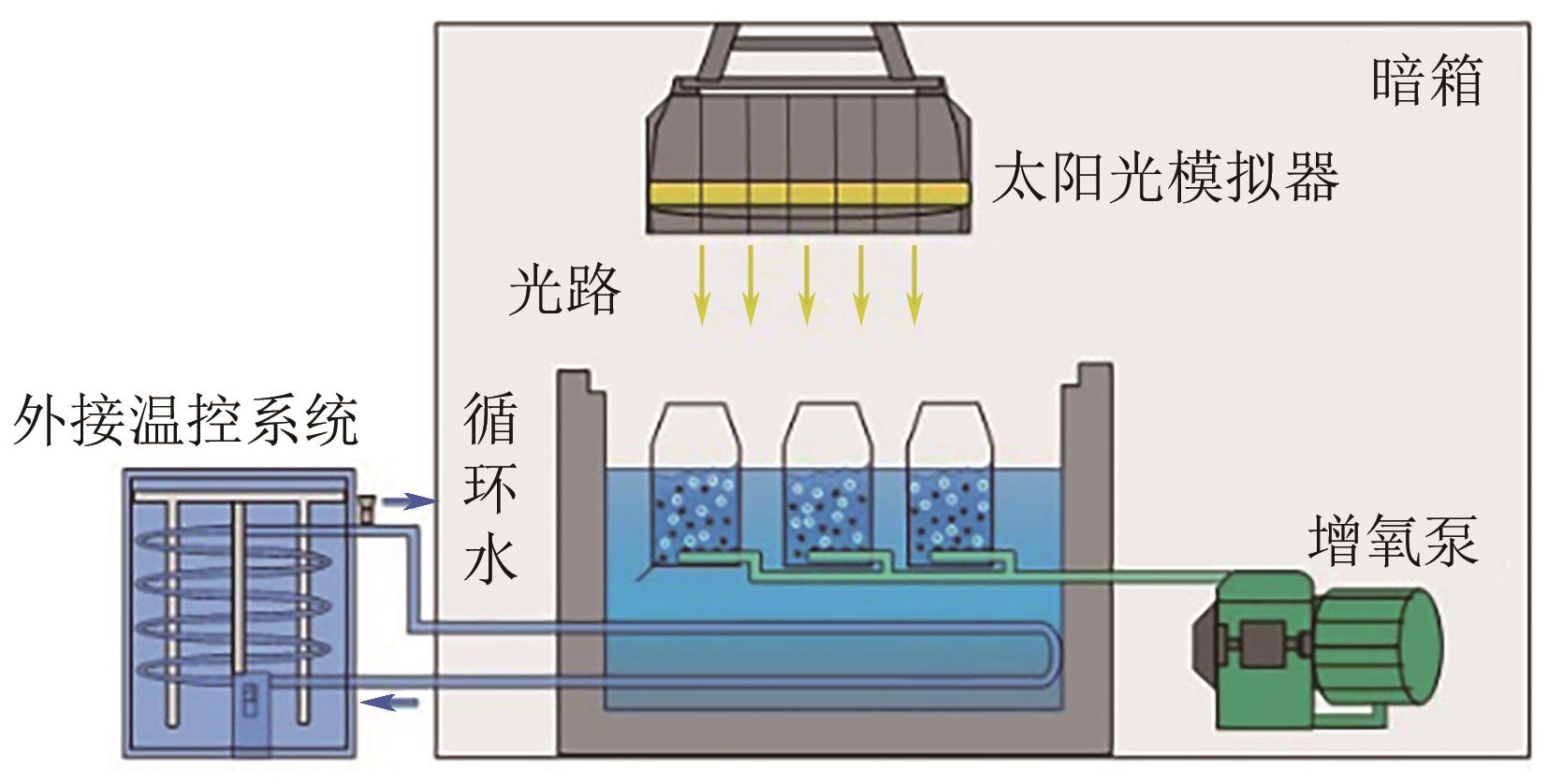
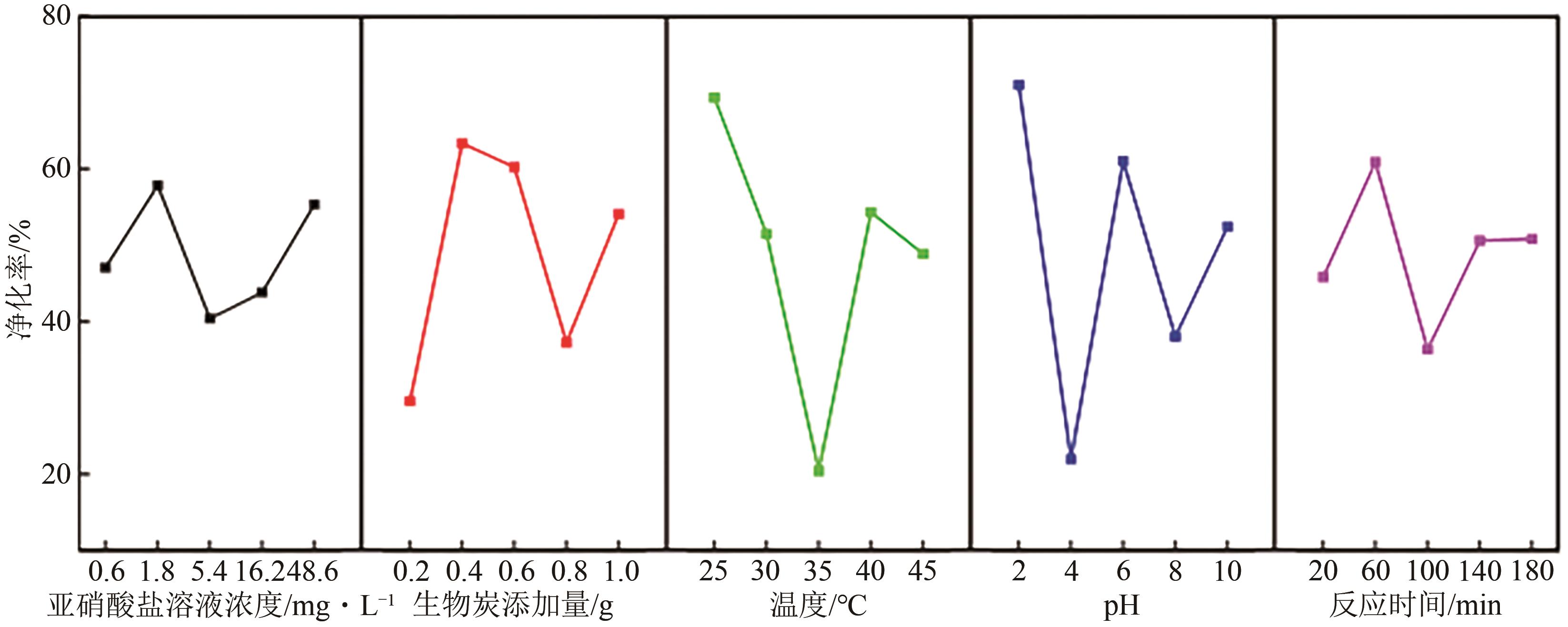
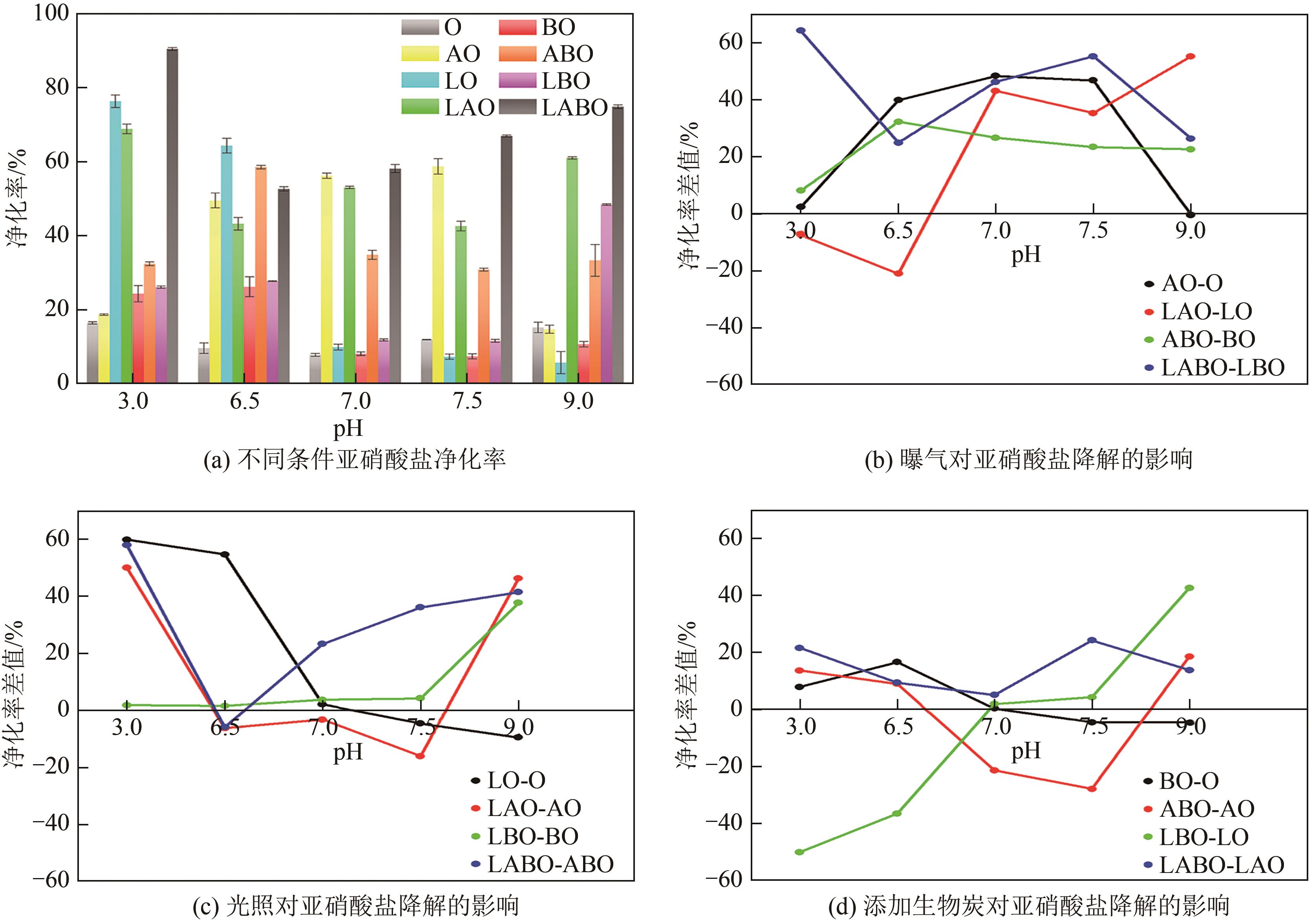
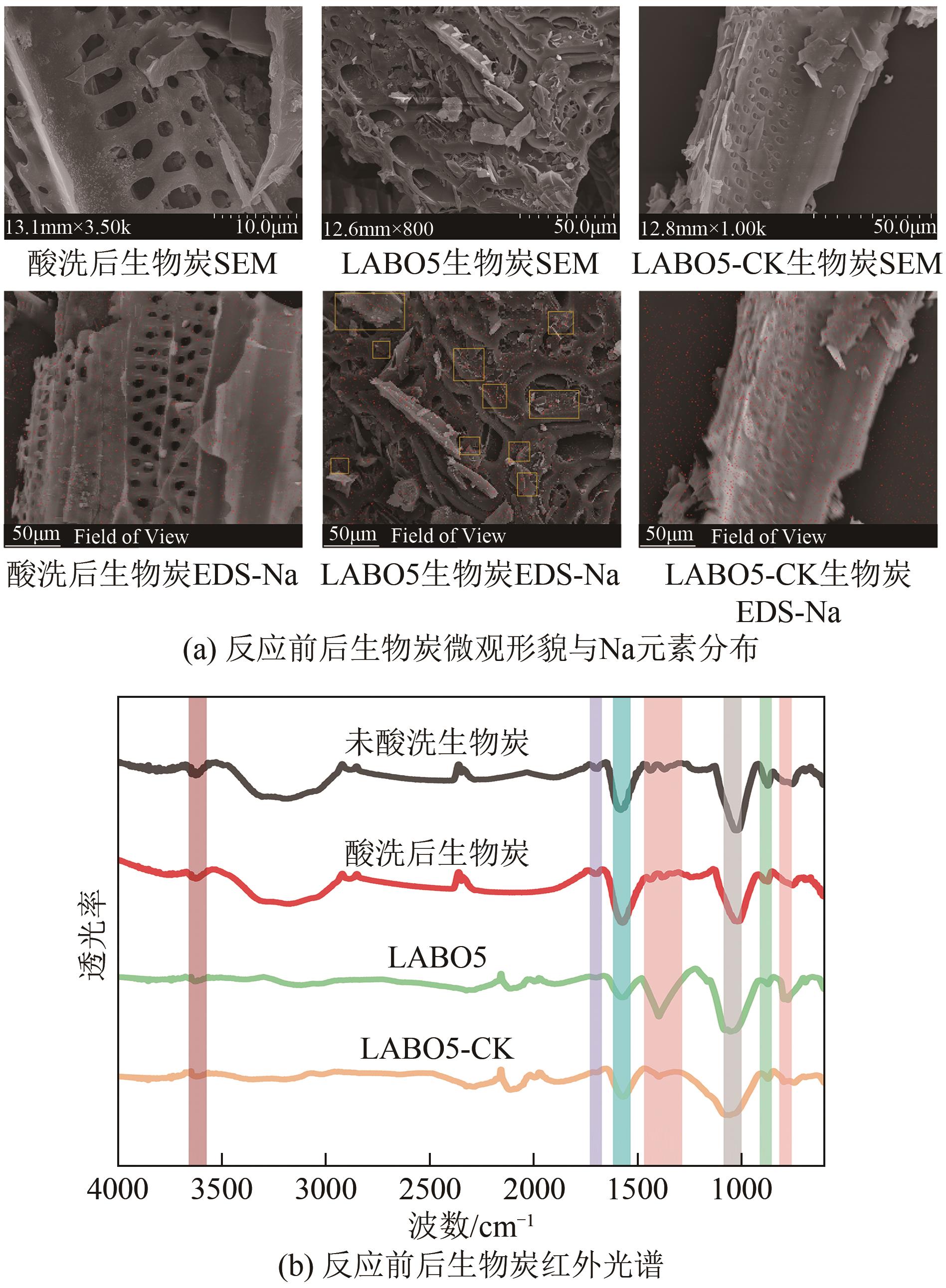
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (8): 4757-4765.DOI: 10.16085/j.issn.1000-6613.2023-1190
• Resources and environmental engineering • Previous Articles
Synergistic biochar photocatalytic oxidation-adsorption for nitrite degradation
MAO Huakai1( ), YU Yang1, ZHANG Yue1, XIA Guangkun1, WU Yuntao1, LOU Leyao1, NIU Wenjuan1,2,3, LIU Nian1,2,3(
), YU Yang1, ZHANG Yue1, XIA Guangkun1, WU Yuntao1, LOU Leyao1, NIU Wenjuan1,2,3, LIU Nian1,2,3( )
)
- 1.College of Engineering, Huazhong Agricultural University, Wuhan 430070, Hubei, China
2.Key Laboratory of Aquaculture Facilities Engineering, Ministry of Agriculture and Rural Affairs, Wuhan 430070, Hubei, China
3.Agricultural Equipment Laboratory of the Middle and Lower Reaches of the Yangtze River, Ministry of Agriculture and Rural Affairs, Wuhan 430070, Hubei, China
-
Received:2023-07-13Revised:2023-09-07Online:2024-09-02Published:2024-08-15 -
Contact:LIU Nian
生物炭光催化氧化-吸附协同降解亚硝酸盐
毛华恺1( ), 余洋1, 张悦1, 夏广坤1, 吴赟韬1, 楼乐瑶1, 牛文娟1,2,3, 刘念1,2,3(
), 余洋1, 张悦1, 夏广坤1, 吴赟韬1, 楼乐瑶1, 牛文娟1,2,3, 刘念1,2,3( )
)
- 1.华中农业大学工学院,湖北 武汉 430070
2.农业农村部水产养殖设施工程重点实验室,湖北 武汉 430070
3.农业农村部长江中下游农业装备重点实验室,湖北 武汉 430070
-
通讯作者:刘念 -
作者简介:毛华恺,(2002—),男,本科生,研究方向为种养废水光催化降解。E-mail:mhk@webmail.hzau.edu.cn。 -
基金资助:国家自然科学基金(32201685);湖北省创新创业训练项目(S202310504186)
CLC Number:
Cite this article
MAO Huakai, YU Yang, ZHANG Yue, XIA Guangkun, WU Yuntao, LOU Leyao, NIU Wenjuan, LIU Nian. Synergistic biochar photocatalytic oxidation-adsorption for nitrite degradation[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4757-4765.
毛华恺, 余洋, 张悦, 夏广坤, 吴赟韬, 楼乐瑶, 牛文娟, 刘念. 生物炭光催化氧化-吸附协同降解亚硝酸盐[J]. 化工进展, 2024, 43(8): 4757-4765.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1190
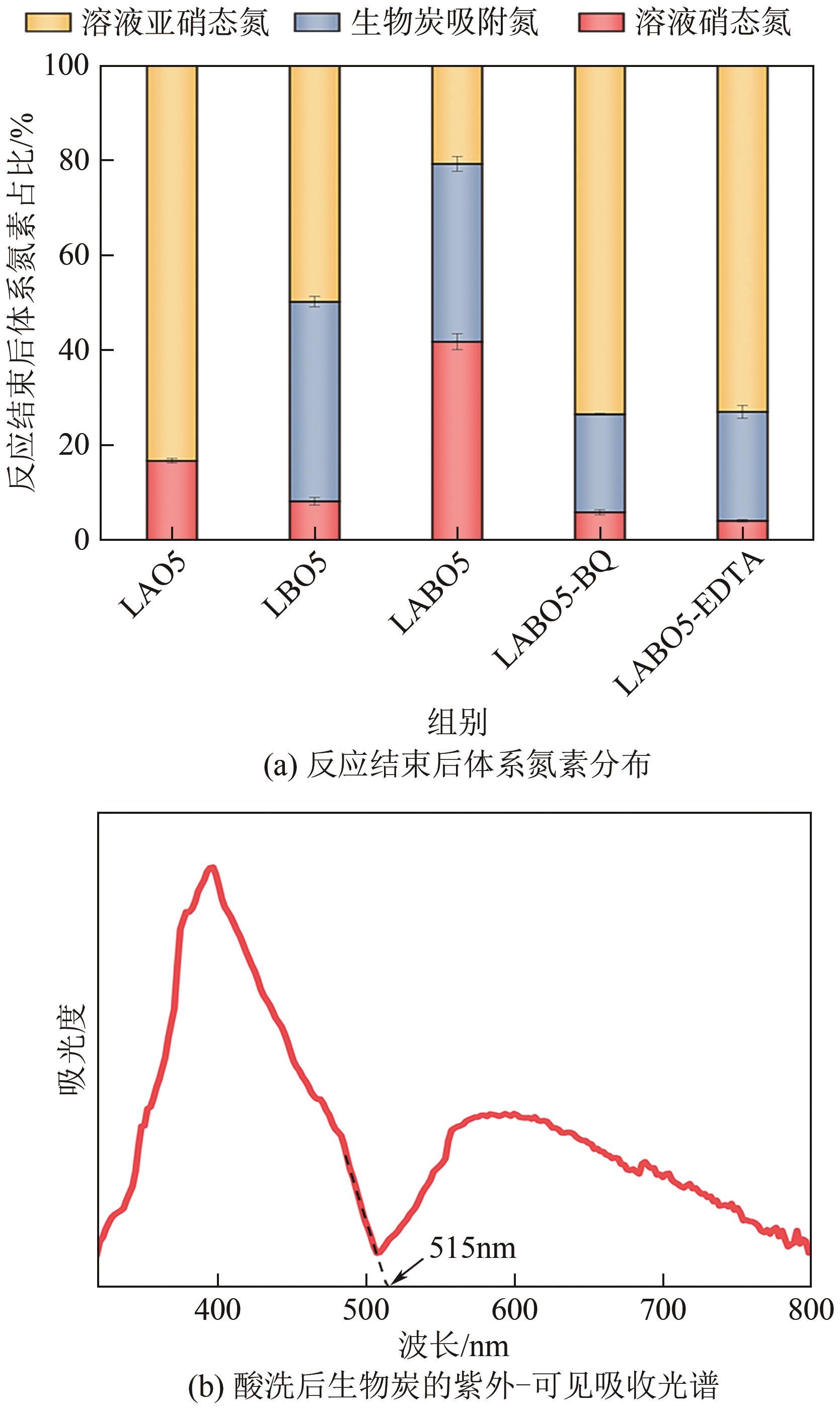
| 元素组成 | 质量分数/% |
|---|---|
| C | 42.72 |
| H | 2.58 |
| O | 47.79 |
| N | 1.20 |
| S | 0.55 |
| K | 1.43 |
| Ca | 0.57 |
| Mg | 0.78 |
| 元素组成 | 质量分数/% |
|---|---|
| C | 42.72 |
| H | 2.58 |
| O | 47.79 |
| N | 1.20 |
| S | 0.55 |
| K | 1.43 |
| Ca | 0.57 |
| Mg | 0.78 |
| 水平 | α/mg·L-1 | β/g | γ/℃ | δ | ε/min |
|---|---|---|---|---|---|
| 1 | 0.6 | 0.2 | 25 | 2 | 20 |
| 2 | 1.8 | 0.4 | 30 | 4 | 60 |
| 3 | 5.4 | 0.6 | 35 | 6 | 100 |
| 4 | 16.2 | 0.8 | 40 | 8 | 140 |
| 5 | 48.6 | 1.0 | 45 | 10 | 180 |
| 水平 | α/mg·L-1 | β/g | γ/℃ | δ | ε/min |
|---|---|---|---|---|---|
| 1 | 0.6 | 0.2 | 25 | 2 | 20 |
| 2 | 1.8 | 0.4 | 30 | 4 | 60 |
| 3 | 5.4 | 0.6 | 35 | 6 | 100 |
| 4 | 16.2 | 0.8 | 40 | 8 | 140 |
| 5 | 48.6 | 1.0 | 45 | 10 | 180 |
| 试验组 | 编号 | L | A | B | pH | 酸碱度 |
|---|---|---|---|---|---|---|
| LABO | LABO1 | √ | √ | √ | 3 | 强酸 |
| LABO | LABO2 | √ | √ | √ | 6.5 | 弱酸 |
| LABO | LABO3 | √ | √ | √ | 7 | 中性 |
| LABO | LABO4 | √ | √ | √ | 7.5 | 弱碱 |
| LABO | LABO5 | √ | √ | √ | 9 | 强碱 |
| ABO | ABO1 | × | √ | √ | 3 | 强酸 |
| ABO | ABO2 | × | √ | √ | 6.5 | 弱酸 |
| ABO | ABO3 | × | √ | √ | 7 | 中性 |
| ABO | ABO4 | × | √ | √ | 7.5 | 弱碱 |
| ABO | ABO5 | × | √ | √ | 9 | 强碱 |
| LBO | LBO1 | √ | × | √ | 3 | 强酸 |
| LBO | LBO2 | √ | × | √ | 6.5 | 弱酸 |
| LBO | LBO3 | √ | × | √ | 7 | 中性 |
| LBO | LBO4 | √ | × | √ | 7.5 | 弱碱 |
| LBO | LBO5 | √ | × | √ | 9 | 强碱 |
| LAO | LAO1 | √ | √ | × | 3 | 强酸 |
| LAO | LAO2 | √ | √ | × | 6.5 | 弱酸 |
| LAO | LAO3 | √ | √ | × | 7 | 中性 |
| LAO | LAO4 | √ | √ | × | 7.5 | 弱碱 |
| LAO | LAO5 | √ | √ | × | 9 | 强碱 |
| BO | BO1 | × | × | √ | 3 | 强酸 |
| BO | BO2 | × | × | √ | 6.5 | 弱酸 |
| BO | BO3 | × | × | √ | 7 | 中性 |
| BO | BO4 | × | × | √ | 7.5 | 弱碱 |
| BO | BO5 | × | × | √ | 9 | 强碱 |
| AO | AO1 | × | √ | × | 3 | 强酸 |
| AO | AO2 | × | √ | × | 6.5 | 弱酸 |
| AO | AO3 | × | √ | × | 7 | 中性 |
| AO | AO4 | × | √ | × | 7.5 | 弱碱 |
| AO | AO5 | × | √ | × | 9 | 强碱 |
| LO | LO1 | √ | × | × | 3 | 强酸 |
| LO | LO2 | √ | × | × | 6.5 | 弱酸 |
| LO | LO3 | √ | × | × | 7 | 中性 |
| LO | LO4 | √ | × | × | 7.5 | 弱碱 |
| LO | LO5 | √ | × | × | 9 | 强碱 |
| O | O1 | × | × | × | 3 | 强酸 |
| O | O2 | × | × | × | 6.5 | 弱酸 |
| O | O3 | × | × | × | 7 | 中性 |
| O | O4 | × | × | × | 7.5 | 弱碱 |
| O | O5 | × | × | × | 9 | 强碱 |
| 试验组 | 编号 | L | A | B | pH | 酸碱度 |
|---|---|---|---|---|---|---|
| LABO | LABO1 | √ | √ | √ | 3 | 强酸 |
| LABO | LABO2 | √ | √ | √ | 6.5 | 弱酸 |
| LABO | LABO3 | √ | √ | √ | 7 | 中性 |
| LABO | LABO4 | √ | √ | √ | 7.5 | 弱碱 |
| LABO | LABO5 | √ | √ | √ | 9 | 强碱 |
| ABO | ABO1 | × | √ | √ | 3 | 强酸 |
| ABO | ABO2 | × | √ | √ | 6.5 | 弱酸 |
| ABO | ABO3 | × | √ | √ | 7 | 中性 |
| ABO | ABO4 | × | √ | √ | 7.5 | 弱碱 |
| ABO | ABO5 | × | √ | √ | 9 | 强碱 |
| LBO | LBO1 | √ | × | √ | 3 | 强酸 |
| LBO | LBO2 | √ | × | √ | 6.5 | 弱酸 |
| LBO | LBO3 | √ | × | √ | 7 | 中性 |
| LBO | LBO4 | √ | × | √ | 7.5 | 弱碱 |
| LBO | LBO5 | √ | × | √ | 9 | 强碱 |
| LAO | LAO1 | √ | √ | × | 3 | 强酸 |
| LAO | LAO2 | √ | √ | × | 6.5 | 弱酸 |
| LAO | LAO3 | √ | √ | × | 7 | 中性 |
| LAO | LAO4 | √ | √ | × | 7.5 | 弱碱 |
| LAO | LAO5 | √ | √ | × | 9 | 强碱 |
| BO | BO1 | × | × | √ | 3 | 强酸 |
| BO | BO2 | × | × | √ | 6.5 | 弱酸 |
| BO | BO3 | × | × | √ | 7 | 中性 |
| BO | BO4 | × | × | √ | 7.5 | 弱碱 |
| BO | BO5 | × | × | √ | 9 | 强碱 |
| AO | AO1 | × | √ | × | 3 | 强酸 |
| AO | AO2 | × | √ | × | 6.5 | 弱酸 |
| AO | AO3 | × | √ | × | 7 | 中性 |
| AO | AO4 | × | √ | × | 7.5 | 弱碱 |
| AO | AO5 | × | √ | × | 9 | 强碱 |
| LO | LO1 | √ | × | × | 3 | 强酸 |
| LO | LO2 | √ | × | × | 6.5 | 弱酸 |
| LO | LO3 | √ | × | × | 7 | 中性 |
| LO | LO4 | √ | × | × | 7.5 | 弱碱 |
| LO | LO5 | √ | × | × | 9 | 强碱 |
| O | O1 | × | × | × | 3 | 强酸 |
| O | O2 | × | × | × | 6.5 | 弱酸 |
| O | O3 | × | × | × | 7 | 中性 |
| O | O4 | × | × | × | 7.5 | 弱碱 |
| O | O5 | × | × | × | 9 | 强碱 |
| 误差源 | SS | df | MS | F | 显著性 |
|---|---|---|---|---|---|
| α | 2275.34 | 4 | 568.84 | 1.51 | - |
| β | 8851.34 | 4 | 2212.84 | 5.87 | - |
| γ | 12785.26 | 4 | 3196.31 | 8.48 | ** |
| δ | 14891.02 | 4 | 3722.75 | 9.88 | ** |
| ε | 3113.10 | 4 | 778.28 | 2.06 | - |
| 空列 | 10879.21 | 4 | 2719.80 | 7.22 | - |
| 误差 | 10922.71 | 25 | 376.65 | — | — |
| 总和 | 52825.84 | 49 | — | — | — |
| 误差源 | SS | df | MS | F | 显著性 |
|---|---|---|---|---|---|
| α | 2275.34 | 4 | 568.84 | 1.51 | - |
| β | 8851.34 | 4 | 2212.84 | 5.87 | - |
| γ | 12785.26 | 4 | 3196.31 | 8.48 | ** |
| δ | 14891.02 | 4 | 3722.75 | 9.88 | ** |
| ε | 3113.10 | 4 | 778.28 | 2.06 | - |
| 空列 | 10879.21 | 4 | 2719.80 | 7.22 | - |
| 误差 | 10922.71 | 25 | 376.65 | — | — |
| 总和 | 52825.84 | 49 | — | — | — |
| 实验 | α/mg·L-1 | β/g | γ/℃ | δ | ε/min | ζ(空列) | η/% | 标准偏差 |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.6 | 0.2 | 25 | 2 | 20 | 1 | 42.50 | 0.000 |
| 2 | 0.6 | 0.4 | 30 | 4 | 60 | 2 | 54.00 | 0.000 |
| 3 | 0.6 | 0.6 | 35 | 6 | 100 | 3 | 29.85 | 0.017 |
| 4 | 0.6 | 0.8 | 40 | 8 | 140 | 4 | 30.14 | 0.014 |
| 5 | 0.6 | 1.0 | 45 | 10 | 180 | 5 | 78.87 | 0.004 |
| 6 | 1.8 | 0.2 | 30 | 6 | 140 | 5 | 76.11 | 0.010 |
| 7 | 1.8 | 0.4 | 35 | 8 | 180 | 1 | 10.16 | 0.006 |
| 8 | 1.8 | 0.6 | 40 | 10 | 20 | 2 | 79.88 | 0.007 |
| 9 | 1.8 | 0.8 | 45 | 2 | 60 | 3 | 80.55 | 0.005 |
| 10 | 1.8 | 1.0 | 25 | 1 | 100 | 4 | 42.50 | 0.000 |
| 11 | 5.4 | 0.2 | 35 | 10 | 60 | 4 | 6.62 | 0.019 |
| 12 | 5.4 | 0.4 | 40 | 2 | 100 | 5 | 91.06 | 0.003 |
| 13 | 5.4 | 0.6 | 45 | 4 | 140 | 1 | 1.77 | 0.011 |
| 14 | 5.4 | 0.8 | 25 | 6 | 180 | 2 | 68.06 | 0.000 |
| 15 | 5.4 | 1.0 | 30 | 8 | 20 | 3 | 34.51 | 0.016 |
| 16 | 16.2 | 0.2 | 40 | 4 | 180 | 3 | 5.23 | 0.005 |
| 17 | 16.2 | 0.4 | 45 | 6 | 20 | 4 | 65.78 | 0.011 |
| 18 | 16.2 | 0.6 | 25 | 8 | 60 | 5 | 97.93 | 0.001 |
| 19 | 16.2 | 0.8 | 30 | 10 | 100 | 1 | 0.97 | 0.009 |
| 20 | 16.2 | 1.0 | 35 | 2 | 140 | 2 | 49.14 | 0.001 |
| 21 | 48.6 | 0.2 | 45 | 8 | 100 | 2 | 17.38 | 0.004 |
| 22 | 48.6 | 0.4 | 25 | 10 | 140 | 3 | 95.78 | 0.000 |
| 23 | 48.6 | 0.6 | 30 | 2 | 180 | 4 | 91.77 | 0.002 |
| 24 | 48.6 | 0.8 | 35 | 4 | 20 | 5 | 6.42 | 0.023 |
| 25 | 48.6 | 1.0 | 40 | 6 | 60 | 1 | 65.34 | 0.000 |
| 实验 | α/mg·L-1 | β/g | γ/℃ | δ | ε/min | ζ(空列) | η/% | 标准偏差 |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.6 | 0.2 | 25 | 2 | 20 | 1 | 42.50 | 0.000 |
| 2 | 0.6 | 0.4 | 30 | 4 | 60 | 2 | 54.00 | 0.000 |
| 3 | 0.6 | 0.6 | 35 | 6 | 100 | 3 | 29.85 | 0.017 |
| 4 | 0.6 | 0.8 | 40 | 8 | 140 | 4 | 30.14 | 0.014 |
| 5 | 0.6 | 1.0 | 45 | 10 | 180 | 5 | 78.87 | 0.004 |
| 6 | 1.8 | 0.2 | 30 | 6 | 140 | 5 | 76.11 | 0.010 |
| 7 | 1.8 | 0.4 | 35 | 8 | 180 | 1 | 10.16 | 0.006 |
| 8 | 1.8 | 0.6 | 40 | 10 | 20 | 2 | 79.88 | 0.007 |
| 9 | 1.8 | 0.8 | 45 | 2 | 60 | 3 | 80.55 | 0.005 |
| 10 | 1.8 | 1.0 | 25 | 1 | 100 | 4 | 42.50 | 0.000 |
| 11 | 5.4 | 0.2 | 35 | 10 | 60 | 4 | 6.62 | 0.019 |
| 12 | 5.4 | 0.4 | 40 | 2 | 100 | 5 | 91.06 | 0.003 |
| 13 | 5.4 | 0.6 | 45 | 4 | 140 | 1 | 1.77 | 0.011 |
| 14 | 5.4 | 0.8 | 25 | 6 | 180 | 2 | 68.06 | 0.000 |
| 15 | 5.4 | 1.0 | 30 | 8 | 20 | 3 | 34.51 | 0.016 |
| 16 | 16.2 | 0.2 | 40 | 4 | 180 | 3 | 5.23 | 0.005 |
| 17 | 16.2 | 0.4 | 45 | 6 | 20 | 4 | 65.78 | 0.011 |
| 18 | 16.2 | 0.6 | 25 | 8 | 60 | 5 | 97.93 | 0.001 |
| 19 | 16.2 | 0.8 | 30 | 10 | 100 | 1 | 0.97 | 0.009 |
| 20 | 16.2 | 1.0 | 35 | 2 | 140 | 2 | 49.14 | 0.001 |
| 21 | 48.6 | 0.2 | 45 | 8 | 100 | 2 | 17.38 | 0.004 |
| 22 | 48.6 | 0.4 | 25 | 10 | 140 | 3 | 95.78 | 0.000 |
| 23 | 48.6 | 0.6 | 30 | 2 | 180 | 4 | 91.77 | 0.002 |
| 24 | 48.6 | 0.8 | 35 | 4 | 20 | 5 | 6.42 | 0.023 |
| 25 | 48.6 | 1.0 | 40 | 6 | 60 | 1 | 65.34 | 0.000 |
| 参数 | α | β | γ | δ | ε | ζ |
|---|---|---|---|---|---|---|
| K1 | 235.36 | 147.83 | 346.77 | 355.01 | 235.36 | 120.74 |
| K2 | 289.18 | 316.77 | 257.36 | 109.92 | 289.18 | 268.46 |
| K3 | 202.01 | 301.20 | 102.19 | 305.13 | 202.01 | 245.92 |
| K4 | 219.06 | 186.13 | 271.64 | 190.12 | 219.06 | 236.80 |
| K5 | 276.68 | 270.37 | 244.34 | 262.11 | 276.68 | 350.39 |
| R | 87.17 | 168.93 | 244.58 | 245.09 | 122.68 | 229.65 |
| 参数 | α | β | γ | δ | ε | ζ |
|---|---|---|---|---|---|---|
| K1 | 235.36 | 147.83 | 346.77 | 355.01 | 235.36 | 120.74 |
| K2 | 289.18 | 316.77 | 257.36 | 109.92 | 289.18 | 268.46 |
| K3 | 202.01 | 301.20 | 102.19 | 305.13 | 202.01 | 245.92 |
| K4 | 219.06 | 186.13 | 271.64 | 190.12 | 219.06 | 236.80 |
| K5 | 276.68 | 270.37 | 244.34 | 262.11 | 276.68 | 350.39 |
| R | 87.17 | 168.93 | 244.58 | 245.09 | 122.68 | 229.65 |
| 1 | BASAK Bikram, BHUNIA Biswanath, DUTTA Subhasish, et al. Enhanced biodegradation of 4-chlorophenol by Candida tropicalis PHB5 via optimization of physicochemical parameters using Taguchi orthogonal array approach[J]. International Biodeterioration & Biodegradation, 2013, 78: 17-23. |
| 2 | AMORIM Camila C, LEÃO Mônica M D, MOREIRA Regina F P M, et al. Performance of blast furnace waste for azo dye degradation through photo-Fenton-like processes[J]. Chemical Engineering Journal, 2013, 224(1): 59-66. |
| 3 | 于兵川, 吴洪特, 张万忠. 光催化纳米材料在环境保护中的应用[J]. 石油化工, 2005, 34(5): 491-495. |
| YU Bingchuan, WU Hongte, ZHANG Wanzhong. Application of nano-photocatalysts in environmental protection[J]. Petrochemical Technology, 2005, 34(5): 491-495. | |
| 4 | WANG Hao, WANG Jing, XIANG Xin, et al. Preparation of PVDF/CdS/Bi2WO6/ZnO hybrid membrane with enhanced visible-light photocatalytic activity for degrading nitrite in water[J]. Environmental Research, 2020, 191: 110036. |
| 5 | BOUKHEMIKHEM Z, REKHILA G, BRAHIMI R, et al. Photocatalytic NO2-oxidation on the hetero-junction Ag(5%)/NiFe2O4 prepared by sol gel route[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2020, 394: 112454. |
| 6 | FU Dongying, HAN Gaoyi, CHANG Yunzhen, et al. The synthesis and properties of ZnO-graphene nano hybrid for photodegradation of organic pollutant in water[J]. Materials Chemistry and Physics, 2012, 132(2/3): 673-681. |
| 7 | 魏宏斌, 徐迪民. 固定相TiO2膜的制备及其光催化活性[J]. 中国给水排水, 2002, 18(7): 57-59. |
| WEI Hongbin, XU Dimin. Preparation and photocatalytic activity of fixed phase TiO2 film[J]. China Water & Wastewater, 2002,18(7): 57-59. | |
| 8 | ZHANG Lei, JIANG Daochuan, IRFAN Rana Muhammad, et al. Highly efficient and selective photocatalytic dehydrogenation of benzyl alcohol for simultaneous hydrogen and benzaldehyde production over Ni-decorated Zn0.5Cd0.5S solid solution[J]. Journal of Energy Chemistry, 2019, 30(3): 71-77. |
| 9 | LI Xin, LIN Jing, ZHANG Di, et al. Material flow analysis of titanium dioxide and sustainable policy suggestion in China[J]. Resources Policy, 2020, 67: 101685. |
| 10 | SAJAN Chimmikuttanda Ponnappa, WAGEH Swelm, AL-GHAMDI Ahmed A, et al. TiO2 nanosheets with exposed {001} facets for photocatalytic applications[J]. Nano Research, 2016, 9(1): 3-27. |
| 11 | LU Haiqiang, ZHAO Jianghong, LI Li, et al. Selective oxidation of sacrificial ethanol over TiO2-based photocatalysts during water splitting[J]. Energy & Environmental Science, 2011, 4(9): 3384-3388. |
| 12 | YASUDA Masahide, MATSUMOTO Tomoko, YAMASHITA Toshiaki. Sacrificial hydrogen production over TiO2-based photocatalysts: Polyols, carboxylic acids, and saccharides[J]. Renewable and Sustainable Energy Reviews, 2018, 81: 1627-1635. |
| 13 | TESTA Juan J, GRELA María A, LITTER Marta I. Heterogeneous photocatalytic reduction of chromium(Ⅵ) over TiO2 particles in the presence of oxalate: Involvement of Cr(Ⅴ) species[J]. Environmental Science & Technology, 2004, 38(5): 1589-1594. |
| 14 | 陈杰, 肖玉婷, 汪楠, 等. 简易合成具有增强电荷转移的Z型CeO2/C3N4异质结用于光催化CO2还原[J]. Science China Materials, 2023, 66(8): 3165-3175. |
| CHEN Jie, XIAO Yuting, WANG Nan, et al. Facile synthesis of a Z-scheme CeO2/C3N4 heterojunction with enhanced charge transfer for CO2 photoreduction[J]. Science China Materials, 2023, 66(8): 3165-3175. | |
| 15 | LUO Jinhua, WU Yaohui, JIANG Mengzhu, et al. Novel ZnFe2O4/BC/ZnO photocatalyst for high-efficiency degradation of tetracycline under visible light irradiation[J]. Chemosphere, 2023, 311(1): 137041. |
| 16 | XIA Qi, HUANG Binbin, YUAN Xingzhong, et al. Modified stannous sulfide nanoparticles with metal-organic framework: Toward efficient and enhanced photocatalytic reduction of chromium (Ⅵ) under visible light[J]. Journal of Colloid and Interface Science, 2018, 530: 481-492. |
| 17 | DJELLABI Ridha, YANG Bo, WANG Yan, et al. Carbonaceous biomass-titania composites with TiOC bonding bridge for efficient photocatalytic reduction of Cr(Ⅵ) under narrow visible light[J]. Chemical Engineering Journal, 2019, 366: 172-180. |
| 18 | Dinh Ngoc Giao NGO, CHUANG Xiangying, HUANG Chin-Pao, et al. Compositional characterization of nine agricultural waste biochars: The relations between alkaline metals and cation exchange capacity with ammonium adsorption capability[J]. Journal of Environmental Chemical Engineering, 2023, 11(3): 110003. |
| 19 | HUFF Matthew D, LEE James W. Biochar-surface oxygenation with hydrogen peroxide[J]. Journal of Environmental Management, 2016, 165: 17-21. |
| 20 | LIU Yurong, PASKEVICIUS Mark, Veronica SOFIANOS M, et al. A SAXS study of the pore structure evolution in biochar during gasification in H2O, CO2 and H2O/CO2 [J]. Fuel, 2021, 292: 120384. |
| 21 | 周石洋, 陈玲. 食品中亚硝酸盐含量测定的研究[J]. 食品安全质量检测学报, 2011, 2(6): 285-289. |
| ZHOU Shiyang, CHEN Ling. Study on the determination of nitrite in food[J]. Journal of Food Safety & Quality, 2011, 2(6): 285-289. | |
| 22 | 中华人民共和国自然资源部. 地下水质分析方法 硝酸盐的测定紫外分光光度法: [S]. 北京: 中国标准出版社, 2021. |
| Ministry of Natural Resources of the People’s Republic of China. Analysis methods for groundwater quality—Determination of nitrate ultraviolet spectrophotometric method: [S]. Beijing: Standards Press of China, 2021. | |
| 23 | 栗秀萍, 于洋, 何旺, 等. 超重力强化AMP-PZ复合溶液脱碳技术及表观动力学[J]. 化工进展, 2022, 41(S1): 22-28. |
| LI Xiuping, YU Yang, HE Wang, et al. High-gravity intensified decarburization process and apparent kinetics of AMP-PZ composite solution[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 22-28. | |
| 24 | 中本一雄, 黄德如. 无机和配位化合物的红外和拉曼光谱[M]. 北京: 化学工业出版社,1986. |
| KAZUO Nakamoto, HUANG Deru. Inrared and Raman spectra of inorganic and coordination compounds[M]. Beijing: Chemical Industry Press, 1986. | |
| 25 | 黄安香, 杨定云, 杨守禄, 等. 改性生物炭对土壤重金属污染修复研究进展[J]. 化工进展, 2020, 39(12): 5266-5274. |
| HUANG Anxiang, YANG Dingyun, YANG Shoulu, et al. Advance in remediation of heavy metal pollution in soil by modified biochar[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 5266-5274. | |
| 26 | 宋江生, 管益东, 师杨杰, 等. 杨木基生物炭吸附去除水溶液中磺胺吡啶[J]. 净水技术, 2023, 42(7): 90-97. |
| SONG Jiangsheng, GUAN Yidong, SHI Yangjie, et al. Poplar based biochars for adsorption and removal of sulfapyridine in aqueous solution[J]. Water Purification Technology, 2023, 42(7): 90-97. | |
| 27 | 钟来元, 廖荣骏, 刘付宇杰, 等. KOH改性花生壳生物炭对盐酸四环素的吸附性能及其机理[J]. 农业环境科学学报, 2023, 42(9): 2038-2048. |
| ZHONG Laiyuan, LIAO Rongjun, LIUFU Yujie, et al. Adsorption of tetracycline hydrochloride by KOH modified peanut shell biochar and its mechanism[J]. Journal of Agro-Environment Science, 2023, 42(9): 2038-2048. | |
| 28 | 杨婷婷, 黄艳艳, 柳维扬, 等. 三种改性小麦秸秆生物炭表征及其对Cu2+的吸附性能[J]. 农业工程学报, 2023, 39(8): 222-230. |
| YANG Tingting, HUANG Yanyan, LIU Weiyang, et al. Characterization of three kinds of modified wheat straw derived biochars and their sorption capacity for Cu2+ [J]. Transactions of the Chinese Society of Agricultural Engineering, 2023, 39(8): 222-230. | |
| 29 | 王金玉, 王赛男, 卢佳宏, 等. 盐酸改性豆渣不溶性膳食纤维对亚硝酸盐的吸附特性及机理[J]. 大豆科学, 2022, 41(4): 463-471. |
| WANG Jinyu, WANG Sainan, LU Jiahong, et al. Adsorption characteristics and mechanism of hydrochloric acid modified okara insoluble dietary fiber to nitrite[J]. Soybean Science, 2022, 41(4): 463-471. | |
| 30 | 庄远红, 刘静娜, 费鹏, 等. 模拟胃环境下柚皮果胶对亚硝酸根的吸附动力学[J]. 西南大学学报(自然科学版), 2018, 40(12): 65-72. |
| ZHUANG Yuanhong, LIU Jingna, FEI Peng, et al. Adsorption kinetics of pectin from pomelo peelon nitrite in a simulated gastric environment[J]. Journal of Southwest University(Natural Science Edition), 2018, 40(12): 65-72. | |
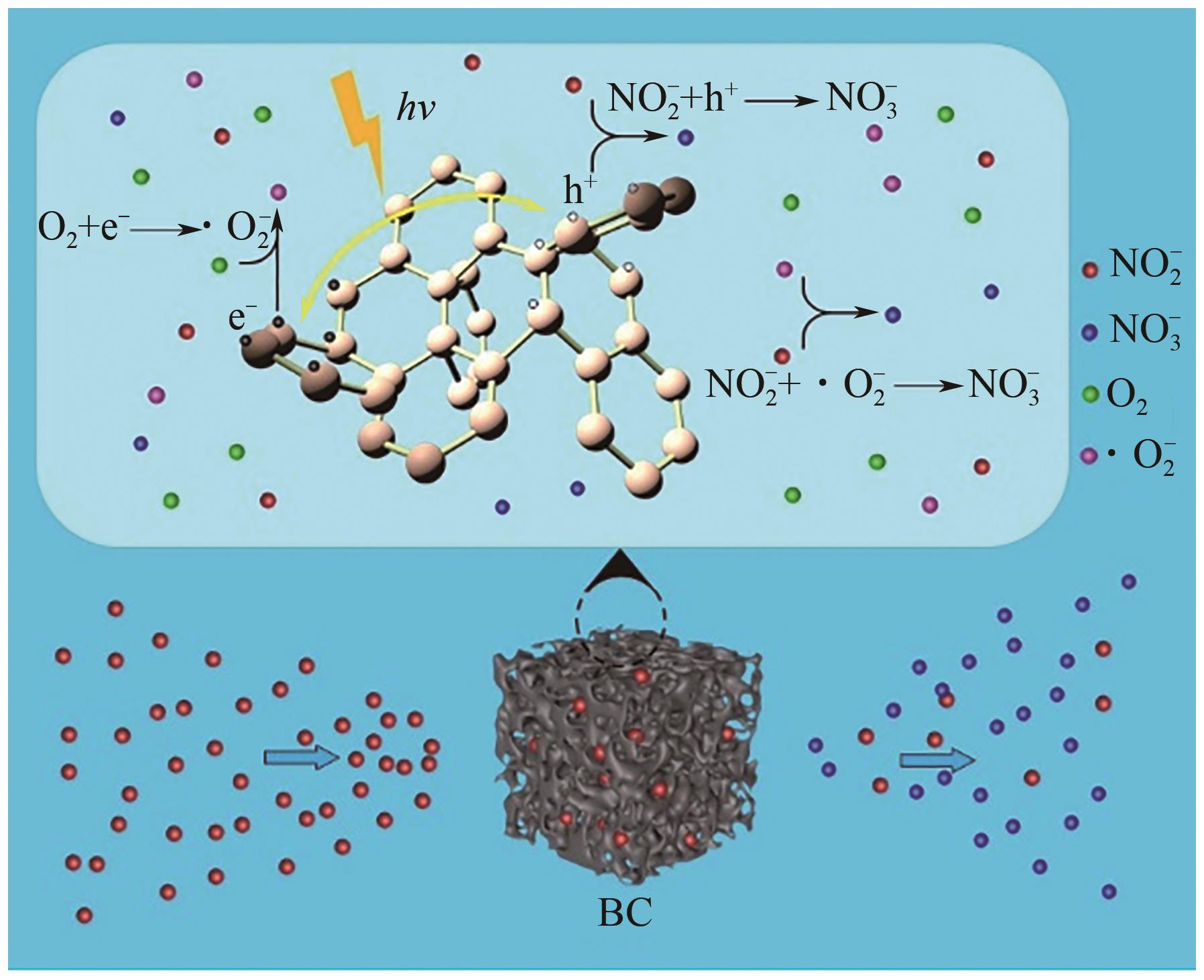
| 31 | 吴丹萍, 陈全, 李东梅, 等. 生物炭含氧官能团的生成溯源及其在污染物吸附-降解过程中的作用[J]. 环境化学, 2021, 40(10): 3190-3198. |
| WU Danping, CHEN Quan, LI Dongmei, et al. Traceability of oxygen-containing functional groups in biochars and their roles in the adsorption-degradation of contaminants[J]. Environmental Chemistry, 2021, 40(10): 3190-3198. | |
| 32 | 向欣. CdS/Bi2S3/Bi2MoO6光催化膜耦合芽孢杆菌协同降解亚硝酸盐[D]. 南宁: 广西大学, 2021. |
| XIANG Xin. Synergistic Degradation of nitrite by CdS/Bi2S3/Bi2MoO6 photocatalytic membrane coupled bacillus[D]. Nanning: Guangxi University, 2021. | |
| 33 | GOMAA Hosam M, SAUDI H A, YAHIA I S, et al. Influence of graphene nanopowder impurities on the structural and optical properties of sodium borate copper-based glass: Towards UV/NIR shielding materials[J]. Solid State Sciences, 2022, 129: 106911. |
| 34 | 李法云, 李佳宇, 吝美霞, 等. 大豆秸秆生物炭负载石墨相氮化碳对土壤石油烃的光催化降解[J]. 应用基础与工程科学学报, 2022, 30(3): 519-529. |
| LI Fayun, LI Jiayu, LIN Meixia, et al. Photocatalytic degradation of soil petroleum hydrocarbons by biochar supported graphite phase carbon nitride[J]. Journal of Basic Science and Engineering, 2022, 30(3): 519-529. | |
| 35 | 曹雪娟, 单柏林, 邓梅, 等. Fe掺杂g-C3N4光催化剂的制备及光催化性能研究[J]. 重庆交通大学学报(自然科学版), 2019, 38(11): 52-57. |
| CAO Xuejuan, SHAN Bailin, DENG Mei, et al. Preparation and photocatalytic properties of Fe-doped g-C3N4 photocatalyst[J]. Journal of Chongqing Jiaotong University(Natural Science), 2019, 38(11): 52-57. | |
| 36 | 高晓明, 付峰, 吕磊, 等. 光催化剂Cu-BiVO4的制备及其光催化降解含酚废水[J]. 化工进展, 2012, 31(5): 1039-1042, 1087. |
| GAO Xiaoming, FU Feng, Lei LYU, et al. Preparation of Cu-BiVO4 photocatalyst and its application in the treatment of phenol-containing wastewater[J]. Chemical Industry and Engineering Progress, 2012, 31(5): 1039-1042, 1087. | |
| 37 | 王宏娟, 郑楠, 董晓丽. 碘掺杂氯氧化铋光催化剂的制备及性能[J]. 大连工业大学学报, 2020, 39(6): 419-423. |
| WANG Hongjuan, ZHENG Nan, DONG Xiaoli. Preparation and properties of I-doped bismuth chloride oxide photocatalyst[J]. Journal of Dalian Polytechnic University, 2020, 39(6): 419-423. | |
| 38 | 李红亮, 张涛, 付贤军, 等. ZnO/g-C3N4复合光催化材料降解四环素研究[J]. 能源与环保, 2023, 45(8): 163-169. |
| LI Hongliang, ZHANG Tao, FU Xianjun, et al. Study on degradation of tetracycline by ZnO/g-C3N4 composite photocatalytic materials[J]. China Energy and Environmental Protection, 2023, 45(8): 163-169. | |
| 39 | 赵文武, 周海静, 黄雁, 等. 稀土掺杂硼酸盐Bi2ZnB2O7∶xDy3+光催化剂的合成及其催化机理[J]. 化工进展, 2022, 41(11): 5843-5849. |
| ZHAO Wenwu, ZHOU Haijing, HUANG Yan, et al. Photocatalytic mechanism and synthesis of rare earth doped borate Bi2ZnB2O7∶xDy3+ photocatalyst[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5843-5849. |
| [1] | WU Zeliang, GUAN Qihui, CHEN Shixia, WANG Jun. Advances in selective hydrogenation of alkynes to alkenes [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4366-4381. |
| [2] | WANG Jia, LI Wencui, WU Fan, GAO Xinqian, LU Anhui. Regulation active components distribution of NiMo/Al2O3 catalysts for hydrodesulfurization [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4393-4402. |
| [3] | FU Tao, LI Li, GAO Lining, ZHU Fuwei, CAO Weiye, CHEN Huaxin. Cement-based boron-doped graphite phase carbon nitride material degrades NO [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4403-4410. |
| [4] | ZHENG Yunxiang, GAO Yilun, LI Yanru, LIU Qinglin, ZHANG Haoteng, WANG Xiangpeng. Preparation and adsorption properties of porous double-network hydrogels modified by nitrilotriacetic acid anhydride [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4542-4549. |
| [5] | ZHANG Xi, LI Haoxin, ZHANG Tianyang, LI Zifu, SUN Wenjun, AO Xiuwei. Degradation of per- and polyfluoroalkyl substances in water by UV-based advanced oxidation or advanced reduction processes [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4587-4600. |
| [6] | SONG Zhanlong, TANG Tao, PAN Wei, ZHAO Xiqiang, SUN Jing, MAO Yanpeng, WANG Wenlong. Micro-nano bubbles enhance ozone oxidation and degradation of wastewater containing phenol [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4614-4623. |
| [7] | LIU Yucan, GAO Zhonglu, XU Xinyi, JI Xianguo, ZHANG Yan, SUN Hongwei, WANG Gang. Adsorption performance and mechanism of diuron from water by calcium-modified water hyacinth-based biochar [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4630-4641. |
| [8] | HU Junjie, HUANG Xingjun, LEI Cheng, YANG Min, LAN Yuanxiao, LUO Jianhong. Advanced treatment of small molecular organic in shale gas produced water [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4674-4680. |
| [9] | GUO Changbin, LI Mengmeng, FENG Menghan, YUAN Tian, ZHANG Keqiang, LUO Yanli, WANG Feng. Preparation of Ce-doped La-based perovskite and its adsorption properties for phosphate and phytic acid in water [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4748-4756. |
| [10] | BIAN Weibai, ZHANG Ruixuan, PAN Jianming. Research progress on preparation methods of inorganic metal lithium ion sieve materials [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4173-4186. |
| [11] | LI Wenzhe, SHEN Miao, WANG Jianqiang. Research progress in the preparation of new two-dimensional layered metal carbon/nitrides by molten salt method [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3660-3671. |
| [12] | HUANG Jun, ZHANG Yingjuan, LIN Yintong, WEI Xuechun, WU Yutong, WU Gaobo, MO Junlin, ZHAO Zhenxia, ZHAO Zhongxing. Preparation of silkworm excrement-based porous biocarbon and synergistic adsorption and slow-release performance for monosultap and dinotefuran [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3964-3971. |
| [13] | YU Lishuang, LI Qingyun, LIU Zhaoming, ZHANG Shuru, LIU Youyan, TANG Aixing. Epoxidation of pinene catalyzed by lipase immobilized on rape pollen biochar [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3996-4004. |
| [14] | ZHANG Shirui, FAN Zhenlian, SONG Huiping, ZHANG Lina, GAO Hongyu, CHENG Shuyan, CHENG Fangqin. Research progress of fly ash supported photocatalytic materials [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4043-4058. |
| [15] | HE Yixue, QIN Xianchao, MA Weifang. Research progress on in situ remediation of halogenated hydrocarbon contamination in groundwater by persulfate-based advanced oxidation process [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4072-4088. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||