerals Engineering, 2016, 94: 29-37. |
| [110] | HUANG Xiaoxue, CHENG Wen, QUAN Xuejun, et al. Catalytic ozonation of biologically treated leachate from municipal solid waste in a microbubble reactor[J]. Ozone: Science & Engineering, 2019, 41(5): 415-426. |
| [111] | AGUILAR CORONADO Miguel, BABAZADEH Mohammad, REZAEI Ebrahim, et al. Complete oxidation of trans-4-pentylcyclohexanecarboxylic acid by catalytic ozonation using alumina supported bi-metallic copper-magnesium oxides[J]. Chemical Engineering Journal, 2024, 497: 154365. |
| [112] | LI Wenbin, SHAO Yuanyuan, ZHU Jesse. Anisotropic turbulent mass transfer model and its application to a gas-particle bubbling fluidized bed[J]. Industrial & Engineering Chemistry Research, 2018, 57(5): 1671-1678. |
| [25] | XIAO Siqi, MA Luming, AN Wenhui, et al. Stability optimization of iron-based catalysts: Application of Cr-enriched alloy steel catalyst in ozonation pharmaceutical wastewater[J]. Separation and Purification Technology, 2025, 354: 129007. |
| [26] | PENG Jiali, YAN Jianfei, CHEN Qixuan, et al. Natural mackinawite catalytic ozonation for N,N-dimethylacetamide (DMAC) degradation in aqueous solution: Kinetic, performance, biotoxicity and mechanism[J]. Chemosphere, 2018, 210: 831-842. |
| [27] | PELALAK Rasool, ALIZADEH Reza, GHARESHABANI Eslam. Enhanced heterogeneous catalytic ozonation of pharmaceutical pollutants using a novel nanostructure of iron-based mineral prepared via plasma technology: A comparative study[J]. Journal of Hazardous Materials, 2020, 392: 122269. |
| [28] | YUAN Lei, SHEN Jimin, YAN Pengwei, et al. Catalytic ozonation of 4-chloronitrobenzene by goethite and Fe2+-modified goethite with low defects: A comparative study[J]. Journal of Hazardous Materials, 2019, 365: 744-750. |
| [29] | LIU Yue, CHEN Zhonglin, DUAN Xuejun, et al. Mechanism of heterogeneous catalytic ozonation of p-chloronitrobenzene in aqueous solution with iron silicate dried at different temperatures[J]. Desalination and Water Treatment, 2016, 57(40): 19002-19009. |
| [30] | OPUTU Ogheneochuko, CHOWDHURY Mahabubur, NYAMAYARO Kudzanai, et al. Catalytic activities of ultra-small β-FeOOH nanorods in ozonation of 4-chlorophenol[J]. Journal of Environmental Sciences, 2015, 35: 83-90. |
| [31] | YUAN Lei, SHEN Jimin, YAN Pengwei, et al. Interface mechanisms of catalytic ozonation with amorphous iron silicate for removal of 4-chloronitrobenzene in aqueous solution[J]. Environmental Science & Technology, 2018, 52(3): 1429-1434. |
| [32] | MOUSSAVI Gholamreza, KHOSRAVI Rasoul, OMRAN Nematollah Rashidnejad. Development of an efficient catalyst from magnetite ore: Characterization and catalytic potential in the ozonation of water toxic contaminants[J]. Applied Catalysis A: General, 2012, 445: 42-49. |
| [33] | WANG Yu-Hsiang, CHEN Kuan-Chung. Removal of disinfection by-products from contaminated water using a synthetic goethite catalyst via catalytic ozonation and a biofiltration system[J]. International Journal of Environmental Research and Public Health, 2014, 11(9): 9325-9344. |
| [34] | SUI Minghao, SHENG Li, LU Kexiang, et al. FeOOH catalytic ozonation of oxalic acid and the effect of phosphate binding on its catalytic activity[J]. Applied Catalysis B: Environmental, 2010, 96(1/2): 94-100. |
| [35] | HUANG Yuanxing, JIANG Jiewen, MA Luming, et al. Iron foam combined ozonation for enhanced treatment of pharmaceutical wastewater[J]. Environmental Research, 2020, 183: 109205. |
| [36] | HUANG Yuanxing, LUO Mengyu, XU Zhihua, et al. Catalytic ozonation of organic contaminants in petrochemical wastewater with iron-nickel foam as catalyst[J]. Separation and Purification Technology, 2019, 211: 269-278. |
| [113] | WEI Chaohai, ZHANG Fengzhen, HU Yun, et al. Ozonation in water treatment: The generation, basic properties of ozone and its practical application[J]. Reviews in Chemical Engineering, 2017, 33(1): 49-89. |
| [114] | WANG He, LIN Xiaozi, HUANG Yuanxing, et al. Two advanced oxidation pathways of modified iron-shavings participation in ozonation[J]. Separation and Purification Technology, 2020, 244: 116838. |
| [115] | SU Li, FENG Jie, ZHOU Ximin, et al. Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles[J]. Analytical Chemistry, 2012, 84(13): 5753-5758. |
| [116] | ZHAO Hui, DONG Yuming, JIANG Pingping, et al. ZnAl2O4 as a novel high-surface-area ozonation catalyst: One-step green synthesis, catalytic performance and mechanism[J]. Chemical Engineering Journal, 2015, 260: 623-630. |
| [117] | YU Deyou, LI Lubiao, WU Minghua, et al. Enhanced photocatalytic ozonation of organic pollutants using an iron-based metal-organic framework[J]. Applied Catalysis B: Environmental, 2019, 251: 66-75. |
| [118] | XIONG Zhaokun, LAI Bo, YANG Ping, et al. Comparative study on the reactivity of Fe/Cu bimetallic particles and zero valent iron (ZVI) under different conditions of N2, air or without aeration[J]. Journal of Hazardous Materials, 2015, 297: 261-268. |
| [119] | LI Xufang, CHEN Weiyu, MA Luming, et al. Characteristics and mechanisms of catalytic ozonation with Fe-shaving-based catalyst in industrial wastewater advanced treatment[J]. Journal of Cleaner Production, 2019, 222: 174-181. |
| [120] | NAWROCKI Jacek, Barbara KASPRZYK-HORDERN. The efficiency and mechanisms of catalytic ozonation[J]. Applied Catalysis B: Environmental, 2010, 99(1/2): 27-42. |
| [121] | YUAN Run, QIN Yihe, HE Can, et al. Fe-Mn-Cu-Ce/Al2O3 as an efficient catalyst for catalytic ozonation of bio-treated coking wastewater: Characteristics, efficiency, and mechanism[J]. Arabian Journal of Chemistry, 2023, 16(1): 104415. |
| [122] | ZHOU Dandan, LI Shilong, CHAI Luyi, et al. Constructing fast mass-transfer channels with efficient catalytic ozonation activity in 2D manganese dioxide membranes by intercalating Fe/Mn bimetallic MOF[J]. Chinese Journal of Chemical Engineering, 2024, 74: 272-286. |
| [123] | YU Liwei, ZHANG Yue, LI Fengmin, et al. Efficient catalytic ozonation for hexazinone degradation by Fe/Ce-doped MOF derivatives[J]. Separation and Purification Technology, 2024, 344: 127277. |
| [124] | XU Anlin, FAN Siyan, MENG Tong, et al. Catalytic ozonation with biogenic Fe-Mn-Co oxides: Biosynthesis protocol and catalytic performance[J]. Applied Catalysis B: Environmental, 2022, 318: 121833. |
| [37] | WEN Chen, XU Xiaoyi, FAN Yunshuang, et al. Pretreatment of water-based seed coating wastewater by combined coagulation and sponge-iron-catalyzed ozonation technology[J]. Chemosphere, 2018,206: 238-247. |
| [38] | XIONG Zhaokun, LAI Bo, YUAN Yue, et al. Degradation of p-nitrophenol (PNP) in aqueous solution by a micro-size Fe0/O3 process (mFe0/O3): Optimization, kinetic, performance and mechanism[J]. Chemical Engineering Journal, 2016, 302: 137-145. |
| [39] | OLEA-MEJIA O, FERNÁNDEZ-MONDRAGÓN M, BARRERA-DÍAZ C, et al. Effect of the Fe nanoparticles generated by pulsed plasma in liquid in the catalyzed ozone removal of phenolphthalein[J]. International Journal of Photoenergy, 2017, 2017(1): 9314764. |
| [40] | BAI Zhiyong, WANG Jianlong, YANG Qi. Catalytic ozonation of dimethyl phthalate by Ce-substituted goethite[J]. International Journal of Environmental Science and Technology, 2017, 14(11): 2379-2388. |
| [41] | XU Zhenzhen, XIE Meiling, Yue BEN, et al. Efficiency and mechanism of atenolol decomposition in Co-FeOOH catalytic ozonation[J]. Journal of Hazardous Materials, 2019, 365: 146-154. |
| [42] | ZHANG Fengzhen, WEI Chaohai, HU Yun, et al. Zinc ferrite catalysts for ozonation of aqueous organic contaminants: Phenol and bio-treated coking wastewater[J]. Separation and Purification Technology, 2015,156: 625-635. |
| [43] | ZHAO Hui, DONG Yuming, WANG Guangli, et al. Novel magnetically separable nanomaterials for heterogeneous catalytic ozonation of phenol pollutant: NiFe2O4 and their performances[J]. Chemical Engineering Journal, 2013, 219: 295-302. |
| [44] | ZHANG Gucheng, ZHANG Jing, ZHANG Yongli, et al. Effect of the composition and surface functional groups of Fe-Ni bimetal oxides catalysts in catalytic ozonation process[J]. Journal of Water Supply: Research and Technology-Aqua, 2018, 67(2): 119-126. |
| [45] | GOUNDEN Asogan N, JONNALAGADDA Sreekanth B, SINGH Sooboo. Debromination of 2,4,6-Tribromophenol and bromate ion minimization in water by catalytic ozonation[J]. Journal of Water Process Engineering, 2019, 31: 100893. |
| [46] | HUANG Yuanxing, YANG Tingting, LIANG Manli, et al. Ni-Fe layered double hydroxides catalized ozonation of synthetic wastewater containing bisphenol A and municipal secondary effluent[J]. Chemosphere, 2019, 235: 143-152. |
| [47] | BAI Zhiyong, YANG Qi, WANG Jianlong. Catalytic ozonation of sulfamethazine antibiotics using Ce0.1Fe0.9OOH: Catalyst preparation and performance[J]. Chemosphere, 2016, 161: 174-180. |
| [48] | LIU Yue, WANG Shiyuan, GONG Weijin, et al. Heterogeneous catalytic ozonation of p-chloronitrobenzene (pCNB) in water with iron silicate doped hydroxylation iron as catalyst[J]. Catalysis Communications, 2017, 89: 81-85. |
| [49] | WU Jin, MA Luming, CHEN Yunlu, et al. Catalytic ozonation of organic pollutants from bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst: Removal and pathways[J]. Water Research, 2016, 92: 140-148. |
| [50] | WU Deli, LIU Ying, HE Hongping, et al. Magnetic pyrite cinder as an efficient heterogeneous ozonation catalyst and synergetic effect of deposited Ce[J]. Chemosphere, 2016, 155: 127-134. |
| [51] | MARTINS Rui C, RAMOS Carina M, QUINTA-FERREIRA Rosa M. Low-cost catalysts to enhance ozone action on the depuration of olive mill wastewaters[J]. Industrial & Engineering Chemistry Research, 2014, 53(40): 15357-15368. |
| [52] | IKHLAQ Amir, MUNIR Hafiz Muhammad Shahzad, KHAN Asifa, et al. Comparative study of catalytic ozonation and Fenton-like processes using iron-loaded rice husk ash as catalyst for the removal of methylene blue in wastewater[J]. Ozone: Science & Engineering, 2019, 41(3): 250-260. |
| [53] | LU Siying, LIU Yongze, FENG Li, et al. Characterization of ferromagnetic sludge-based activated carbon and its application in catalytic ozonation of p-chlorobenzoic acid[J]. Environmental Science and Pollution Research, 2018, 25(6): 5086-5094. |
| [54] | VAN Huu Tap, NGUYEN Lan Huong, HOANG Trung Kien, et al. Using FeO-constituted iron slag wastes as heterogeneous catalyst for Fenton and ozonation processes to degrade Reactive Red 24 from aqueous solution[J]. Separation and Purification Technology, 2019, 224: 431-442. |
| [55] | MENG Fanqing, MA Wei, ZONG Panpan, et al. Synthesis of a novel catalyst based on Fe(Ⅱ)/Fe(Ⅲ) oxide and high alumina coal fly ash for the degradation of o-methylphenol[J]. Journal of Cleaner Production, 2016, 133: 986-993. |
| [56] | HOU Sen, JIA Shengyong, JIA Jinjin, et al. Fe3O4 nanoparticles loading on cow dung based activated carbon as an efficient catalyst for catalytic microbubble ozonation of biologically pretreated coal gasification wastewater[J]. Journal of Environmental Management, 2020, 267: 110615. |
| [57] | WU Jie, GAO Hong, YAO Shuo, et al. Degradation of Crystal Violet by catalytic ozonation using Fe/activated carbon catalyst[J]. Separation and Purification Technology, 2015, 147: 179-185. |
| [58] | XIONG Wei, CHEN Nan, FENG Chuanping, et al. Ozonation catalyzed by iron- and/or manganese-supported granular activated carbons for the treatment of phenol[J]. Environmental Science and Pollution Research, 2019, 26(20): 21022-21033. |
| [59] | BAI Zhiyong, YANG Qi, WANG Jianlong. Fe3O4/multi-walled carbon nanotubes as an efficient catalyst for catalytic ozonation of p-hydroxybenzoic acid[J]. International Journal of Environmental Science and Technology, 2016, 13(2): 483-492. |
| [60] | CHEN Chunmao, CHEN Hongshuo, GUO Xuan, et al. Advanced ozone treatment of heavy oil refining wastewater by activated carbon supported iron oxide[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 2782-2791. |
| [125] | YAN Liqiang, BING Jishuai, WU Hecheng. The behavior of ozone on different iron oxides surface sites in water[J]. Scientific Reports, 2019, 9(1): 14752. |
| [126] | WANG Cheng, LI Aimin, SHUANG Chendong. The effect on ozone catalytic performance of prepared-FeOOH by different precursors[J]. Journal of Environmental Management, 2018, 228: 158-164. |
| [127] | ZHANG Tao, LI Chunjuan, MA Jun, et al. Surface hydroxyl groups of synthetic α-FeOOH in promoting OH generation from aqueous ozone: Property and activity relationship[J]. Applied Catalysis B: Environmental, 2008, 82(1/2): 131-137. |
| [128] | ZHANG Yanqing, LI Meng, ZHANG Qian. Silicon-modified ferric hydroxide for catalytic ozonation of nitrobenzene in aqueous solution[J]. Desalination and Water Treatment, 2015, 54(10): 2902-2908. |
| [129] | REN Yueming, CHEN Yu, ZENG Teng, et al. Influence of Bi-doping on Mn1- x Bi x Fe2O4 catalytic ozonation of di-n-butyl phthalate[J]. Chemical Engineering Journal, 2016, 283: 622-630. |
| [130] | LIU Dan, WANG Chunrong, SONG Yifan, et al. Effective mineralization of quinoline and bio-treated coking wastewater by catalytic ozonation using CuFe2O4/Sepiolite catalyst: Efficiency and mechanism[J]. Chemosphere, 2019, 227: 647-656. |
| [131] | CHENG Chen, LI Jianping, WEN Yuzhen, et al. Deactivation mechanism of Fe/Al2O3 catalyst during the ozonation of reverse osmosis concentrates (ROCs): Effect of silicate[J]. Chemical Engineering Journal Advances, 2020, 1: 100003. |
| [132] | TAN Xuefei, WANG Shengzhe, MA Lei, et al. Catalytic ozone oxidation of m-cresol by spirulina biochar-modified perovskite: Catalyst deactivation and in situ regeneration[J]. Separation and Purification Technology, 2024, 329: 124994. |
| [133] | KONG Xiangtong, GARG Shikha, CHEN Guifeng, et al. Investigation of the deactivation and regeneration of an Fe2O3/Al2O3·SiO2 catalyst used in catalytic ozonation of coal chemical industry wastewater[J]. Journal of Hazardous Materials, 2023, 451: 131194. |
| [61] | LI Xukai, CHEN Weirui, LI Laisheng. Catalytic ozonation of oxalic acid in the presence of Fe2O3-loaded activated carbon[J]. Ozone: Science & Engineering, 2018, 40(6): 448-456. |
| [62] | HUANG Yuanxing, CUI Chenchen, ZHANG Daofang, et al. Heterogeneous catalytic ozonation of dibutyl phthalate in aqueous solution in the presence of iron-loaded activated carbon[J]. Chemosphere, 2015, 119: 295-301. |
| [63] | TANG Shoufeng, YUAN Deling, ZHANG Qi, et al. Fe-Mn bi-metallic oxides loaded on granular activated carbon to enhance dye removal by catalytic ozonation[J]. Environmental Science and Pollution Research International, 2016, 23(18): 18800-18808. |
| [64] | BAI Zhiyong, YANG Qi, WANG Jianlong. Catalytic ozonation of sulfamethazine antibiotics using Fe3O4/multiwalled carbon nanotubes[J]. Environmental Progress & Sustainable Energy, 2018, 37(2): 678-685. |
| [1] | ZHAO Yulong, CAI Kai, YU Shanqing. Influence of pore structure of alumina on the adsorption, diffusion and reactivity of hydrocarbon molecules in catalytic cracking [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 213-221. |
| [2] | LI Junliang, LI Yue, SUN Daolai. Hydrodeoxygenation of 1,2-butanediol to 1-butanol over Cu/SiO2-Al2O3 catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 222-231. |
| [3] | LIU Chao, DING Chengao, WU Baoshun, LEI Xinyu, WANG Guangying, YU Zhengwei. Effect of TiO2 support particle size on the denitrification and water/sulfur poisoning resistance of RuO x -V2O5-WO3/TiO2 catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 232-242. |
| [4] | ZHANG Hanlin, YUE Xuehai, LIU Junxi, YIN Fengjun. Fabrication of high stability electrocatalyst for oxygen evolution reaction by Ru-Sr-Ir electrodeposition [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 243-251. |
| [5] | MA Yun, CUI Jiahao, DU Jie, ZHANG Fan, SHAN Qiaoli, NIU Ruize, BAI Haitao. Prospect of research on advanced oxidation processes for fracturing flowback fluids in oil and gas fields [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 441-450. |
| [6] | LIU Zhe, ZHOU Shunli, LI Yongxiang, ZHANG Chengxi, LIU Yipeng. Research progress on alkyl naphthalene synthesis catalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 144-158. |
| [7] | LIN Yijie, QIAO Peng, LI Xinrui, ZHANG Hongbin, WANG Xueqin. Construction and application of heterostructures of photocatalyst TiO2 nanomaterials [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 159-177. |
| [8] | WANG Tao, ZHANG Xuebing, ZHANG Qi, CHEN Qiang, ZHANG Kui, MEN Zhuowu. Effects of reduction-carburization temperature and inlet CO concentration on industrial precipitated iron-based catalyst for Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 178-184. |
| [9] | BAO Xinde, LIU Biye, HUANG Renwei, HONG Yuhao, GUAN Xin, LIN Jinguo. Preparation of biomass-based@CuNiOS composite catalysts for the reduction of organic dye [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 185-196. |
| [10] | ZHAO Siyang, LI Chenran, LIU Yang. Process optimization for regulating diene selectivity of MTO regenerated catalyst through pre-carbon deposition using C4 by-product [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 205-212. |
| [11] | SUN Mengyuan, LU Shijian, LIU Ling, XUE Yanyang, ZHANG Yunrong, DONG Qi, KANG Guojun. Research progress of MOF and their derivatives in carbon capture [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5339-5350. |
| [12] | WANG Wenjun, LIU Ruixin, WANG Jun, ZHANG Qinglei, HOU Li’an. Research progress of visible light degradation of indoor VOCs by titanium dioxide materials [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5351-5362. |
| [13] | CAO Jiangfei, LEI Xiaotong, HUANG Zhiyi, HUANG Jiankai, CHEN Fan, YANG Pianpian, XIE Chunsheng. Preparation of iron-nitrogen doped carbon microspheres and their activation for PS degradation of rhodamine B [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5406-5415. |
| [14] | ZENG Jin, GAO Yan, WANG Zhaopeng, XIE Yuyun, LIU Jun, LIANG Qi, WANG Chunying. Degradation mechanism of 2,4-dichlorophenoxyacetic acid by NaYF4:Yb,Tm composite TiO2/Bi2WO6 photocatalyst and evaluation of products toxicity [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5416-5431. |
| [15] | CHEN Zizhao, HE Fangshu, HU Qiang, YANG Yang, CHEN Hanping, YANG Haiping. Research progress on anti-carbon deposition Ni-based catalysts for dry reforming of methane [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4968-4978. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||