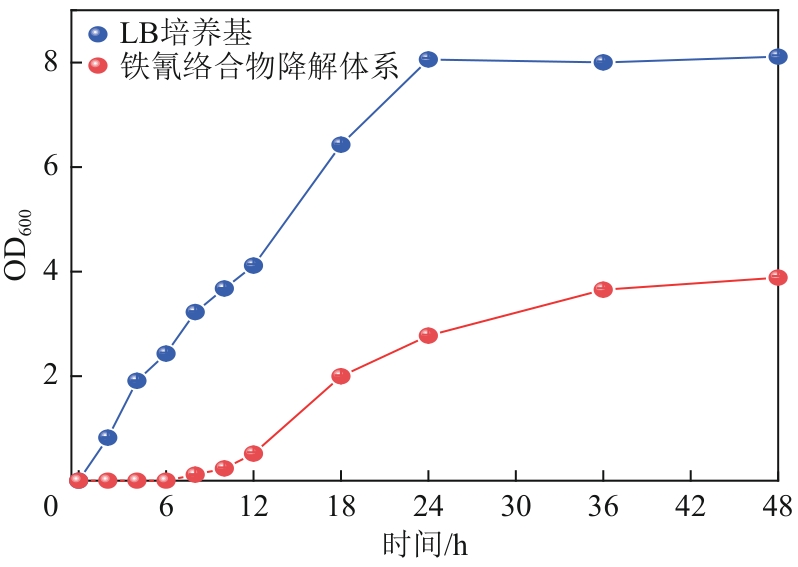
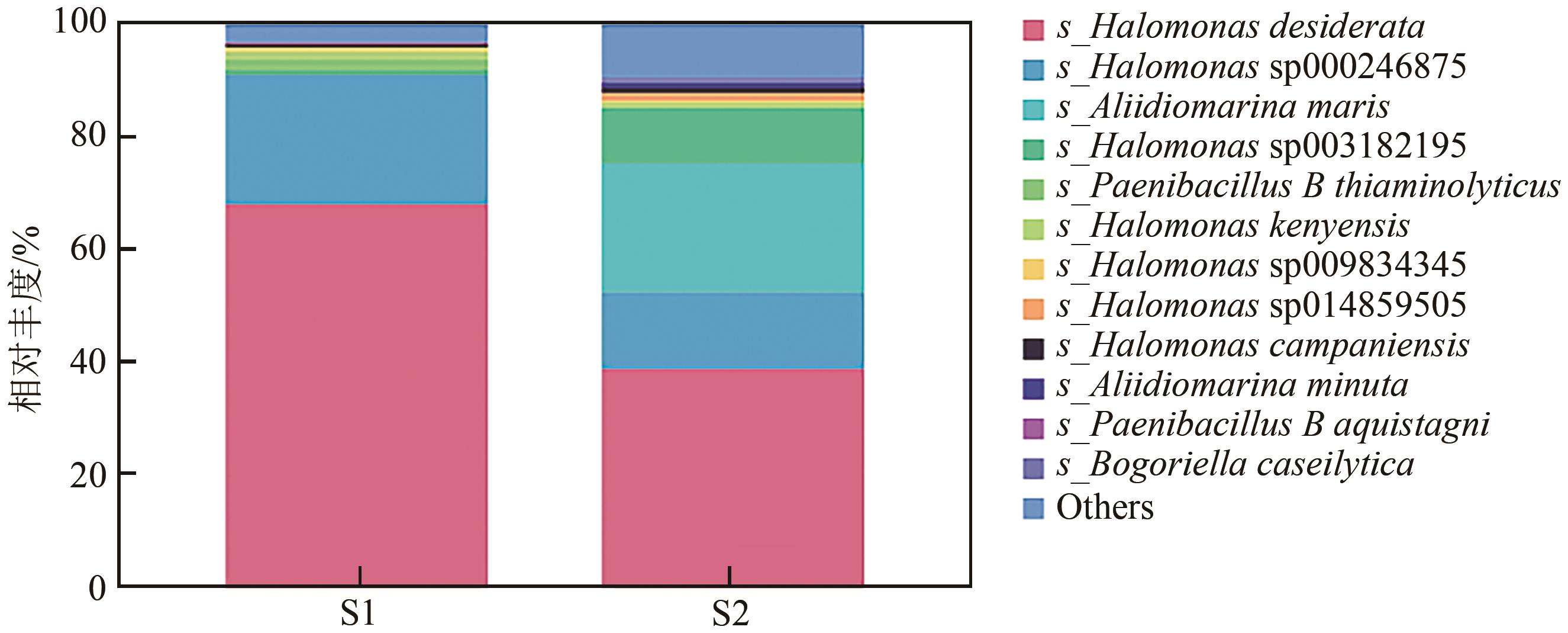
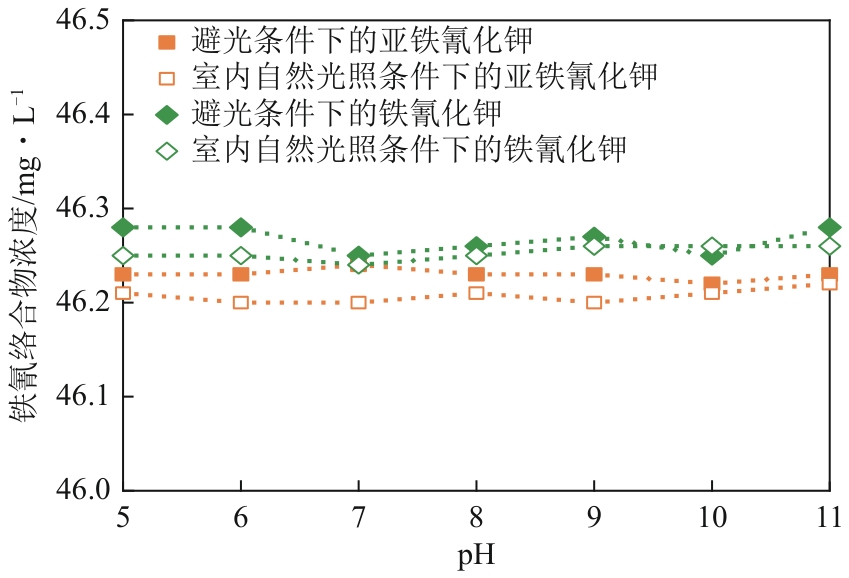
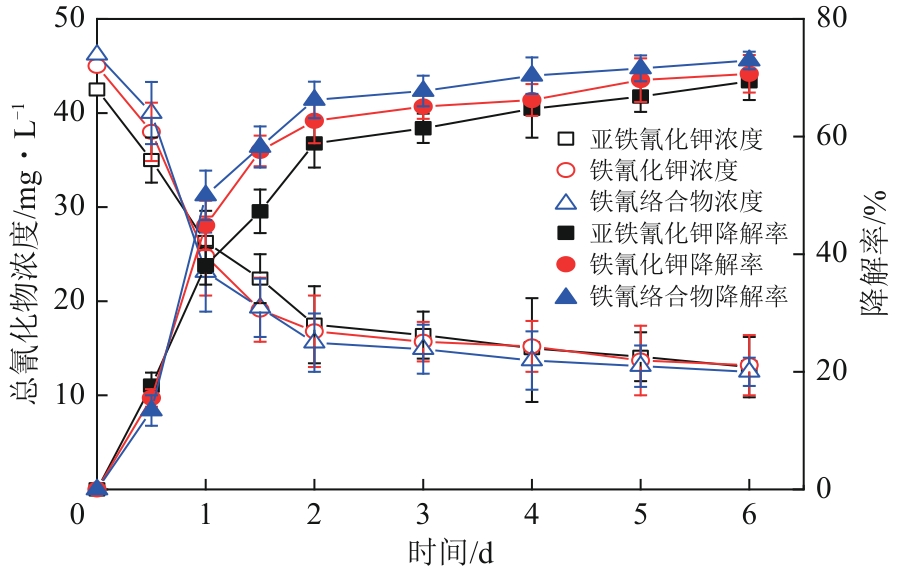
| 1 |
孙培杰, 王林平, 徐乐瑾. 焦化废水中氰化物的处理技术研究进展[J]. 化工进展, 2021, 40(S1): 386-396.
|
|
SUN Peijie, WANG Linping, XU Lejin. Advances in the treatment of cyanide in coking wastewater[J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 386-396.
|
| 2 |
DASH Rajesh Roshan, DASH Rakesh Roshan, BALOMAJUMDAR Chandrajit. Treatment of cyanide bearing effluents by adsorption, biodegradation and combined processes: Effect of process parameters[J]. Desalination and Water Treatment, 2014, 52(16/17/18): 3355-3366.
|
| 3 |
MAMELKINA Maria A, Miguel HERRAIZ-CARBONÉ, COTILLAS Salvador, et al. Treatment of mining wastewater polluted with cyanide by coagulation processes: A mechanistic study[J]. Separation and Purification Technology, 2020, 237: 116345.
|
| 4 |
Angélica ALVILLO-RIVERA, Sofía GARRIDO-HOYOS, Germán BUITRÓN, et al. Biological treatment for the degradation of cyanide: A review[J]. Journal of Materials Research and Technology, 2021, 12: 1418-1433.
|
| 5 |
AGARWAL Bhumica, BALOMAJUMDER Chandrajit, THAKUR Prabhat Kumar. Simultaneous co-adsorptive removal of phenol and cyanide from binary solution using granular activated carbon[J]. Chemical Engineering Journal, 2013, 228: 655-664.
|
| 6 |
韩雯雯, 赵荣欣, 杨洪英, 等. 黄金氰渣尾矿浆脱氰技术综述[J]. 中国有色冶金, 2023, 52(3): 41-49.
|
|
HAN Wenwen, ZHAO Rongxin, YANG Hongying, et al. Review on cyanide removal technologies of gold cyanide residue slurry[J]. China Nonferrous Metallurgy, 2023, 52(3): 41-49.
|
| 7 |
周虹励. 纳米铁改性玉米秸秆生物炭对水体中氰化物的吸附行为及其机理研究[D]. 重庆: 重庆大学, 2022.
|
|
ZHOU Hongli. Adsorption behavior and mechanism of nano-iron modified corn stover biochar on cyanide in water[D]. Chongqing: Chongqing University, 2022.
|
| 8 |
王祎, 崔韬, 谷青青, 等. 管式电化学反应器中试处理高浓度含氰废水与经济分析[J]. 环境工程学报, 2021, 15(8): 2639-2650.
|
|
WANG Yi, CUI Tao, GU Qingqing, et al. Pilot-scale treatment of high-concentration cyanide-containing wastewater by a tubular electrochemical reactor and economic analysis[J]. Chinese Journal of Environmental Engineering, 2021, 15(8): 2639-2650.
|
| 9 |
GSCHWENDTNER Silvia, MANSFELDT Tim, KUBLIK Susanne, et al. Long-term ferrocyanide application via deicing salts promotes the establishment of Actinomycetales assimilating ferrocyanide-derived carbon in soil[J]. Microbial Biotechnology, 2016, 9(4): 502-513.
|
| 10 |
DIMITROVA Tsvetelina, REPMANN Frank, FREESE Dirk. Degradation of ferrocyanide by natural isolated bacteria[J]. International Journal of Phytoremediation, 2020, 22(1): 20-28.
|
| 11 |
LUQUE-ALMAGRO Víctor M, HUERTAS María-J, Manuel MARTÍNEZ-LUQUE, et al. Bacterial degradation of cyanide and its metal complexes under alkaline conditions[J]. Applied and Environmental Microbiology, 2005, 71(2): 940-947.
|
| 12 |
RISHI Saloni, KAUR Ispreet, NASEEM Mariya, et al. Development of immobilized novel fungal consortium for the efficient remediation of cyanide-contaminated wastewaters[J]. Bioresource Technology, 2023, 373: 128750.
|
| 13 |
EBBS Stephen. Biological degradation of cyanide compounds[J]. Current Opinion in Biotechnology, 2004, 15(3): 231-236.
|
| 14 |
WELMAN-PURCHASE Megan D, CASTILLO Julio, Alba GOMEZ-ARIAS, et al. First insight into the natural biodegradation of cyanide in a gold tailings environment enriched in cyanide compounds[J]. Science of the Total Environment, 2024, 906: 167174.
|
| 15 |
陈昱光, 李青云, 沈慧婷, 等. 铝工业大修渣总氰化物的无害化处理[J]. 中国环境科学, 2023, 43(11): 5884-5891.
|
|
CHEN Yuguang, LI Qingyun, SHEN Huiting, et al. The harmless disposal of total cyanide in the spent pot lining derived from electrolytic aluminum industry[J]. China Environmental Science, 2023, 43(11): 5884-5891.
|
| 16 |
丘能, 林宏飞, 周郁文, 等. 用氯化亚铁从高盐固废浸出液中去除氰化物试验研究[J]. 湿法冶金, 2022, 41(3): 274-277.
|
|
QIU Neng, LIN Hongfei, ZHOU Yuwen, et al. Removal of cyanide from high salt solid waste leaching solution using ferrous chloride[J]. Hydrometallurgy of China, 2022, 41(3): 274-277.
|
| 17 |
中华人民共和国环境保护部. 土壤 氰化物和总氰化物的测定 分光光度法: [S]. 北京: 中国环境科学出版社, 2015.
|
|
Ministry of Environmental Protection of the People’s Republic of China. Soil-Determination of cyanide and total cyanide-Spectrometric method: [S]. Beijing: China Environmental Science Press, 2015.
|
| 18 |
环境保护部. 水质 氨氮的测定 纳氏试剂分光光度法: [S]. 北京: 中国环境科学出版社, 2010.
|
|
Ministry of Environmental Protection of the People’s Republic of China. Water quality-Determinatiion of ammonia nitrogen-Nesslers reagent spectrophotometry: [S]. Beijing: China Environmental Science Press, 2010.
|
| 19 |
LI Qingyun, LU Hui, YIN Yexing, et al. Synergic effect of adsorption and biodegradation enhance cyanide removal by immobilized Alcaligenes sp. strain DN25[J]. Journal of Hazardous Materials, 2019, 364: 367-375.
|
| 20 |
杨宗政, 孙炜, 张天宇, 等. 一株耐盐甲醛降解菌Achromobacter sp.WY0的分离及降解特性研究[J]. 生物学杂志, 2023, 40(6): 25-31, 52.
|
|
YANG Zongzheng, SUN Wei, ZHANG Tianyu, et al. The study of isolation and degradation characteristics of a salt-tolerance, formaldehyde degrading bacterium, Achromobacter sp. WY0[J]. Journal of Biology, 2023, 40(6): 25-31, 52.
|
| 21 |
闫敬民. 氧化金矿氰化浸出渣的无害化处置[D]. 北京: 中国科学院大学(中国科学院过程工程研究所), 2021.
|
|
YAN Jingmin. Harmless treatment of cyanide leaching residue from oxidized gold ore[D]. Beijing: Institute of Process Engineering, Chinese Academy of Sciences, 2021.
|
| 22 |
KHAMAR Z, MAKHDOUMI-KAKHKI A, MAHMUDY GHARAIE M H. Remediation of cyanide from the gold mine tailing pond by a novel bacterial co-culture[J]. International Biodeterioration & Biodegradation, 2015, 99: 123-128.
|
| 23 |
MALMIR Narges, ZAMANI Mohammadreza, MOTALLEBI Mostafa, et al. Cyanide biodegradation by Trichoderma harzianum and cyanide hydratase network analysis[J]. Molecules, 2022, 27(10): 3336.
|
| 24 |
WEI Yunmei, CHEN Shuang, REN Tingting, et al. Effectiveness and mechanism of cyanide remediation from contaminated soils using thermally activated persulfate[J]. Chemosphere, 2022, 292: 133463.
|
| 25 |
张亚珍, 向媛羚, 张庆明, 等. 外源刺激剂强化芽孢杆菌高效修复Cr(Ⅵ)污染水体[J]. 农业环境科学学报, 2024, 43(5): 1100-1113.
|
|
ZHANG Yazhen, XIANG Yuanling, ZHANG Qingming, et al. Exogenous stimulants enhance the efficacy of remediation of Cr(Ⅵ)-contaminated water by Bacillus sp.[J]. Journal of Agro-Environment Science, 2024, 43(5): 1100-1113.
|
| 26 |
LI Jiannan, FENG Yujie, QIU Ye, et al. Recovery of electron and carbon source from agricultural waste corncob by microbial electrochemical system to enhance wastewater denitrification[J]. Science of the Total Environment, 2023, 878: 162926.
|
| 27 |
BIANCO F, RACE M, PAPIRIO S, et al. Removal of polycyclic aromatic hydrocarbons during anaerobic biostimulation of marine sediments[J]. Science of the Total Environment, 2020, 709: 136141.
|
 ), LI Qingyun1(
), LI Qingyun1( ), LI Mei1, FANG Yiyan1, SHEN Huiting1, LIN Hongfei2
), LI Mei1, FANG Yiyan1, SHEN Huiting1, LIN Hongfei2
 ), 李青云1(
), 李青云1( ), 李梅1, 方艺燕1, 沈慧婷1, 林宏飞2
), 李梅1, 方艺燕1, 沈慧婷1, 林宏飞2