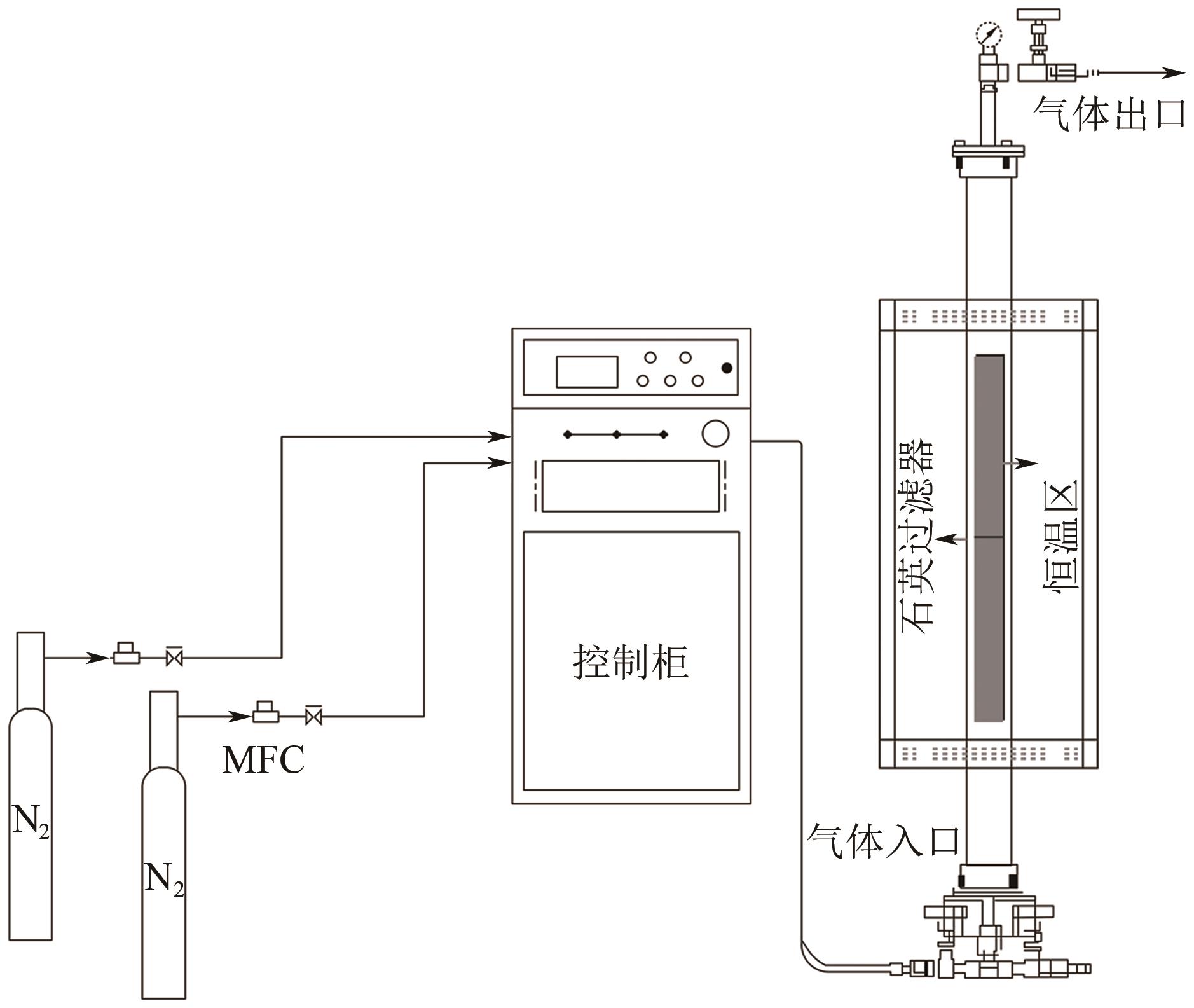
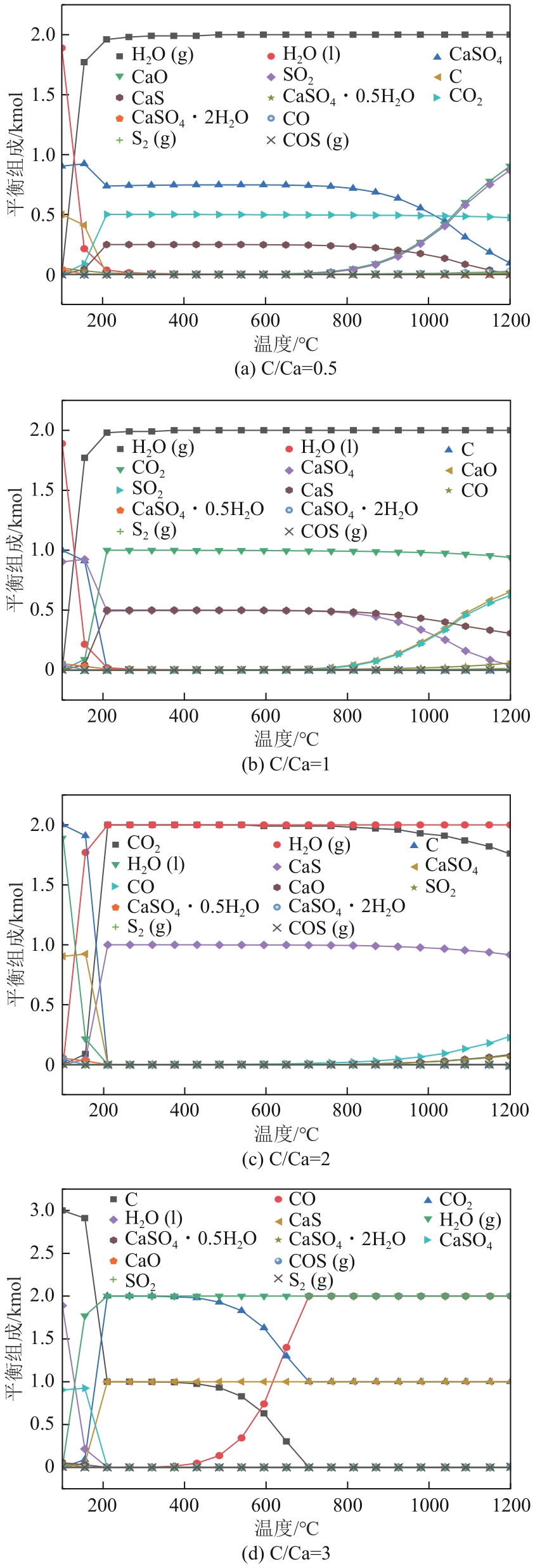
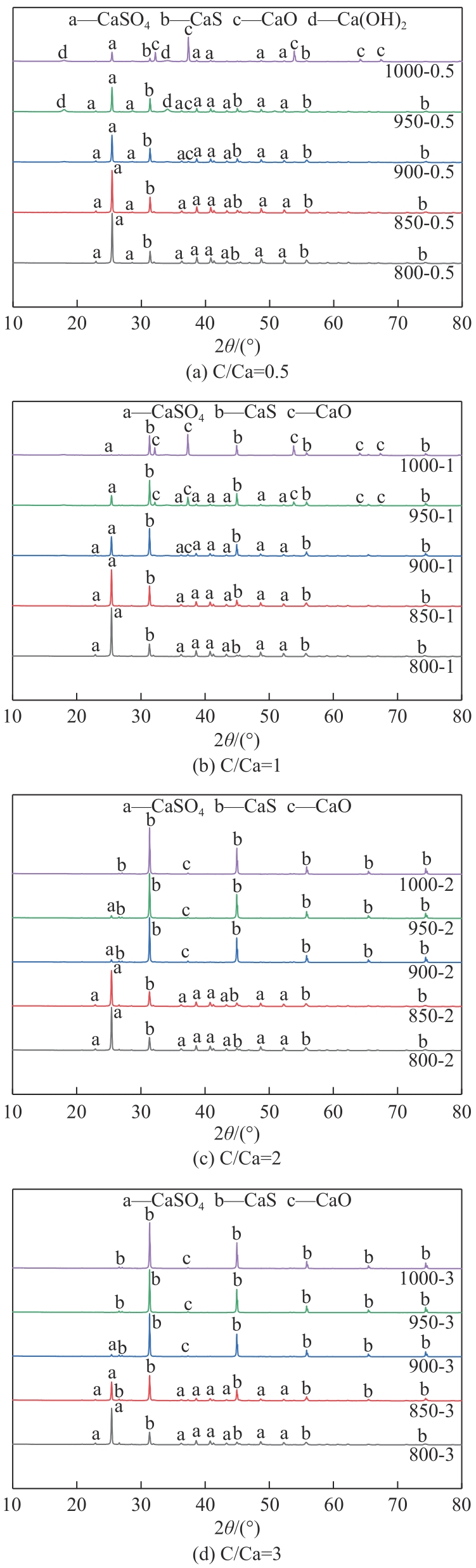
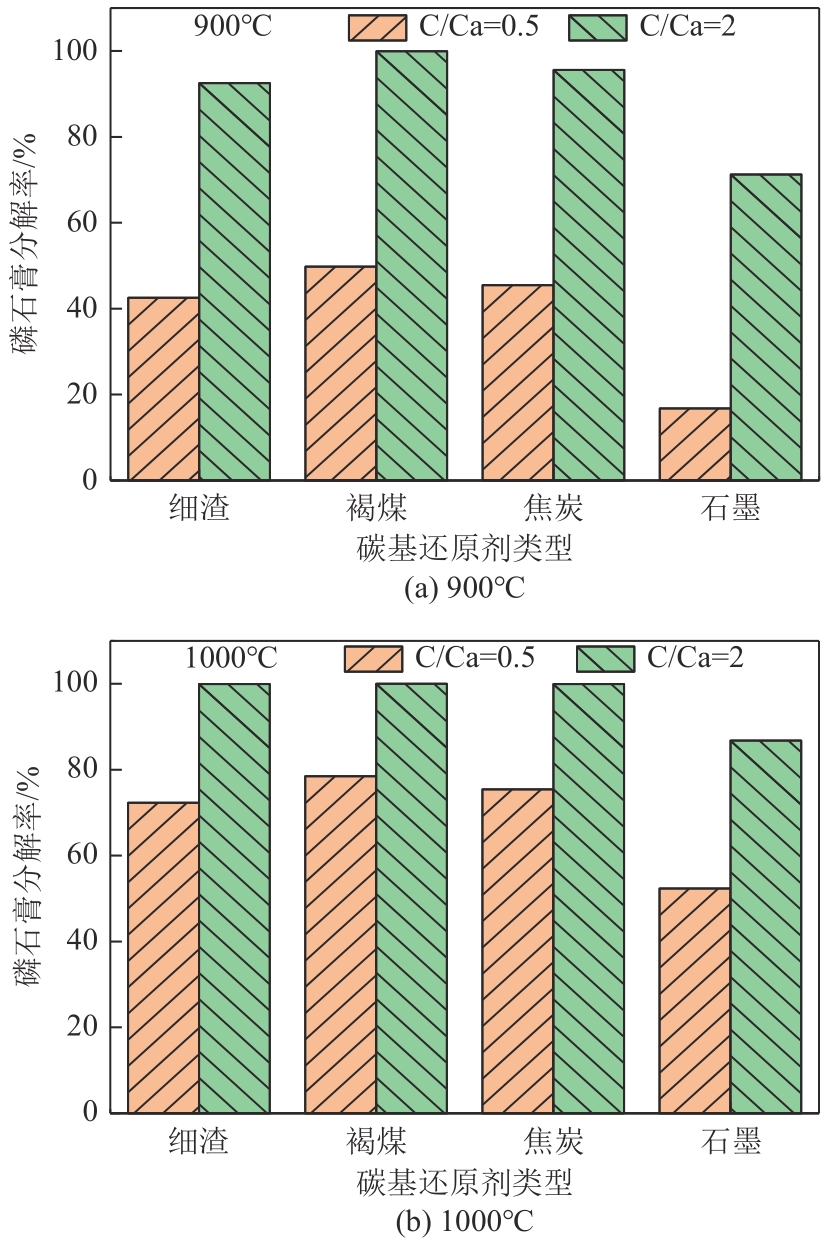
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (6): 3479-3491.DOI: 10.16085/j.issn.1000-6613.2023-0883
• Resources and environmental engineering • Previous Articles
Analysis of high temperature reduction process of phosphogypsum by coal gasification fine slag in fluidized bed
MA Dong1( ), XIE Guilin1, TIAN Zhihua1, WANG Qinhui1(
), XIE Guilin1, TIAN Zhihua1, WANG Qinhui1( ), ZHANG Jianguo2, SONG Huilin2, ZHONG Jin2
), ZHANG Jianguo2, SONG Huilin2, ZHONG Jin2
- 1.State Key Laboratory of Clean Energy Utilization, Zhejiang University, Hangzhou 310027, Zhejiang, China
2.Yunnan Yuntianhua Environmental Protection Technology Co. , Ltd. , Kunming 650300, Yunnan, China
-
Received:2023-05-29Revised:2023-07-03Online:2024-07-02Published:2024-06-15 -
Contact:WANG Qinhui
流化床中煤气化细渣高温还原磷石膏过程
马栋1( ), 解桂林1, 田治华1, 王勤辉1(
), 解桂林1, 田治华1, 王勤辉1( ), 张建国2, 宋慧林2, 钟晋2
), 张建国2, 宋慧林2, 钟晋2
- 1.浙江大学能源清洁利用国家重点实验室,浙江 杭州 310027
2.云南云天化环保科技有限公司,云南 昆明 650300
-
通讯作者:王勤辉 -
作者简介:马栋(1992—),男,博士研究生,研究方向为流化床固废处理等。E‑mail:1054140162@qq.com。 -
基金资助:中央高校基本科研业务费专项(2022ZFJH004)
CLC Number:
Cite this article
MA Dong, XIE Guilin, TIAN Zhihua, WANG Qinhui, ZHANG Jianguo, SONG Huilin, ZHONG Jin. Analysis of high temperature reduction process of phosphogypsum by coal gasification fine slag in fluidized bed[J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3479-3491.
马栋, 解桂林, 田治华, 王勤辉, 张建国, 宋慧林, 钟晋. 流化床中煤气化细渣高温还原磷石膏过程[J]. 化工进展, 2024, 43(6): 3479-3491.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0883
| SiO2 | Al2O3 | SO3 | Fe2O3 | CaO | MgO | K2O | Na2O | P2O5 | F | TiO2 | 结晶水 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.42 | — | 43.12 | 0.17 | 30.28 | 0.21 | 0.01 | 0.09 | 0.87 | 0.16 | 0.08 | 19.21 |
| SiO2 | Al2O3 | SO3 | Fe2O3 | CaO | MgO | K2O | Na2O | P2O5 | F | TiO2 | 结晶水 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.42 | — | 43.12 | 0.17 | 30.28 | 0.21 | 0.01 | 0.09 | 0.87 | 0.16 | 0.08 | 19.21 |
| 样品 | 工业分析 | 元素分析 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | C | H | O① | N | St | |
| 脱灰前 | 0.45 | 67.62 | 1.96 | 29.97 | 26.64 | 0.38 | 4.43 | 0.12 | 0.36 |
| 脱灰后 | 3.24 | 4.86 | 12.37 | 79.53 | 75.69 | 3.24 | 11.71 | 0.31 | 0.95 |
| 样品 | 工业分析 | 元素分析 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | C | H | O① | N | St | |
| 脱灰前 | 0.45 | 67.62 | 1.96 | 29.97 | 26.64 | 0.38 | 4.43 | 0.12 | 0.36 |
| 脱灰后 | 3.24 | 4.86 | 12.37 | 79.53 | 75.69 | 3.24 | 11.71 | 0.31 | 0.95 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O |
|---|---|---|---|---|---|---|
| 42.37 | 21.65 | 12.65 | 9.64 | 1.52 | 1.78 | 3.62 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O |
|---|---|---|---|---|---|---|
| 42.37 | 21.65 | 12.65 | 9.64 | 1.52 | 1.78 | 3.62 |
| 反应模型 | 代号 | G (α) | f (α) |
|---|---|---|---|
| 相界控制的反应(一维) | R1 | α | 1 |
| 相界控制的反应(二维) | R2 | 1-(1-α)1/2 | 2(1-α)1/2 |
| 相界控制的反应(三维) | R3 | 1-(1-α)1/3 | 3(1-α)2/3 |
| 能量最低模型 | P2/3 | α3/2 | 2/3α-1/2 |
| 能量最低模型 | P2 | α1/2 | 2α1/2 |
| 能量最低模型 | P3 | α1/3 | 3α2/3 |
| 一维扩散模型 | D1 | α2 | 1/(2α) |
| 二维扩散模型 | D2 | (1-α)ln(1-α)+α | [-ln(1-α)]-1 |
| 三维扩散模型 | D3 | [1-(1-α)1/3]2 | (3/2)(1-α)2/3[1-(1-α)1/3]-1 |
| 缩核模型 (n=1) | A1 | -ln(1-α) | 1-α |
| 缩核模型(n=1/2) | A2 | [-ln(1-α)]1/2 | 2(1-α)[-ln(1-α)]1/2 |
| 缩核模型(n=1/3) | A3 | [-ln(1-α)]1/3 | 3(1-α)[-ln(1-α)]2/3 |
| 缩核模型(n=1/4) | A4 | [-ln(1-α)]1/4 | 4(1-α)[-ln(1-α)]3/4 |
| 缩核模型(n=2/3) | A5 | [-ln(1-α)]2/3 | 3/2(1-α)[-ln(1-α)]1/3 |
| 缩核模型(n=2) | A6 | [-ln(1-α)]2 | 0.5(1-α)[-ln(1-α)]-1 |
| 缩核模型(n =1.5) | A1.5 | [-ln(1-α)]3/2 | 2/3(1-α)[-ln(1-α)]-0.5 |
| 反应模型 | 代号 | G (α) | f (α) |
|---|---|---|---|
| 相界控制的反应(一维) | R1 | α | 1 |
| 相界控制的反应(二维) | R2 | 1-(1-α)1/2 | 2(1-α)1/2 |
| 相界控制的反应(三维) | R3 | 1-(1-α)1/3 | 3(1-α)2/3 |
| 能量最低模型 | P2/3 | α3/2 | 2/3α-1/2 |
| 能量最低模型 | P2 | α1/2 | 2α1/2 |
| 能量最低模型 | P3 | α1/3 | 3α2/3 |
| 一维扩散模型 | D1 | α2 | 1/(2α) |
| 二维扩散模型 | D2 | (1-α)ln(1-α)+α | [-ln(1-α)]-1 |
| 三维扩散模型 | D3 | [1-(1-α)1/3]2 | (3/2)(1-α)2/3[1-(1-α)1/3]-1 |
| 缩核模型 (n=1) | A1 | -ln(1-α) | 1-α |
| 缩核模型(n=1/2) | A2 | [-ln(1-α)]1/2 | 2(1-α)[-ln(1-α)]1/2 |
| 缩核模型(n=1/3) | A3 | [-ln(1-α)]1/3 | 3(1-α)[-ln(1-α)]2/3 |
| 缩核模型(n=1/4) | A4 | [-ln(1-α)]1/4 | 4(1-α)[-ln(1-α)]3/4 |
| 缩核模型(n=2/3) | A5 | [-ln(1-α)]2/3 | 3/2(1-α)[-ln(1-α)]1/3 |
| 缩核模型(n=2) | A6 | [-ln(1-α)]2 | 0.5(1-α)[-ln(1-α)]-1 |
| 缩核模型(n =1.5) | A1.5 | [-ln(1-α)]3/2 | 2/3(1-α)[-ln(1-α)]-0.5 |
| 1 | SHEN Zhongjie, NIKOLIC H, CAUDILL L S, et al. A deep insight on the coal ash-to-slag transformation behavior during the entrained flow gasification process[J]. Fuel, 2021, 289: 119953. |
| 2 | LIU Xiaodong, JIN Zhengwei, JING Yunhuan, et al. Review of the characteristics and graded utilisation of coal gasification slag[J]. Chinese Journal of Chemical Engineering, 2021, 35: 92-106. |
| 3 | PAN Chanchan, LIANG Qinfeng, GUO Xiaolei, et al. Characteristics of different sized slag particles from entrained-flow coal gasification[J]. Energy & Fuels, 2016, 30(2): 1487-1495. |
| 4 | GUO Yang, ZHANG Yixin, ZHAO Xu, et al. Multifaceted evaluation of distribution, occurrence, and leaching features of typical heavy metals in different-sized coal gasification fine slag from Ningdong region, China: A case study[J]. Science of the Total Environment, 2022, 831: 154726. |
| 5 | DUAN Liangyan, HU Xiude, SUN Deshuai, et al. Rapid removal of low concentrations of mercury from wastewater using coal gasification slag[J]. Korean Journal of Chemical Engineering, 2020, 37(7): 1166-1173. |
| 6 | LI Jiawei, CHEN Zhichao, YUAN Linxuan, et al. Effects of flotation and acid treatment on unburned carbon recovery from atmospheric circulating fluidized bed coal gasification fine ash and application evaluation of residual carbon[J]. Waste Management, 2021, 136: 283-294. |
| 7 | SUN Jian, LIU Wenqiang, WANG Wenyu, et al. Optimizing synergy between phosphogypsum disposal and cement plant CO2 capture by the calcium looping process[J]. Energy & Fuels, 2016, 30(2): 1256-1265. |
| 8 | NEDELCIU C E, RAGNARSDOTTIR K V, SCHLYTER P, et al. Global phosphorus supply chain dynamics: Assessing regional impact to 2050[J]. Global Food Security, 2020, 26: 100426. |
| 9 | MA D, WANG Qinhui. Experimental study of CaS preparation from lignite reduced phosphogypsum in fluidized bed[J]. Journal of Chemical Technology and Biotechnology, 2023, 98(3): 756-72. |
| 10 | 刘雨浩, 毛宏勃, 宋文强, 等. 磷石膏-硫酸渣煤基还原焙烧物相转化研究[J]. 非金属矿, 2023, 46(1): 11-15. |
| LIU Yuhao, MAO Hongbo, SONG Wenqiang, et al. Study on phase transformation of phosphogypsum-pyrite cinder under coal based reduction roasting[J]. Non-Metallic Mines, 2023, 46(1): 11-15. | |
| 11 | 吴泳霖, 张伟, 奠波, 等. 磷石膏热分解研究现状[J]. 硅酸盐通报, 2022, 41(9): 3129-3137. |
| WU Yonglin, ZHANG Wei, DIAN Bo, et al. Research status of thermal decomposition of phosphogypsum[J]. Bulletin of the Chinese Ceramic Society, 2022, 41(9): 3129-3137. | |
| 12 | BI Yongxiang, XU Li, YANG Min, et al. Study on the effect of the activity of anthracite on the decomposition of phosphogypsum[J]. Industrial & Engineering Chemistry Research, 2022, 61(19): 6311-6321. |
| 13 | ANTAR K, JEMAL M. A thermogravimetric study into the effects of additives and water vapor on the reduction of gypsum and Tunisian phosphogypsum with graphite or coke in a nitrogen atmosphere[J]. Journal of Thermal Analysis and Calorimetry, 2018, 132(1): 113-125. |
| 14 | 朱凯, 解桂林, 陈昭睿, 等. 磷石膏在一氧化碳气氛下的还原反应特性[J]. 硅酸盐学报, 2013, 41(11): 1540-1545. |
| ZHU Kai, XIE Guilin, CHEN Zhaorui, et al. Reaction characteristics of phosphogypsum under carbon monoxide atmosphere[J]. Journal of the Chinese Ceramic Society, 2013, 41(11): 1540-1545. | |
| 15 | 陈延信, 王聪, 赵博, 等. 气体硫磺预还原磷石膏制贝利特硫铝酸盐水泥[J]. 非金属矿, 2021, 44(1): 9-12. |
| CHEN Yanxin, WANG Cong, ZHAO Bo, et al. Preparation of sulphoaluminate cement by pre-reduction of phosphogypsum with gas sulfur[J]. Non-Metallic Mines, 2021, 44(1): 9-12. | |
| 16 | 连艳, 马丽萍, 刘红盼, 等. 磷石膏与硫化氢气体反应制硫化钙的实验研究[J]. 化学工程, 2016, 44(8): 48-52. |
| LIAN Yan, MA Liping, LIU Hongpan, et al. Experimental study on preparation of calcium sulfide via phosphogypsum and hydrogen sulfide reaction[J]. Chemical Engineering (China), 2016, 44(8): 48-52. | |
| 17 | 王烨, 王俊哲, 张志业, 等. 不同气氛对硫铁矿与磷石膏反应过程软化温度的影响研究[J]. 无机盐工业, 2017, 49(12): 57-60. |
| WANG Ye, WANG Junzhe, ZHANG Zhiye, et al. Research on effects of different atmospheres on softening temperature of reaction process between pyrite and phosphogypsum[J]. Inorganic Chemicals Industry, 2017, 49(12): 57-60. | |
| 18 | 杨秀山, 邓少刚, 黄鹏辉, 等. 硫化钙磷石膏固固反应的热分析动力学[J]. 高校化学工程学报, 2016, 30(3): 588-596. |
| YANG Xiushan, DENG Shaogang, HUANG Penghui, et al. Thermal analysis kinetic studies on solid-solid reaction of calcium sulfide and phosphogypsum[J]. Journal of Chemical Engineering of Chinese Universities, 2016, 30(3): 588-596. | |
| 19 | XIA Xiao, ZHANG Liqiang, LI Zhanyao, et al. Recovery of CaO from CaSO4 via CO reduction decomposition under different atmospheres[J]. Journal of Environmental Management, 2022, 301: 113855. |
| 20 | 李晓亚, 周托, 那永洁, 等. CaS氧化和CaS与CaSO4固固反应机理[J]. 中国粉体技术, 2019, 25(2): 18-24. |
| LI Xiaoya, ZHOU Tuo, NA Yongjie, et al. Mechanism of oxidation reaction of CaS and solid-solid reaction of CaS and CaSO4 [J]. China Powder Science and Technology, 2019, 25(2): 18-24. | |
| 21 | 宋谦石, 王潇伟, 张威, 等. 无机元素对生物质焦炭-CO2气化反应性的催化/抑制作用研究及模型构建[J]. 化工学报, 2022, 73(11): 5240-5250. |
| SONG Qianshi, WANG Xiaowei, ZHANG Wei, et al. Study on catalytic/inhibitory effect of inorganic elements on gasification reactivity of biomass coke-CO2 and model construction[J]. CIESC Journal, 2022, 73(11): 5240-5250. | |
| 22 | 刘琦, 敖先权, 陈前林, 等. 煤矸石对磷石膏高温还原分解反应影响及机理[J]. 非金属矿, 2021, 44(3): 5-8. |
| LIU Qi, AO Xianquan, CHEN Qianlin, et al. Effect of coal gangue on reduction decomposition of phosphogypsum at high temperature and its mechanism[J]. Non-Metallic Mines, 2021, 44(3): 5-8. | |
| 23 | JIA Xin, WANG Qinhui, CEN Kefa, et al. An experimental study of CaSO4 decomposition during coal pyrolysis[J]. Fuel, 2016, 163: 157-165. |
| 24 | LIU Qi, AO Xianquan, CHEN Qianlin, et al. Reaction characteristics and kinetics of phosphogypsum decomposition via synergistic reduction effect of composite reducing agent[J]. Journal of Material Cycles and Waste Management, 2022, 24(2): 595-605. |
| 25 | QU Yi, LI Aimin, WANG Dong, et al. Kinetic study of the effect of in situ mineral solids on pyrolysis process of oil sludge[J]. Chemical Engineering Journal, 2019, 374: 338-346. |
| 26 | HE Qing, DING Lu, GONG Yan, et al. Effect of torrefaction on pinewood pyrolysis kinetics and thermal behavior using thermogravimetric analysis[J]. Bioresource Technology, 2019, 280: 104-111. |
| 27 | SOBEK S, WERLE S. Isoconversional determination of the apparent reaction models governing pyrolysis of wood, straw and sewage sludge, with an approach to rate modelling[J]. Renewable Energy, 2020, 161: 972-987. |
| [1] | ZHOU Qiuming, NIU Congcong, LYU Shuaishuai, LI Hongwei, WEN Fuli, XU Run, LI Mingfeng. Promoting CO2 hydrogenation to methanol through product transformation and separation [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2776-2785. |
| [2] | PANG Shuxin, WANG Hao, WANG Jianyu, ZHU Kake, LIU Zhicheng. Thermodynamic calculation of methane combined reforming to synthesis gas process based on Aspen Plus [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2890-2900. |
| [3] | ZHU Yanni, WANG Wei, SUN Yanchenhao, WEI Gang, ZHANG Dawei. Numerical simulation of centrifugal spray drying based on single-droplet evaporation [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1700-1710. |
| [4] | WANG Debin, LIN Mengyu, YANG Xue, DONG Dianquan. Preparation and adsorption properties of zinc-doped titanium-based cesium ion sieves [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1953-1961. |
| [5] | HUANG Meng, SUN Zhigao, XU Wenchao, ZHANG Huanran, YANG Yang. Promotion of HCFC-141b hydrate production by lactone sophorolipids [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1199-1205. |
| [6] | WANG Kexu, ZHANG Xiangping, WANG Hongyan, BAI Yan, WANG Hui. Progress on current-responsive catalysts and their applications in intensifying typical reactions [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 49-59. |
| [7] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [8] | WANG Peng, ZHANG Yang, FAN Bingqiang, HE Dengbo, SHEN Changshuai, ZHANG Hedong, ZHENG Shili, ZOU Xing. Process and kinetics of hydrochloric acid leaching of high-carbon ferrochromium [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 510-517. |
| [9] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [10] | DONG Jiayu, WANG Simin. Experimental on ultrasound enhancement of para-xylene crystallization characteristics and regulation mechanism [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4504-4513. |
| [11] | WANG Junjie, PAN Yanqiu, NIU Yabin, YU Lu. Molecular level catalytic reforming model construction and application [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3404-3412. |
| [12] | DING Wenjin, LIU Zhuoqi, LU Haichen, SUN Hongjuan, PENG Tongjiang. Preparation of high-purity CaCO3 from phosphogypsum for CO2 mineralization in CH3COONa-NH4OH-H2O system [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3824-3833. |
| [13] | YANG Xuzhao, LI Qing, YUAN Kangkang, ZHANG Yingying, HAN Jingli, WU Shide. Thermodynamic properties of Gemini ionic liquid based deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3123-3129. |
| [14] | MA Runmei, YANG Haichao, LI Zhengda, LI Shuangxi, ZHAO Xiang, ZHANG Guoqing. Influence analysis of coating on deformation and frictional wear of mechanical seal end for high-speed bearing cavity [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1688-1697. |
| [15] | GE Weitong, LIAO Yalong, LI Mingyuan, JI Guangxiong, XI Jiajun. Preparation and dechlorination kinetics of Pd-Fe/MWCNTs bimetallic catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1885-1894. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||