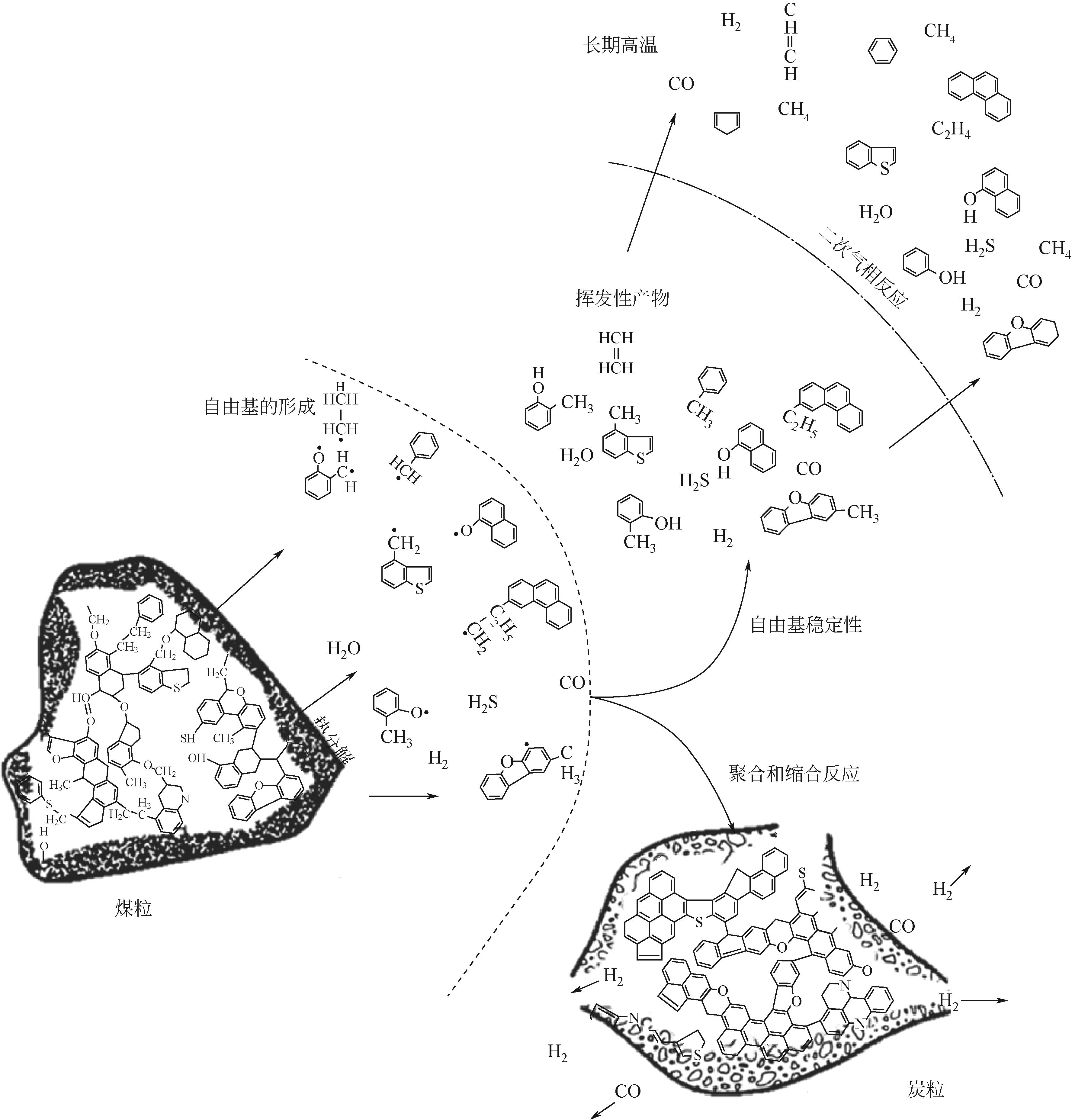
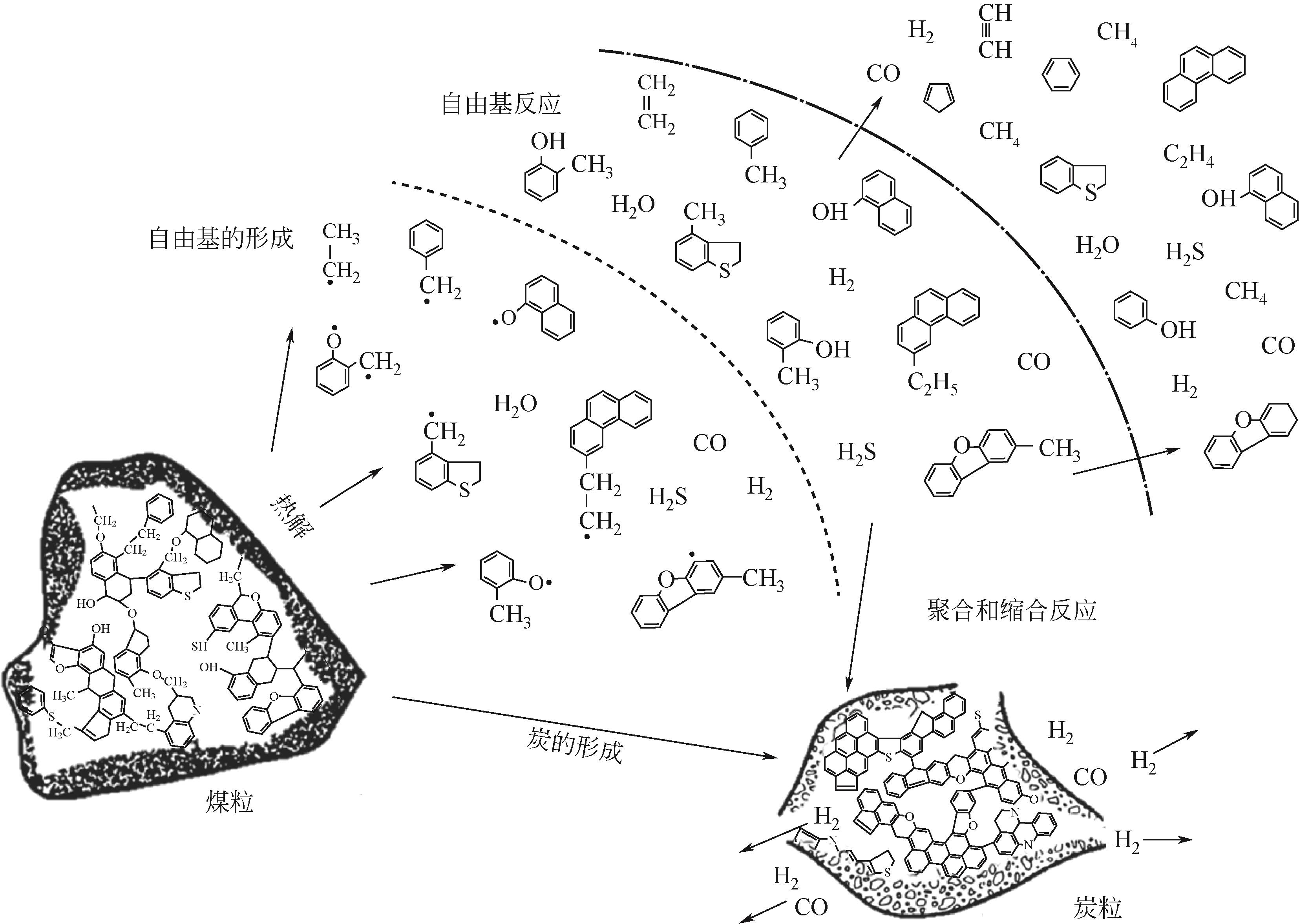
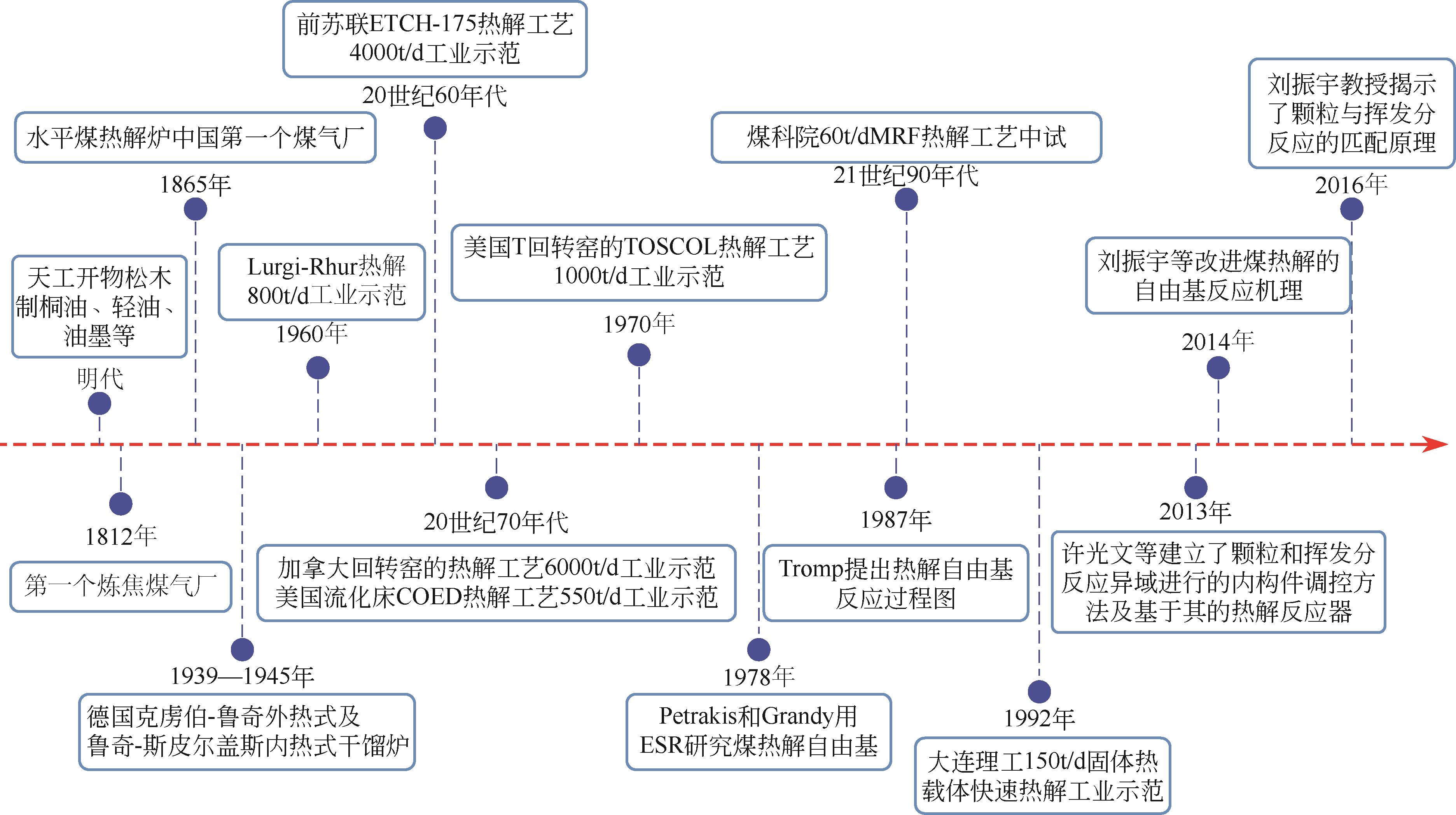
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (7): 3620-3636.DOI: 10.16085/j.issn.1000-6613.2024-0753
• Column: Thermochemical Reaction Engineering Technology • Previous Articles Next Articles
Research progress and development history of coal pyrolysis
CAO Jingpei( ), YAO Naiyu, PANG Xinbo, ZHAO Xiaoyan, ZHAO Jingping, CAI Shijie, XU Min, FENG Xiaobo, YI Fengjiao
), YAO Naiyu, PANG Xinbo, ZHAO Xiaoyan, ZHAO Jingping, CAI Shijie, XU Min, FENG Xiaobo, YI Fengjiao
- Jiangsu Province Engineering Research Center of Fine Utilization of Carbon Resources, China University of Mining & Technology, Xuzhou 221116, Jiangsu, China
-
Received:2024-05-07Revised:2024-06-19Online:2024-08-14Published:2024-07-25 -
Contact:CAO Jingpei
煤热解研究进展及其发展历程
曹景沛( ), 姚乃瑜, 庞新博, 赵小燕, 赵静平, 蔡士杰, 徐敏, 冯晓博, 伊凤娇
), 姚乃瑜, 庞新博, 赵小燕, 赵静平, 蔡士杰, 徐敏, 冯晓博, 伊凤娇
- 中国矿业大学,江苏省碳资源精细化利用工程研究中心,江苏 徐州 221116
-
通讯作者:曹景沛 -
作者简介:曹景沛(1983—),男,教授,研究方向为煤与生物质定向热转化。E-mail:caojingpei@cumt.edu.cn。 -
基金资助:国家自然科学基金(22178374)
CLC Number:
Cite this article
CAO Jingpei, YAO Naiyu, PANG Xinbo, ZHAO Xiaoyan, ZHAO Jingping, CAI Shijie, XU Min, FENG Xiaobo, YI Fengjiao. Research progress and development history of coal pyrolysis[J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3620-3636.
曹景沛, 姚乃瑜, 庞新博, 赵小燕, 赵静平, 蔡士杰, 徐敏, 冯晓博, 伊凤娇. 煤热解研究进展及其发展历程[J]. 化工进展, 2024, 43(7): 3620-3636.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0753
| 化学键 | 键能/kJ·mol-1 | 化学键 | 键能/kJ·mol-1 |
|---|---|---|---|
| Car—Car | 2057 | 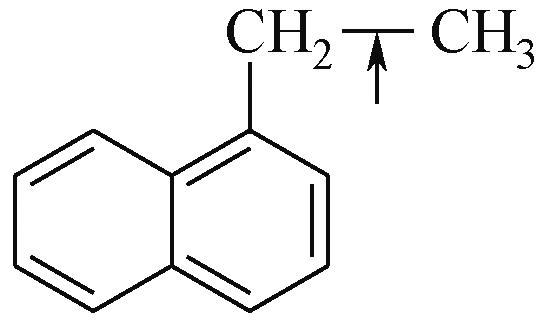 | 284 |
| Car—H | 425 | ||
| Cal—H | 392 | 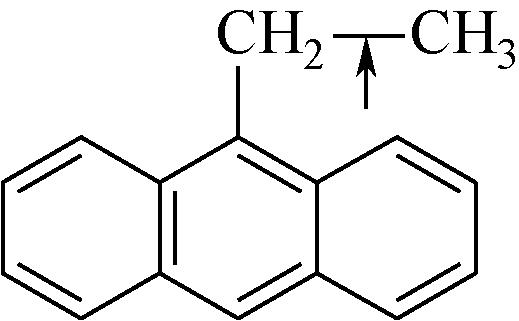 | 251 |
| Car—Cal | 332 | ||
| Cal—O | 314 | 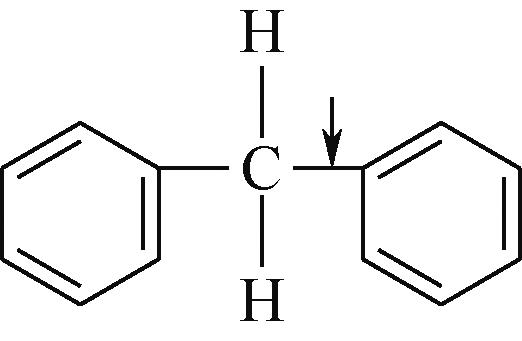 | 339 |
| Cal—Cal | 297 | ||
 | 301 | 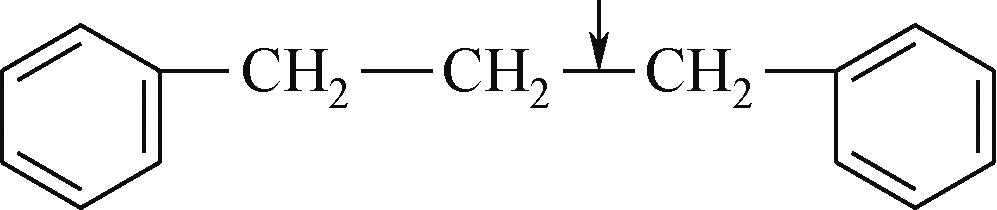 | 284 |
| 化学键 | 键能/kJ·mol-1 | 化学键 | 键能/kJ·mol-1 |
|---|---|---|---|
| Car—Car | 2057 |  | 284 |
| Car—H | 425 | ||
| Cal—H | 392 |  | 251 |
| Car—Cal | 332 | ||
| Cal—O | 314 |  | 339 |
| Cal—Cal | 297 | ||
 | 301 | 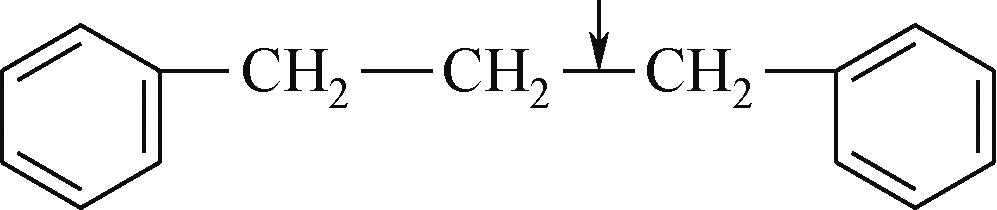 | 284 |
| 煤化程度 | 产物 | ||
|---|---|---|---|
| 半焦/焦炭 | 焦油/热解水 | 热解气 | |
| 褐煤 | 59.5 | 31.5 | 9.0 |
| 长焰煤 | 64.7 | 29.0 | 6.3 |
| 气煤 | 64.4 | 29.2 | 6.4 |
| 焦煤 | 73.3 | 21.7 | 5.0 |
| 无烟煤 | 95.2 | 2.1 | 2.7 |
| 煤化程度 | 产物 | ||
|---|---|---|---|
| 半焦/焦炭 | 焦油/热解水 | 热解气 | |
| 褐煤 | 59.5 | 31.5 | 9.0 |
| 长焰煤 | 64.7 | 29.0 | 6.3 |
| 气煤 | 64.4 | 29.2 | 6.4 |
| 焦煤 | 73.3 | 21.7 | 5.0 |
| 无烟煤 | 95.2 | 2.1 | 2.7 |
| 煤化程度 | 焦油 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 芳香族 | 脂肪族 | 酚类 | 酮类 | 醇类 | 醛类 | 酸类/酯类 | 含氮化合物 | 含硫化合物 | 其他含氧化合物 | |
| 褐煤 | 28.8 | 17.1 | 32.2 | 3.1 | 7.4 | 0.3 | 1.5 | 2.9 | 1.2 | — |
| 长焰煤 | 26.5 | 27.1 | 35.1 | 1.8 | 4.8 | 0.3 | 0.9 | 1.2 | 0.08 | 1.9 |
| 气煤 | 19.5 | 31.8 | 31.1 | 2.5 | 2.3 | 0.4 | 1.3 | 6.1 | 2.9 | 2.1 |
| 焦煤 | 65.0 | 7.6 | 6.6 | 1.0 | 3.8 | 0.4 | 0.8 | 4.3 | 7.3 | 3.3 |
| 无烟煤 | 44.8 | 1.5 | 0 | — | 28.4 | — | 3.8 | 11.7 | — | 4.4 |
| 煤化程度 | 焦油 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 芳香族 | 脂肪族 | 酚类 | 酮类 | 醇类 | 醛类 | 酸类/酯类 | 含氮化合物 | 含硫化合物 | 其他含氧化合物 | |
| 褐煤 | 28.8 | 17.1 | 32.2 | 3.1 | 7.4 | 0.3 | 1.5 | 2.9 | 1.2 | — |
| 长焰煤 | 26.5 | 27.1 | 35.1 | 1.8 | 4.8 | 0.3 | 0.9 | 1.2 | 0.08 | 1.9 |
| 气煤 | 19.5 | 31.8 | 31.1 | 2.5 | 2.3 | 0.4 | 1.3 | 6.1 | 2.9 | 2.1 |
| 焦煤 | 65.0 | 7.6 | 6.6 | 1.0 | 3.8 | 0.4 | 0.8 | 4.3 | 7.3 | 3.3 |
| 无烟煤 | 44.8 | 1.5 | 0 | — | 28.4 | — | 3.8 | 11.7 | — | 4.4 |
| 煤种 | H2 | CO | CO2 | CH4 | C2H6 | C2H4 | C3H8 | C3H6 |
|---|---|---|---|---|---|---|---|---|
| 褐煤 | 4.17 | 3.00 | 4.99 | 2.33 | 0.22 | 0.25 | 0.05 | 0.14 |
| 长焰煤 | 4.70 | 2.67 | 2.69 | 3.36 | 0.34 | 0.18 | 0.07 | 0.08 |
| 气煤 | 6.01 | 1.22 | 3.40 | 4.40 | 0.44 | 0.23 | 0.10 | 0.13 |
| 焦煤 | 21.24 | 0.41 | 0.28 | 9.17 | 0.95 | 0.25 | 0.17 | 0.12 |
| 无烟煤 | 18.77 | 0.78 | 1.45 | 9.89 | 0.19 | 0.05 | 0.02 | 0.02 |
| 煤种 | H2 | CO | CO2 | CH4 | C2H6 | C2H4 | C3H8 | C3H6 |
|---|---|---|---|---|---|---|---|---|
| 褐煤 | 4.17 | 3.00 | 4.99 | 2.33 | 0.22 | 0.25 | 0.05 | 0.14 |
| 长焰煤 | 4.70 | 2.67 | 2.69 | 3.36 | 0.34 | 0.18 | 0.07 | 0.08 |
| 气煤 | 6.01 | 1.22 | 3.40 | 4.40 | 0.44 | 0.23 | 0.10 | 0.13 |
| 焦煤 | 21.24 | 0.41 | 0.28 | 9.17 | 0.95 | 0.25 | 0.17 | 0.12 |
| 无烟煤 | 18.77 | 0.78 | 1.45 | 9.89 | 0.19 | 0.05 | 0.02 | 0.02 |
| 组成 | 神府原煤 | 镜质组 | 惰质组 |
|---|---|---|---|
| 酚类 | 38.7 | 41.6 | 21.1 |
| 苯类 | 16.6 | 11.1 | 23.8 |
| 萘类 | 18.9 | 24.9 | 14.4 |
| 长链烃类 | 6.0 | 17.8 | 3.8 |
| 含氧化合物 | 10.7 | 2.8 | 24.8 |
| 其他 | 9.2 | 1.8 | 12.2 |
| 组成 | 神府原煤 | 镜质组 | 惰质组 |
|---|---|---|---|
| 酚类 | 38.7 | 41.6 | 21.1 |
| 苯类 | 16.6 | 11.1 | 23.8 |
| 萘类 | 18.9 | 24.9 | 14.4 |
| 长链烃类 | 6.0 | 17.8 | 3.8 |
| 含氧化合物 | 10.7 | 2.8 | 24.8 |
| 其他 | 9.2 | 1.8 | 12.2 |
| 压力 | 煤种 | 反应条件 | CH4/% | H2/% | CO/% | 焦油(daf)/% | 水(daf)/% |
|---|---|---|---|---|---|---|---|
| 1MPa | 唐山烟煤 | 600℃,N2气氛 | 1.1 | 1.2 | 0.25 | 9.0 | 4.7 |
| 2MPa | 1.4 | 1.0 | 0.23 | 12.5 | 4.6 | ||
| 3MPa | 1.0 | 0.8 | 0.75 | 11.0 | 4.9 | ||
| 压力 | 煤种 | 反应条件 | CH4(daf)/L·g-1 | H2(daf)/L·g-1 | CO(daf)/L·g-1 | 焦油(daf)/% | 气体(daf)/% |
| 0.1MPa | 西部烟煤 | 700℃,N2气氛 | 0.038 | 0.072 | 0.054 | 3.20 | 10.5 |
| 0.5MPa | 0.039 | 0.065 | 0.048 | 4.05 | 9.2 | ||
| 1.0MPa | 0.042 | 0.055 | 0.043 | 4.45 | 8.2 | ||
| 1.5MPa | 0.045 | 0.054 | 0.042 | 4.40 | 8.0 |
| 压力 | 煤种 | 反应条件 | CH4/% | H2/% | CO/% | 焦油(daf)/% | 水(daf)/% |
|---|---|---|---|---|---|---|---|
| 1MPa | 唐山烟煤 | 600℃,N2气氛 | 1.1 | 1.2 | 0.25 | 9.0 | 4.7 |
| 2MPa | 1.4 | 1.0 | 0.23 | 12.5 | 4.6 | ||
| 3MPa | 1.0 | 0.8 | 0.75 | 11.0 | 4.9 | ||
| 压力 | 煤种 | 反应条件 | CH4(daf)/L·g-1 | H2(daf)/L·g-1 | CO(daf)/L·g-1 | 焦油(daf)/% | 气体(daf)/% |
| 0.1MPa | 西部烟煤 | 700℃,N2气氛 | 0.038 | 0.072 | 0.054 | 3.20 | 10.5 |
| 0.5MPa | 0.039 | 0.065 | 0.048 | 4.05 | 9.2 | ||
| 1.0MPa | 0.042 | 0.055 | 0.043 | 4.45 | 8.2 | ||
| 1.5MPa | 0.045 | 0.054 | 0.042 | 4.40 | 8.0 |
| 反应气氛 | 煤种 | 反应条件 | CO2/mmol·g-1 | CO/mmol·g-1 | CH4/mmol·g-1 | H2/mmol·g-1 | 焦油/% | 气体/% |
|---|---|---|---|---|---|---|---|---|
| N2 | 神府原煤 | 650℃,无催化剂,气体流速50mL/min | 0.65 | 0.05 | 0.83 | 0.43 | 4.10 | 27.24 |
| H2 | 0.86 | 0.02 | 3.61 | — | 7.39 | 19.99 | ||
| CH4 | 0.11 | 0.20 | — | 7.68 | 9.37 | 13.45 | ||
| H2/CO | 3.50 | — | 2.23 | — | 7.44 | 19.89 | ||
| 反应气氛 | 煤种 | 反应条件 | CO2/% | CO/% | CH4/% | H2/% | C n H m /% | 焦油/% |
| N2 | 平朔煤 | 600℃,气体流速800mL/min | 5.2 | 8.5 | 29.3 | 23.2 | 3.0 | 9.5 |
| H2 | 4.8 | 10.3 | 39.64 | 41.1 | 2.5 | 11.5 | ||
| CH4/CO2 | 10.8 | 12.8 | 43.2 | 28.2 | 4.3 | 12.0 |
| 反应气氛 | 煤种 | 反应条件 | CO2/mmol·g-1 | CO/mmol·g-1 | CH4/mmol·g-1 | H2/mmol·g-1 | 焦油/% | 气体/% |
|---|---|---|---|---|---|---|---|---|
| N2 | 神府原煤 | 650℃,无催化剂,气体流速50mL/min | 0.65 | 0.05 | 0.83 | 0.43 | 4.10 | 27.24 |
| H2 | 0.86 | 0.02 | 3.61 | — | 7.39 | 19.99 | ||
| CH4 | 0.11 | 0.20 | — | 7.68 | 9.37 | 13.45 | ||
| H2/CO | 3.50 | — | 2.23 | — | 7.44 | 19.89 | ||
| 反应气氛 | 煤种 | 反应条件 | CO2/% | CO/% | CH4/% | H2/% | C n H m /% | 焦油/% |
| N2 | 平朔煤 | 600℃,气体流速800mL/min | 5.2 | 8.5 | 29.3 | 23.2 | 3.0 | 9.5 |
| H2 | 4.8 | 10.3 | 39.64 | 41.1 | 2.5 | 11.5 | ||
| CH4/CO2 | 10.8 | 12.8 | 43.2 | 28.2 | 4.3 | 12.0 |
| 催化剂 | 原料 | 催化剂 | 载气 | 结果 | 催化类型 | 参考文献 |
|---|---|---|---|---|---|---|
| 金属类 | 霍林河褐煤 | 金属氯化物 (CaCl2、KCl、NiCl2、CoCl2和ZnCl2) | N2 | 促进有机物更多的转化为轻质物质 | 直接催化热解 | [ |
| 低阶煤 | 熔融碳酸盐 (Li2CO3、Na2CO3和K2CO3) | Ar | 增加烃类含量从而增加油的质量 | 直接催化热解 | [ | |
| 褐煤、亚烟煤和烟煤 | 碱金属及碱土金属 | He | 对酚类化合物和缩合芳烃分解为轻质芳烃具有良好的催化活性 | 直接催化热解 | [ | |
| 内蒙古和新疆褐煤 | 钼基和铁基催化剂 | N2 | 有利于煤的解聚反应,焦油产率提高 | 直接催化热解 | [ | |
| 褐煤 | 铁矿石 (褐铁矿、赤铁矿、菱铁矿和磁铁矿) | N2 | 褐铁矿在轻质芳烃生产方面有最好的性能 | 热解挥发分催化热解 | [ | |
| 半焦类 | 神东长焰煤 | 半焦催化剂 | N2 | 可为焦油的分解提供活性表面和催化无机组分,有利于焦油的提质升级 | 直接催化热解 | [ |
| 山西烟煤 | 半焦和金属浸渍半焦催化剂 (Co-char、Ni-char、Cu-char和Zn-char) | N2 | 提高焦油中轻质焦油比例,降低N、S含量,提高焦油H/C比例 | 热解挥发分催化热解 | [ | |
| 呼伦贝尔褐煤 | 生物质半焦 | N2 | 可为裂解重馏分煤焦油提供催化活性位点,热解产生的轻质焦油比例提高 | 热解挥发分催化热解 | [ | |
| 神木亚烟煤 | 半焦催化剂和商用椰壳活性炭 | N2 | 提高煤焦油的质量,增加轻焦油的含量和气体产率 | 热解挥发分催化热解 | [ | |
| 山西烟煤 | 金属改性半焦催化剂 (Ni-char、Fe-Ni-char、Mg-Ni-char、Ce-Ni-char和Zr-Ni-char) | N2 | 焦油中轻质焦油产率提高,S和N的含量降低 | 热解挥发分催化热解 | [ | |
| 分子筛类 | 宁夏烟煤 | Y型 | He | BTEXN的产率提高,焦油质量提高 | 热解挥发分催化热解 | [ |
| 汾西烟煤 | USY | He | 苯的含量增加了500%,甲苯、乙苯、二甲苯和萘等化合物的含量也有不同程度的增加 | 热解挥发分催化热解 | [ | |
| 宁夏烟煤 | 多级孔Y型 | — | BTEXN等轻芳烃的总量从5600ng/mg(原煤热解)增加到18800ng/mg | 热解挥发分催化热解 | [ | |
| 胜利褐煤 | HZSM-5 | Ar | 高铝含量的ZSM-5(SiO2/Al2O3=50)产生BTEXN效果更好 | 热解挥发分催化热解 | [ | |
| 胜利褐煤 | 金属改性ZSM-5 (Co、Mo、Ni改性) | Ar | 金属的引入使焦油中芳烃含量增加,有机氧含量明显降低 | 热解挥发分催化热解 | [ | |
| 神东煤 | 多级孔HZSM-5 | Ar | 多级孔ZSM-5可以促进脂肪烃的裂解环化和酚池的解离,提高了焦油中轻质芳烃的收率 | 直接催化热解 | [ | |
金属负载 ZSM-22 (Co、Mo) | 榆林煤 | H2/N2 | 选择性地提高了脂肪族、芳烃和酚类的相对含量,而抑制了醇类和含氮化合物的相对含量 | 热解挥发分催化热解 | [ |
| 催化剂 | 原料 | 催化剂 | 载气 | 结果 | 催化类型 | 参考文献 |
|---|---|---|---|---|---|---|
| 金属类 | 霍林河褐煤 | 金属氯化物 (CaCl2、KCl、NiCl2、CoCl2和ZnCl2) | N2 | 促进有机物更多的转化为轻质物质 | 直接催化热解 | [ |
| 低阶煤 | 熔融碳酸盐 (Li2CO3、Na2CO3和K2CO3) | Ar | 增加烃类含量从而增加油的质量 | 直接催化热解 | [ | |
| 褐煤、亚烟煤和烟煤 | 碱金属及碱土金属 | He | 对酚类化合物和缩合芳烃分解为轻质芳烃具有良好的催化活性 | 直接催化热解 | [ | |
| 内蒙古和新疆褐煤 | 钼基和铁基催化剂 | N2 | 有利于煤的解聚反应,焦油产率提高 | 直接催化热解 | [ | |
| 褐煤 | 铁矿石 (褐铁矿、赤铁矿、菱铁矿和磁铁矿) | N2 | 褐铁矿在轻质芳烃生产方面有最好的性能 | 热解挥发分催化热解 | [ | |
| 半焦类 | 神东长焰煤 | 半焦催化剂 | N2 | 可为焦油的分解提供活性表面和催化无机组分,有利于焦油的提质升级 | 直接催化热解 | [ |
| 山西烟煤 | 半焦和金属浸渍半焦催化剂 (Co-char、Ni-char、Cu-char和Zn-char) | N2 | 提高焦油中轻质焦油比例,降低N、S含量,提高焦油H/C比例 | 热解挥发分催化热解 | [ | |
| 呼伦贝尔褐煤 | 生物质半焦 | N2 | 可为裂解重馏分煤焦油提供催化活性位点,热解产生的轻质焦油比例提高 | 热解挥发分催化热解 | [ | |
| 神木亚烟煤 | 半焦催化剂和商用椰壳活性炭 | N2 | 提高煤焦油的质量,增加轻焦油的含量和气体产率 | 热解挥发分催化热解 | [ | |
| 山西烟煤 | 金属改性半焦催化剂 (Ni-char、Fe-Ni-char、Mg-Ni-char、Ce-Ni-char和Zr-Ni-char) | N2 | 焦油中轻质焦油产率提高,S和N的含量降低 | 热解挥发分催化热解 | [ | |
| 分子筛类 | 宁夏烟煤 | Y型 | He | BTEXN的产率提高,焦油质量提高 | 热解挥发分催化热解 | [ |
| 汾西烟煤 | USY | He | 苯的含量增加了500%,甲苯、乙苯、二甲苯和萘等化合物的含量也有不同程度的增加 | 热解挥发分催化热解 | [ | |
| 宁夏烟煤 | 多级孔Y型 | — | BTEXN等轻芳烃的总量从5600ng/mg(原煤热解)增加到18800ng/mg | 热解挥发分催化热解 | [ | |
| 胜利褐煤 | HZSM-5 | Ar | 高铝含量的ZSM-5(SiO2/Al2O3=50)产生BTEXN效果更好 | 热解挥发分催化热解 | [ | |
| 胜利褐煤 | 金属改性ZSM-5 (Co、Mo、Ni改性) | Ar | 金属的引入使焦油中芳烃含量增加,有机氧含量明显降低 | 热解挥发分催化热解 | [ | |
| 神东煤 | 多级孔HZSM-5 | Ar | 多级孔ZSM-5可以促进脂肪烃的裂解环化和酚池的解离,提高了焦油中轻质芳烃的收率 | 直接催化热解 | [ | |
金属负载 ZSM-22 (Co、Mo) | 榆林煤 | H2/N2 | 选择性地提高了脂肪族、芳烃和酚类的相对含量,而抑制了醇类和含氮化合物的相对含量 | 热解挥发分催化热解 | [ |
| 106 | ZHAO Zongbin, LI Wen, QIU Jieshan, et al. Effect of Na, Ca and Fe on the evolution of nitrogen species during pyrolysis and combustion of model chars[J]. Fuel, 2003, 82(15/16/17): 1839-1844. |
| 107 | LIU Huan, YI Linlin, HU Hongyun, et al. Emission control of NO x precursors during sewage sludge pyrolysis using an integrated pretreatment of Fenton peroxidation and CaO conditioning[J]. Fuel, 2017, 195: 208-216. |
| 108 | DENG Lei, JIN Xi, ZHANG Yu, et al. Release of nitrogen species during rapid pyrolysis of model coals[J]. Energy & Fuels, 2013, 27(1): 430-439. |
| 109 | WANG Meijun, HU Yongfeng, WANG Jiancheng, et al. Transformation of sulfur during pyrolysis of inertinite-rich coals and correlation with their characteristics[J]. Journal of Analytical and Applied Pyrolysis, 2013, 104: 585-592. |
| 110 | RAMMLER R W, K L. Synthetic fuels from Lurgi coal pyrolysis[J]. Energy Progress, 1982, 2: 121-129. |
| 111 | HUGHMARK Gordon A. Power requirements and interfacial area in gas-liquid turbine agitated systems[J]. Industrial & Engineering Chemistry Process Design and Development, 1980, 19(4): 638-641. |
| 112 | 郭树才. 煤化工工艺学[M]. 2版. 北京: 化学工业出版社, 2006. |
| GUO Shucai. Coal chemical technology[M]. 2nd ed. Beijing: Chemical Industry Press, 2006. | |
| 113 | 陈硕翼, 朱卫东, 唐明生, 等. 煤炭直接转化技术发展现状与趋势[J]. 科技中国, 2018(10): 8-11. |
| CHEN Shuoyi, ZHU Weidong, TANG Mingsheng, et al. Development status and trend of coal direct conversion technology[J]. China Scitechnology Business, 2018(10): 8-11. | |
| 114 | ATWOOD M T, SCHULMAN B L. The toscoal process pyrolysis of westerncoals and lignites for char and oil production[J]. Preprints of Papers American Chemical Society Division of Fuel Chemical, 1977, 22: 233-252. |
| 115 | H S A, T E R. COED plant for coal conversion[J]. Chemical Engineering Progress, 1971, 67: 75-80. |
| 1 | HUANG Yongming, XUE Lian, KHAN Zeeshan. What abates carbon emissions in China: Examining the impact of renewable energy and green investment[J]. Sustainable Development, 2021, 29(5): 823-834. |
| 2 | WEN Huwei, LIANG Weitao, LEE Chien-Chiang. China’s progress toward sustainable development in pursuit of carbon neutrality: Regional differences and dynamic evolution[J]. Environmental Impact Assessment Review, 2023, 98: 106959. |
| 3 | DUAN Hongbo, MO Jianlei, FAN Ying, et al. Achieving China’s energy and climate policy targets in 2030 under multiple uncertainties[J]. Energy Economics, 2018, 70: 45-60. |
| 4 | XU Guangwen, BAI Dingrong, XU Chunming, et al. Challenges and opportunities for engineering thermochemistry in carbon-neutralization technologies[J]. National Science Review, 2022, 10(9): nwac217. |
| 5 | KLEIN Michael L, SHINODA Wataru. Large-scale molecular dynamics simulations of self-assembling systems[J]. Science, 2008, 321(5890): 798-800. |
| 6 | 袁亮. 我国煤炭工业高质量发展面临的挑战与对策[J]. 中国煤炭, 2020, 46(1): 6-12. |
| YUAN Liang. Challenges and countermeasures for high quality development of China’s coal industry[J]. China Coal, 2020, 46(1): 6-12. | |
| 7 | 郑化安. 中低温煤热解技术研究进展及产业化方向[J]. 洁净煤技术, 2018, 24(1): 13-18. |
| ZHENG Huaan. Research status and industrial direction of middle and low temperature coal pyrolysis technology[J]. Clean Coal Technology, 2018, 24(1): 13-18. | |
| 8 | SHI Lei, LIU Qingya, GUO Xiaojin, et al. Pyrolysis behavior and bonding information of coal—A TGA study[J]. Fuel Processing Technology, 2013, 108: 125-132. |
| 9 | MOULIJN J A, TROMP P J J. Coal pyrolysis[M]//FIGUEIREDO J L, MOULIJN J A. Carbon and coal gasification. Dordrecht: Springer, 1986: 455-484. |
| 10 | 刘振宇. 煤化学的前沿与挑战: 结构与反应[J]. 中国科学: 化学, 2014, 44(9): 1431-1439. |
| LIU Zhenyu. Advancement in coal chemistry: Structure and reactivity[J]. Scientia Sinica Chimica, 2014, 44(9): 1431-1439. | |
| 11 | Anton FEENSTRA K, HESS Berk, BERENDSEN Herman J C. Improving efficiency of large time-scale molecular dynamics simulations of hydrogen-rich systems[J]. Journal of Computational Chemistry, 1999, 20(8): 786-798. |
| 12 | RUSSO Michael F, LI Rong, MENCH Matthew, et al. Molecular dynamic simulation of aluminum-water reactions using the ReaxFF reactive force field[J]. International Journal of Hydrogen Energy, 2011, 36(10): 5828-5835. |
| 13 | ZHENG Mo, LI Xiaoxia, GUO Li. Dynamic trends for char/soot formation during secondary reactions of coal pyrolysis by large-scale reactive molecular dynamics[J]. Journal of Analytical and Applied Pyrolysis, 2021, 155: 105048. |
| 14 | HONG Dikun, GUO Xin. Molecular dynamics simulations of Zhundong coal pyrolysis using reactive force field[J]. Fuel, 2017, 210: 58-66. |
| 15 | SALMON Elodie, VAN DUIN Adri C T, LORANT François, et al. Early maturation processes in coal. Part 2: Reactive dynamics simulations using the ReaxFF reactive force field on Morwell Brown coal structures[J]. Organic Geochemistry, 2009, 40(12): 1195-1209. |
| 16 | Fidel CASTRO-MARCANO, RUSSO Michael F, VAN DUIN Adri C T, et al. Pyrolysis of a large-scale molecular model for Illinois no. 6 coal using the ReaxFF reactive force field[J]. Journal of Analytical and Applied Pyrolysis, 2014, 109: 79-89. |
| 17 | XU Fang, PAN Shuo, LIU Chunguang, et al. Construction and evaluation of chemical structure model of Huolinhe lignite using molecular modeling[J]. RSC Advances, 2017, 7(66): 41512-41519. |
| 18 | XU Fang, LIU Hui, WANG Qing, et al. ReaxFF-based molecular dynamics simulation of the initial pyrolysis mechanism of lignite[J]. Fuel Processing Technology, 2019, 195: 106147. |
| 19 | XU Fang, LIU Hui, WANG Qing, et al. Study of non-isothermal pyrolysis mechanism of lignite using ReaxFF molecular dynamics simulations[J]. Fuel, 2019, 256: 115884. |
| 20 | BAI Hongcun, MAO Ning, WANG Ruihan, et al. Kinetic characteristics and reactive behaviors of HSW vitrinite coal pyrolysis: A comprehensive analysis based on TG-MS experiments, kinetics models and ReaxFF MD simulations[J]. Energy Reports, 2021, 7: 1416-1435. |
| 21 | ZHENG Mo, LI Xiaoxia, LIU Jian, et al. Pyrolysis of Liulin coal simulated by GPU-based ReaxFF MD with cheminformatics analysis[J]. Energy & Fuels, 2014, 28(1): 522-534. |
| 116 | 戴秋菊, 唐道武, 常万林. 采用多段回转炉热解工艺综合利用年青煤[J]. 煤炭加工与综合利用, 1999(3): 22-23. |
| DAI Qiuju, TANG Daowu, CHANG Wanlin. Comprehensive utilization of low-rank coal by pyrolysis technique in multi-stage rotary kiln[J]. Coal Processing & Comprehensive Utilization, 1999(3): 22-23. | |
| 117 | 王勤辉, 骆仲泱, 方梦祥, 等. 12兆瓦热电气多联产装置的开发[J]. 燃料化学学报, 2002, 30(2): 141-146. |
| WANG Qinhui, LUO Zhongyang, FANG Mengxiang, et al. Development of a 12MW multi-generation of gas, steam and power[J]. Journal of Fuel Chemistry and Technology, 2002, 30(2): 141-146. | |
| 118 | WANG Jieguang, LU Xuesong, YAO Jianzhong, et al. Experimental study of coal topping process in a downer reactor[J]. Industrial & Engineering Chemistry Research, 2005, 44(3): 463-470. |
| 119 | ZHANG Chun, WU Rongcheng, XU Guangwen. Coal pyrolysis for high-quality tar in a fixed-bed pyrolyzer enhanced with internals[J]. Energy & Fuels, 2014, 28(1): 236-244. |
| 120 | ZHANG Chun, WU Rongcheng, HU Erfeng, et al. Coal pyrolysis for high-quality tar and gas in 100 kg fixed bed enhanced with internals[J]. Energy & Fuels, 2014, 28(11): 7294-7302. |
| 121 | 刘振宇. 煤快速热解制油技术问题的化学反应工程根源: 逆向传热与传质[J]. 化工学报, 2016, 67(1): 1-5. |
| LIU Zhenyu. Origin of common problems in fast coal pyrolysis technologies for tar: The countercurrent flow of heat and volatiles[J]. CIESC Journal, 2016, 67(1): 1-5. | |
| 22 | ZHENG Mo, LI Xiaoxia, NIE Fengguang, et al. Investigation of overall pyrolysis stages for Liulin bituminous coal by large-scale ReaxFF molecular dynamics[J]. Energy & Fuels, 2017, 31(4): 3675-3683. |
| 23 | VYAZOVKIN Sergey, ACHILIAS Dimitris, Xavier FERNANDEZ-FRANCOS, et al. ICTAC Kinetics Committee recommendations for analysis of thermal polymerization kinetics[J]. Thermochimica Acta, 2022, 714: 179243. |
| 24 | JIN Chengchao, LIU Daiming, HU Jing, et al. The role of microstructure in piezocatalytic degradation of organic dye pollutants in wastewater[J]. Nano Energy, 2019, 59: 372-379. |
| 25 | ZHAO Dongting, WANG Xianhua, MILLER James B, et al. The chemistry and kinetics of polyethylene pyrolysis: A process to produce fuels and chemicals[J]. ChemSusChem, 2020, 13(7): 1764-1774. |
| 26 | ZAKER Ali, CHEN Zhi, Mohammed ZAHEER-UDDIN, et al. Co-pyrolysis of sewage sludge and low-density polyethylene—A thermogravimetric study of thermo-kinetics and thermodynamic parameters[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104554. |
| 27 | Jun Sheng TEH, TEOH Yew Heng, Heoy Geok HOW, et al. Thermal analysis technologies for biomass feedstocks: A state-of-the-art review[J]. Processes, 2021, 9(9): 1610. |
| 28 | HAO Junhui, FENG Wen, QIAO Yingyun, et al. Thermal cracking behaviors and products distribution of oil sand bitumen by TG-FTIR and Py-GC/TOF-MS[J]. Energy Conversion and Management, 2017, 151: 227-239. |
| 29 | SONG Huijuan, LIU Guangrui, ZHANG Jinzhi, et al. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method[J]. Fuel Processing Technology, 2017, 156: 454-460. |
| 30 | JIANG Yuan, ZONG Peijie, TIAN Bin, et al. Pyrolysis behaviors and product distribution of Shenmu coal at high heating rate: A study using TG-FTIR and Py-GC/MS[J]. Energy Conversion and Management, 2019, 179: 72-80. |
| 31 | 刘钦甫, 徐占杰, 崔晓南, 等. 不同煤化程度煤的热解及氮的释放行为[J]. 煤炭学报, 2015, 40(2): 450-455. |
| LIU Qinfu, XU Zhanjie, CUI Xiaonan, et al. Release behavior of nitrogen in different rank coals during pyrolysis[J]. Journal of China Coal Society, 2015, 40(2): 450-455. | |
| 32 | SONIBARE Oluwadayo O, HAEGER Tobias, FOLEY Stephen F. Structural characterization of nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy[J]. Energy, 2010, 35(12): 5347-5353. |
| 33 | DING Yanming, EZEKOYE Ofodike A, LU Shouxiang, et al. Thermal degradation of beech wood with thermogravimetry/Fourier transform infrared analysis[J]. Energy Conversion and Management, 2016, 120: 370-377. |
| 34 | ZHU Hongqing, ZHAO Hongru, WEI Hongyi, et al. Investigation into the thermal behavior and FTIR micro-characteristics of re-oxidation coal[J]. Combustion and Flame, 2020, 216: 354-368. |
| 35 | Kwang-Hyun KO, RAWAL Aditya, SAHAJWALLA Veena. Analysis of thermal degradation kinetics and carbon structure changes of co-pyrolysis between macadamia nut shell and PET using thermogravimetric analysis and 13C solid state nuclear magnetic resonance[J]. Energy Conversion and Management, 2014, 86: 154-164. |
| 36 | MA Zhongqing, CHEN Dengyu, GU Jie, et al. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods[J]. Energy Conversion and Management, 2015, 89: 251-259. |
| 37 | ABOULKAS A, HARFI K EL, BOUADILI A EL. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms[J]. Energy Conversion and Management, 2010, 51(7): 1363-1369. |
| 38 | PICKARD S, DAOOD S S, POURKASHANIAN M, et al. Robust extension of the coats-redfern technique: Reviewing rapid and realiable reactivity analysis of complex fuels decomposing in inert and oxidizing thermogravimetric analysis atmospheres[J]. Energy & Fuels, 2013, 27(5): 2818-2826. |
| 39 | BADZIOCH Stanley, HAWKSLEY Peter G W. Kinetics of thermal decomposition of pulverized coal particles[J]. Industrial & Engineering Chemistry Process Design and Development, 1970, 9(4): 521-530. |
| 40 | ARENILLAS A, RUBIERA F, PEVIDA C, et al. A comparison of different methods for predicting coal devolatilisation kinetics[J]. Journal of Analytical and Applied Pyrolysis, 2001, 58: 685-701. |
| 41 | JAIN Ankit A, MEHRA Anurag, RANADE Vivek V. Processing of TGA data: Analysis of isoconversional and model fitting methods[J]. Fuel, 2016, 165: 490-498. |
| 42 | LIU Xuguang, LI Baoqing, MIURA Kouichi. Analysis of pyrolysis and gasification reactions of hydrothermally and supercritically upgraded low-rank coal by using a new distributed activation energy model[J]. Fuel Processing Technology, 2001, 69(1): 1-12. |
| 43 | ZHANG Jinzhi, CHEN Tianju, WU Jingli, et al. A novel Gaussian-DAEM-reaction model for the pyrolysis of cellulose, hemicellulose and lignin[J]. RSC Advances, 2014, 4(34): 17513-17520. |
| 44 | WANG Junli, LI Peng, LIANG Litong, et al. Kinetics modeling of low-rank coal pyrolysis based on a three-Gaussian distributed activation energy model (DAEM) reaction model[J]. Energy & Fuels, 2016, 30(11): 9693-9702. |
| 45 | ANTHONY D B, HOWARD J B. Coal devoiatiiization and hydrogasification[J]. American Institute of Chemical Engineers Journals, 1976, 22: 625-656. |
| 46 | MIURA Kouichi, MAKI Taisuke. A simple method for estimating f(E) and k0(E) in the distributed activation energy model[J]. Energy & Fuels, 1998, 12(5): 864-869. |
| 47 | DE CAPRARIIS Benedetta, DE FILIPPIS Paolo, HERCE Carlos, et al. Double-Gaussian distributed activation energy model for coal devolatilization[J]. Energy & Fuels, 2012, 26(10): 6153-6159. |
| 48 | GAVALAS George R. Coal pyrolysis[M]. Amsterdam: Elsevier Scientific Pub. Co., 1982. |
| 49 | 谢克昌. 煤的结构与反应性[M]. 北京: 科学出版社, 2002. |
| XIE Kechang. Coal structure and its reactivity[M]. Beijing: Science Press, 2002. | |
| 50 | 李雅. 低阶煤热解特性及其产物分布研究[D]. 大连: 大连理工大学, 2018. |
| LI Ya. Pyrolysis characteristics and products distribution of low-rank coal[D]. Dalian: Dalian University of Technology, 2018. | |
| 51 | 张双全. 煤化学[M]. 4版. 徐州: 中国矿业大学出版社, 2017. |
| ZHANG Shuangquan. Coal chemistry[M]. 4th ed. Xuzhou, China: China University of Mining & Technology Press, 2017. | |
| 52 | 姜雨, 徐秀丽, 孟庆波, 等. 不同煤化度煤热解焦油的组分分析[J]. 煤化工, 2016, 44(6): 6-10, 18. |
| JIANG Yu, XU Xiuli, MENG Qingbo, et al. Analysis of tar compositions derived from coal pyrolysis at different degree of coalification[J]. Coal Chemical Industry, 2016, 44(6): 6-10, 18. | |
| 53 | GAO Meiqi, WANG Yulong, DONG Jie, et al. Release behavior and formation mechanism of polycyclic aromatic hydrocarbons during coal pyrolysis[J]. Chemosphere, 2016, 158: 1-8. |
| 54 | VAN HEEK K H, HODEK W. Structure and pyrolysis behaviour of different coals and relevant model substances[J]. Fuel, 1994, 73(6): 886-896. |
| 55 | REN Xueyu, ZHAO Shixuan, CAO Jingpei, et al. Effect of coal ranks on light aromatics production during reforming of pyrolysis volatiles over HZSM-5 under Ar and H2-assisted atmospheres[J]. Journal of Analytical and Applied Pyrolysis, 2020, 152: 104958. |
| 56 | ZHAO Yunpeng, HU Haoquan, JIN Lijun, et al. Pyrolysis behavior of vitrinite and inertinite from Chinese Pingshuo coal by TG-MS and in a fixed bed reactor[J]. Fuel Processing Technology, 2011, 92(4): 780-786. |
| 57 | 赵伟, 张晓欠, 周安宁, 等. 神府煤煤岩显微组分的浮选分离及富集物的低温热解产物特性研究[J]. 燃料化学学报, 2014, 42(5): 527-533. |
| ZHAO Wei, ZHANG Xiaoqian, ZHOU Anning, et al. Flotation separation of Shenfu coal macerals and low temperature pyrolysis characteristics of different maceral concentrate[J]. Journal of Fuel Chemistry and Technology, 2014, 42(5): 527-533. | |
| 58 | 刘巧霞, 张月明, 刘丹, 等. 煤热解焦油收率影响因素[J]. 煤炭加工与综合利用, 2018(8): 75-78, 81, 9. |
| LIU Qiaoxia, ZHANG Yueming, LIU Dan, et al. Influence factors of the tar yield in coal pyrolysis[J]. Coal Processing & Comprehensive Utilization, 2018(8): 75-78, 81, 9. | |
| 59 | 刘壮, 田宜水, 胡二峰, 等. 低阶煤热解影响因素及其工艺技术研究进展[J]. 洁净煤技术, 2021, 27(1): 50-59. |
| LIU Zhuang, TIAN Yishui, HU Erfeng, et al. Research progress on influencing factors and technology of low-rank coal pyrolysis[J]. Clean Coal Technology, 2021, 27(1): 50-59. | |
| 60 | 商铁成. 热解温度对低阶煤热解性能影响研究[J]. 洁净煤技术, 2014, 20(6): 28-31. |
| SHANG Tiecheng. Influence of temperature on pyrolysis properties of low rank coal[J]. Clean Coal Technology, 2014, 20(6): 28-31. | |
| 61 | LIU Tianlong, CAO Jingpei, ZHAO Xiaoyan, et al. In situ upgrading of Shengli lignite pyrolysis vapors over metal-loaded HZSM-5 catalyst[J]. Fuel Processing Technology, 2017, 160: 19-26. |
| 62 | HAYASHI Jun-ichiro, TAKAHASHI Hiroshi, Satoshi DOI, et al. Reactions in brown coal pyrolysis responsible for heating rate effect on tar yield[J]. Energy & Fuels, 2000, 14(2): 400-408. |
| 63 | WU Dun, LIU Guijian, CHEN Shancheng, et al. An experimental investigation on heating rate effect in the thermal behavior of perhydrous bituminous coal during pyrolysis[J]. Journal of Thermal Analysis and Calorimetry, 2015, 119(3): 2195-2203. |
| 64 | YU Yanxu, KONG Jiao, WANG Meijun, et al. Structure and oxidation reactivity of char: Effects of pyrolysis heating rate and pressure[J]. Journal of Fuel Chemistry and Technology, 2018, 46(9): 1025-1035. |
| 65 | XU Shipei, ZENG Xi, HAN Zhennan, et al. Quick pyrolysis of a massive coal sample via rapid infrared heating[J]. Applied Energy, 2019, 242: 732-740. |
| 66 | 吴洁, 狄佐星, 罗明生, 等. N2气氛下温度和压力对煤热解的影响[J]. 化工进展, 2019, 38(S1): 116-121. |
| WU Jie, DI Zuoxing, LUO Mingsheng, et al. Effects of temperature and pressure on coal pyrolysis in N2 atmosphere[J]. Chemical Industry and Engineering Progress, 2019, 38(S1): 116-121. | |
| 67 | LUO Kang, ZHANG Chun, ZHU Shenghua, et al. Tar formation during coal pyrolysis under N2 and CO2 atmospheres at elevated pressures[J]. Journal of Analytical and Applied Pyrolysis, 2016, 118: 130-135. |
| 68 | ANTHONY Donald B, HOWARD Jack B, HOTTEL Hoyt C, et al. Rapid devolatilization and hydrogasification of bituminous coal[J]. Fuel, 1976, 55(2): 121-128. |
| 69 | 孙鸣, 代晓敏, 姚一, 等. 呼伦贝尔褐煤负压热解特性研究[J]. 中国矿业大学学报, 2015, 44(3): 483-488. |
| SUN Ming, DAI Xiaomin, YAO Yi, et al. Pyrolysis characteristics of Hulunbeir lignite under negative-pressure[J]. Journal of China University of Mining & Technology, 2015, 44(3): 483-488. | |
| 70 | J-i HAYASHI, TAKAHASHI H, IWATSUKI M, et al. Rapid conversion of tar and char from pyrolysis of a brown coal by reactions with steam in a drop-tube reactor[J]. Fuel, 2000, 79(3/4): 439-447. |
| 71 | 雷玉. 神府煤在不同气氛下的催化热解反应性研究[D]. 西安: 西安科技大学, 2010. |
| LEI Yu. Study on reactivity of catalytic pyrolysis of Shenfu coal in different reactive gas[D]. Xi’an: Xi’an University of Science and Technology, 2010. | |
| 72 | 史雪君, 汪勤亚, 马委元, 等. 反应气氛对平朔煤热解反应性能的影响[J]. 煤炭转化, 2014, 37(3): 5-9. |
| SHI Xuejun, WANG Qinya, MA Weiyuan, et al. Study on reactivity of pyrolysis of pingshuo coal in different reactive gas[J]. Coal Conversion, 2014, 37(3): 5-9. | |
| 73 | ZOU Xianwu, YAO Jianzhong, YANG Xuemin, et al. Catalytic effects of metal chlorides on the pyrolysis of lignite[J]. Energy & Fuels, 2007, 21(2): 619-624. |
| 74 | RIZKIANA Jenny, GUAN Guoqing, WIDAYATNO Wahyu Bambang, et al. Oil production from mild pyrolysis of low-rank coal in molten salts media[J]. Applied Energy, 2015, 154: 944-950. |
| 75 | YAN Lunjing, BAI Yonghui, KONG Xiaojun, et al. Effects of alkali and alkaline earth metals on the formation of light aromatic hydrocarbons during coal pyrolysis[J]. Journal of Analytical and Applied Pyrolysis, 2016, 122: 169-174. |
| 76 | LIANG Litong, HUANG Wei, GAO Fuxing, et al. Mild catalytic depolymerization of low rank coals: A novel way to increase tar yield[J]. RSC Advances, 2015, 5(4): 2493-2503. |
| 77 | HE Lu, HUI Helong, LI Songgeng, et al. Production of light aromatic hydrocarbons by catalytic cracking of coal pyrolysis vapors over natural iron ores[J]. Fuel, 2018, 216: 227-232. |
| 78 | LI Xiaohong, MA Jiangshan, LI Lili, et al. Semi-coke as solid heat carrier for low-temperature coal tar upgrading[J]. Fuel Processing Technology, 2016, 143: 79-85. |
| 79 | HAN Jiangze, WANG Xingdong, YUE Junrong, et al. Catalytic upgrading of coal pyrolysis tar over char-based catalysts[J]. Fuel Processing Technology, 2014, 122: 98-106. |
| 80 | FU Daqing, LI Xiaohong, LI Wenying, et al. Catalytic upgrading of coal pyrolysis products over bio-char[J]. Fuel Processing Technology, 2018, 176: 240-248. |
| 81 | JIN Lijun, BAI Xiaoyu, LI Yang, et al. In-situ catalytic upgrading of coal pyrolysis tar on carbon-based catalyst in a fixed-bed reactor[J]. Fuel Processing Technology, 2016, 147: 41-46. |
| 82 | HAN Jiangze, LIU Xiaoxing, YUE Junrong, et al. Catalytic upgrading of in situ coal pyrolysis tar over Ni-char catalyst with different additives[J]. Energy & Fuels, 2014, 28(8): 4934-4941. |
| 83 | LIU Yujie, YAN Lunjing, BAI Yonghui, et al. Catalytic upgrading of volatile from coal pyrolysis over faujasite zeolites[J]. Journal of Analytical and Applied Pyrolysis, 2018, 132: 184-189. |
| 84 | YAN Lunjing, KONG Xiaojun, ZHAO Ruifang, et al. Catalytic upgrading of gaseous tars over zeolite catalysts during coal pyrolysis[J]. Fuel Processing Technology, 2015, 138: 424-429. |
| 85 | LV Peng, YAN Lunjing, LIU Yan, et al. Catalytic conversion of coal pyrolysis vapors to light aromatics over hierarchical Y-type zeolites[J]. Journal of the Energy Institute, 2020, 93(4): 1354-1363. |
| 86 | REN Xueyu, CAO Jingpei, ZHAO Xiaoyan, et al. Catalytic conversion of lignite pyrolysis volatiles to light aromatics over ZSM-5: SiO2/Al2O3 ratio effects and mechanism insights[J]. Journal of Analytical and Applied Pyrolysis, 2019, 139: 22-30. |
| 87 | REN Xueyu, CAO Jingpei, ZHAO Xiaoyan, et al. Catalytic upgrading of pyrolysis vapors from lignite over mono/bimetal-loaded mesoporous HZSM-5[J]. Fuel, 2018, 218: 33-40. |
| 88 | ZHANG Zhuangzhuang, LIU Nan, AN Chongxin, et al. Effect of hierarchical ZSM-5 zeolites on product distribution of low rank coal fast pyrolysis in a fluidized bed[J]. Journal of Fuel Chemistry and Technology, 2021, 49(4): 407-414. |
| 89 | LI Qing, FENG Xiuyan, WANG Xiao, et al. Pyrolysis of Yulin coal over ZSM-22 supported catalysts for upgrading coal tar in fixed bed reactor[J]. Journal of Analytical and Applied Pyrolysis, 2017, 126: 390-396. |
| 90 | SONG Huijuan, LIU Guangrui, WU Jinhu. Pyrolysis characteristics and kinetics of low rank coals by distributed activation energy model[J]. Energy Conversion and Management, 2016, 126: 1037-1046. |
| 91 | WU Hongwei, HAYASHI Jun-ichiro, CHIBA Tadatoshi, et al. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. Part Ⅴ. Combined effects of Na concentration and char structure on char reactivity[J]. Fuel, 2004, 83(1): 23-30. |
| 92 | LIN Xiongchao, LUO Meng, LI Shouyi, et al. The evolutionary route of coal matrix during integrated cascade pyrolysis of a typical low-rank coal[J]. Applied Energy, 2017, 199: 335-346. |
| 93 | YU Junqin, GUO Qinghua, DING Lu, et al. Study on the effect of inherent AAEM on char structure evolution during coal pyrolysis by in situ Raman and TG[J]. Fuel, 2021, 292: 120406. |
| 94 | DU Zhonghua, LI Wu. The catalytic effect from alkaline elements on the tar-rich coal pyrolysis[J]. Catalysts, 2022, 12(4): 376. |
| 95 | HU Song, JIANG Long, WANG Yi, et al. Effects of inherent alkali and alkaline earth metallic species on biomass pyrolysis at different temperatures[J]. Bioresource Technology, 2015, 192: 23-30. |
| 96 | SATHE Chirag, HAYASHI Jun-ichiro, LI Chunzhu, et al. Combined effects of pressure and ion-exchangeable metallic species on pyrolysis of Victorian lignite[J]. Fuel, 2003, 82(3): 343-350. |
| 97 | HAYASHI Jun-ichiro, MORI Takao, AMAMOTO Shinobu, et al. Flash pyrolysis of brown coal modified by alcohol-vapor explosion treatment[J]. Energy & Fuels, 1996, 10(5): 1099-1107. |
| 98 | TYLER Ralph J, SCHAFER Harry N S. Flash pyrolysis of coals: Influence of cations on the devolatilization behaviour of brown coals[J]. Fuel, 1980, 59(7): 487-494. |
| 99 | WORNAT Mary J, NELSON Peter F. Effects of ion-exchanged calcium on brown coal tar composition as determined by Fourier transform infrared spectroscopy[J]. Energy & Fuels, 1992, 6(2): 136-142. |
| 100 | CALKINS William H. The chemical forms of sulfur in coal: A review[J]. Fuel, 1994, 73(4): 475-484. |
| 101 | LI Chunzhu, TAN Li lian. Formation of NO x and SO x precursors during the pyrolysis of coal and biomass. Part III. Further discussion on the formation of HCN and NH3 during pyrolysis[J]. Fuel, 2000, 79(15): 1899-1906. |
| 102 | XIE Zongli, FENG Jie, ZHAO Wei, et al. Formation of NO x and SO x precursors during the pyrolysis of coal and biomass. Part IV. Pyrolysis of a set of Australian and Chinese coals[J]. Fuel, 2001, 80(15): 2131-2138. |
| 103 | GLARBORG P, JENSEN A D, JOHNSSON J E. Fuel nitrogen conversion in solid fuel fired systems[J]. Progress in Energy and Combustion Science, 2003, 29(2): 89-113. |
| 104 | ZHOU Hao, HUANG Yan, MO Guiyuan, et al. Experimental investigations of the conversion of fuel-N, volatile-N and char-N to NO x and N2O during single coal particle fluidized bed combustion[J]. Journal of the Energy Institute, 2017, 90(1): 62-72. |
| 105 | ZHENG Mo, LI Xiaoxia, GUO Li. Investigation of N behavior during coal pyrolysis and oxidation using ReaxFF molecular dynamics[J]. Fuel, 2018, 233: 867-876. |
| [1] | YANG Shini, XU Yudong. Preparation of gypsum whisker from the gypsum sludge derived in a close-loop treatment process for leachate nanofiltration concentrate [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5450-5459. |
| [2] | WANG Lanxin, LI Fei, QIAN Yanan, TIAN Yujie, SHEN Jun, WANG Wei. Numerical simulation of coal pyrolysis with different moisture content in fixed-bed reactor [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4513-4525. |
| [3] | DU Lei, CAO Zhitao, XU Lang, ZHANG Yingjie, SUN Baochang, ZOU Haikui, CHU Guangwen, CHEN Jianfeng. Progress research in preparation of adiponitrile [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3683-3696. |
| [4] | SHI Xiuding, WANG Yongquan, ZENG Jing, SU Chang, HONG Junming. Nanotubular Co-N-C activated percarbonate for tetracycline degradation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3041-3052. |
| [5] | WU Zhanhua, KONG Debao, TIAN Junjun, LIANG Rujun, LU Penghui, SHENG Min. Evaluation method of the reactivity safety based on "1+N" mode [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3199-3207. |
| [6] | WANG Peigan, LI Leli, XIE Songzhuan, SONG Bingbing, KONG Qiaoping, LIU Gaige, MA Weiwei, SHI Xueqing. Phosphate adsorption mechanism of sludge-based FeCa-ALE composite material [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2365-2373. |
| [7] | JIN Zhihao, WANG Yunfan, TIAN Zhenyu. Strategies for combustion kinetics of afterburning in rocket engine exhaust plumes [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1776-1780. |
| [8] | ZUO Ji, LUO Li, XIE Yongkai, CHEN Wenyao, QIAN Gang, ZHOU Xinggui, DUAN Xuezhi. Effect of Cu catalyst particle size on methanol nonoxidative dehydrogenation to formaldehyde [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1347-1354. |
| [9] | LI Zhuoyu, YU Meiqi, CHEN Xiaoyan, HU Ruohui, WANG Qinghong, CHEN Chunmao, ZHAN Yali. Effects and mechanism on the removal of nitrobenzene from water by adsorption of refining waste catalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(2): 1076-1087. |
| [10] | WU Guojie, LIU Quanyu, PENG Cheng, XIA Siqi, HUANG Dongfang, ZHOU Quanbao, LYU Peng, WANG Xuegang. Research progress on hydrogen absorption and desorption performance of metal oxide catalyzed solid-state hydrogen storage materials [J]. Chemical Industry and Engineering Progress, 2025, 44(12): 6996-7018. |
| [11] | CHEN Xinyue, CHEN Binjian, MAO Yudong, YAN Min, XUE Lu. Research progress and prospect of hydrogen evolution catalysts for alkaline water electrolysis [J]. Chemical Industry and Engineering Progress, 2025, 44(11): 6334-6349. |
| [12] | ZHANG Qiang, SUN Nan, ZHENG Junjie, WU Qiang, LIU Chuanhai, LI Yuanji. Effect of mixed thermodynamic promoters on kinetic and recovery study of hydration separation coal mine gas [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 192-201. |
| [13] | YIN Chenyang, LIU Yongfeng, CHEN Ruizhe, ZHANG Lu, SONG Jin’ou, LIU Haifeng. Kinetic simulation of n-hexane pyrolysis reaction based on quantitative calculations [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4273-4282. |
| [14] | HE Haixia, WAN Yameng, LI Fanfan, NIU Xinyu, ZHANG Jingwen, LI Tao, REN Baozeng. Kinetics and crystallization process of naphazoline hydrochloride in methanol-ethyl acetate system [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4230-4245. |
| [15] | LIU Yucan, GAO Zhonglu, XU Xinyi, JI Xianguo, ZHANG Yan, SUN Hongwei, WANG Gang. Adsorption performance and mechanism of diuron from water by calcium-modified water hyacinth-based biochar [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4630-4641. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||