Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (10): 5771-5788.DOI: 10.16085/j.issn.1000-6613.2024-1299
• Materials science and technology • Previous Articles
Research progress and prospect of SF6 adsorption by metal-organic frame materials
YANG Yu( ), ZHAO Wenbo, XU Zhiyong(
), ZHAO Wenbo, XU Zhiyong( )
)
- College of Chemical Engineering, Kunming University of Science and Technology, Kunming 650000, Yunnan, China
-
Received:2024-08-07Revised:2024-10-07Online:2025-11-10Published:2025-10-25 -
Contact:XU Zhiyong
金属有机骨架材料吸附SF6的研究进展及展望
- 昆明理工大学化学工程学院,云南 昆明 650000
-
通讯作者:徐志勇 -
作者简介:杨宇(2000—),男,硕士研究生,研究方向为酸性和含氟电子特种气体的捕集。E-mail:919697671@qq.com。 -
基金资助:国家自然科学基金(22278198);国家自然科学基金(21068016)
CLC Number:
Cite this article
YANG Yu, ZHAO Wenbo, XU Zhiyong. Research progress and prospect of SF6 adsorption by metal-organic frame materials[J]. Chemical Industry and Engineering Progress, 2025, 44(10): 5771-5788.
杨宇, 赵文波, 徐志勇. 金属有机骨架材料吸附SF6的研究进展及展望[J]. 化工进展, 2025, 44(10): 5771-5788.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1299
| 吸附气体 | 全球变暖潜能值 | 动力学直径/Å | 标准熔点/℃ | 标准沸点/℃ | 极化率/cm3 | 四极矩/esu·cm2 |
|---|---|---|---|---|---|---|
| SF6 | 22800 | 5.13 | -64 | -50 | 6.54×10-24 | 0 |
| N2 | 0 | 3.64 | -198 | -210 | 1.74×10-24 | 1.52×10-26 |
| CO2 | 1 | 3.30 | -78 | — | 2.91×10-24 | 4.30×10-26 |
| 吸附气体 | 全球变暖潜能值 | 动力学直径/Å | 标准熔点/℃ | 标准沸点/℃ | 极化率/cm3 | 四极矩/esu·cm2 |
|---|---|---|---|---|---|---|
| SF6 | 22800 | 5.13 | -64 | -50 | 6.54×10-24 | 0 |
| N2 | 0 | 3.64 | -198 | -210 | 1.74×10-24 | 1.52×10-26 |
| CO2 | 1 | 3.30 | -78 | — | 2.91×10-24 | 4.30×10-26 |
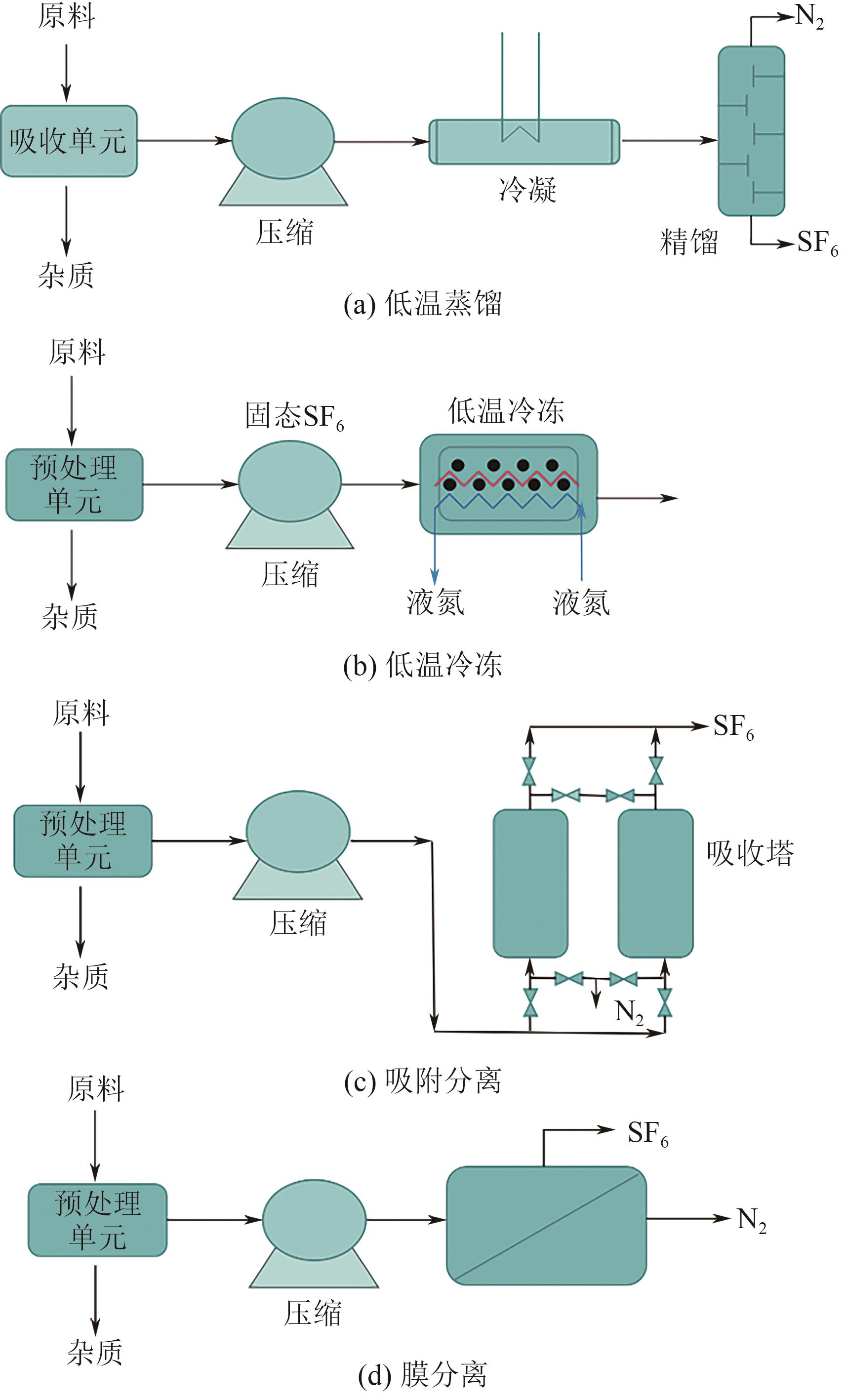
| 分离方法 | 工作原理 | 典型工况 | SF6回收率/% | 所用材料/溶剂 | 优点 | 缺点 | 已工业化的技术 | 文献 |
|---|---|---|---|---|---|---|---|---|
| 低温蒸馏技术 | SF6和N2之间的沸点差异 | 0.4MPa,-30℃ | 99.0 | 冷媒 | 可以达到较高的SF6纯度(99.999%,体积分数);通过热集成可以提高能源效率 | 投资和运营成本高,设备庞大,能耗高 | 由KEPCO开发的模型 | [ |
| 低温冷冻技术 | SF6和N2之间的熔点差异 | -196℃ | 99.0 | 液氮 | SF6冻结后融化成液相,其他杂质可以通过过滤器去除 | 要将SF6从气体转变为固体,需要采用非常特殊的低温 | 由ABB开发的模型 由KEPCO开发的模型 | [ |
| 膜分离技术 | 利用混合气体在通过膜时传递速率的不同 | 101325Pa或更高 | 90.0 | 聚合物膜、无机膜、混合基质膜 | 由于不涉及相变,具有较高的膜比表面积 | 进料中的杂质会降低分离效率 | 膜分离装置(Solvay Fluor and Derivate GmbH) | [ |
| 离子液体吸收技术 | 独特的阴阳离子与气体分子之间的相互作用 | — | 90.0 | 离子溶剂 | 高稳定性、溶解性能可调节、不易挥发、可再生 | 成本高、黏度高、制备工艺复杂 | 离子液体烟气脱硫 | [ |
| 吸附分离技术 | 通过吸附剂与气体混合物各组分之间的相互作用力 | 101325Pa或更高 | 80.0~90.0 | 沸石分子筛、金属有机骨架、活性炭 | 使用高比表面积的吸附剂可以吸附大量的SF6 | SF6吸附-解吸循环可能需要较高的吸附压力 | 沸石13X(Solvay Fluor and Derivate GmbH) | [ |
| 分离方法 | 工作原理 | 典型工况 | SF6回收率/% | 所用材料/溶剂 | 优点 | 缺点 | 已工业化的技术 | 文献 |
|---|---|---|---|---|---|---|---|---|
| 低温蒸馏技术 | SF6和N2之间的沸点差异 | 0.4MPa,-30℃ | 99.0 | 冷媒 | 可以达到较高的SF6纯度(99.999%,体积分数);通过热集成可以提高能源效率 | 投资和运营成本高,设备庞大,能耗高 | 由KEPCO开发的模型 | [ |
| 低温冷冻技术 | SF6和N2之间的熔点差异 | -196℃ | 99.0 | 液氮 | SF6冻结后融化成液相,其他杂质可以通过过滤器去除 | 要将SF6从气体转变为固体,需要采用非常特殊的低温 | 由ABB开发的模型 由KEPCO开发的模型 | [ |
| 膜分离技术 | 利用混合气体在通过膜时传递速率的不同 | 101325Pa或更高 | 90.0 | 聚合物膜、无机膜、混合基质膜 | 由于不涉及相变,具有较高的膜比表面积 | 进料中的杂质会降低分离效率 | 膜分离装置(Solvay Fluor and Derivate GmbH) | [ |
| 离子液体吸收技术 | 独特的阴阳离子与气体分子之间的相互作用 | — | 90.0 | 离子溶剂 | 高稳定性、溶解性能可调节、不易挥发、可再生 | 成本高、黏度高、制备工艺复杂 | 离子液体烟气脱硫 | [ |
| 吸附分离技术 | 通过吸附剂与气体混合物各组分之间的相互作用力 | 101325Pa或更高 | 80.0~90.0 | 沸石分子筛、金属有机骨架、活性炭 | 使用高比表面积的吸附剂可以吸附大量的SF6 | SF6吸附-解吸循环可能需要较高的吸附压力 | 沸石13X(Solvay Fluor and Derivate GmbH) | [ |
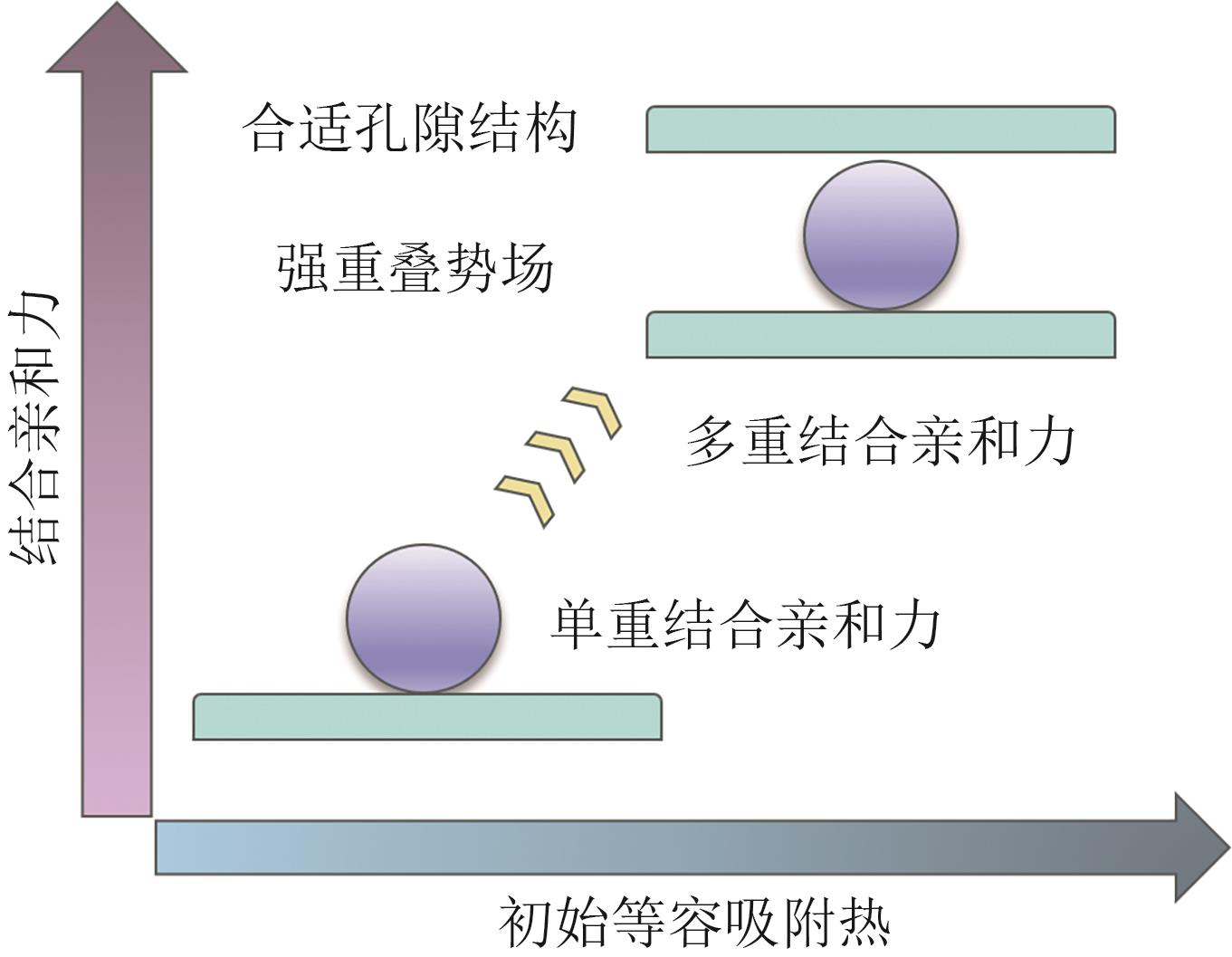
| 材料 | BET比表面积/m2·g-1 | IAST选择性SF6/N2(10∶90) | SF6吸附量(10kPa)/mmol·g-1 | SF6吸附量(100kPa)/mmol·g-1 | Qst/kJ·mol-1 | 参考文献 |
|---|---|---|---|---|---|---|
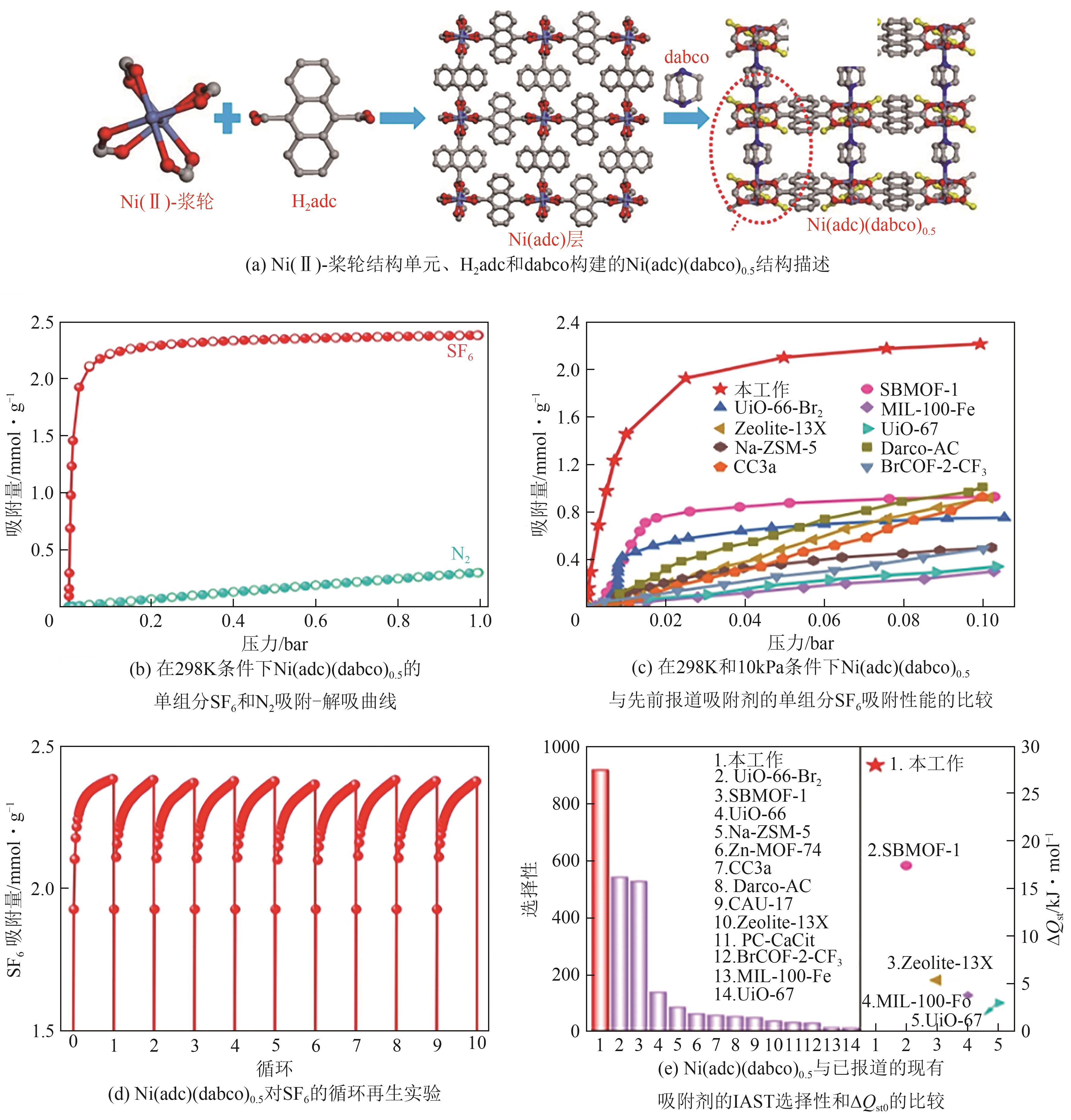
| Ni(adc)(dabco)0.5 | 743.9 | 948.2 | 2.23 | 2.38 | 47.6 | [ |
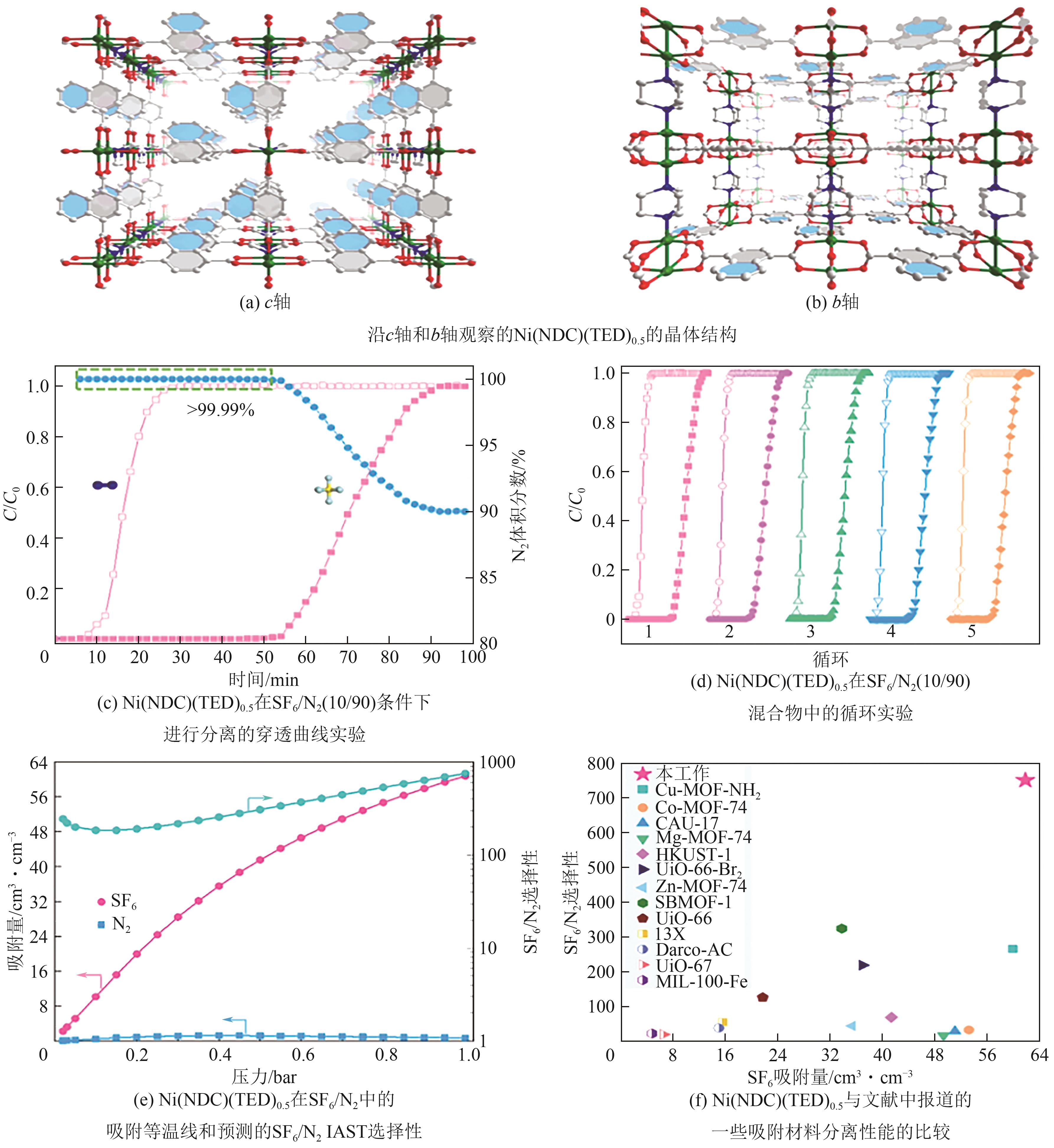
| Ni(NDC)(TED)0.5 | 1306.6 | 748 | 2.76 | 4.37 | 32.15 | [ |
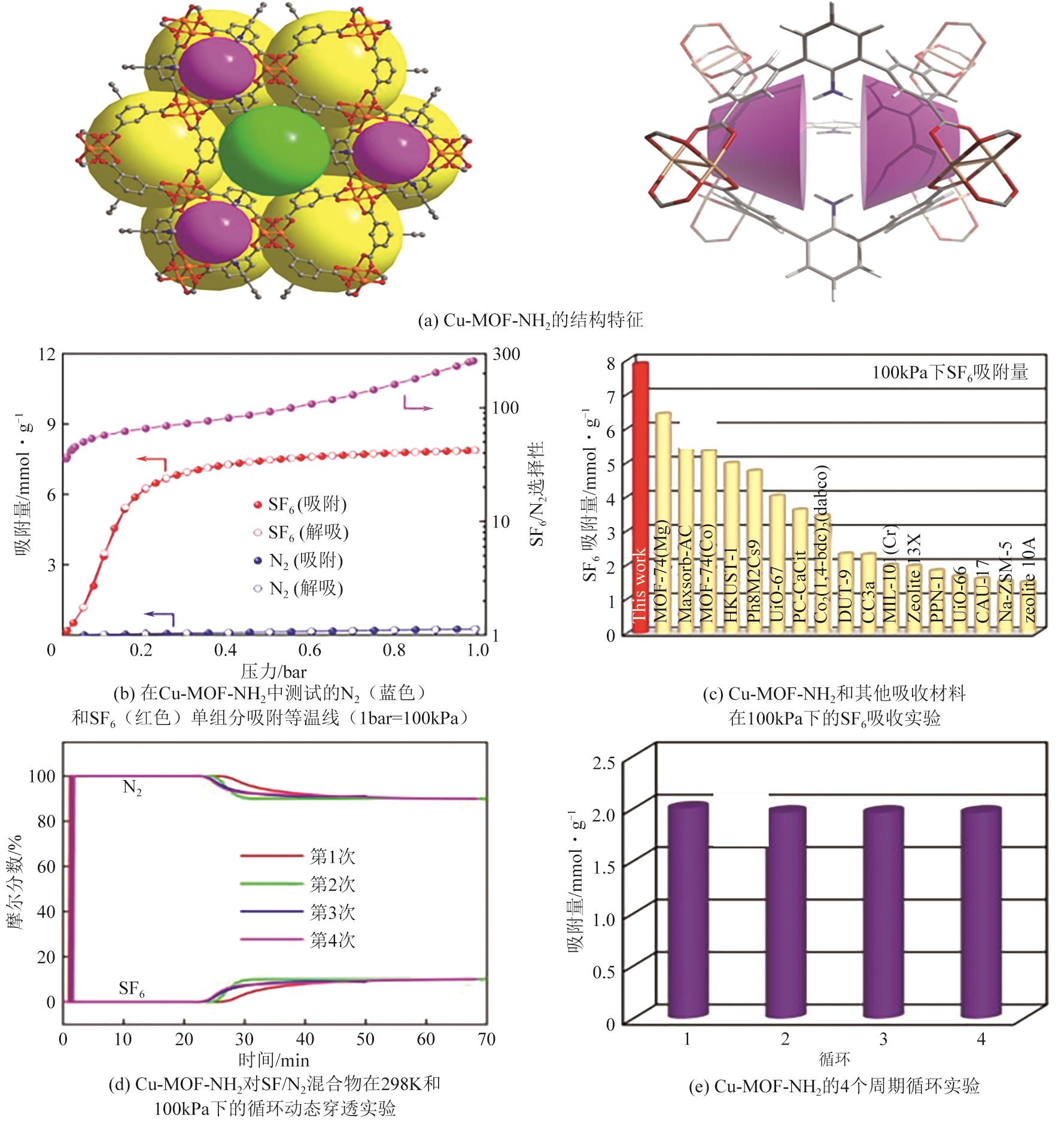
| Cu-MOF-NH2 | 2145 | 266 | 3.39 | 7.88 | 55.2 | [ |
| V-TCPB | 1107 | 360.7 | 2.29 | 3.07 | 30.08 | [ |
| GA-TCPB | 1144 | 418.5 | 2.26 | 2.95 | 30.44 | [ |
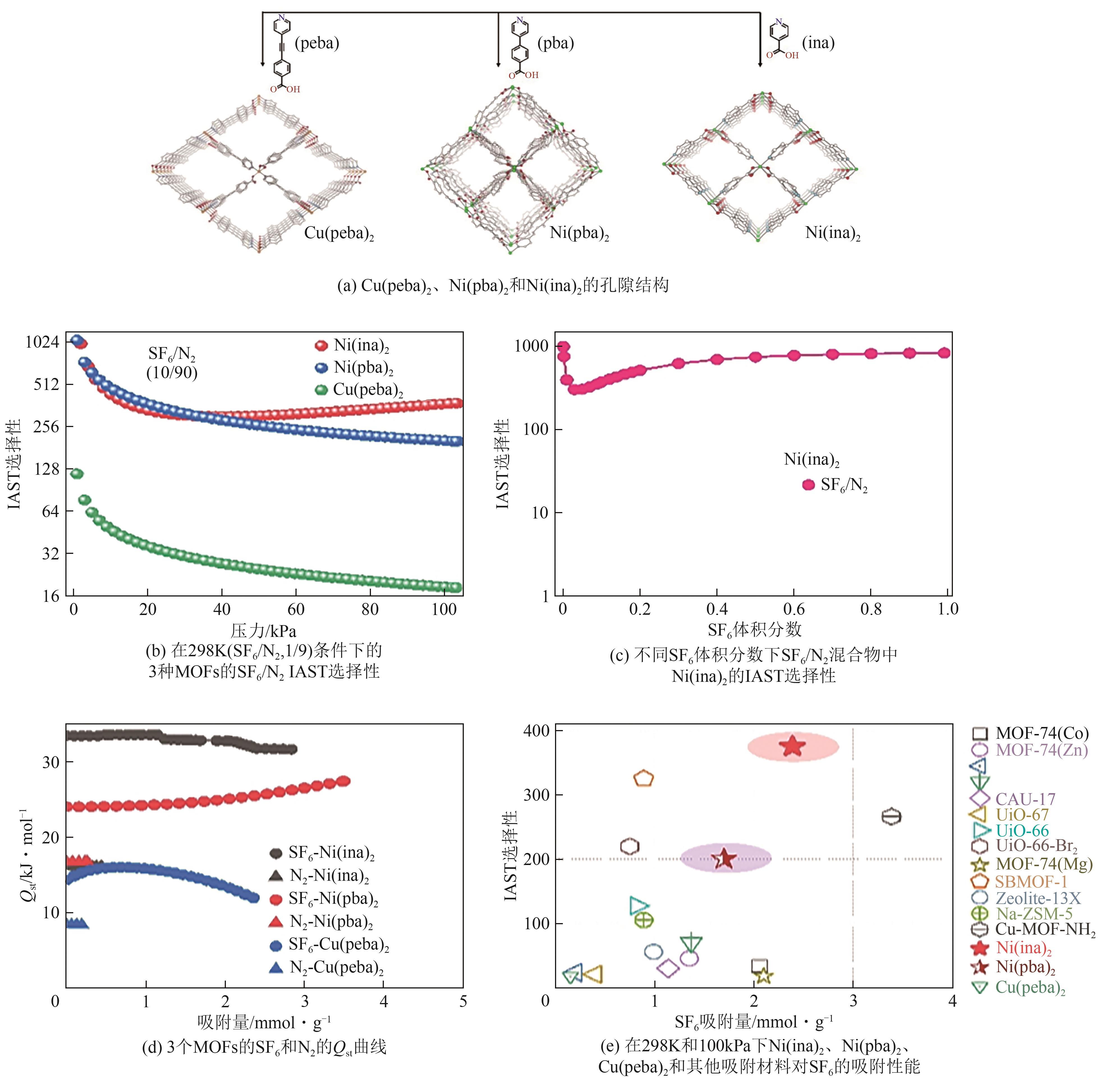
| Ni(ina)2 | 470 | 375.1 | 2.39 | 2.84 | 33.4 | [ |
| SBMOF-1 | 150 | 325.0 | 0.93 | 1.02 | 32.5 | [ |
| Ni(3-mpba)2 | 835.1 | 221 | — | 2.83 | 38.4 | [ |
| Ni(pba)2 | 807.2 | 156.5 | 1.69 | 3.47 | 38.2 | [ |
| CAU-10-H | 684.4 | 122.6 | 0.68 | 1.00 | 24.9 | [ |
| CAU-10-Py | 935.9 | 203.6 | 1.13 | 1.76 | 32.6 | [ |
| HBU-21 | 381.44 | 184.05 | 1.34 | — | 24.52 | [ |
| MIL-101 | — | 70 | — | 2.23 | — | [ |
| DUT-9 | — | 70 | — | 2.32 | — | [ |
| Mg-MOF-74 | 1631 | 19 | 1.34 | 6.42 | — | [ |
| Co-MOF-74 | 1219 | 37 | 1.32 | 5.34 | — | [ |
| Zn-MOF-74 | 992 | 45 | 1.02 | 3.76 | 42.3 | [ |
| HKUST-1a | 1090 | 37 | 0.86 | 3.46 | 57.4 | [ |
| HKUST-1b | 1135 | 49 | 1.32 | 4.12 | 34.5 | [ |
| HKUST-1c | 1328 | 70 | 1.50 | 4.98 | 30.2 | [ |
| CAU-17 | 530 | 31 | — | 1.61 | 27.6 | [ |
| SU-100 | 385 | 36 | — | 2.07 | 20.4 | [ |
| ZIF-7-8s | 1624 | 40 | — | 2.08 | — | [ |
| UiO-66-Br2 | 616 | 220 | 0.8 | 1.63 | 45 | [ |
| Zn(TMBDC)(DABCO)0.5 | 975.9 | 239 | 2.48 | 4.61 | 46 | [ |
| UiO-66-Br2@PS/DVB | 59 | — | — | 0.18 | — | [ |
| YTU-29-NH2 | 1269.5 | 36.7 | — | 4.26 | 28.9 | [ |
| Ni(3-min)(bdc)0.5 | 628 | 91 | — | 2.25 | 31.3 | [ |
| GNU-3a | 930.08 | 317.6 | — | 2.63 | 22.9 | [ |
| SNNU-204 | 2170 | 49 | — | 6 | 21.0 | [ |
| YTU-30 | 714.0 | 68 | — | 1.65 | 27 | [ |
| Sc-cage-MOF | 580 | 22.7 | — | 1.59 | 30.7 | [ |
| UU-200 | 115 | 44.81 | — | 1.19 | — | [ |
| BUT-53 | 866 | 2485 | — | 3.62 | 23.8 | [ |
| 材料 | BET比表面积/m2·g-1 | IAST选择性SF6/N2(10∶90) | SF6吸附量(10kPa)/mmol·g-1 | SF6吸附量(100kPa)/mmol·g-1 | Qst/kJ·mol-1 | 参考文献 |
|---|---|---|---|---|---|---|
| Ni(adc)(dabco)0.5 | 743.9 | 948.2 | 2.23 | 2.38 | 47.6 | [ |
| Ni(NDC)(TED)0.5 | 1306.6 | 748 | 2.76 | 4.37 | 32.15 | [ |
| Cu-MOF-NH2 | 2145 | 266 | 3.39 | 7.88 | 55.2 | [ |
| V-TCPB | 1107 | 360.7 | 2.29 | 3.07 | 30.08 | [ |
| GA-TCPB | 1144 | 418.5 | 2.26 | 2.95 | 30.44 | [ |
| Ni(ina)2 | 470 | 375.1 | 2.39 | 2.84 | 33.4 | [ |
| SBMOF-1 | 150 | 325.0 | 0.93 | 1.02 | 32.5 | [ |
| Ni(3-mpba)2 | 835.1 | 221 | — | 2.83 | 38.4 | [ |
| Ni(pba)2 | 807.2 | 156.5 | 1.69 | 3.47 | 38.2 | [ |
| CAU-10-H | 684.4 | 122.6 | 0.68 | 1.00 | 24.9 | [ |
| CAU-10-Py | 935.9 | 203.6 | 1.13 | 1.76 | 32.6 | [ |
| HBU-21 | 381.44 | 184.05 | 1.34 | — | 24.52 | [ |
| MIL-101 | — | 70 | — | 2.23 | — | [ |
| DUT-9 | — | 70 | — | 2.32 | — | [ |
| Mg-MOF-74 | 1631 | 19 | 1.34 | 6.42 | — | [ |
| Co-MOF-74 | 1219 | 37 | 1.32 | 5.34 | — | [ |
| Zn-MOF-74 | 992 | 45 | 1.02 | 3.76 | 42.3 | [ |
| HKUST-1a | 1090 | 37 | 0.86 | 3.46 | 57.4 | [ |
| HKUST-1b | 1135 | 49 | 1.32 | 4.12 | 34.5 | [ |
| HKUST-1c | 1328 | 70 | 1.50 | 4.98 | 30.2 | [ |
| CAU-17 | 530 | 31 | — | 1.61 | 27.6 | [ |
| SU-100 | 385 | 36 | — | 2.07 | 20.4 | [ |
| ZIF-7-8s | 1624 | 40 | — | 2.08 | — | [ |
| UiO-66-Br2 | 616 | 220 | 0.8 | 1.63 | 45 | [ |
| Zn(TMBDC)(DABCO)0.5 | 975.9 | 239 | 2.48 | 4.61 | 46 | [ |
| UiO-66-Br2@PS/DVB | 59 | — | — | 0.18 | — | [ |
| YTU-29-NH2 | 1269.5 | 36.7 | — | 4.26 | 28.9 | [ |
| Ni(3-min)(bdc)0.5 | 628 | 91 | — | 2.25 | 31.3 | [ |
| GNU-3a | 930.08 | 317.6 | — | 2.63 | 22.9 | [ |
| SNNU-204 | 2170 | 49 | — | 6 | 21.0 | [ |
| YTU-30 | 714.0 | 68 | — | 1.65 | 27 | [ |
| Sc-cage-MOF | 580 | 22.7 | — | 1.59 | 30.7 | [ |
| UU-200 | 115 | 44.81 | — | 1.19 | — | [ |
| BUT-53 | 866 | 2485 | — | 3.62 | 23.8 | [ |
| [1] | LEE How Ming, CHANG Moo Been, WU KUAN Yu. Abatement of sulfur hexafluoride emissions from the semiconductor manufacturing process by atmospheric-pressure plasmas[J]. Journal of the Air & Waste Management Association, 2004, 54(8): 960-970. |
| [2] | GUPTA Nishesh Kumar, VIKRANT Kumar, KIM Kwang Soo, et al. Regeneration strategies for metal-organic frameworks post acidic gas capture[J]. Coordination Chemistry Reviews, 2022, 467: 214629. |
| [3] | SEEGER Martin. Perspectives on research on high voltage gas circuit breakers[J]. Plasma Chemistry and Plasma Processing, 2015, 35(3): 527-541. |
| [4] | MAISS Manfred, BRENNINKMEIJER Carl A M. Atmospheric SF6: Trends, sources, and prospects[J]. Environmental Science and Technology, 1998, 32(20): 3077-3086. |
| [5] | FANG Xuekun, HU Xia, Greet JANSSENS-MAENHOUT, et al. Sulfur hexafluoride (SF6) emission estimates for China: An inventory for 1990—2010 and a projection to 2020[J]. Environmental Science & Technology, 2013, 47(8): 3848-3855. |
| [6] | HASELL Tom, MIKLITZ Marcin, STEPHENSON Andrew, et al. Porous organic cages for sulfur hexafluoride separation[J]. Journal of the American Chemical Society, 2016, 138(5): 1653-1659. |
| [7] | SKARMOUTSOS Ioannis, KOUKARAS Emmanuel N, GALIOTIS Costas, et al. Porous carbon nanotube networks and pillared graphene materials exhibiting high SF6 adsorption uptake and separation selectivity of SF6/N2 fluid mixtures: A comparative molecular simulation study[J]. Microporous and Mesoporous Materials, 2020, 307: 110464. |
| [8] | WANG Shaomin, MU Xuantong, LIU Haoran, et al. Pore-structure control in metal-organic frameworks (MOFs) for capture of the greenhouse gas SF6 with record separation[J]. Angewandte Chemie International Edition, 2022, 61(33): e202207066. |
| [9] | BEROUAL Abderrahmane, HADDAD Abderrahmane Manu. Recent advances in the quest for a new insulation gas with a low impact on the environment to replace sulfur hexafluoride (SF6) gas in high-voltage power network applications[J]. Energies, 2017, 10(8): 1216. |
| [10] | INAMI Kiyoshi, MAEDA Yasuhiro, HABUCHI Yoshitaka, et al. Problems of the application of N2/SF6 mixtures to gas-insulated bus[J]. Electrical Engineering in Japan, 2001, 137(4): 25-31. |
| [11] | FU Hongru, JIANG Yuying, LUO Jiahua, et al. A robust heterometallic Cd(Ⅱ)/Ba(Ⅱ)-organic framework with exposed amino group and active sites exhibiting excellent CO2/CH4 and C2H2/CH4 separation[J]. Chinese Journal of Structural Chemistry, 2022, 41(3): 2203287-2203292. |
| [12] | Michelle ÅHLÉN, AMOMBO NOA Francoise M, Lars ÖHRSTRÖM, et al. Pore size effect of 1,3,6,8-tetrakis(4-carboxyphenyl)pyrene-based metal-organic frameworks for enhanced SF6 adsorption with high selectivity[J]. Microporous and Mesoporous Materials, 2022, 343: 112161. |
| [13] | FURMANIAK Sylwester, TERZYK Artur P, GAUDEN Piotr A, et al. Simulation of SF6 adsorption on the bundles of single walled carbon nanotubes[J]. Microporous and Mesoporous Materials, 2012, 154: 51-55. |
| [14] | YAN Le, ZHENG Hui-Ting, SONG Liang, et al. Methyl-functionalized flexible ultra-microporous MOF for efficient SF6/N2 mixture separation[J]. Chemical Engineering Journal, 2023, 472: 145145. |
| [15] | YANG Shanqing, HU Tongliang, CHEN Banglin. Microporous metal-organic framework materials for efficient capture and separation of greenhouse gases[J]. Science China Chemistry, 2023, 66(8): 2181-2203. |
| [16] | ZHAO Yanlong, ZHANG Xin, LI Muzi, et al. Non-CO2 greenhouse gas separation using advanced porous materials[J]. Chemical Society Reviews, 2024, 53(4): 2056-2098. |
| [17] | LI Jianrong, KUPPLER Ryan J, ZHOU Hongcai. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
| [18] | SIRCAR Shivaji. Basic research needs for design of adsorptive gas separation processes[J]. Industrial & Engineering Chemistry Research, 2006, 45(16): 5435-5448. |
| [19] | BAKER Richard W. Membrane technology and applications[M]. Membrane Technology & Applications, 2004, 39(7): 85. |
| [20] | SHI Qi, WANG Jing, SHANG Hua, et al. Effective CH4 enrichment from N2 by SIM-1 via a strong adsorption potential SOD cage[J]. Separation and Purification Technology, 2020, 230: 115850. |
| [21] | MA Shengqian, SUN Daofeng, WANG Xisen, et al. A mesh-adjustable molecular sieve for general use in gas separation[J]. Angewandte Chemie International Edition, 2007, 46(14): 2458-2462. |
| [22] | LI Tong, JIA Xiaoxia, CHEN Hui, et al. Tuning the pore environment of MOFs toward efficient CH4/N2 separation under humid conditions[J]. ACS Applied Materials & Interfaces, 2022, 14(13): 15830-15839. |
| [23] | ISMAIL N H, SALLEH W N W, ISMAIL A F, et al. Hydrophilic polymer-based membrane for oily wastewater treatment: A review[J]. Separation and Purification Technology, 2020, 233: 116007. |
| [24] | BAKER Richard W, LOKHANDWALA Kaaeid. Natural gas processing with membranes: An overview[J]. Industrial & Engineering Chemistry Research, 2008, 47(7): 2109-2121. |
| [25] | REINE Travis A, Bruce ELDRIDGE R. Absorption equilibrium and kinetics for ethylene-ethane separation with a novel solvent[J]. Industrial & Engineering Chemistry Research, 2005, 44(19): 7505-7510. |
| [26] | MA Chen, WANG Xingjie, WANG Xun, et al. Novel glucose-based adsorbents (Glc-As) with preferential adsorption of ethane over ethylene and high capacity[J]. Chemical Engineering Science, 2017, 172: 612-621. |
| [27] | HAMEDI Homa, KARIMI Iftekhar A, GUNDERSEN Truls. Optimal cryogenic processes for nitrogen rejection from natural gas[J]. Computers & Chemical Engineering, 2018, 112: 101-111. |
| [28] | 李晓波, 胡保安, 顾平. 动态膜分离技术研究进展[J]. 膜科学与技术, 2007, 27(4): 91-95. |
| LI Xiaobo, HU Baoan, GU Ping. Research progress of dynamic membrane separation technology[J]. Science and technology of Membrane, 2007, 27(4): 91-95. | |
| [29] | LI Yuxiao, WANG Ting, LIU Dahuanet al. Fabrication of ultrathin membranes using 2D-MOF nanosheets for tunable gas separation[J]. Chemistry: An Asian Journal, 2021, 16(21): 3413-3418. |
| [30] | 林刚, 陈晓惠, 金石, 等. 气体膜分离原理、动态与展望[J]. 低温与特气, 2003, 21(2): 13-18. |
| LIN Gang, CHEN Xiaohui, JIN Shi, et al. Principle, dynamics and prospect of gas membrane separation[J]. Low Temperature and Specialty Gases, 2003, 21(2): 13-18. | |
| [31] | HAO Shuang, JIA Zhiqian, WEN Jianping, et al. Progress in adsorptive membranes for separation—A review[J]. Separation and Purification Technology, 2021, 255: 117772. |
| [32] | KIM Kyeongsook, KIM Kwang Sin, LEE Jeong Eun, et al. Status of SF6 separation/refining technology development for electric industry in Korea[J]. Separation and Purification Technology, 2018, 200: 29-35. |
| [33] | Sung-Hwan BAE, KIM Ja-Hee, Han-Seung LIM. A study on new power business model using power information technology[J]. Journal of Electrical Engineering & Technology, 2010, 5(3): 379-388. |
| [34] | XIONG Wenjie, SHI Mingzhen, ZHANG Xiaomin, et al. The efficient conversion of H2S into mercaptan alcohols mediated in protic ionic liquids under mild conditions[J]. Green Chemistry, 2021, 23(20): 7969-7975. |
| [35] | ZHANG Xiaomin, XIONG Wenjie, SHI Mingzhen, et al. Task-specific ionic liquids as absorbents and catalysts for efficient capture and conversion of H2S into value-added mercaptan acids[J]. Chemical Engineering Journal, 2021, 408: 127866. |
| [36] | SOSA Julio E, SANTIAGO Rubén, Daniel HOSPITAL-BENITO, et al. Process evaluation of fluorinated ionic liquids as F-gas absorbents[J]. Environmental Science & Technology, 2020, 54(19): 12784-12794. |
| [37] | JI Jialan, XIONG Wenjie, ZHANG Xiaomin, et al. Reversible absorption of NF3 with high solubility in Lewis acidic ionic liquids[J]. Chemical Engineering Journal, 2022, 440: 135902. |
| [38] | LIU Ping, CAI Kaixing, LIU Mao, et al. Deep eutectic solvents with multiple hydroxyl sites for efficient and reversible absorption of SF6 [J]. Journal of Molecular Liquids, 2022, 356: 119052. |
| [39] | CHIN Cynthia, KAMIN Zykamilia, BAHRUN Mohd Hardyianto Vai, et al. The production of industrial-grade oxygen from air by pressure swing adsorption[J]. International Journal of Chemical Engineering, 2023, 2023: 2308227. |
| [40] | CHUAH Chong Yang, LEE Yechan, Tae-Hyun BAE. Potential of adsorbents and membranes for SF6 capture and recovery: A review[J]. Chemical Engineering Journal, 2021, 404: 126577. |
| [41] | ROMERO M D, OVEJERO G, RODRÍGUEZ A, et al. Enhancement of the basic properties in FAU zeolites by impregnation with cesium hydroxide[J]. Microporous and Mesoporous Materials, 2005, 81(1/2/3): 313-320. |
| [42] | SUN Xin, KING Jonathan, ANTHONY Jennifer L. Molecular sieve synthesis in the presence of tetraalkylammonium and dialkylimidazolium molten salts[J]. Chemical Engineering Journal, 2009, 147(1): 2-5. |
| [43] | MATITO-MARTOS I, MARTIN-CALVO A, GUTIÉRREZ-SEVILLANO J J, et al. Zeolite screening for the separation of gas mixtures containing SO2, CO2 and CO[J]. Physical Chemistry Chemical Physics, 2014, 16(37): 19884-19893. |
| [44] | BERNAL M P, CORONAS J, MENÉNDEZ M, et al. Separation of CO2/N2 mixtures using MFI-type zeolite membranes[J]. AIChE Journal, 2004, 50(1): 127-135. |
| [45] | HIMENO Shuji, TOMITA Toshihiro, SUZUKI Kenji, et al. Synthesis and permeation properties of a DDR-type zeolite membrane for separation of CO2/CH4 gaseous mixtures[J]. Industrial & Engineering Chemistry Research, 2007, 46(21): 6989-6997. |
| [46] | GARCÍA-PÉREZ E, PARRA J B, ANIA C O, et al. A computational study of CO2, N2, and CH4 adsorption in zeolites[J]. Adsorption, 2007, 13(5): 469-476. |
| [47] | CHEN Sirui, SHEN Yuanhui, GUAN Zhongbo, et al. Adsorption properties of SF6 on zeolite NaY, 13X, activated carbon, and silica gel[J]. Journal of Chemical & Engineering Data, 2020, 65(8): 4044-4051. |
| [48] | MATITO-MARTOS I, ÁLVAREZ-OSSORIO J, GUTIÉRREZ-SEVILLANO J J, et al. Zeolites for the selective adsorption of sulfur hexafluoride[J]. Physical Chemistry Chemical Physics, 2015, 17(27): 18121-18130. |
| [49] | JAGIELLO Jacek, BANDOSZ Teresa J, PUTYERA Karol, et al. Adsorption near ambient temperatures of methane, carbon tetrafluoride, and sulfur hexafluoride on commercial activated carbons[J]. Journal of Chemical & Engineering Data, 1995, 40(6): 1288-1292. |
| [50] | SUN Rui, TAI Cheuk-Wai, Maria STRØMME, et al. Hierarchical porous carbon synthesized from novel porous amorphous calcium or magnesium citrate with enhanced SF6 uptake and SF6/N2 selectivity[J]. ACS Applied Nano Materials, 2019, 2(2): 778-789. |
| [51] | CHIANG Yuchun, WU Poyun. Adsorption equilibrium of sulfur hexafluoride on multi-walled carbon nanotubes[J]. Journal of Hazardous Materials, 2010, 178(1/2/3): 729-738. |
| [52] | KANG Dong Won, KANG Minjung, HONG Chang Seop, et al. Post-synthetic modification of porous materials: Superprotonic conductivities and membrane applications in fuel cells[J]. Journal of Materials Chemistry A, 2020, 8(16): 7474-7494. |
| [53] | LEE Daeyeon, LEE Sangho, Younghu SON, et al. Uncoordinated tetrazole ligands in metal-organic frameworks for proton-conductivity studies[J]. Bulletin of the Korean Chemical Society, 2022, 43(7): 912-917. |
| [54] | KANG Dong Won, Minki JUN, KIM Jun, et al. Double hypercrosslinked porous organic polymer-derived electrocatalysts for a water splitting device[J]. ACS Applied Energy Materials, 2022, 5(3): 3269-3274. |
| [55] | Seyoung KOO, KANG Dong Won. Emerging porous solids and sonochemistry[J]. Crystengcomm, 2023, 25(43): 5994-6005. |
| [56] | WANG Luyao, HUANG Hengcong, ZHANG Xiaoyu, et al. Designed metal-organic frameworks with potential for multi-component hydrocarbon separation[J]. Coordination Chemistry Reviews, 2023, 484: 215111. |
| [57] | SUN Zhiqiang, LIAO Yiren, ZHAO Shilin, et al. Research progress in metal-organic frameworks (MOFs) in CO2 capture from post-combustion coal-fired flue gas: Characteristics, preparation, modification and applications[J]. Journal of Materials Chemistry A, 2022, 10(10): 5174-5211. |
| [58] | YOON Jung Heum, LEE Woo Ram, LEE Jeong Tae, et al. Design and synthesis of novel lanthanide MOFs by unique in situ organic and inorganic reactions[J]. Bulletin of the Korean Chemical Society, 2022, 43(9): 1136-1140. |
| [59] | CHOI In-Hwan, GU Ja-Min, KIM Hyun-Chul, et al. Gas sorption properties of a new Zn-BTB metal-organic framework with permanent porosity[J]. Bulletin of the Korean Chemical Society, 2023, 44(9): 780-787. |
| [60] | SENKOVSKA Irena, BAREA Elisa, NAVARRO Jorge Andrés Rodríguez, et al. Adsorptive capturing and storing greenhouse gases such as sulfur hexafluoride and carbon tetrafluoride using metal-organic frameworks[J]. Microporous & Mesoporous Materials, 2012, 156: 115-120. |
| [61] | HORCAJADA Patricia, Suzy SURBLÉ, SERRE Christian, et al. Synthesis and catalytic properties of MIL-100(Fe), an iron(Ⅲ) carboxylate with large pores[J]. Chemical Communications, 2007(27): 2820-2822. |
| [62] | Jinhee BAE, PARK Sun Ho, MOON Dohyun, et al. Crystalline hydrogen bonding of water molecules confined in a metal-organic framework[J]. Communications Chemistry, 2022, 5: 51. |
| [63] | SONG Dahae, Jinhee BAE, JI Hoon, et al. Coordinative Reduction of metal nodes enhances the hydrolytic stability of a paddlewheel metal-organic framework[J]. Journal of the American Chemical Society, 2019, 141(19): 7853-7864. |
| [64] | CHUI Stephen S Y, Samuel M F LO, CHARMANT Jonathan P H, et al. A chemically functionalizable nanoporous material[Cu3(TMA)2(H2O)3] n [J]. Science, 1999, 283(5405): 1148-1150. |
| [65] | WANG Hepeng, Jürgen GETZSCHMANN, SENKOVSKA Irena, et al. Structural transformation and high pressure methane adsorption of Co2(1,4-bdc)2dabco[J]. Microporous and Mesoporous Materials, 2008, 116(1/2/3): 653-657. |
| [66] | KIM Hyojin, HONG Chang Seop. MOF-74-type frameworks: Tunable pore environment and functionality through metal and ligand modification[J]. CrystEngComm, 2021, 23(6): 1377-1387. |
| [67] | KIM Min-Bum, LEE Seung-Joon, LEE Chang Yeon, et al. High SF6 selectivities and capacities in isostructural metal-organic frameworks with proper pore sizes and highly dense unsaturated metal sites[J]. Microporous & Mesoporous Materials, 2014, 190: 356-361. |
| [68] | CHUAH Chong Yang, Kunli GOH, Tae-Hyun BAE. Hierarchically structured HKUST-1 nanocrystals for enhanced SF6 capture and recovery[J]. The Journal of Physical Chemistry C, 2017, 121(12): 6748-6755. |
| [69] | KIM Min-Bum, KIM Kyung-Min, Hoon KIM-Tea, et al. Highly selective adsorption of SF6 over N2 in a bromine-functionalized zirconium-based metal-organic framework[J]. Chemical Engineering Journal, 2018, 339: 223-229. |
| [70] | MIAO Xiaoyu, SUI Jincheng, WENG Sen, et al. Construction of hierarchical porous UiO-66-Br2@PS/DVB-packed columns by high internal phase emulsion strategy for enhanced separation of CF4/N2 and SF6/N2 [J]. ACS Applied Materials & Interfaces, 2024,16(18): 24083-24093. |
| [71] | REN Jiahao, CHANG Miao, ZENG Wenjiang, et al. Computer-aided discovery of MOFs with calixarene-analogous microenvironment for exceptional SF6 capture[J]. Chemistry of Materials, 2021, 33(13): 5108-5114. |
| [72] | LI Yongpeng, NI Jingjing, ZHANG Xiaojie, et al. Pore environmental modification by amino groups in robust microporous MOFs for SF6 capturing and SF6/N2 separation[J]. Inorganic Chemistry, 2024, 63(29): 13568-13575. |
| [73] | HU Yongqi, WANG Lingyao, Ruihan NAN, et al. Pore engineering in cost-effective and stable Al-MOFs for efficient capture of the greenhouse gas SF6 [J]. Chemical Engineering Journal, 2023, 471: 144851. |
| [74] | ZHENG Sutao, JIANG Runyuan, JIANG Yu, et al. Methyl-functionalized microporous metal-organic framework for efficient SF6/N2 separation[J]. Separation and Purification Technology, 2023, 318: 123957. |
| [75] | LIU Hao ran, WANG Shao min, DONG Yong li, et al. Control of pore environment in nickel-based metal-organic frameworks for SF6/N2 separation[J]. Chinese Journal of Structural Chemistry, 2023, 42(2): 100022. |
| [76] | CHANG Miao, WEI Yan, LIU Dahuan, et al. a general strategy for instantaneous and continuous synthesis of ultrasmall metal-organic framework nanoparticles[J]. Angewandte Chemie International Edition, 2021, 60(50): 26390-26396. |
| [77] | TOYODA M, MURASE H, IMAI T, et al. SF6 Reclaimer from SF6/N2 mixtures by gas separation with molecular sieving effect[J]. IEEE Power Engineering Review, 2002, 22(6): 61. |
| [78] | WANG Tongge, CHANG Miao, YAN Tongan, et al. Calcium-based metal-organic framework for efficient capture of sulfur hexafluoride at low concentrations[J]. Industrial & Engineering Chemistry Research, 2021, 60(16): 5976-5983. |
| [79] | CHANG Miao, YAN Tongan, WEI Yan, et al. Metal-organic framework-based single-molecule SF6 trap for record SF6 capture[J]. Chemistry of Materials, 2022, 34(20): 9134-9143. |
| [80] | YANG Mingshan, CHANG Miao, YAN Tongan, et al. A nickel-based metal-organic framework for efficient SF6/N2 separation with record SF6 uptake and SF6/N2 selectivity[J]. Separation and Purification Technology, 2022, 295: 121340. |
| [81] | LI Shi ming, ZHANG Qiang, JIANG Hong chan, et al. Constructing local nanomolecular trap in a scalable, low-cost, and ultramicroporous metal-organic framework for efficient capture of greenhouse gases SF6 and CO2 [J]. Chemical Engineering Journal, 2024, 496: 154026. |
| [82] | LI Yong peng, NI Jing jing, LI Shuo, et al. Rational pore-window size control in three Cu-MOFs with different pore environments for efficient capture of the greenhouse gas SF6 [J]. Journal of Solid State Chemistry, 2024, 329: 124443. |
| [83] | LI Yong peng, ZHANG Xiaojie, NI Jing jing, et al. Design of a highly-stable cobalt (Ⅱ) porous framework based on aromatic stacking strategy for efficient SF6 capture and SF6/N2 mixture separation[J]. Separation and Purification Technology, 2024, 343: 126995. |
| [84] | Milan KÖPPEN, DHAKSHINAMOORTHY Amarajothi, Ken INGE A, et al. Synthesis, transformation, catalysis, and gas sorption investigations on the bismuth metal-organic framework CAU-17[J]. European Journal of Inorganic Chemistry, 2018, 2018(30): 3496-3503. |
| [85] | GRAPE Erik, ROOTH Victoria, XU Hongyi, et al. Breathing metal-organic frameworks based on flexible inorganic building units[J]. Acta Crystallographica Section A: Foundations and Advances, 2019, 75(a2): e478. |
| [86] | WANG Hao, SHI Le, XIONG Zhangyi, et al. A two-dimensional metal-organic framework assembled from scandium-based cages for the selective capture of sulfur hexafluoride[J]. Chemical Communications, 2024, 60(17): 2397-2400. |
| [87] | Michelle ÅHLÉN, JAWORSKI Aleksander, Maria STRØMME, et al. Selective adsorption of CO2 and SF6 on mixed-linker ZIF-7-8s: The effect of linker substitution on uptake capacity and kinetics[J]. Chemical Engineering Journal, 2021, 422(21): 130117. |
| [88] | LIU Ping, ZHAO Tianxiang, CAI Kaixing, et al. Rapid mechanochemical construction of HKUST-1 with enhancing water stability by hybrid ligands assembly strategy for efficient adsorption of SF6 [J]. Chemical Engineering Journal, 2022, 437: 135364. |
| [89] | Jae-Hoon CHA, Seong-Bin GA, LEE Seung-Jun, et al. Integrated material and process evaluation of metal-organic frameworks database for energy-efficient SF6/N2 separation[J]. Chemical Engineering Journal, 2021, 426: 131787. |
| [90] | AMOMBO NOA Francoise M, CHEUNG Ocean, Michelle ÅHLÉN, et al. A hexagon based Mn(Ⅱ) rod metal-organic framework-structure, SF6 gas sorption, magnetism and electrochemistry[J]. Chemical Communications, 2023, 59(15): 2106-2109. |
| [91] | YAN Jiangwen, GANG Shuqi, LIU Ziyue,et al. An In(Ⅲ)-MOF based on pore engineering for efficient capture SF6 from SF6/N2 mixture[J]. Separation and Purification Technology, 2023, 327: 124929. |
| [92] | Michelle ÅHLÉN, ZHOU Yi, HEDBOM Daniel, et al. Efficient SF6 capture and separation in robust gallium-and vanadium-based metal-organic frameworks[J]. Journal of Materials Chemistry A, 2023, 11(48): 26435-26441. |
| [93] | Michelle ÅHLÉN, KAPACA Elina, HEDBOM Daniel, et al. Gas sorption properties and kinetics of porous bismuth-based metal-organic frameworks and the selective CO2 and SF6 sorption on a new bismuth trimesate-based structure UU-200[J]. Microporous and Mesoporous Materials, 2022, 329: 111548. |
| [94] | ZHANG Xin, ZHAO Yanlong, LI Xiangyu, et al. Recovery of high-purity SF6 from humid SF6/N2 mixture within a Co(Ⅱ)-Pyrazolate framework[J]. Journal of the American Chemical Society, 2024, 146(28): 19303-19309. |
| [95] | RIDDELL Imogen A, SMULDERS Maarten M J, CLEGG Jack K, et al. Encapsulation, storage and controlled release of sulfur hexafluoride from a metal-organic capsule[J]. Chemical Communications, 2011, 47(1): 457-459. |
| [96] | KITAO Takashi, ZHANG Yuanyuan, KITAGAWA Susumu, et al. Hybridization of MOFs and polymers[J]. Chemical Society Reviews, 2017, 46(11): 3108-3133. |
| [97] | TIAN Jian, THALLAPALLY Praveen K, Peter MCGRAIL B. Porous organic molecular materials[J]. CrystEngComm, 2012, 14(6): 1909-1919. |
| [98] | ZHANG Gang, MASTALERZ Michael. Organic cage compounds-from shape-persistency to function[J]. Chemical Society Reviews, 2014, 43(6): 1934-1947. |
| [99] | MASTALERZ Michael. Shape-persistent organic cage compounds by dynamic covalent bond formation[J]. Angewandte Chemie International Edition, 2010, 49(30): 5042-5053. |
| [100] | AVELLANEDA Antonio, VALENTE Peter, BURGUN Alexandre, et al. Kinetically controlled porosity in a robust organic cage material[J]. Angewandte Chemie International Edition, 2013, 52(13): 3746-3749. |
| [101] | HASELL Tom, MIKLITZ Marcin, STEPHENSON Andrew, et al. Porous organic cages for sulfur hexafluoride separation[J]. Journal of the American Chemical Society, 2016, 138(5): 1653-1659. |
| [102] | LI Xinle, YANG Chongqing, SUN Bing, et al. Expeditious synthesis of covalent organic frameworks: A review[J]. Journal of Materials Chemistry A, 2020, 8(32): 16045-16060. |
| [103] | LIANG Rongran, JIANG Shuyan, Ru-Han A, et al. Two-dimensional covalent organic frameworks with hierarchical porosity[J]. Chemical Society Reviews, 2020, 49(12): 3920-3951. |
| [104] | SUN Tao, XIE Jian, GUO Wei, et al. Covalent-organic frameworks: Advanced organic electrode materials for rechargeable batteries[J]. Advanced Energy Materials, 2020, 10(19): 1904199. |
| [105] | FURUKAWA Hiroyasu, YAGHI Omar M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications[J]. Journal of the American Chemical Society, 2009, 131(25): 8875-8883. |
| [106] | ZHENG Xianqiang, SHEN Yanlong, WANG Shitao, et al. Selective adsorption of SF6 in covalent- and metal-organic frameworks[J]. Chinese Journal of Chemical Engineering, 2021, 39: 88-95. |
| [107] | WANG Shanshan, WU Yue, ZHANG Ying, et al. HF resistant porous aromatic frameworks for electronic special gases separation[J]. Langmuir, 2022, 38(28): 8667-8676. |
| [1] | YANG Zhenglu, YANG Lifeng, LU Xiaofei, SUO Xian, ZHANG Anyun, CUI Xili, XING Huabin. Advances in machine learning accelerating the screening and discovery of porous adsorbents [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4288-4301. |
| [2] | LIANG Shuwei, YU Jie, XIE Zhongyin, PEI Jianlu, LIN Zhongxin, CHEN Zexiang. Covalent organic frameworks for radioactive gaseous iodine adsorption [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3965-3975. |
| [3] | LI Peiyi, SUN Bolong, LIU Ruiyan, ZHOU Xinyao, LIU Ruilin, HU Yuanyuan, XU Gongtao, LI Xinping. Preparation of sodium alginate/titanium dioxide composite porous material and its application in oil-water separation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3053-3061. |
| [4] | HAN Pei, LI Jinjian, KE Tian, ZHANG Zhiguo, BAO Zongbi, REN Qilong, YANG Qiwei. Advances in adsorption separation of sulfur hexafluoride/nitrogen by novel porous materials [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3592-3617. |
| [5] | GENG Xiumei, ZHANG Feng, ZHANG Xiang, SHAN Meixia, ZHANG Yatao. Research progress on the stability of Pebax-based mixed matrix membranes for CO2 separation [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4996-5012. |
| [6] | SHI Jiabo, ZHANG Yuxuan, CHEN Xuefeng, TAN Jiaojun. Preparation and oil-water separation property of tannic acid-nanoclay synergistically modified collagen fiber-based porous materials [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4624-4629. |
| [7] | WANG Lina, WU Jinsheng. Research progress of synthesis and application of covalent organic frameworks [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3834-3856. |
| [8] | FENG Bangman, YUE Chengguang, WANG Mei-Yan, WANG Yue, MA Xinbin. Fabrication of heterogeneous tannic acid-zirconium mesoporous material and the catalytic performance on cycloaddition of CO2 with epoxide [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2803-2810. |
| [9] | LU Guangjun, HAN Jingang, CHEN Ying, MA Zhibin. Preparation of magnesium slag-based porous materials and their performance for Pb2+ adsorption in wastewater [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2126-2134. |
| [10] | JIN Binhao, ZHU Xiaoqian, KE Tian, ZHANG Zhiguo, BAO Zongbi, REN Qilong, SU Baogen, YANG Qiwei. Advances in adsorbents for aromatics/cycloalkanes separation [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1863-1881. |
| [11] | HE Lan, GAO Zhuwei, QI Xinyu, LI Chengxin, WANG Shihao, LIU Zhongxin. Research progress in hydrophobic modification of melamine sponge and its application in oil-water separation field [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 984-1000. |
| [12] | WEN Zhipeng, KE Siyin, YANG Huilin, LI Yong, YU Chuanming, LIAO Mingneng. Superhydrophobic porous foams constructed based on the high internal phase emulsion template method [J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5633-5641. |
| [13] | CHEN Le, CHONG Hailing, ZHANG Zhihui, HE Mingyang, CHEN Qun. Synthesis of Cu-BTC modified by CTAB and its adsorption and separation of xylene isomers [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 455-464. |
| [14] | ZHANG Jie, WANG Fangfang, XIA Zhonglin, ZHAO Guangjin, MA Shuangchen. Current SF6 emission, emission reduction and future prospects under “carbon peaking and carbon neutrality” [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 447-460. |
| [15] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||