Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (10): 5837-5856.DOI: 10.16085/j.issn.1000-6613.2024-0795
• Resources and environmental engineering • Previous Articles
A review on the adsorption of organic pollutants by covalent organic frameworks (COFs) from environmental remediation
HOU Linli1( ), ZHANG Mengling1, LANG Fengxiang2, ZHENG Xiyi1, LIU Limin1(
), ZHANG Mengling1, LANG Fengxiang2, ZHENG Xiyi1, LIU Limin1( )
)
- 1.College of Chemistry and Chemical Engineering, Jinggangshan University, Ji’an 343000, Jiangxi, China
2.Hydrology and Water Resources Monitoring Center, Middle Reaches of Ganjiang River, Ji’an 343100, Jiangxi, China
-
Received:2024-05-13Revised:2024-06-13Online:2024-10-29Published:2024-10-15 -
Contact:LIU Limin
共价有机框架材料在环境修复领域中吸附有机污染物的研究进展
候林丽1( ), 张梦玲1, 郎锋祥2, 郑希怡1, 刘利民1(
), 张梦玲1, 郎锋祥2, 郑希怡1, 刘利民1( )
)
- 1.井冈山大学化学化工学院,江西 吉安 343000
2.赣江中游水文水资源监测中心,江西 吉安 343100
-
通讯作者:刘利民 -
作者简介:候林丽(1984—),女,博士研究生,研究方向为传感吸附。E-mail:2832300996@qq.com。 -
基金资助:国家自然科学基金(22266016);江西省自然科学基金(20232BAB203018);江西省教育厅科技项目重点项目(GJJ2201601);吉安市科技局项目(吉市科计字〔2022〕6号9)
CLC Number:
Cite this article
HOU Linli, ZHANG Mengling, LANG Fengxiang, ZHENG Xiyi, LIU Limin. A review on the adsorption of organic pollutants by covalent organic frameworks (COFs) from environmental remediation[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5837-5856.
候林丽, 张梦玲, 郎锋祥, 郑希怡, 刘利民. 共价有机框架材料在环境修复领域中吸附有机污染物的研究进展[J]. 化工进展, 2024, 43(10): 5837-5856.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0795
| COFs | 比表面积/m2·g-1 | 目标污染物 | 最佳 pH | 最大吸附容量/mg·g-1 | 平衡时间/min | 循环利用次数/次 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 氮基键COFs | |||||||
| TFPPy-CB | 284.5 | 木樨草素 槲皮素 芦丁 | 66.32 67.85 10.69 | 5 5 5 | 5 5 5 | [ [ [ | |
| TFPPy-ODH | 667.6 | 木樨草素 槲皮素 芦丁 | 73.20 70.83 9.76 | 5 5 5 | 5 5 5 | [ [ [ | |
TpPa-1 TpTph TpBD | 1179 715 592 | 磺胺嘧啶 磺胺嘧啶 磺胺嘧啶 | 4~7 4~7 4~7 | 82.9 150 188 | 240 240 240 | 5 5 5 | [ [ [ |
| TpBch | 652.7 | 盐酸阿霉素 | 7.4~8.5 | 137.4 | 90 | 4 | [ |
| 离子型COFs | |||||||
| TFP-DgCl | 12.70 | 双氯芬酸钠 | 5 | 1100.08 | 60 | [ | |
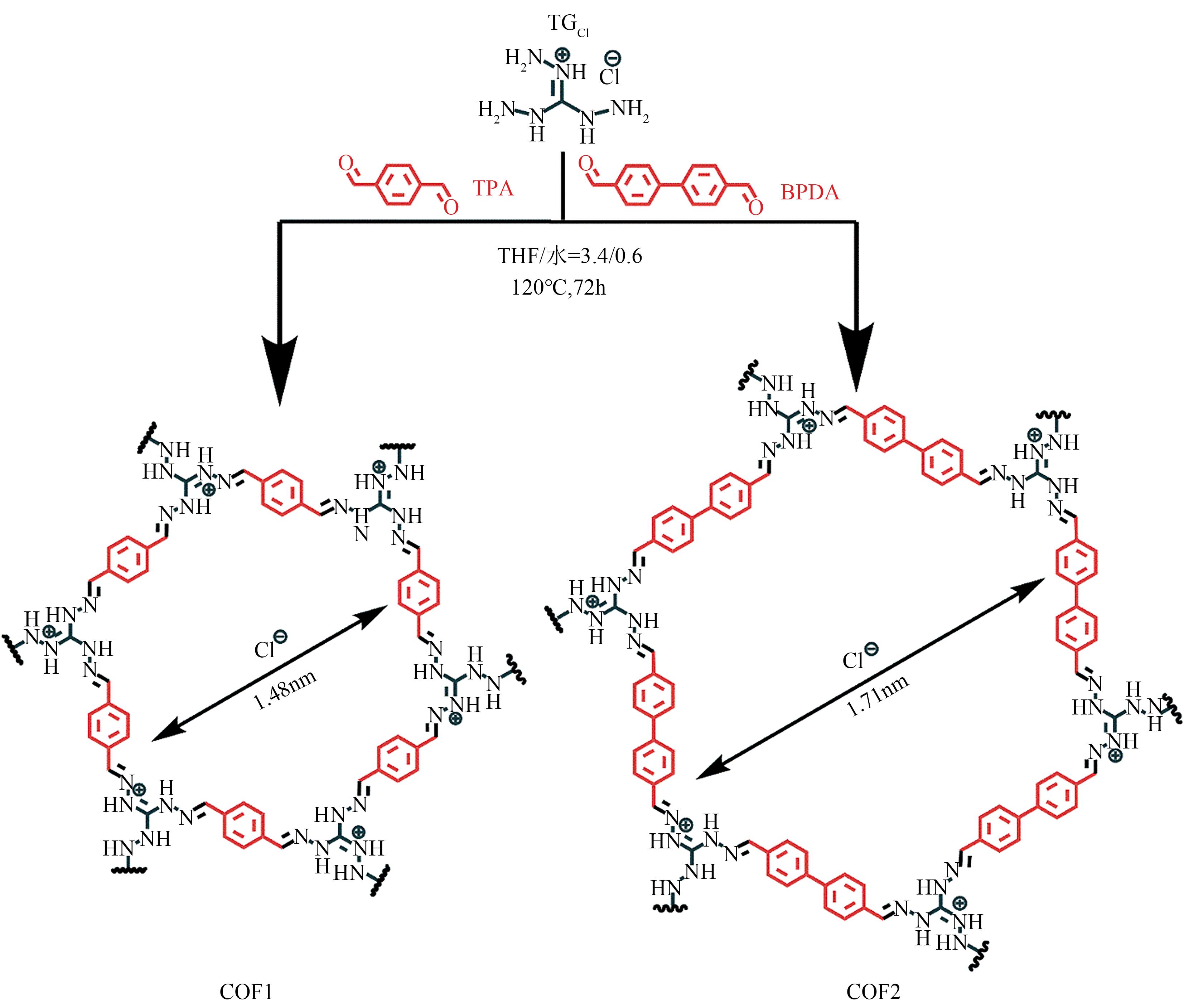
| TPA-TGCl | 26.8 | 双氯芬酸钠 酮洛芬 | 5.7 4.3 | 724.64 240.96 | 180 180 | 5 5 | [ [ |
| TpPa-SO3H | 102 | 磺胺嘧啶 | 7 | 174 | 120 | 4 | [ |
| 接枝官能团COFs | |||||||
| COF-NH2 | 282 | 酮洛芬 布洛芬 萘普生 | 33 18 16 | 180 180 180 | [ [ [ | ||
| COF-NO2 | 679 | 酮洛芬 布洛芬 萘普生 | 70 94 80 | 120 120 120 | [ [ [ | ||
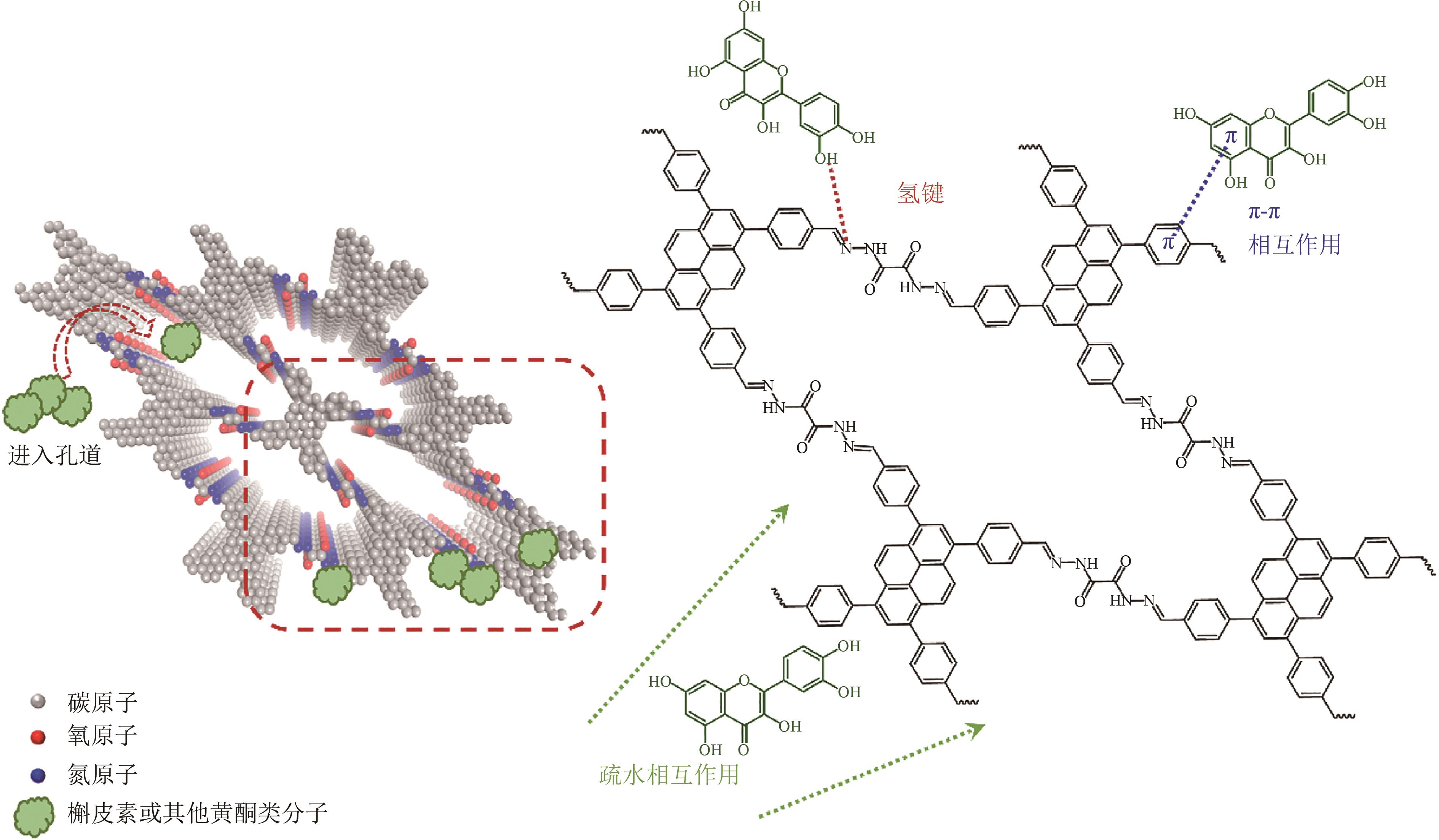
| TzDa-PBA | 274.3 | 木犀草素 槲皮素 黄芩素 杨梅素 | 8 8 8 8 | 219.3 213.0 189.0 134.0 | 20 20 20 20 | 5 5 5 5 | [ [ [ [ |
| HP-COFs | 743 | 磺胺嘧啶 | 6 | 168 | 240 | 5 | [ |
| COF-3-NH2 | 114 | 双氯芬酸钠 | 5 | 410.0 | 30 | 4 | [ |
| 复合功能化COFs | |||||||
| COF@MXene | 131.26 | 左氧氟沙星 洛美沙星 环丙沙星 加替沙星 莫西沙星 | 7~8 7~8 7~8 7~8 7~8 | 84.20 54.63 71.89 57.04 147.91 | 90 90 90 90 90 | 6 6 6 6 6 | [ [ [ [ [ |
| Tb@TpPa-SO3H | 236 | 诺氟沙星 环丙沙星 恩诺沙星 | 8 8 8 | 989 956 998 | 2 2 2 | 5 5 5 | [ [ [ |
| Fe3O4@COF-TpDBd | 265.24 | 环丙沙星 诺氟沙星 | 7 7 | 219.25 225.31 | 30 30 | 5 5 | [ [ |
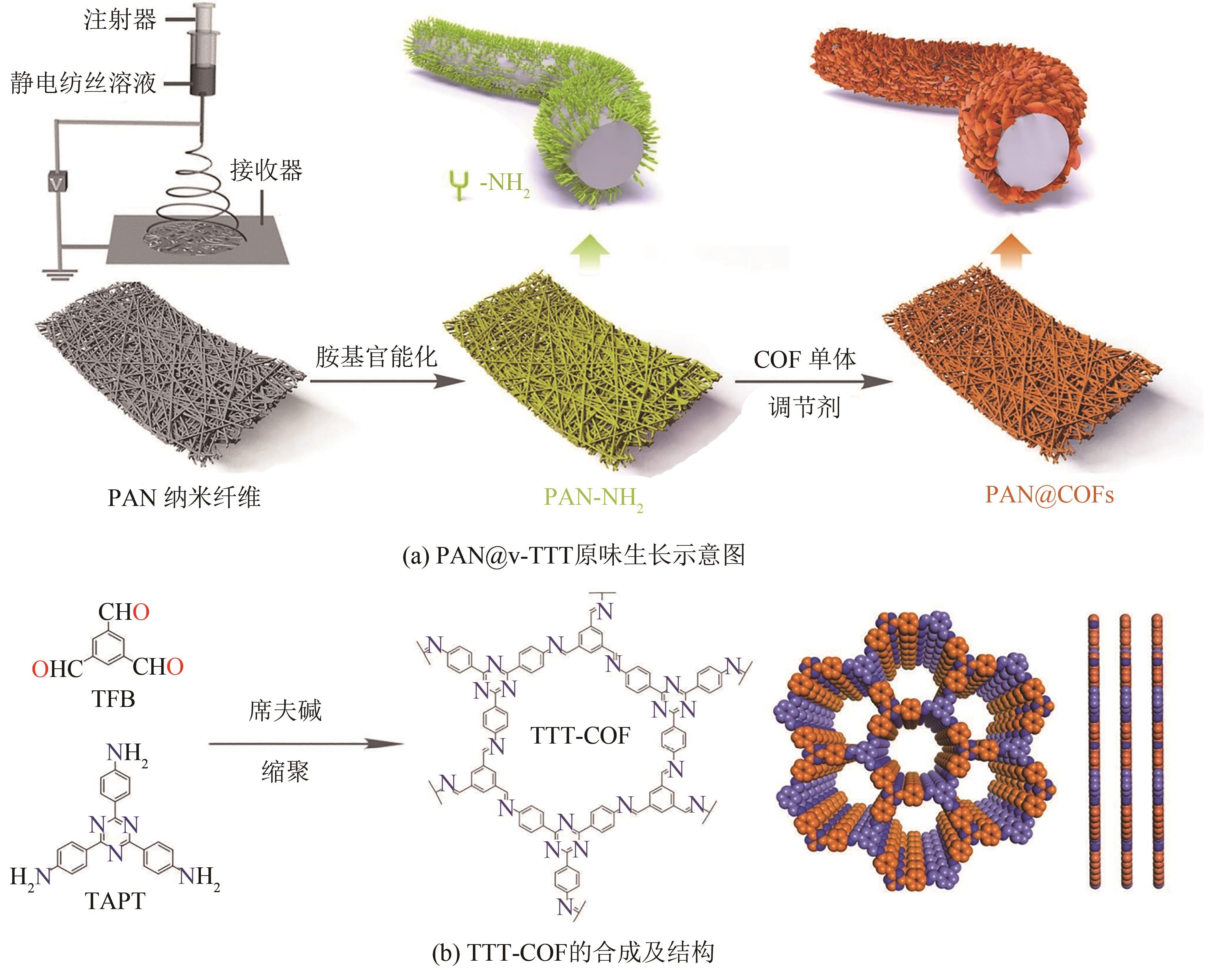
| PAN@v-TTT | 氧氟沙星 | 236 | [ | ||||
| OH-MCOF | 89.80 | 双氯芬酸钠 | 5 | 203.4 | 30 | 5 | [ |
| MCC/COF/PANI | 69.86 | 吲哚美辛 双氯芬酸钠 | 149.1 112.5 | 60 60 | 5 5 | [ [ |
| COFs | 比表面积/m2·g-1 | 目标污染物 | 最佳 pH | 最大吸附容量/mg·g-1 | 平衡时间/min | 循环利用次数/次 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 氮基键COFs | |||||||
| TFPPy-CB | 284.5 | 木樨草素 槲皮素 芦丁 | 66.32 67.85 10.69 | 5 5 5 | 5 5 5 | [ [ [ | |
| TFPPy-ODH | 667.6 | 木樨草素 槲皮素 芦丁 | 73.20 70.83 9.76 | 5 5 5 | 5 5 5 | [ [ [ | |
TpPa-1 TpTph TpBD | 1179 715 592 | 磺胺嘧啶 磺胺嘧啶 磺胺嘧啶 | 4~7 4~7 4~7 | 82.9 150 188 | 240 240 240 | 5 5 5 | [ [ [ |
| TpBch | 652.7 | 盐酸阿霉素 | 7.4~8.5 | 137.4 | 90 | 4 | [ |
| 离子型COFs | |||||||
| TFP-DgCl | 12.70 | 双氯芬酸钠 | 5 | 1100.08 | 60 | [ | |
| TPA-TGCl | 26.8 | 双氯芬酸钠 酮洛芬 | 5.7 4.3 | 724.64 240.96 | 180 180 | 5 5 | [ [ |
| TpPa-SO3H | 102 | 磺胺嘧啶 | 7 | 174 | 120 | 4 | [ |
| 接枝官能团COFs | |||||||
| COF-NH2 | 282 | 酮洛芬 布洛芬 萘普生 | 33 18 16 | 180 180 180 | [ [ [ | ||
| COF-NO2 | 679 | 酮洛芬 布洛芬 萘普生 | 70 94 80 | 120 120 120 | [ [ [ | ||
| TzDa-PBA | 274.3 | 木犀草素 槲皮素 黄芩素 杨梅素 | 8 8 8 8 | 219.3 213.0 189.0 134.0 | 20 20 20 20 | 5 5 5 5 | [ [ [ [ |
| HP-COFs | 743 | 磺胺嘧啶 | 6 | 168 | 240 | 5 | [ |
| COF-3-NH2 | 114 | 双氯芬酸钠 | 5 | 410.0 | 30 | 4 | [ |
| 复合功能化COFs | |||||||
| COF@MXene | 131.26 | 左氧氟沙星 洛美沙星 环丙沙星 加替沙星 莫西沙星 | 7~8 7~8 7~8 7~8 7~8 | 84.20 54.63 71.89 57.04 147.91 | 90 90 90 90 90 | 6 6 6 6 6 | [ [ [ [ [ |
| Tb@TpPa-SO3H | 236 | 诺氟沙星 环丙沙星 恩诺沙星 | 8 8 8 | 989 956 998 | 2 2 2 | 5 5 5 | [ [ [ |
| Fe3O4@COF-TpDBd | 265.24 | 环丙沙星 诺氟沙星 | 7 7 | 219.25 225.31 | 30 30 | 5 5 | [ [ |
| PAN@v-TTT | 氧氟沙星 | 236 | [ | ||||
| OH-MCOF | 89.80 | 双氯芬酸钠 | 5 | 203.4 | 30 | 5 | [ |
| MCC/COF/PANI | 69.86 | 吲哚美辛 双氯芬酸钠 | 149.1 112.5 | 60 60 | 5 5 | [ [ |
| COFs | 比表面积/m2·g-1 | 目标污染物 | 最佳 pH | 最大吸附容量/mg·g-1 | 平衡时间/min | 循环利用次数/次 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 接枝官能团COFs | |||||||
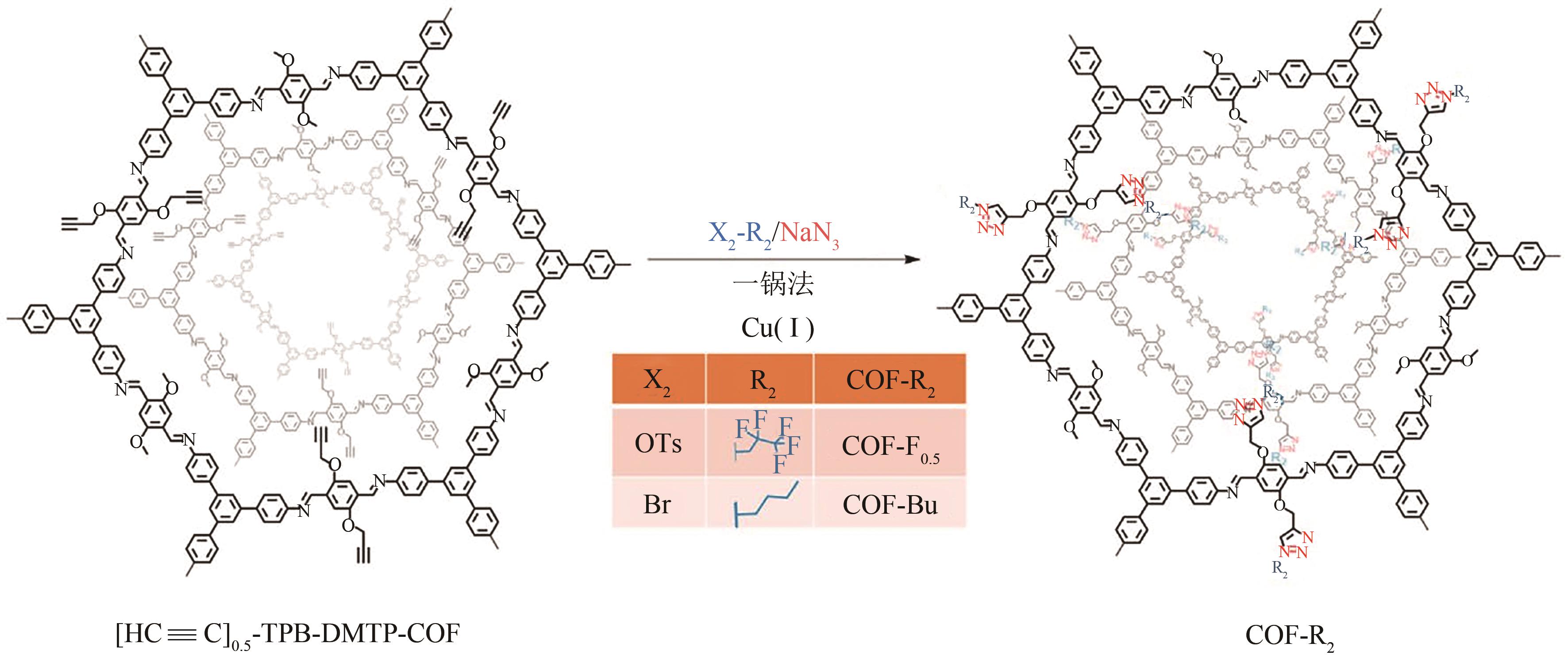
COF-Bu COF-F COF-F0.5 | 1516 354 1573 | 氟虫腈 氟虫腈 氟虫腈 | 122.4 149.8 194.7 | [ [ [ | |||
| COF-(CF3)2 | 1533 | 氟虫灵 氟虫腈 | 151 171 | [ [ | |||
| mTpBD-Me2 | 亲脂性毒死蜱 阿特拉津 | 270 54 | 120 120 | 5 5 | [ [ | ||
| COF-F1N5 | 190.2 | 全氟己磺酸盐 全氟辛烷磺酸 | 6 6 | 300.2μmol/g 879.8μmol/g | 1440 1440 | 5 5 | [ [ |
| COF-300-乙烷 | 全氟辛酸 | 2 | 250 | 1080 | [ | ||
| 复合功能化COFs | |||||||
| 磁性COF | 1543 | 哒嗪硫磷 辛硫磷 嘧硫磷 甲拌磷 | 163.9 172.4 175.4 178.6 | 30 30 30 30 | 10 10 10 10 | [ [ [ [ |
| COFs | 比表面积/m2·g-1 | 目标污染物 | 最佳 pH | 最大吸附容量/mg·g-1 | 平衡时间/min | 循环利用次数/次 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 接枝官能团COFs | |||||||
COF-Bu COF-F COF-F0.5 | 1516 354 1573 | 氟虫腈 氟虫腈 氟虫腈 | 122.4 149.8 194.7 | [ [ [ | |||
| COF-(CF3)2 | 1533 | 氟虫灵 氟虫腈 | 151 171 | [ [ | |||
| mTpBD-Me2 | 亲脂性毒死蜱 阿特拉津 | 270 54 | 120 120 | 5 5 | [ [ | ||
| COF-F1N5 | 190.2 | 全氟己磺酸盐 全氟辛烷磺酸 | 6 6 | 300.2μmol/g 879.8μmol/g | 1440 1440 | 5 5 | [ [ |
| COF-300-乙烷 | 全氟辛酸 | 2 | 250 | 1080 | [ | ||
| 复合功能化COFs | |||||||
| 磁性COF | 1543 | 哒嗪硫磷 辛硫磷 嘧硫磷 甲拌磷 | 163.9 172.4 175.4 178.6 | 30 30 30 30 | 10 10 10 10 | [ [ [ [ |
| COFs | 比表面积/m2·g-1 | 目标污染物 | 最佳pH | 最大吸附容量/mg·g-1 | 平衡时间/min | 循环利用次数/次 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 氮基键 COFs | |||||||
BFTB-PyTA BFTB-BFTB BFTB-BCTA | 1133 1040 834 | 罗丹明B 罗丹明B 罗丹明B | 2127 1854 1605 | 5 5 5 | 5 5 5 | [ [ [ | |
| TzDBd | 162.3 | 龙胆紫 亮绿 | 6 6 | 307.7 276.1 | 2 2 | [ [ | |
| COF-Z1 | 512.52 | 罗丹明B 亚甲基蓝 龙胆紫 刚果红 | 7 7 7 7 | 344 510 564 425 | 120 120 120 120 | 4 4 4 4 | [ [ [ [ |
| COF-Z2 | 549.44 | 罗丹明B 亚甲基蓝 龙胆紫 刚果红 | 7 7 7 7 | 430 315 395 483 | 120 120 120 120 | 4 4 4 4 | [ [ [ [ |
| PI-based COFs | 亚甲基蓝 | 10 | 315.6 | 250 | [ | ||
| TzTPT-COF | 亚甲基蓝 | 572.484 | 20 | [ | |||
| 苯并咪唑-COF | 240 | 甲基橙 亚甲基蓝 | 3 11 | 256 185 | 160 160 | 5 5 | [ [ |
| 离子型COFs | |||||||
| TpBd(SO3H)2-COF | 32.4 | 亚甲基蓝 | 6~7 | 166 ± 13 | [ | ||
| TFPB-Pa-SO3H COF | 105.4 | 结晶紫 亚甲基蓝 罗丹明B | 9 9 9 | 1559 1174 1062 | 80 120 240 | 5 5 5 | [ [ [ |
| TFPB-BDSA COF | 127.8 | 结晶紫 亚甲基蓝 罗丹明B | 9 9 9 | 1288 1166 1054 | 100 150 350 | 5 5 5 | [ [ [ |
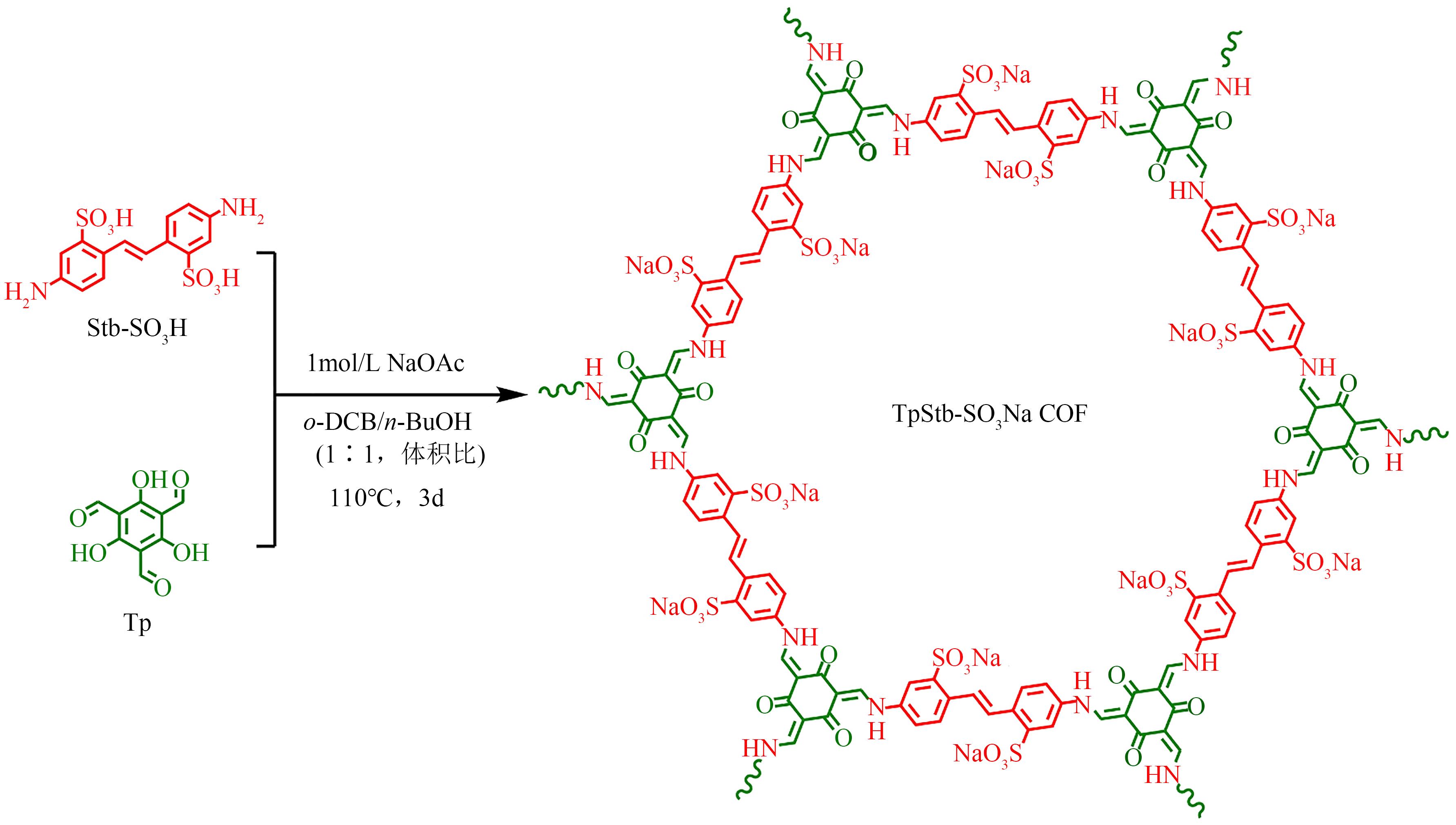
| TpStb-SO3Na | 15.4 | 亚甲基蓝 结晶紫 孔雀石绿 黄花绿B | 1078 1861 5857 1339 | 10 10 10 10 | 5 5 5 5 | [ [ [ [ | |
| 接枝官能团COFs | |||||||
| COF-TPDD-COOH | 亚甲基蓝 结晶紫 孔雀石绿 | 10 10 10 | 102.89 88.02 128.64 | 60 60 60 | [ [ [ | ||
| COF-OH | 252.74 | 铬黑T 铬蓝黑 刚果红 | 229.12 90.71 158.39 | 20 20 20 | 3 3 3 | [ [ [ | |
| 复合功能化COFs | |||||||
| MOF-5/COF | 7.025 | 金胺O 罗丹明B | 9.5 9.5 | 17.95 16.18 | 8 8 | 6 6 | [ [ |
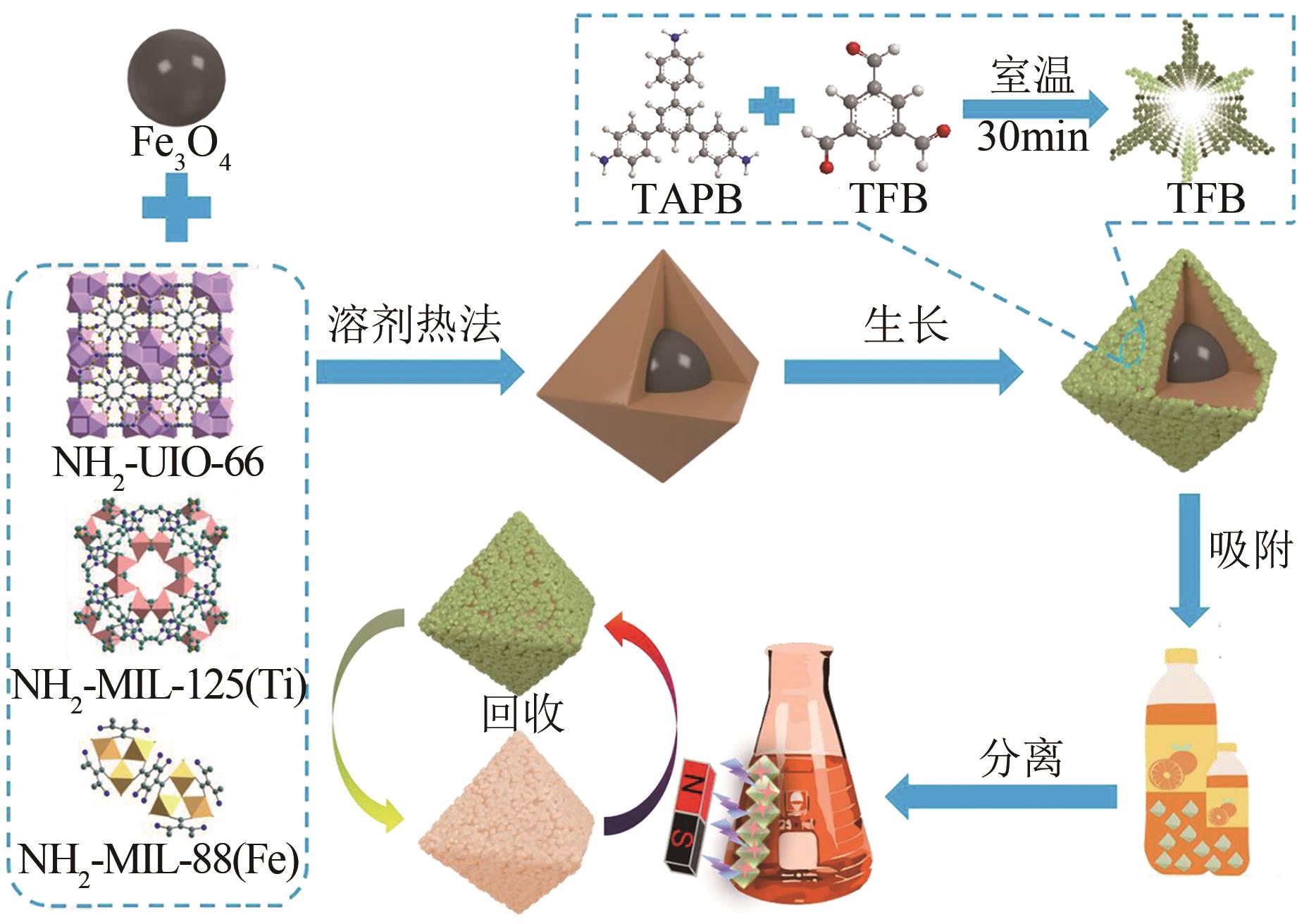
Fe3O4@MOF(Fe)@COF Fe3O4@MOF(Zr)@COF | 808.35 347.62 | 刚果红 刚果红 | 5 5 | 1250.02 625.13 | 120 120 | 7 7 | [ [ |
| Fe3O4@MOF(Ti)@COF | 63.50 | 黄果苷 | 5 | 294.12 | 120 | 7 | [ |
| BiPy-MCOF | 192.87 | 甲基橙 | 3.1 | 440.990 μmol/g | 180 | 4 | [ |
| COFs | 比表面积/m2·g-1 | 目标污染物 | 最佳pH | 最大吸附容量/mg·g-1 | 平衡时间/min | 循环利用次数/次 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 氮基键 COFs | |||||||
BFTB-PyTA BFTB-BFTB BFTB-BCTA | 1133 1040 834 | 罗丹明B 罗丹明B 罗丹明B | 2127 1854 1605 | 5 5 5 | 5 5 5 | [ [ [ | |
| TzDBd | 162.3 | 龙胆紫 亮绿 | 6 6 | 307.7 276.1 | 2 2 | [ [ | |
| COF-Z1 | 512.52 | 罗丹明B 亚甲基蓝 龙胆紫 刚果红 | 7 7 7 7 | 344 510 564 425 | 120 120 120 120 | 4 4 4 4 | [ [ [ [ |
| COF-Z2 | 549.44 | 罗丹明B 亚甲基蓝 龙胆紫 刚果红 | 7 7 7 7 | 430 315 395 483 | 120 120 120 120 | 4 4 4 4 | [ [ [ [ |
| PI-based COFs | 亚甲基蓝 | 10 | 315.6 | 250 | [ | ||
| TzTPT-COF | 亚甲基蓝 | 572.484 | 20 | [ | |||
| 苯并咪唑-COF | 240 | 甲基橙 亚甲基蓝 | 3 11 | 256 185 | 160 160 | 5 5 | [ [ |
| 离子型COFs | |||||||
| TpBd(SO3H)2-COF | 32.4 | 亚甲基蓝 | 6~7 | 166 ± 13 | [ | ||
| TFPB-Pa-SO3H COF | 105.4 | 结晶紫 亚甲基蓝 罗丹明B | 9 9 9 | 1559 1174 1062 | 80 120 240 | 5 5 5 | [ [ [ |
| TFPB-BDSA COF | 127.8 | 结晶紫 亚甲基蓝 罗丹明B | 9 9 9 | 1288 1166 1054 | 100 150 350 | 5 5 5 | [ [ [ |
| TpStb-SO3Na | 15.4 | 亚甲基蓝 结晶紫 孔雀石绿 黄花绿B | 1078 1861 5857 1339 | 10 10 10 10 | 5 5 5 5 | [ [ [ [ | |
| 接枝官能团COFs | |||||||
| COF-TPDD-COOH | 亚甲基蓝 结晶紫 孔雀石绿 | 10 10 10 | 102.89 88.02 128.64 | 60 60 60 | [ [ [ | ||
| COF-OH | 252.74 | 铬黑T 铬蓝黑 刚果红 | 229.12 90.71 158.39 | 20 20 20 | 3 3 3 | [ [ [ | |
| 复合功能化COFs | |||||||
| MOF-5/COF | 7.025 | 金胺O 罗丹明B | 9.5 9.5 | 17.95 16.18 | 8 8 | 6 6 | [ [ |
Fe3O4@MOF(Fe)@COF Fe3O4@MOF(Zr)@COF | 808.35 347.62 | 刚果红 刚果红 | 5 5 | 1250.02 625.13 | 120 120 | 7 7 | [ [ |
| Fe3O4@MOF(Ti)@COF | 63.50 | 黄果苷 | 5 | 294.12 | 120 | 7 | [ |
| BiPy-MCOF | 192.87 | 甲基橙 | 3.1 | 440.990 μmol/g | 180 | 4 | [ |
| COFs | 比表面积/m2·g-1 | 目标污染物 | 最佳pH | 最大吸附容量/mg·g-1 | 平衡时间/min | 循环利用次数/次 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 氮基键COFs | |||||||
| COF-1 | 190 | 双酚S 双酚A | 4~7 4~7 | 157 125 | 60 60 | 4 4 | [ [ |
| COF-2 | 51 | 双酚S 双酚A | 4~7 4~7 | 195 145 | 60 60 | 4 4 | [ [ |
| TFPB-TTA COF | 1175.8 | 2,4,6-三硝基苯酚 2,4-二硝基苯酚 4-硝基苯酚 | 3 3 3 | 1045.53 255.75 862.08 | 45 45 45 | 9 9 9 | [ [ [ |
| TTA-DVA-COF | 2-硝基苯酚 | 3 | 2277.97 | 120 | 5 | [ | |
| 接枝官能团COFs | |||||||
| COF-TADH | 4-硝基苯酚 | 1402 | 5 | [ | |||
| BA-COF | 282.76 | 腺苷 | 8 | 33.2 | 20 | 5 | [ |
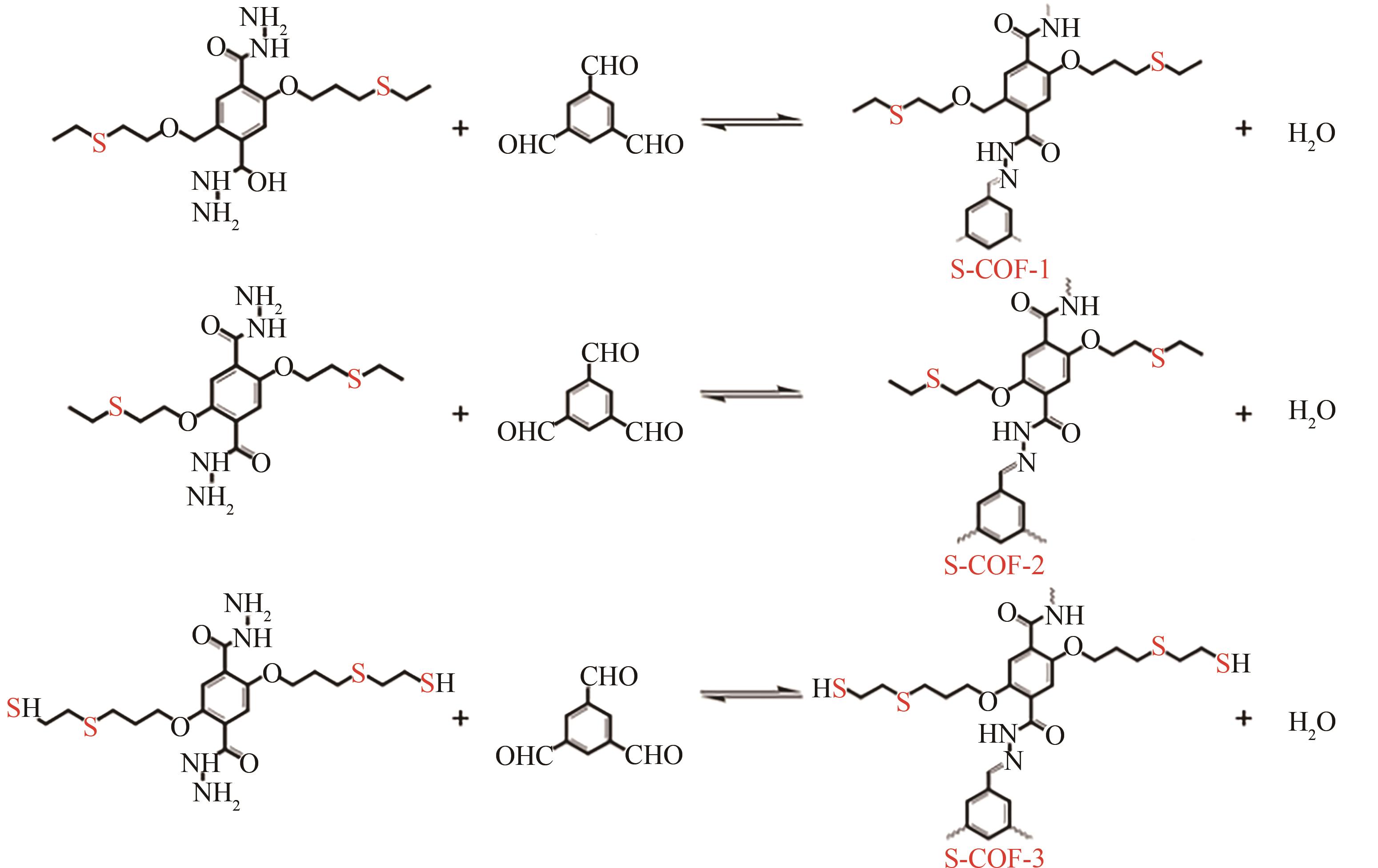
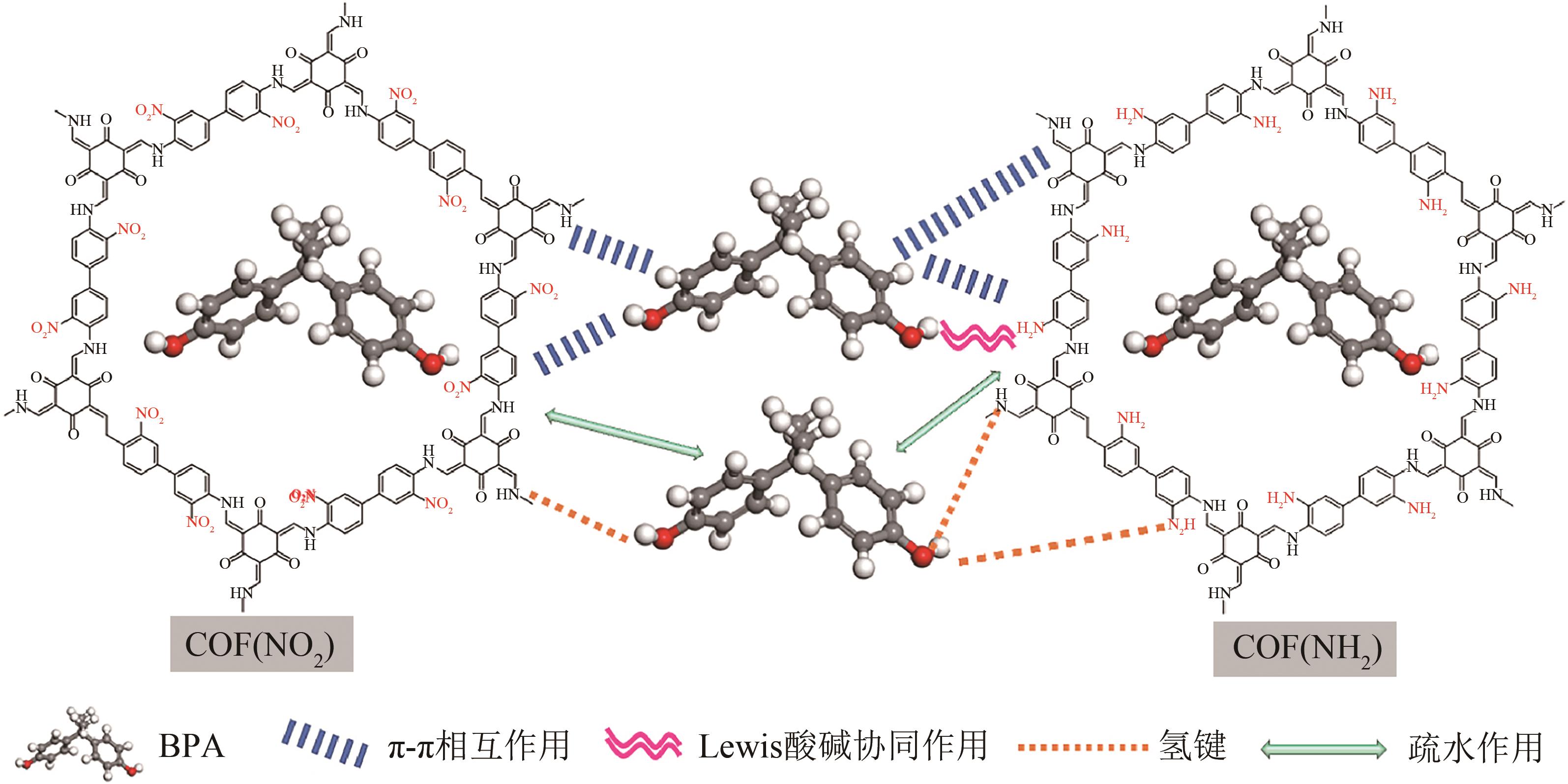
COF(NH2) COF(NO2) | 81 287 | 双酚A 双酚A | <5 2~7 | 190.22 155.14 | 180 180 | 5 5 | [ [ |
| 复合功能化COFs | |||||||
| Fe3O4@COF | 335.23 | 双酚A 双酚AF | 7 7 | 140 290.4 | 120 120 | 5 5 | [ [ |
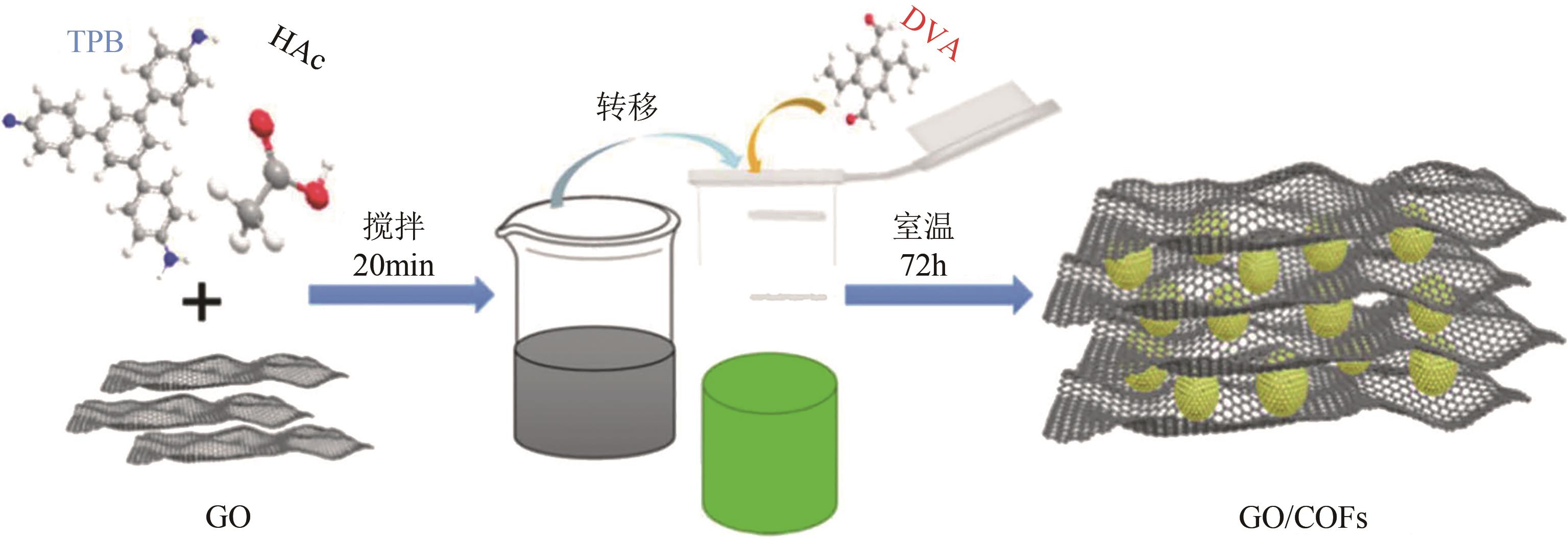
| GO/COFs | 236.04 | 萘 1-萘胺 1-萘酚 | 4 8 | 211 110 98.2 | 120 120 120 | 5 5 5 | [ [ [ |
| Fe3O4@COFs | 55.71 | 三氯生 三氯卡班 | 6~9 6~9 | 3500μg/g 1500μg/g | 20 20 | 10 10 | [ [ |
| COFs | 比表面积/m2·g-1 | 目标污染物 | 最佳pH | 最大吸附容量/mg·g-1 | 平衡时间/min | 循环利用次数/次 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 氮基键COFs | |||||||
| COF-1 | 190 | 双酚S 双酚A | 4~7 4~7 | 157 125 | 60 60 | 4 4 | [ [ |
| COF-2 | 51 | 双酚S 双酚A | 4~7 4~7 | 195 145 | 60 60 | 4 4 | [ [ |
| TFPB-TTA COF | 1175.8 | 2,4,6-三硝基苯酚 2,4-二硝基苯酚 4-硝基苯酚 | 3 3 3 | 1045.53 255.75 862.08 | 45 45 45 | 9 9 9 | [ [ [ |
| TTA-DVA-COF | 2-硝基苯酚 | 3 | 2277.97 | 120 | 5 | [ | |
| 接枝官能团COFs | |||||||
| COF-TADH | 4-硝基苯酚 | 1402 | 5 | [ | |||
| BA-COF | 282.76 | 腺苷 | 8 | 33.2 | 20 | 5 | [ |
COF(NH2) COF(NO2) | 81 287 | 双酚A 双酚A | <5 2~7 | 190.22 155.14 | 180 180 | 5 5 | [ [ |
| 复合功能化COFs | |||||||
| Fe3O4@COF | 335.23 | 双酚A 双酚AF | 7 7 | 140 290.4 | 120 120 | 5 5 | [ [ |
| GO/COFs | 236.04 | 萘 1-萘胺 1-萘酚 | 4 8 | 211 110 98.2 | 120 120 120 | 5 5 5 | [ [ [ |
| Fe3O4@COFs | 55.71 | 三氯生 三氯卡班 | 6~9 6~9 | 3500μg/g 1500μg/g | 20 20 | 10 10 | [ [ |
| 1 | LI Wenkui, REN Peng, ZHOU Yiwan, et al. Europium(Ⅲ) functionalized 3D covalent organic framework for quinones adsorption and sensing investigation[J]. Journal of Hazardous Materials, 2020, 388: 121740. |
| 2 | GHAHARI Afsaneh, RAISSI Heidar, PASBAN Samaneh, et al. Proposing two-dimensional covalent organic frameworks material for the capture of phenol molecules from wastewaters[J]. NPJ Clean Water, 2022, 5: 28. |
| 3 | ZHAO Chaofeng, SUN Lu, AI Yuejie, et al. Adsorption dynamics and intrinsic mechanism of POPs on corrole-based COF: A computational study[J]. Journal of Cleaner Production, 2022, 338: 130566. |
| 4 | ROMERO Vanesa, FERNANDES Soraia P S, GONÇALVES Liliana P L, et al. One-step synthesis of magnetic covalent organic framework composite for the adsorption of marine toxin okadaic acid[J]. CrystEngComm, 2023, 25(16): 2456-2462. |
| 5 | SUN Wenjing, HU Xiaoyu, XIANG Yuhong, et al. Adsorption behavior and mechanism of sulfonamides on controllably synthesized covalent organic frameworks[J]. Environmental Science and Pollution Research International, 2022, 29(13): 18680-18688. |
| 6 | WANG Wei, JIA Ye, ZHOU Shuangxi, et al. Removal of typical PFAS from water by covalent organic frameworks with different pore sizes[J]. Journal of Hazardous Materials, 2023, 460: 132522. |
| 7 | KOJO Acquah Ebenezer, CHO Wansu, PARK Chiyoung. Mildly oxidized porous covalent triazine frameworks with rapid and high adsorption capability for aqueous organic micropollutants[J]. Journal of Industrial and Engineering Chemistry, 2022, 116: 250-256. |
| 8 | LIU Zhongshan, WANG Hongwei, Junjie OU, et al. Construction of hierarchically porous monoliths from covalent organic frameworks (COFs) and their application for bisphenol A removal[J]. Journal of Hazardous Materials, 2018, 355: 145-153. |
| 9 | ZHUANG Shuting, CHEN Rong, LIU Yong, et al. Magnetic COFs for the adsorptive removal of diclofenac and sulfamethazine from aqueous solution: Adsorption kinetics, isotherms study and DFT calculation[J]. Journal of Hazardous Materials, 2020, 385: 121596. |
| 10 | ZHANG Shijuan, WU Xia, MA Chong, et al. Cationic surfactant modified 3D COF and its application in the adsorption of UV filters and alkylphenols from food packaging material migrants[J]. Journal of Agricultural and Food Chemistry, 2020, 68(11): 3663-3669. |
| 11 | LIU Ruiqi, YANG Juan, LIU Rui, et al. Effects of nanopore size on the adsorption of sulfamerazine from aqueous solution by β-ketoenamine covalent organic frameworks[J]. ACS Applied Nano Materials, 2022, 5(12): 17851-17858. |
| 12 | MO Peiying, FU Daijun, CHEN Ping, et al. Ionic covalent organic frameworks for non-steroidal anti-inflammatory drugs (NSAIDs) removal from aqueous solution: Adsorption performance and mechanism[J]. Separation and Purification Technology, 2021, 278: 119238. |
| 13 | LI Rui, TANG Xihao, WU Jialin, et al. A sulfonate-functionalized covalent organic framework for record-high adsorption and effective separation of organic dyes[J]. Chemical Engineering Journal, 2023, 464: 142706. |
| 14 | LIN Xiaogeng, LI Chen, HE Yasan, et al. A thermally promoted homogenous-floating-concentrating strategy synthesizing highly crystalline triazine/hydroxyl-rich COFs for 4-nitrophenol adsorption[J]. Macromolecular Rapid Communications, 2023, 44(11): e2200786. |
| 15 | YANG Yulian, LIU Qiuyi, ZOU Yuemeng, et al. Covalent assembly synthesis of covalent organic framework and MXene based composite for the adsorption of fluoroquinolones[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 110975. |
| 16 | DI Siyuan, ZHANG Mengqi, SHI Chunxiang, et al. Thoughtful design of a covalent organic framework with tailor-made polarity and pore size for the enrichment of bisphenols and their derivatives: Extraction performance, adsorption mechanism and toxicity evaluation[J]. Environmental Pollution, 2023, 326: 121475. |
| 17 | ZHAO Yuxiang, SUI Zhuyin, CHANG Zhaosen, et al. A trifluoromethyl-grafted ultra-stable fluorescent covalent organic framework for adsorption and detection of pesticides[J]. Journal of Materials Chemistry A, 2020, 8(47): 25156-25164. |
| 18 | LI Zhentao, JIANG Min, HU Zhuang, et al. Synthesis of phenylboronic acid-functionalized covalent organic framework via thiol-ene “click” reaction for highly efficient adsorption of cis-diol containing flavonoids[J]. Microporous and Mesoporous Materials, 2023, 362: 112798. |
| 19 | EL-MAHDY Ahmed F M, ZAKARIA Mohamed Barakat, WANG Haoxin, et al. Heteroporous bifluorenylidene-based covalent organic frameworks displaying exceptional dye adsorption behavior and high energy storage[J]. Journal of Materials Chemistry A, 2020, 8(47): 25148-25155. |
| 20 | WEI Xiaohang, HUANG Dongdong, PEI Dong, et al. Synthesis of imine-linked covalent organic frameworks and their adsorption properties for flavonoids[J]. Microporous and Mesoporous Materials, 2023, 348: 112333. |
| 21 | CHEN Jiaqing, ZHENG Qiongqing, XIAO Saijin, et al. Construction of two-dimensional fluorescent covalent organic framework nanosheets for the detection and removal of nitrophenols[J]. Analytical Chemistry, 2022, 94(5): 2517-2526. |
| 22 | KUMAR Shubham, KUMARI Kusum, SINGH Saurabh K, et al. Amorphous tetrazine-triazine-functionalized covalent organic framework for adsorption and removal of dyes[J]. New Journal of Chemistry, 2023, 47(29): 13676-13686. |
| 23 | ZHENG Wang, LI Anran, WANG Xiuwen, et al. Construction of hydrophilic covalent organic frameworks and their fast and efficient adsorption of cationic dyes from aqueous solution[J]. New Journal of Chemistry, 2022, 46(46): 22185-22194. |
| 24 | LIN Zili, JIN Yuhan, CHEN Yongxian, et al. Leaf-like ionic covalent organic framework for the highly efficient and selective removal of non-steroidal anti-inflammatory drugs: Adsorption performance and mechanism insights[J]. Journal of Colloid and Interface Science, 2023, 645: 943-955. |
| 25 | WANG Shunli, YUAN Ning, DAI Tingting, et al. Surface post-functionalization of COFs by economical strategy via multiple-component one-pot tandem reactions and their application in adsorption of pesticides[J]. Advanced Composites and Hybrid Materials, 2022, 5(2): 1439-1449. |
| 26 | WANG Wei, ZHOU Shuangxi, JIANG Xiangzhe, et al. Fluorinated quaternary ammonium covalent organic frameworks for selective and efficient removal of typical per- and polyfluoroalkyl substances[J]. Chemical Engineering Journal, 2023, 474: 145629. |
| 27 | WANG Luchun, TAO Yongqing, WANG Junji, et al. A novel hydroxyl-riched covalent organic framework as an advanced adsorbent for the adsorption of anionic azo dyes[J]. Analytica Chimica Acta, 2022, 1227: 340329. |
| 28 | LIU Shaochi, YANG Lijuan, QUAN Tian, et al. Glutathione-functionalized highly crystalline fluorescent covalent organic framework as a fluorescence-sensing and adsorption double platform for cationic dyes[J]. Separation and Purification Technology, 2022, 288: 120673. |
| 29 | ZHANG Anrui, LIU Xuewei, HONG Jiahui, et al. A mussel-pearl side chain interaction in mercury(Ⅱ) and phenol removal by sulfur-functionalized covalent organic frameworks: A DFT study[J]. The Science of the Total Environment, 2022, 838(Pt 2): 156082. |
| 30 | ZEPPUHAR Andrea N, ROLLINS Devin S, HUBER Dale L, et al. Linkage transformations in a three-dimensional covalent organic framework for high-capacity adsorption of perfluoroalkyl substances[J]. ACS Applied Materials & Interfaces, 2023: 15 (45): 52622-52630. |
| 31 | ZHANG Menghan, WANG Wei, ZHANG Qianxin, et al. Pore surface engineering of covalent organic frameworks by simultaneously appending amine group and tailoring pore size for efficient adsorption of diclofenac sodium[J]. Chemical Engineering Journal, 2023, 459: 141561. |
| 32 | LIANG Ying, FENG Lijuan, LIU Xin, et al. Enhanced selective adsorption of NSAIDs by covalent organic frameworks via functional group tuning[J]. Chemical Engineering Journal, 2021, 404: 127095. |
| 33 | XIONG Mingyang, WANG Bing, WANG Haiyan, et al. Probing the adsorption behavior and mechanism of NO2 and NH2 functionalized covalent organic frameworks (COFs) for removal of bisphenol A[J]. Microporous and Mesoporous Materials, 2022, 346: 112299. |
| 34 | ZHAO Yue, FENG Chenghong, TIAN Chenhao, et al. Enhanced adsorption selectivity of bisphenol analogues by tuning the functional groups of covalent organic frameworks (COFs)[J]. Separation and Purification Technology, 2022, 297: 121489. |
| 35 | SHANG Shengcong, LIU Youxing, LIU Minghui, et al. Studying the adsorption mechanisms of nanoplastics on covalent organic frameworks via molecular dynamics simulations[J]. Journal of Hazardous Materials, 2022, 421: 126796. |
| 36 | FERNANDES Soraia P S, Petr KOVÁŘ, Milan PŠENIČKA, et al. Selection of covalent organic framework pore functionalities for differential adsorption of microcystin toxin analogues[J]. ACS Applied Materials & Interfaces, 2021, 13(13): 15053-15063. |
| 37 | WANG Yuhua, WANG Weihua, ZHANG Zhiqiang, et al. Adsorption performance of the functional COFs for removal of bisphenol A: Molecular dynamic simulations[J]. Journal of Molecular Liquids, 2023, 392: 123475. |
| 38 | WANG Ziyi, XU Li, ZOU Ting, et al. Fabrication of boronic acid-functionalized covalent organic framework for the selective adsorption of cis-diol-containing compounds[J]. Applied Surface Science, 2024, 644: 158698. |
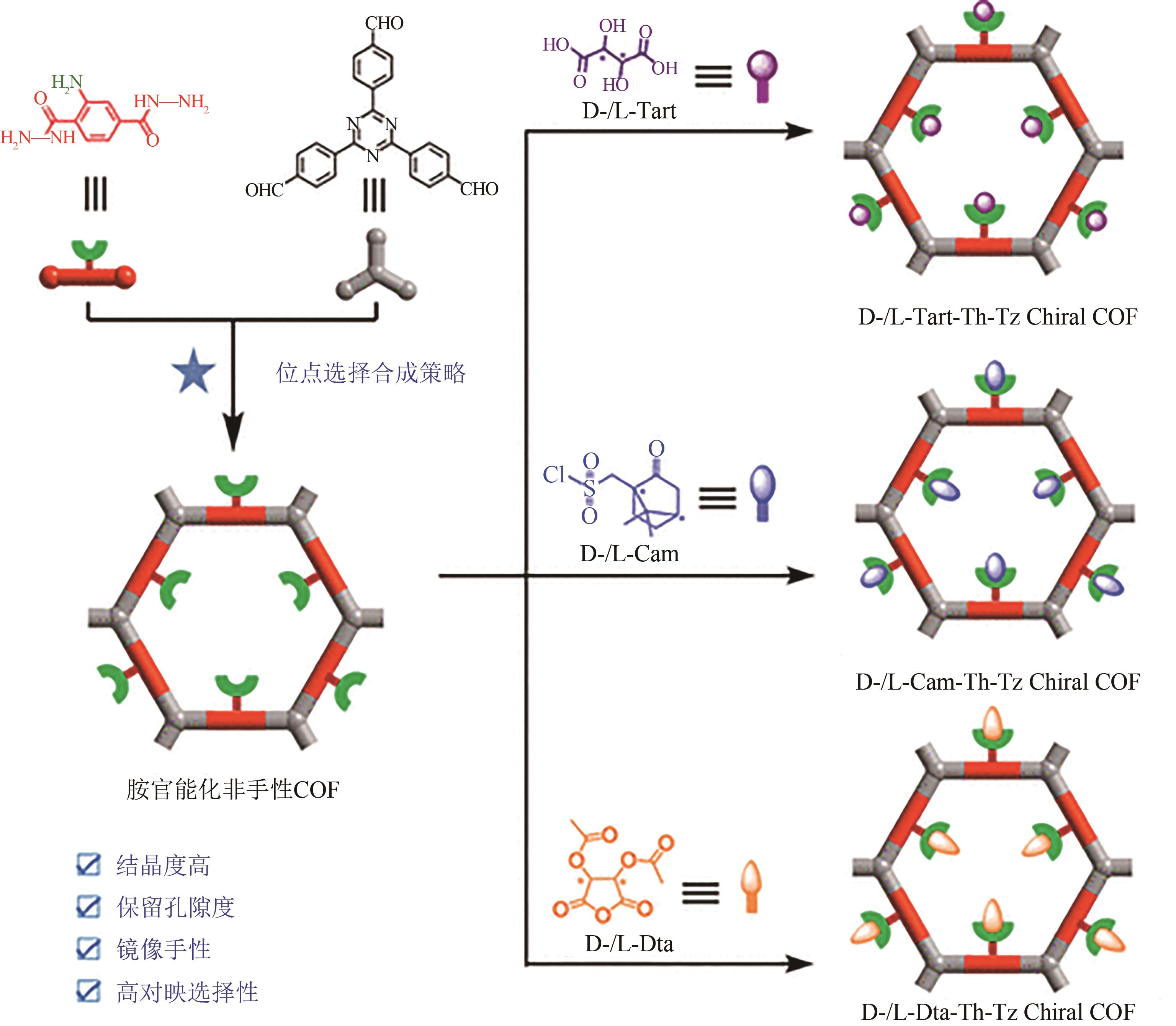
| 39 | ZHUO Siqi, WANG Xuehua, LI Lingyu, et al. Chiral carboxyl-functionalized covalent organic framework for enantioselective adsorption of amino acids[J]. ACS Applied Materials & Interfaces, 2021, 13(26): 31059-31065. |
| 40 | TANG Xihao, YANG Yixuan, LI Xinle, et al. Postmodification of an amine-functionalized covalent organic framework for enantioselective adsorption of tyrosine[J]. ACS Applied Materials & Interfaces, 2023, 15(20): 24836-24845. |
| 41 | FIROOZI Maryam, RAFIEE Zahra, DASHTIAN Kheibar. New MOF/COF hybrid as a robust adsorbent for simultaneous removal of auramine O and rhodamine B dyes[J]. ACS Omega, 2020, 5(16): 9420-9428. |
| 42 | LIANG Li, CHEN Jia, CHEN Xuwei, et al. In situ synthesis of a GO/COFs composite with enhanced adsorption performance for organic pollutants in water[J]. Environmental Science: Nano, 2022, 9(2): 554-567. |
| 43 | JIANG Wei, CUI Weirong, LIANG Ruping, et al. Difunctional covalent organic framework hybrid material for synergistic adsorption and selective removal of fluoroquinolone antibiotics[J]. Journal of Hazardous Materials, 2021, 413: 125302. |
| 44 | ZHAO Xiaojia, LI Qun, PACHFULE Pradip, et al. Construction of covalent organic framework nanofiber membranes for efficient adsorption of antibiotics[J]. Small, 2023, 19(26): e2301200. |
| 45 | QI Peipei, WANG Jiao, LI Hongping, et al. Fluffy ball-like magnetic covalent organic frameworks for adsorption and removal of organothiophosphate pesticides[J]. The Science of the Total Environment, 2022, 840: 156529. |
| 46 | BU Fan, HUANG Wei, XIAN Mo, et al. Magnetic carboxyl-functionalized covalent organic frameworks for adsorption of quinolones with high capacities, fast kinetics and easy regeneration[J]. Journal of Cleaner Production, 2022, 336: 130485. |
| 47 | ZHANG Xue, ZHU Donghai, WANG Shiyi, et al. Efficient adsorption and degradation of dyes from water using magnetic covalent organic frameworks with a pyridinic structure[J]. Environmental Science and Pollution Research International, 2023, 30(12): 34669-34683. |
| 48 | WANG Qinzhi, ZHAO Yijian, SHI Zhan, et al. Magnetic amino-functionalized-MOF(M=Fe, Ti, Zr)@COFs with superior biocompatibility: Performance and mechanism on adsorption of azo dyes in soft drinks[J]. Chemical Engineering Journal, 2021, 420: 129955. |
| 49 | LI Yuanyuan, FENG Jingbo, WANG Rui, et al. The efficient removal of diclofenac and indomethacin with novel polyaniline-modified microcrystalline cellulose/covalent organic framework nanocomposites[J]. Journal of the TaiwanInstitute of Chemical Engineers, 2023, 145: 104834. |
| 50 | JI Shilei, XIAO Shanshan, WANG Luliang, et al. Synthesis of hydrazone-linked covalent organic framework for pH-mediated adsorption and removal of doxorubicin hydrochloride[J]. European Polymer Journal, 2023, 201: 112572. |
| 51 | LIU Ruiqi, HUANG Lijin, TAO Hui, et al. Microenvironment engineering of covalent organic frameworks for the efficient removal of sulfamerazine from aqueous solution[J]. Journal of Environmental Chemical Engineering, 2022, 10(2): 107300. |
| 52 | LIU Ruiqi, YAN Qian, TANG Yumeng, et al. NaCl template-assisted synthesis of self-floating COFs foams for the efficient removal of sulfamerazine[J]. Journal of Hazardous Materials, 2022, 421: 126702. |
| 53 | MI Xin, ZHOU Shuangxi, ZHOU Ziming, et al. Adsorptive removal of diclofenac sodium from aqueous solution by magnetic COF: Role of hydroxyl group on COF[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 603: 125238. |
| 54 | ROMERO Vanesa, FERNANDES Soraia P S, Petr KOVÁŘ, et al. Efficient adsorption of endocrine-disrupting pesticides from water with a reusable magnetic covalent organic framework[J]. Microporous and Mesoporous Materials, 2020, 307: 110523. |
| 55 | LI Yang, YANG Chengxiong, QIAN Hailong, et al. Carboxyl-functionalized covalent organic frameworks for the adsorption and removal of triphenylmethane dyes[J]. ACS Applied Nano Materials, 2019, 2(11): 7290-7298. |
| 56 | ZHAO Jie, SHEN Xinyu, LIU Yifan, et al. (3, 3)-Connected triazine-based covalent organic frameworks for efficient CO2 separation over N2 and dye adsorption[J]. Langmuir, 2023, 39(46): 16367-16373. |
| 57 | HSU Ya-Chung, Manik Chandra SIL, LIN Ching-Hsuan, et al. Modification of covalent organic framework by hydrolysis for efficient and selective removal of organic dye[J]. Applied Surface Science, 2023, 612: 155890. |
| 58 | XU Shixian, YAO Zhaoquan, ZHANG Yinghui. A covalent organic framework exhibiting amphiphilic selective adsorption toward ionic organic dyes tuned by pH value[J]. European Polymer Journal, 2020, 133: 109764. |
| 59 | MACHADO Tiago F, SANTOS Filipa A, PEREIRA Rui F P, et al. β-Ketoenamine covalent organic frameworks-effects of functionalization on pollutant adsorption[J]. Polymers, 2022, 14(15): 3096. |
| 60 | FU Daijun, ZHANG Qianxin, CHEN Ping, et al. Efficient removal of bisphenol pollutants on imine-based covalent organic frameworks: Adsorption behavior and mechanism[J]. RSC Advances, 2021, 11(30): 18308-18320. |
| 61 | WEN Qiulin, SHE Wenzhi, LIU Jinzhou, et al. Synthesis of size-controllable urchin-like covalent organic frameworks for adsorption of nitrophenols[J]. Microporous and Mesoporous Materials, 2023, 357: 112624. |
| 62 | YOU Lijun, XU Ke, DING Guanjun, et al. Facile synthesis of Fe3O4@COF covalent organic frameworks for the adsorption of bisphenols from aqueous solution[J]. Journal of Molecular Liquids, 2020, 320: 114456. |
| 63 | LI Yanxia, ZHANG Hongna, CHEN Yiting, et al. Core-shell structured magnetic covalent organic framework nanocomposites for triclosan and triclocarban adsorption[J]. ACS Applied Materials & Interfaces, 2019, 11(25): 22492-22500. |
| 64 | ZHAO Yanli, TAO Xinfeng, LIN Jiaping, et al. Azobenzene functionalized organic covalent frameworks: Controlled morphologies and photo-regulated adsorption[J]. Advanced Functional Materials, 2023, 33(38): 2302225. |
| 65 | GENDY Eman Abdelnasser, OYEKUNLE Daniel Temitayo, IFTHIKAR Jerosha, et al. A review on the adsorption mechanism of different organic contaminants by covalent organic framework (COF) from the aquatic environment[J]. Environmental Science and Pollution Research International, 2022, 29(22): 32566-32593. |
| 66 | ZANGO Zakariyya Uba, BINZOWAIMIL Ayed M, ALDAGHRI Osamah A, et al. Applications of covalent organic frameworks for the elimination of dyes from wastewater: A state-of-the-arts review[J]. Chemosphere, 2023, 343: 140223. |
| 67 | ASLAM Awais ALI, IRSHAD Adnan, NAZIR Muhammad Shahid, et al. A review on covalent organic frameworks as adsorbents for organic pollutants[J]. Journal of Cleaner Production, 2023, 400: 136737. |
| 68 | HOU Kun, GU Haiping, YANG Yafeng, et al. Recent progress in advanced covalent organic framework composites for environmental remediation[J]. Advanced Composites and Hybrid Materials, 2023, 6(6): 199. |
| 69 | 许春树, 姚庆达, 梁永贤, 等. 共价有机框架材料功能化策略及其对Hg(Ⅵ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| XU Chunshu, YAO Qingda, LIANG Yongxian, et al. Research progress on functionalization strategies of covalent organic framework materials and their adsorption properties for Hg(Ⅱ) and Cr(Ⅵ)[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. | |
| 70 | 陈琦, 王文涛, 张志鹏, 等. 共价有机框架材料对放射性核素吸附的研究进展[J]. 化工进展, 2021, 40(S2): 241-255. |
| CHEN Qi, WANG Wentao, ZHANG Zhipeng, et al. Progress of covalent framework for radionuclides absorption[J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 241-255. | |
| 71 | RASHEED Tahir. Covalent organic frameworks as promising adsorbent paradigm for environmental pollutants from aqueous matrices: Perspective and challenges[J]. Science of the Total Environment, 2022, 833: 155279. |
| 72 | LIU Xiaolu, PANG Hongwei, LIU Xuewei, et al. Orderly porous covalent organic frameworks-based materials: Superior adsorbents for pollutants removal from aqueous solutions[J]. The Innovation, 2021, 2(1): 100076. |
| 73 | SKORJANC Tina, SHETTY Dinesh, TRABOLSI Ali. Pollutant removal with organic macrocycle-based covalent organic polymers and frameworks[J]. Chem, 2021, 7(4): 882-918. |
| 74 | ZHANG Ning, ISHAG Alhadi, LI Ying, et al. Recent investigations and progress in environmental remediation by using covalent organic framework-based adsorption method: A review[J]. Journal of Cleaner Production, 2020, 277: 123360. |
| 75 | GENDY Eman A, IFTHIKAR Jerosha, Jawad ALI, et al. Removal of heavy metals by covalent organic frameworks (COFs): A review on its mechanism and adsorption properties[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105687. |
| [1] | LIU Li, FENG Bo, WEN Yang, GU Qixiong. Research progress in synthesis, functionalization and metal adsorption of silica-based mesoporous materials [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5063-5078. |
| [2] | CAO Shuyang, SHI Jingbo, DONG Youming, LYU Jianxiong. Water adsorption and desorption isotherms and thermodynamic properties of Eucalyptus obliqua woods at different temperatures [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5095-5105. |
| [3] | WU Yuqi, LI Jiangtao, DING Jianzhi, SONG Xiulan, SU Bingqin. Calcined Mg/Al hydrotalcites for CO2 removal in anaerobic digestion biogas: Performances and mechanisms [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5250-5261. |
| [4] | YANG Xinheng, JI Zhiyong, GUO Zhiyuan, LIU Qi, ZHANG Panpan, WANG Jing, LIU Jie, BI Jingtao, ZHAO Yingying, YUAN Junsheng. Preparation of lithium aluminum layered double hydroxides and their lithium deintercalation performance [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5262-5274. |
| [5] | BIAN Weibai, ZHANG Ruixuan, PAN Jianming. Research progress on preparation methods of inorganic metal lithium ion sieve materials [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4173-4186. |
| [6] | WANG Jia, LI Wencui, WU Fan, GAO Xinqian, LU Anhui. Regulation active components distribution of NiMo/Al2O3 catalysts for hydrodesulfurization [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4393-4402. |
| [7] | ZHENG Yunxiang, GAO Yilun, LI Yanru, LIU Qinglin, ZHANG Haoteng, WANG Xiangpeng. Preparation and adsorption properties of porous double-network hydrogels modified by nitrilotriacetic acid anhydride [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4542-4549. |
| [8] | LIU Yucan, GAO Zhonglu, XU Xinyi, JI Xianguo, ZHANG Yan, SUN Hongwei, WANG Gang. Adsorption performance and mechanism of diuron from water by calcium-modified water hyacinth-based biochar [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4630-4641. |
| [9] | HU Junjie, HUANG Xingjun, LEI Cheng, YANG Min, LAN Yuanxiao, LUO Jianhong. Advanced treatment of small molecular organic in shale gas produced water [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4674-4680. |
| [10] | GUO Changbin, LI Mengmeng, FENG Menghan, YUAN Tian, ZHANG Keqiang, LUO Yanli, WANG Feng. Preparation of Ce-doped La-based perovskite and its adsorption properties for phosphate and phytic acid in water [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4748-4756. |
| [11] | MAO Huakai, YU Yang, ZHANG Yue, XIA Guangkun, WU Yuntao, LOU Leyao, NIU Wenjuan, LIU Nian. Synergistic biochar photocatalytic oxidation-adsorption for nitrite degradation [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4757-4765. |
| [12] | GONG Decheng, SHEN Qian, ZHU Xianqing, HUANG Yun, XIA Ao, ZHANG Jingmiao, ZHU Xun, LIAO Qiang. Recent progress in the production of hydrogen-rich syngas via supercritical water gasification of microalgae [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3709-3728. |
| [13] | HUANG Jun, ZHANG Yingjuan, LIN Yintong, WEI Xuechun, WU Yutong, WU Gaobo, MO Junlin, ZHAO Zhenxia, ZHAO Zhongxing. Preparation of silkworm excrement-based porous biocarbon and synergistic adsorption and slow-release performance for monosultap and dinotefuran [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3964-3971. |
| [14] | ZHANG Shirui, FAN Zhenlian, SONG Huiping, ZHANG Lina, GAO Hongyu, CHENG Shuyan, CHENG Fangqin. Research progress of fly ash supported photocatalytic materials [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4043-4058. |
| [15] | LIU Kefeng, LIU Taoran, CAI Yong, HU Xuesheng, DONG Weigang, ZHOU Huaqun, GAO Fei. Progress in research and engineering demonstration of CO2 capture technology [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2901-2914. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||