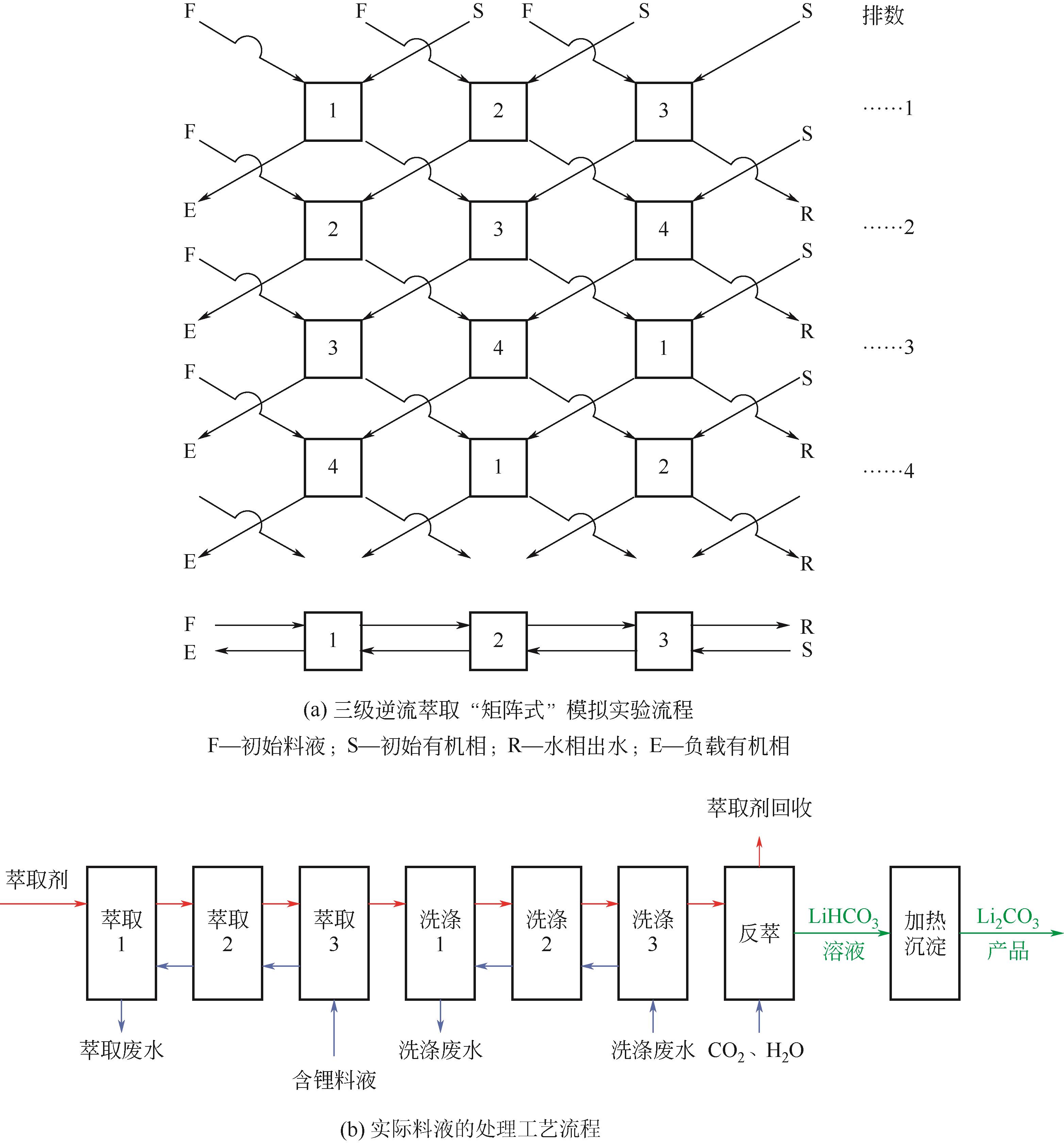
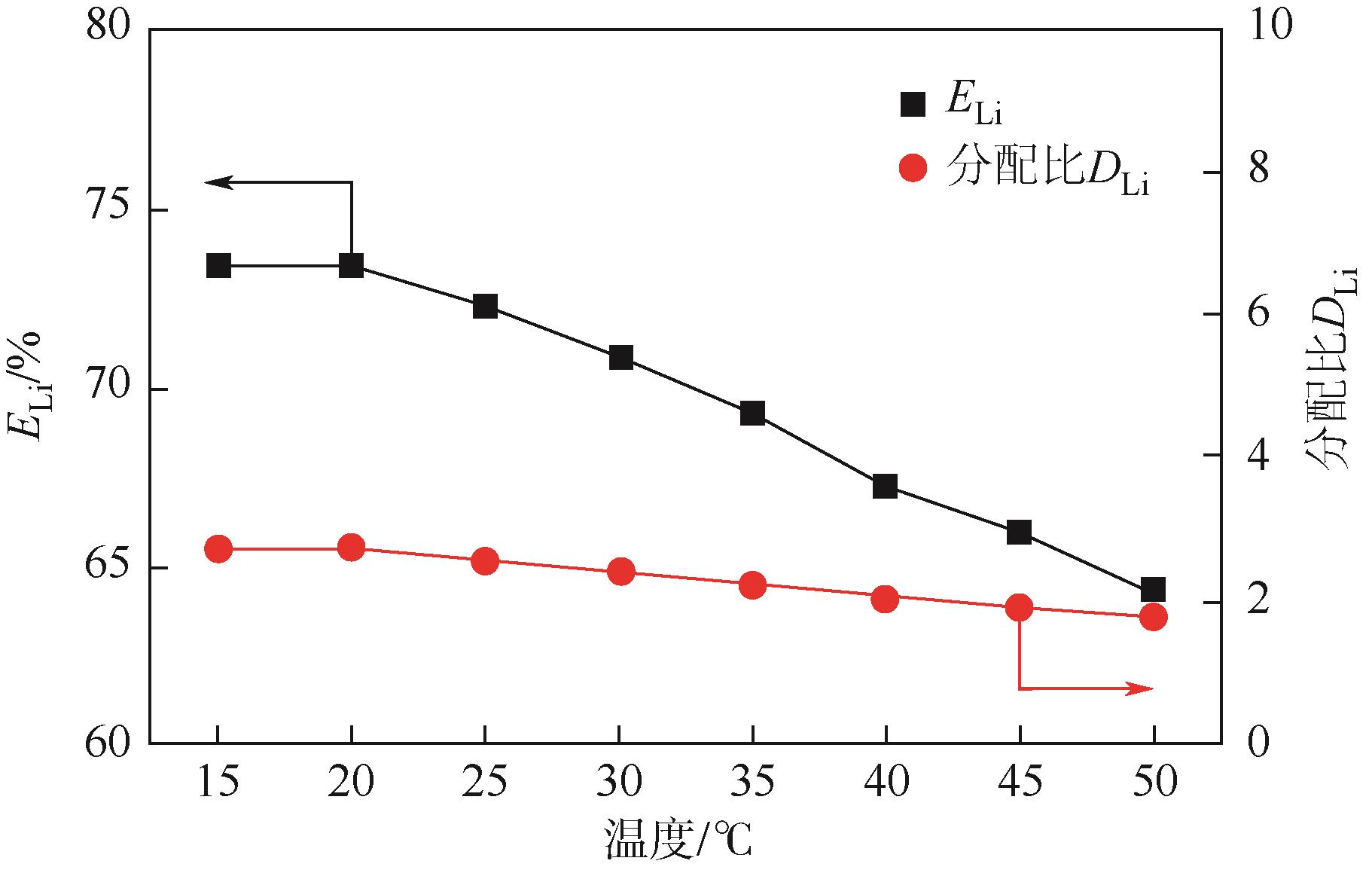
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (S1): 627-639.DOI: 10.16085/j.issn.1000-6613.2024-0784
• Resources and environmental engineering • Previous Articles Next Articles
Application of IPE-23 extractant in the recovery of lithium from lithium-containing waste liquors
HE Fang1( ), XU Gaojie2, PEI Xiang2, SUN Dezhi1, NING Pengge2, CAO Hongbin1,2(
), XU Gaojie2, PEI Xiang2, SUN Dezhi1, NING Pengge2, CAO Hongbin1,2( )
)
- 1.College of Environmental Science & Engineering, Beijing Forestry University, Beijing 100083, China
2.Institute of Process Engineering, Chinese Academy of Sciences, Chemistry & Chemical Engineering Date Center, CAS, Beijing 100190, China
-
Received:2024-05-10Revised:2024-08-21Online:2024-12-06Published:2024-11-20 -
Contact:CAO Hongbin
IPE-23萃取剂在含锂废液中回收锂的应用
何方1( ), 许高洁2, 裴翔2, 孙德智1, 宁朋歌2, 曹宏斌1,2(
), 许高洁2, 裴翔2, 孙德智1, 宁朋歌2, 曹宏斌1,2( )
)
- 1.北京林业大学环境科学与工程学院,北京 100083
2.中国科学院过程工程研究所,中国科学院化学化工科学 数据中心,北京 100190
-
通讯作者:曹宏斌 -
作者简介:何方(2000—),男,硕士研究生,研究方向为废旧锂电池资源回收。E-mail:eminemleonard@163.com。 -
基金资助:中国科学院战略性先导科技专项(XDA0430105)
CLC Number:
Cite this article
HE Fang, XU Gaojie, PEI Xiang, SUN Dezhi, NING Pengge, CAO Hongbin. Application of IPE-23 extractant in the recovery of lithium from lithium-containing waste liquors[J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 627-639.
何方, 许高洁, 裴翔, 孙德智, 宁朋歌, 曹宏斌. IPE-23萃取剂在含锂废液中回收锂的应用[J]. 化工进展, 2024, 43(S1): 627-639.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0784
| 参数 | 数值 |
|---|---|
| pH | 12.68 |
| COD/mg·L-1 | 70 |
| 盐度/g·L-1 | 31.05 |
| 金属离子浓度/mg·L-1 | |
| Li | 6490 |
| Na | 12550 |
| K | 58 |
| B | 22 |
| Si | 10 |
| Ca | 8 |
| 参数 | 数值 |
|---|---|
| pH | 12.68 |
| COD/mg·L-1 | 70 |
| 盐度/g·L-1 | 31.05 |
| 金属离子浓度/mg·L-1 | |
| Li | 6490 |
| Na | 12550 |
| K | 58 |
| B | 22 |
| Si | 10 |
| Ca | 8 |
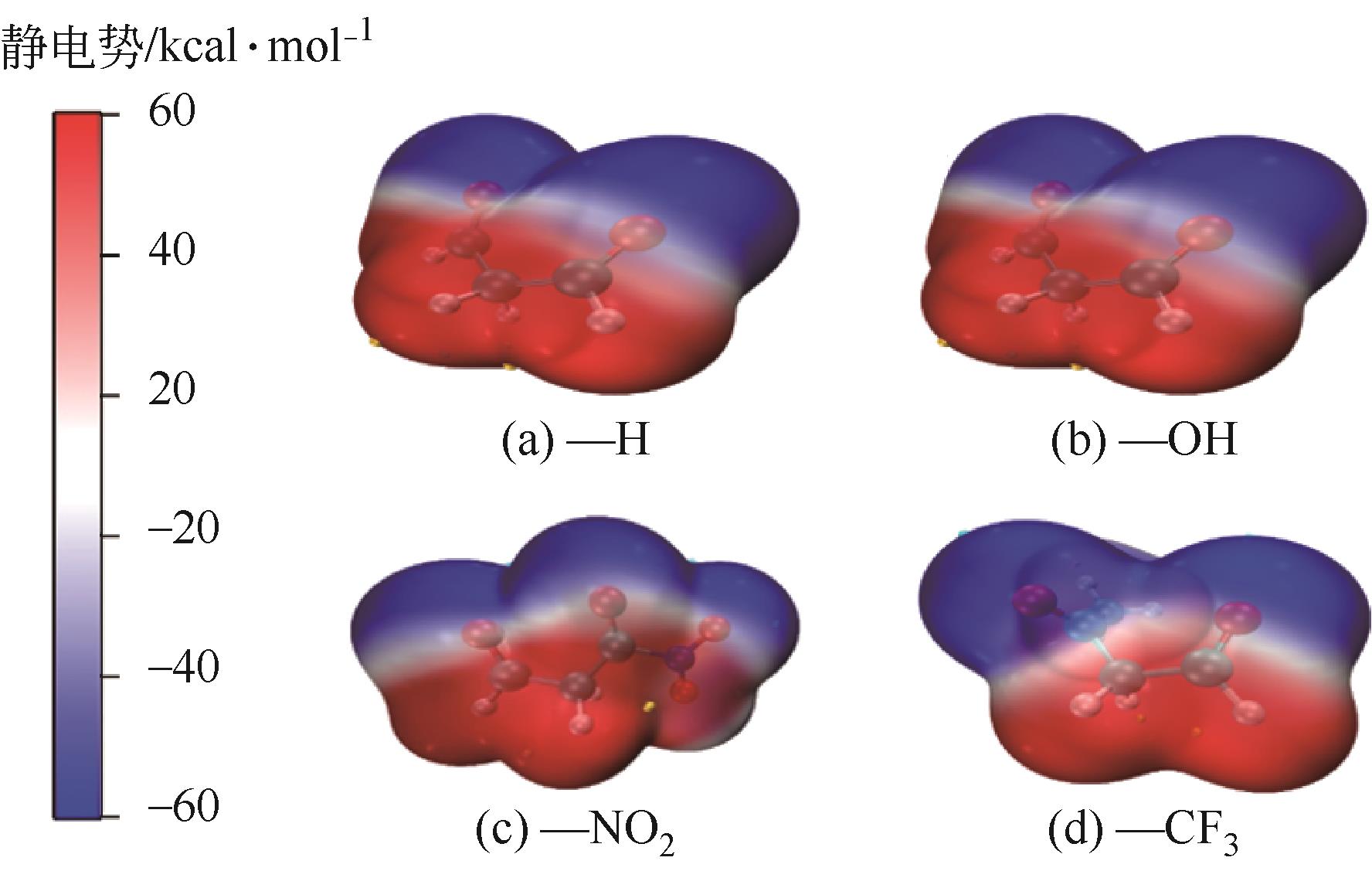
| R1取代基 | ΔG /kcal·mol-1 | ΔG-ΔG-H /kcal·mol-1 | vdW静电势 极小值 /kcal·mol-1 | vdW静电势极大值 /kcal·mol-1 |
|---|---|---|---|---|
| —H | 112.6575 | 0 | -66.19 | 43.06 |
| —OH | 116.4849 | 3.8274 | -42.05 | 60.23 |
| —NO2 | 107.5073 | -5.1502 | -43.07 | 56.57 |
| —F | 111.9443 | -0.7132 | -57.03 | 51.1 |
| —CN | 195.6406 | 82.9831 | -45.3 | 44.09 |
| —CF3 | 108.7517 | -3.9058 | -38.03 | 50.08 |
| —Ph | 114.3678 | 1.7103 | -47.09 | 44.32 |
| —OCH3 | 117.8343 | 5.1768 | -45.05 | 39.65 |
| —N(CH3)2 | 118.5974 | 5.9399 | -59.32 | 44.9 |
| —CH3 | 115.0102 | 2.3527 | -47.26 | 41.9 |
—CH2CH CH2 CH2 | 115.2835 | 2.6260 | -49.17 | 43.41 |
| —CH2CH3 | 115.6481 | 2.9906 | -48.28 | 41.61 |
| —C(CH3)3 | 115.3681 | 2.7106 | -50.27 | 43.24 |
| R1取代基 | ΔG /kcal·mol-1 | ΔG-ΔG-H /kcal·mol-1 | vdW静电势 极小值 /kcal·mol-1 | vdW静电势极大值 /kcal·mol-1 |
|---|---|---|---|---|
| —H | 112.6575 | 0 | -66.19 | 43.06 |
| —OH | 116.4849 | 3.8274 | -42.05 | 60.23 |
| —NO2 | 107.5073 | -5.1502 | -43.07 | 56.57 |
| —F | 111.9443 | -0.7132 | -57.03 | 51.1 |
| —CN | 195.6406 | 82.9831 | -45.3 | 44.09 |
| —CF3 | 108.7517 | -3.9058 | -38.03 | 50.08 |
| —Ph | 114.3678 | 1.7103 | -47.09 | 44.32 |
| —OCH3 | 117.8343 | 5.1768 | -45.05 | 39.65 |
| —N(CH3)2 | 118.5974 | 5.9399 | -59.32 | 44.9 |
| —CH3 | 115.0102 | 2.3527 | -47.26 | 41.9 |
—CH2CH CH2 CH2 | 115.2835 | 2.6260 | -49.17 | 43.41 |
| —CH2CH3 | 115.6481 | 2.9906 | -48.28 | 41.61 |
| —C(CH3)3 | 115.3681 | 2.7106 | -50.27 | 43.24 |
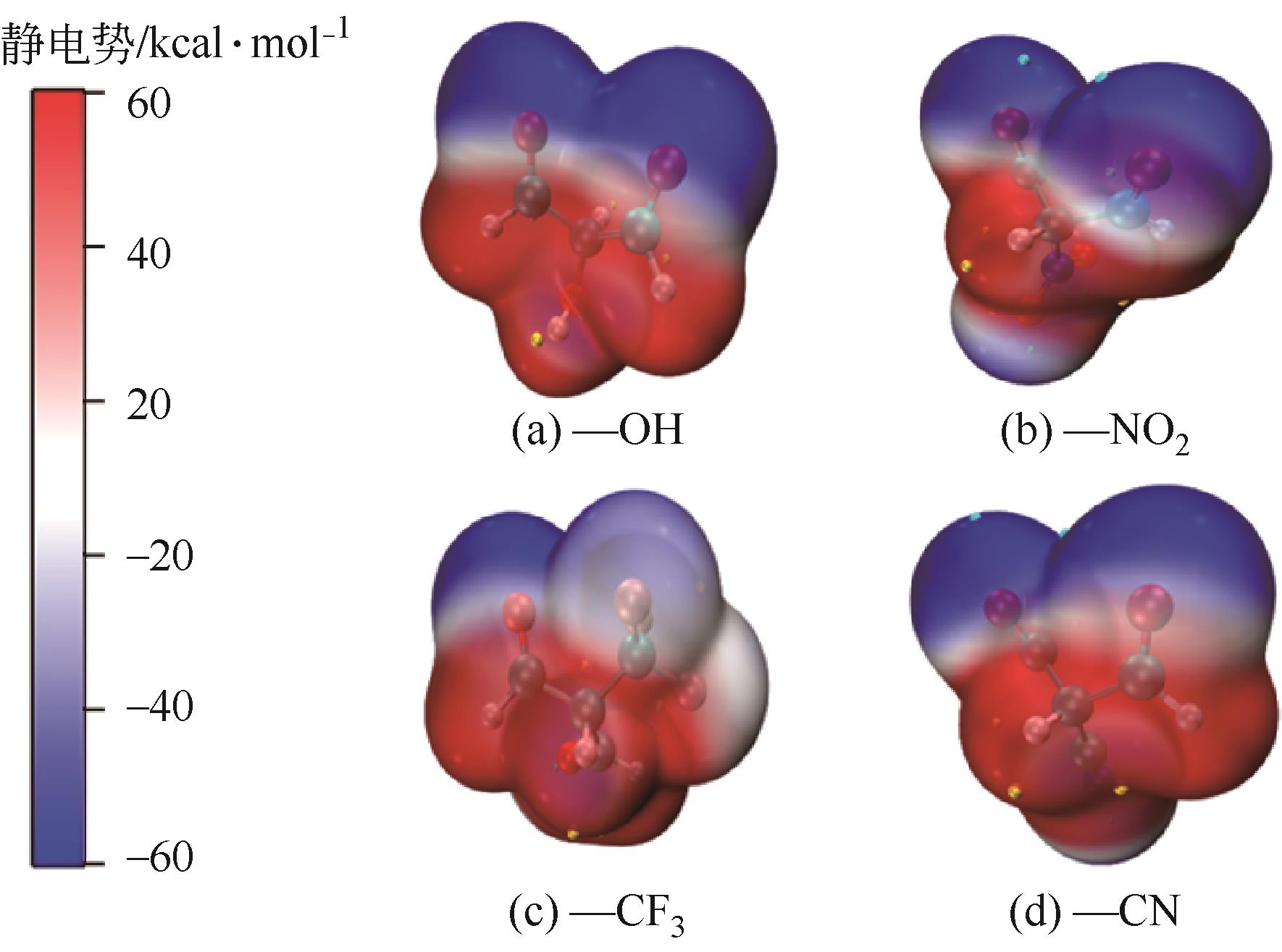
| R3取代基 | ΔG /kcal·mol-1 | ΔG-ΔG-H /kcal·mol-1 | vdW静电势极小值 /kcal·mol-1 | vdW静电势极大值 /kcal·mol-1 |
|---|---|---|---|---|
| —H | 112.6575 | 0 | -66.19 | 43.06 |
| —OH | 112.5267 | -0.1308 | -54.68 | 82.18 |
| —NO2 | 98.6403 | -14.0172 | -30.79 | 51.39 |
| —F | 113.9283 | 1.2708 | -46.79 | 44.25 |
| —CN | 99.1364 | -13.5211 | -36.31 | 53.17 |
| —CF3 | 106.1881 | -6.4694 | -39.01 | 54.52 |
| —Ph | 111.7872 | -0.8703 | -46.4 | 43.59 |
| —OCH3 | 113.6880 | 1.0305 | -53 | 35.08 |
| —N(CH3)2 | 112.0872 | -0.5703 | -46.89 | 43.85 |
| —CH3 | 116.0881 | 3.4306 | -42.61 | 42.23 |
—CH2CH CH2 CH2 | 111.9502 | -0.7073 | -63.42 | 42.08 |
| —CH2CH3 | 112.7878 | 0.1303 | -63.3 | 40.46 |
| —C(CH3)3 | 115.5021 | 2.8446 | -50.27 | 43.24 |
| R3取代基 | ΔG /kcal·mol-1 | ΔG-ΔG-H /kcal·mol-1 | vdW静电势极小值 /kcal·mol-1 | vdW静电势极大值 /kcal·mol-1 |
|---|---|---|---|---|
| —H | 112.6575 | 0 | -66.19 | 43.06 |
| —OH | 112.5267 | -0.1308 | -54.68 | 82.18 |
| —NO2 | 98.6403 | -14.0172 | -30.79 | 51.39 |
| —F | 113.9283 | 1.2708 | -46.79 | 44.25 |
| —CN | 99.1364 | -13.5211 | -36.31 | 53.17 |
| —CF3 | 106.1881 | -6.4694 | -39.01 | 54.52 |
| —Ph | 111.7872 | -0.8703 | -46.4 | 43.59 |
| —OCH3 | 113.6880 | 1.0305 | -53 | 35.08 |
| —N(CH3)2 | 112.0872 | -0.5703 | -46.89 | 43.85 |
| —CH3 | 116.0881 | 3.4306 | -42.61 | 42.23 |
—CH2CH CH2 CH2 | 111.9502 | -0.7073 | -63.42 | 42.08 |
| —CH2CH3 | 112.7878 | 0.1303 | -63.3 | 40.46 |
| —C(CH3)3 | 115.5021 | 2.8446 | -50.27 | 43.24 |
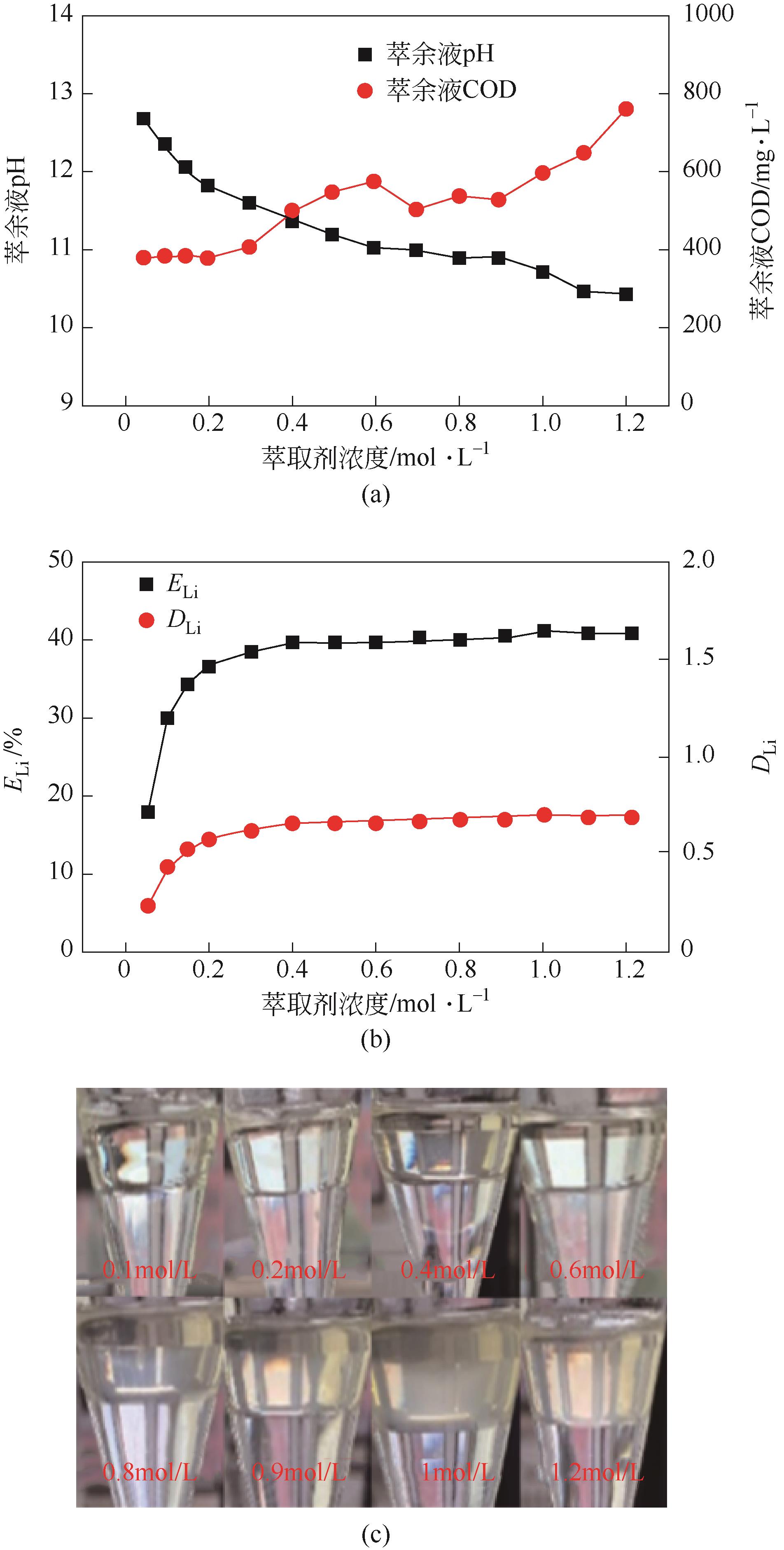
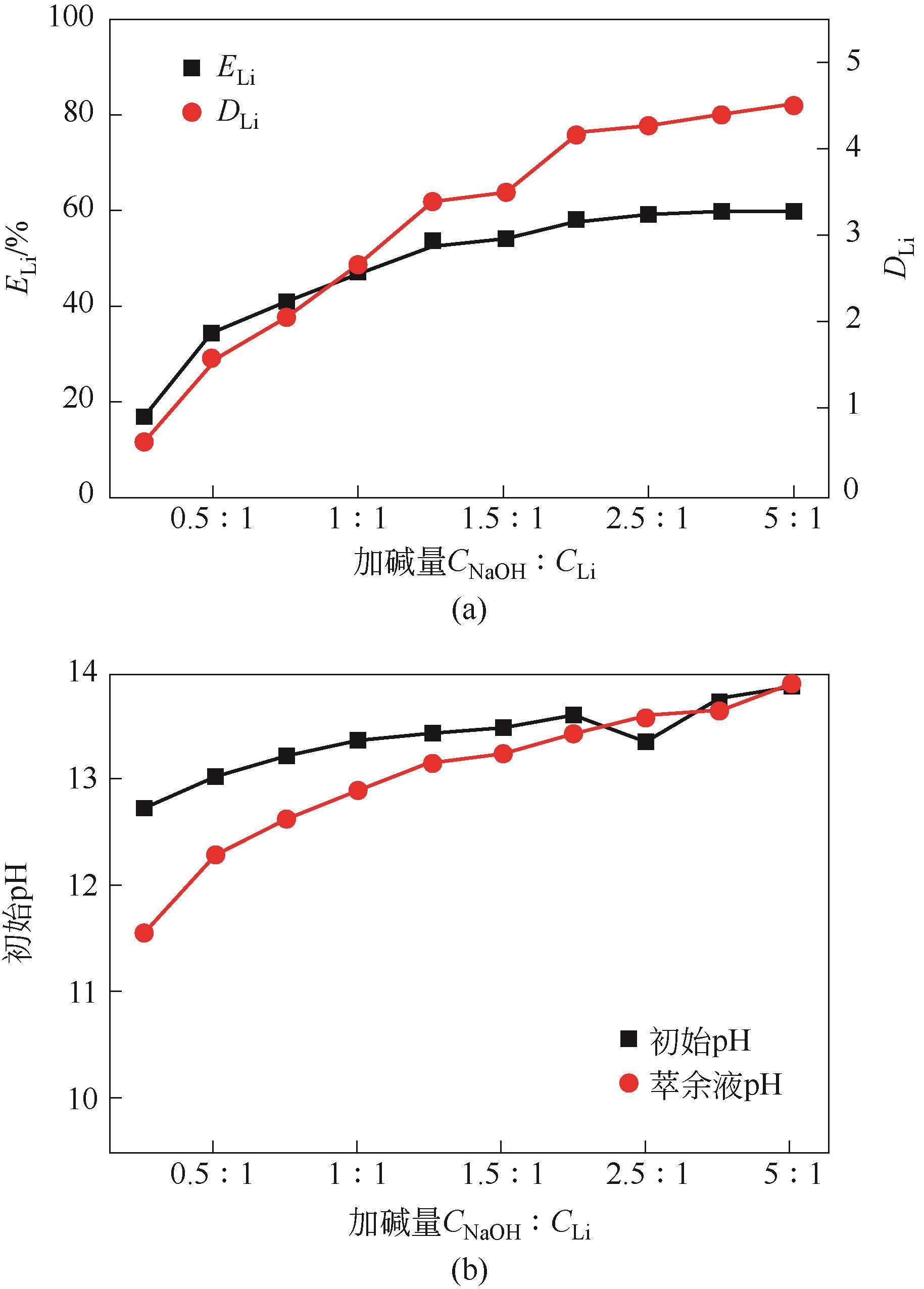
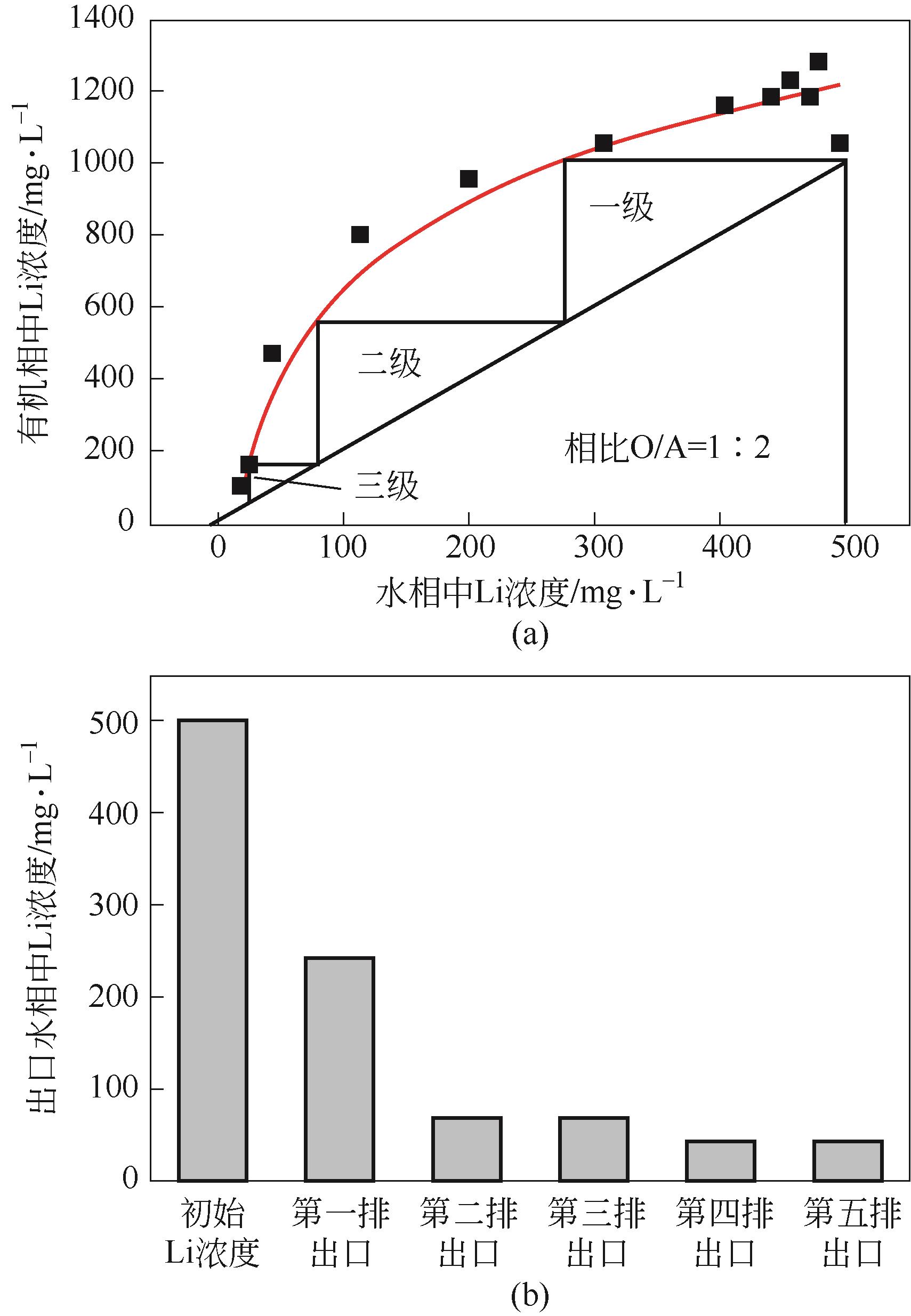
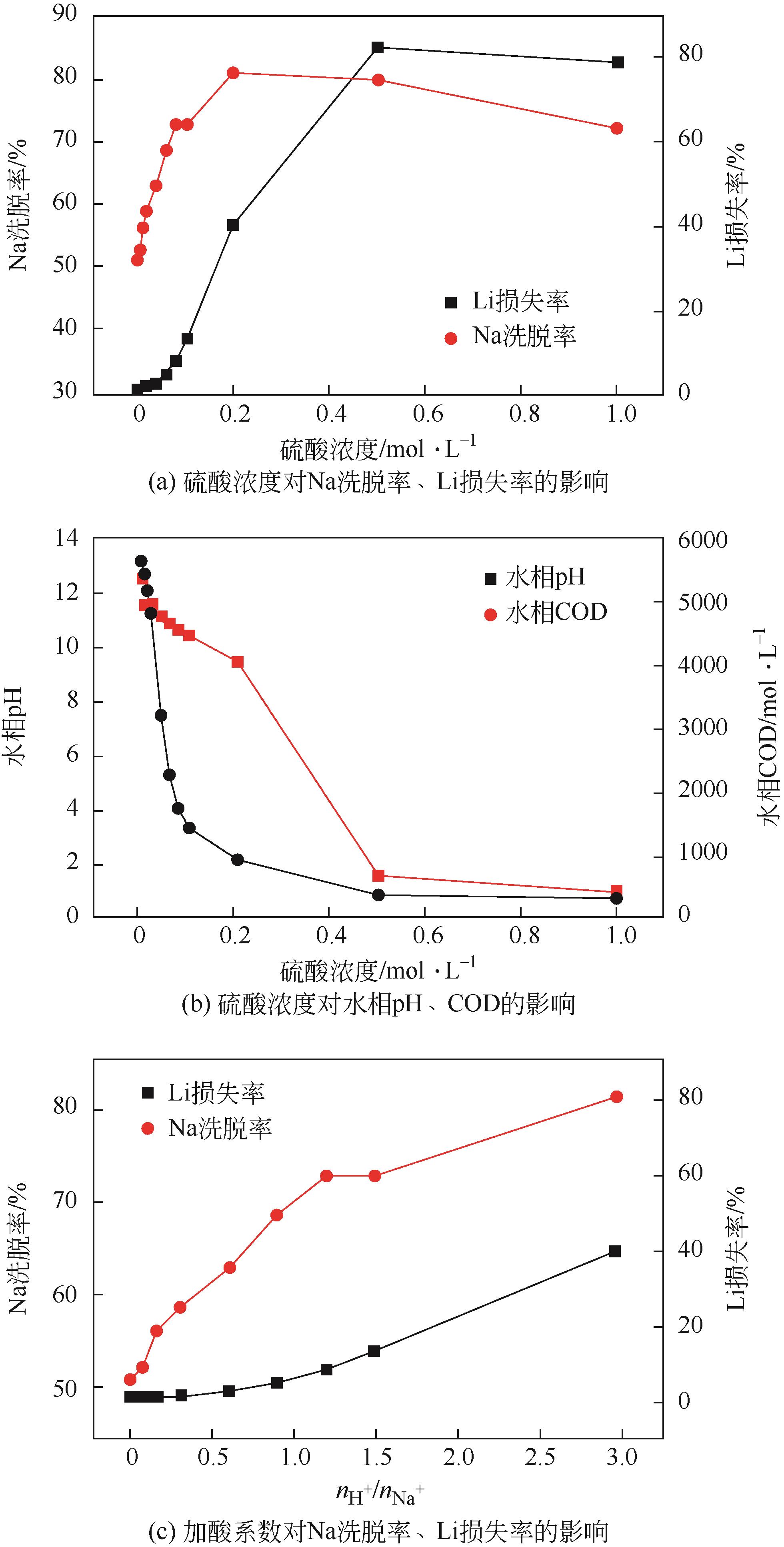
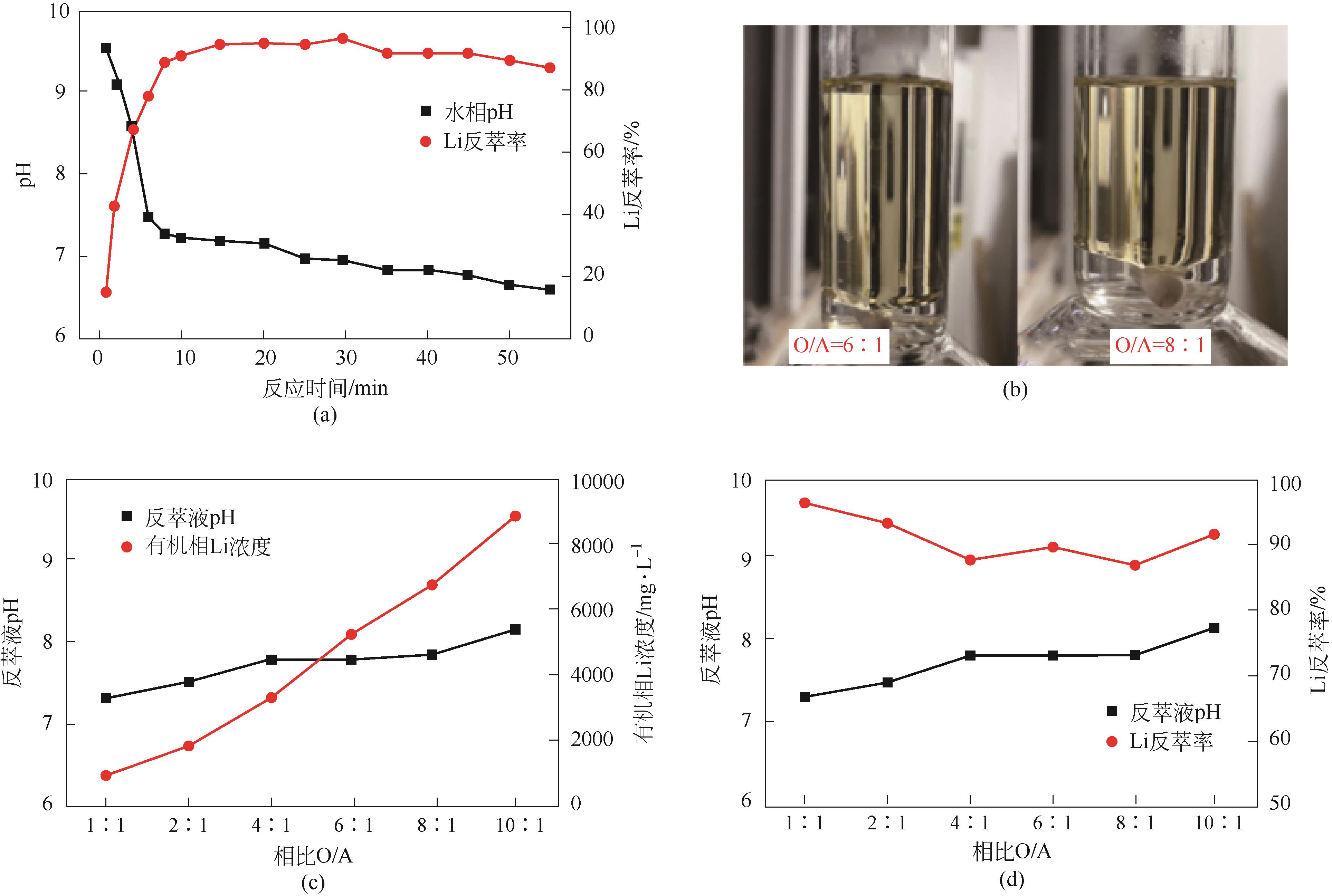
| 工艺流程 | 水相中金属离子浓度/mg·L-1 | 有机相中金属离子浓度/mg·L-1 | 分离比DLi | 锂钠分离系数 | Na洗脱率/% | Li损失率/% | Li反萃率/% | ||
|---|---|---|---|---|---|---|---|---|---|
| Li | Na | Li | Na | ||||||
| 萃取1 | 211.5 | 12440 | 564 | 440 | 2.67 | 75.39 | |||
| 萃取2 | 51 | 12390 | 885 | 400 | 17.35 | 537.51 | |||
| 萃取3 | 35.5 | 12350 | 916 | 620 | 25.81 | 513.97 | |||
| 洗涤1 | 410 | 3780 | 870.75 | 191.5 | 69.11 | 4.94 | |||
| 洗涤2 | 614 | 4430 | 845.25 | 110.25 | 82.22 | 7.72 | |||
| 洗涤3 | 902 | 4710 | 809.25 | 75.25 | 87.86 | 11.65 | |||
| 反萃 | 6490 | 372 | 100.00 | ||||||
| 工艺流程 | 水相中金属离子浓度/mg·L-1 | 有机相中金属离子浓度/mg·L-1 | 分离比DLi | 锂钠分离系数 | Na洗脱率/% | Li损失率/% | Li反萃率/% | ||
|---|---|---|---|---|---|---|---|---|---|
| Li | Na | Li | Na | ||||||
| 萃取1 | 211.5 | 12440 | 564 | 440 | 2.67 | 75.39 | |||
| 萃取2 | 51 | 12390 | 885 | 400 | 17.35 | 537.51 | |||
| 萃取3 | 35.5 | 12350 | 916 | 620 | 25.81 | 513.97 | |||
| 洗涤1 | 410 | 3780 | 870.75 | 191.5 | 69.11 | 4.94 | |||
| 洗涤2 | 614 | 4430 | 845.25 | 110.25 | 82.22 | 7.72 | |||
| 洗涤3 | 902 | 4710 | 809.25 | 75.25 | 87.86 | 11.65 | |||
| 反萃 | 6490 | 372 | 100.00 | ||||||
| 1 | YANG Sixie, ZHANG Fan, DING Huaiping, et al. Lithium metal extraction from seawater[J]. Joule, 2018, 2(9): 1648-1651. |
| 2 | KANAGASUNDARAM Thines, MURPHY Olivia, HAJI Maha N, et al. The recovery and separation of lithium by using solvent extraction methods[J]. Coordination Chemistry Reviews, 2024, 509: 215727. |
| 3 | 吕峥, 宋佳丽, 孙峙, 等. 中国锂离子电池回收技术知识产权分析[J]. 清华大学学报(自然科学版), 2019, 59(7): 551-557. |
| Zheng LYU, SONG Jiali, SUN Zhi, et al. Intellectual property analysis of lithium-ion battery recycling methods in China[J]. Journal of Tsinghua University (Science and Technology), 2019, 59(7): 551-557. | |
| 4 | MROZIK Wojciech, RAJAEIFAR Mohammad ALI, HEIDRICH Oliver, et al. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries[J]. Energy & Environmental Science, 2021, 14(12): 6099-6121. |
| 5 | FAN Ersha, LI Li, WANG Zhenpo, et al. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects[J]. Chemical Reviews, 2020, 120(14): 7020-7063. |
| 6 | 林娇, 刘春伟, 曹宏斌, 等. 基于高温化学转化的废旧锂离子电池资源化技术[J]. 化学进展, 2018, 30(9): 1445-1454. |
| LIN Jiao, LIU Chunwei, CAO Hongbin, et al. Recovery of spent lithium ion batteries based on high temperature chemical conversion[J]. Progress in Chemistry, 2018, 30(9): 1445-1454. | |
| 7 | 宋佳丽, 孙峙, 高文芳, 等. 锂离子电池正极废料中有价元素的选择性回收及其动力学模型[J]. 过程工程学报, 2017, 17(4): 845-852. |
| SONG Jiali, SUN Sun, GAO Wenfang, et al. Selective recovery and kinetics of valuable elements from waste lithium-ion battery cathodes[J]. The Chinese Journal of Process Engineering, 2017, 17(4): 845-852. | |
| 8 | 曹宏斌. 锂动力电池高效循环与污染控制[J]. 高科技与产业化, 2023, 29(6): 18-21. |
| CAO Hongbin. Efficient cycling and pollution control of lithium power batteries[J]. High-Technology & Commercialization, 2023, 29(6): 18-21. | |
| 9 | ZHENG Xiaohong, ZHU Zewen, LIN Xiao, et al. A mini-review on metal recycling from spent lithium ion batteries[J]. Engineering, 2018, 4(3): 361-370. |
| 10 | LI Yukun, Weiguang LYU, HUANG Hanlin, et al. Recycling of spent lithium-ion batteries in view of green chemistry[J]. Green Chemistry, 2021, 23(17): 6139-6171. |
| 11 | 李丽娟, 彭小五, 时东, 等. 含锂卤水中锂资源高效利用与绿色分离的新型萃取体系[J]. 盐湖研究, 2019, 26(4): 1-10. |
| LI Lijuan, PENG Xiaowu, SHI Dong, et al. Eco-friendly separation and effective applications of lithium resources from various brine with lithium: Their extractant and extraction system[J]. Journal of Salt Lake Research, 2019, 26(4): 1-10. | |
| 12 | 许庆仁. 从氯化镁饱和溶液中萃取锂的研究[J]. 有机化学, 1979(1): 13-33. |
| XU Qingren. Study on extracting lithium from saturated solution of magnesium chloride[J]. Chinese Journal of Organic CHEMISTRY, 1979(1): 13-33. | |
| 13 | 张金才, 王敏, 戴静. 卤水提锂的萃取体系概述[J]. 盐湖研究, 2005, 13(1): 42-48, 54. |
| ZHANG Jincai, WANG Min, DAI Jing. Summarization of the lithium extraction system[J]. Journal of Salt Lake Research, 2005, 13(1): 42-48, 54. | |
| 14 | 时东, 李晋峰, 张波, 等. N523-TBP-磺化煤油萃取体系从饱和氯化镁卤水中萃取锂的工艺研究[J]. 盐湖研究, 2013, 21(2): 52-57. |
| SHI Dong, LI Jinfeng, ZHANG Bo, et al. Process study on N523-TBP-sulfonated kerosene extraction system for extraction of lithium from brine saturated by magnesium chloride[J]. Journal of Salt Lake Research, 2013, 21(2): 52-57. | |
| 15 | 时东, 李丽娟, 宋富根, 等. N523-TBP混合萃取体系从盐湖卤水中萃取锂的机理研究[J]. 盐湖研究, 2017, 25(1): 57-63. |
| SHI Dong, LI Lijuan, SONG Fugen, et al. Mechanism study of extracting lithium from brine with N523-TBP mixed extraction system[J]. Journal of Salt Lake Research, 2017, 25(1): 57-63. | |
| 16 | 束玉珍, 吴继宗, 邓惟勤. 冠状化合物在锂同位素分离中的应用[J]. 核化学与放射化学, 2015, 37(1): 12-17. |
| SHU Yuzhen, WU Jizong, DENG Weiqin. Application of crown compounds in lithium isotopes separation[J]. Journal of Nuclear and Radiochemistry, 2015, 37(1): 12-17. | |
| 17 | 王苏. 不同侧链冠醚-离子液体萃取分离锂同位素的研究[D]. 西宁: 中国科学院大学(中国科学院青海盐湖研究所), 2018. |
| WANG Su. Study on extraction and separation of lithium isotopes from different side chain crown ether - ionic liquids[D]. Xining: Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, 2018. | |
| 18 | 石成龙. 离子液体体系用于盐湖卤水中提取锂的研究[D]. 西宁: 中国科学院大学(中国科学院青海盐湖研究所), 2017. |
| SHI Chenglong. Study on the application of ionic liquid systems for extraction oflithium from salt lake brine[D]. Xining: Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, 2017. | |
| 19 | 肖江, 贾永忠, 姚颖, 等. 溶剂萃取法分离锂同位素研究进展[J]. 盐湖研究, 2016, 24(3): 62-72. |
| XIAO Jiang, JIA Yongzhong, YAO Ying, et al. A review of separation of lithium isotopes by solvent extraction[J]. Journal of Salt Lake Research, 2016, 24(3): 62-72. | |
| 20 | ZHANG Licheng, LI Lijuan, SHI Dong, et al. Selective extraction of lithium from alkaline brine using HBTA-TOPO synergistic extraction system[J]. Separation and Purification Technology, 2017, 188: 167-173. |
| 21 | PRANOLO Yoko, ZHU Zhaowu, CHENG CHU yong. Separation of lithium from sodium in chloride solutions using SSX systems with LIX 54 and Cyanex 923[J]. Hydrometallurgy, 2015, 154: 33-39. |
| 22 | ISHIMORI Ken-ichiro, MORI Seiji, ITO Yuji, et al. Equilibrium and ab initio computational studies on the adduct formation of 1,3-diketonato-lithium(Ⅰ), -sodium(Ⅰ) and -potassium(Ⅰ) with 1,10-phenanthroline and its 2,9-dimethyl derivatives[J]. Talanta, 2009, 78(4/5): 1272-1279. |
| 23 | 苏慧. 多组分协同溶剂萃取体系应用于高镁盐湖卤水提锂的研究[D]. 北京: 中国科学院大学(中国科学院过程工程研究所), 2021. |
| SU Hui. Study on the lithium recovery from salt lake brines containing high megnesium concentration using muitiple component synergistic solvent extraction system[D]. Beijing: Institute of Process Engineering, Chinese Academy of Sciences, 2021. | |
| 24 | 赵晓乐, 梁渠, 蔡静. 二苯并-14-冠-4超声波合成及其用于锂镁分离的研究[J]. 化学研究与应用, 2016, 28(8): 1098-1102. |
| ZHAO Xiaole, LIANG Qu, CAI Jing. Study on ultrasonic-aid synthetic method of dibenzo-14-crown-4 and the application in separating lithium and magnesium[J]. Chemical Research and Application, 2016, 28(8): 1098-1102. | |
| 25 | 王万航. 新型杂多酸类共萃剂用于盐湖卤水萃取提锂的应用基础研究[D]. 北京: 北京化工大学, 2022. |
| WANG Wanhang. Application fundamental research on lithium extraction from salt lake brines with novel heteropoly acid co-extraction agent[D]. Beijing: Beijing University of Chemical Technology, 2022. | |
| 26 | ZHANG Licheng, LI Lijuan, SHI Dong, et al. Recovery of lithium from alkaline brine by solvent extraction with β - d i k e t o n e [J]. Hydrometallurgy, 2018, 175: 35-42. |
| 27 | 张旭堃, 张健, 苏慧, 等. 硫酸锂沉锂母液中锂的回收[J]. 稀有金属, 2022, 46(1): 67-77. |
| ZHANG Xukun, ZHANG Jian, SU Hui, et al. Recovery of lithium from the mother solution of lithium precipitation in lithium ore processing[J]. Chinese Journal of Rare Metals, 2022, 46(1): 67-77. |
| [1] | YANG Junhui, YUAN Jun, ZHANG Jida, WANG Jinhai, QIAO Hongbin, CAI Zhenyi, MA Zhongcheng. Structural design and performance analysis of a new type of heat accumulator [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 282-294. |
| [2] | XIE Yulin, RAU Jui-yeh, HUANG Jian, HAO Jiayi, WANG Youyi, HUANG Qi. Preparation of continuous ZIF-8 membrane and its progress in hydrogen separation [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 403-418. |
| [3] | ZHOU Yu, XIA Taiyang, WEI Qi, TANG Tian, TIAN Lei. Optimization of micro-channel coupled reverse osmosis membrane series treatment of methanol to olefin wastewater [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 43-51. |
| [4] | XUE Lixin, TU Longdou, LI Shiyang, ZHENG Chenchen, CAI Dajian, GAO Congjie. High efficient dye desalting mixed matrix nanofiltration membranes containing in-situ grown ZIF-L particles in polyethyleneimine (PEI) coating before interface polymerization [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 431-442. |
| [5] | GENG Xiumei, ZHANG Feng, ZHANG Xiang, SHAN Meixia, ZHANG Yatao. Research progress on the stability of Pebax-based mixed matrix membranes for CO2 separation [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4996-5012. |
| [6] | LI Zhenwu, PU Di, XIONG Yachun, WU Dingying, JIN Cheng, GUO Yongjun. Research progress of nanomaterials for oil displacement in enhancing oil recovery [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5035-5048. |
| [7] | ZHANG Yufeng, PANG Yuqian, PEI Haonan, FAN Xiaoqing. Three-way rod metal-organic frameworks for purifying of C2—C3 from natural gas [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5185-5192. |
| [8] | MA Hongzhou, DANG Yubo, WANG Yaoning, ZENG Jinyang, ZHAO Xiaojun, SHI Jianwei. Zinc-based desulfurizer scrap and copper-zinc-based catalyst synergistic vacuum carbothermal extraction of zinc [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5275-5281. |
| [9] | JIAO Wenlei, LIU Zhen, CHEN Junxian, ZHANG Tianyu, JI Zhongli. Structure and performance influencing factors of vane separation components: The reviews [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4187-4202. |
| [10] | SONG Jiakai, KONG Lingzhen, CHEN Jiaqing, SUN Huan, LI Qi, LI Changhe, WANG Sicheng, KONG Biao. Liquid film flow and separation characteristics in the swirl separation section of a tubular deliquidiser [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4297-4306. |
| [11] | GAO Xinyue, FAN Gaofeng, LIU Aiping, WANG Chang'an, HOU Yujie, ZHANG Jinming, XU Jie, CHE Defu. Research progress on waste heat recovery technology for flue gas and slurry after wet desulphurization [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4307-4319. |
| [12] | SUN Yan, FENG Qianying, XIE Xiaoyang, HE Jiaojie, YANG Liwei, BAI Bo. Research progress on the cyclodextrin-based thin film composite membranes [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4464-4476. |
| [13] | ZHANG Rui, JIANG Jing, XU Hongfei, YANG Shengkai, LI Yahong, ZHOU Jingyuan, ZENG Jianxian, HUANG Xiaoping, LIU Pengfei, ZHANG Mingming, LI Zhiqiang. Progress of ceramic membrane separation technology and its application in bio-manufacturing field [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4550-4561. |
| [14] | LI Weijie, LU Leilei, LI Deke, WANG Chunhang, ZHANG Zuming, TAN Qiang. Lithium-ion battery disassembly and recycling technology and progress [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4601-4613. |
| [15] | SHI Jiabo, ZHANG Yuxuan, CHEN Xuefeng, TAN Jiaojun. Preparation and oil-water separation property of tannic acid-nanoclay synergistically modified collagen fiber-based porous materials [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4624-4629. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||