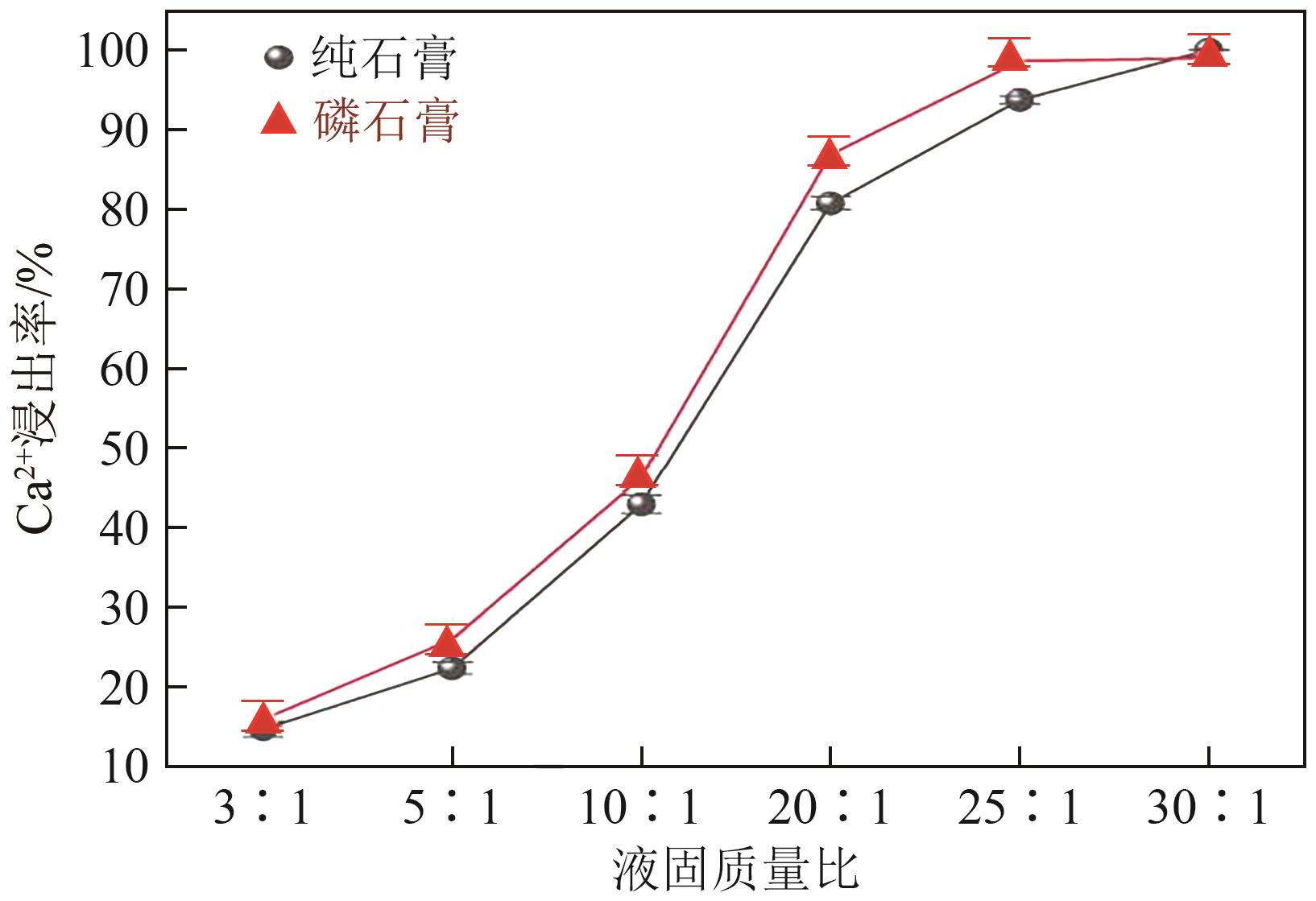
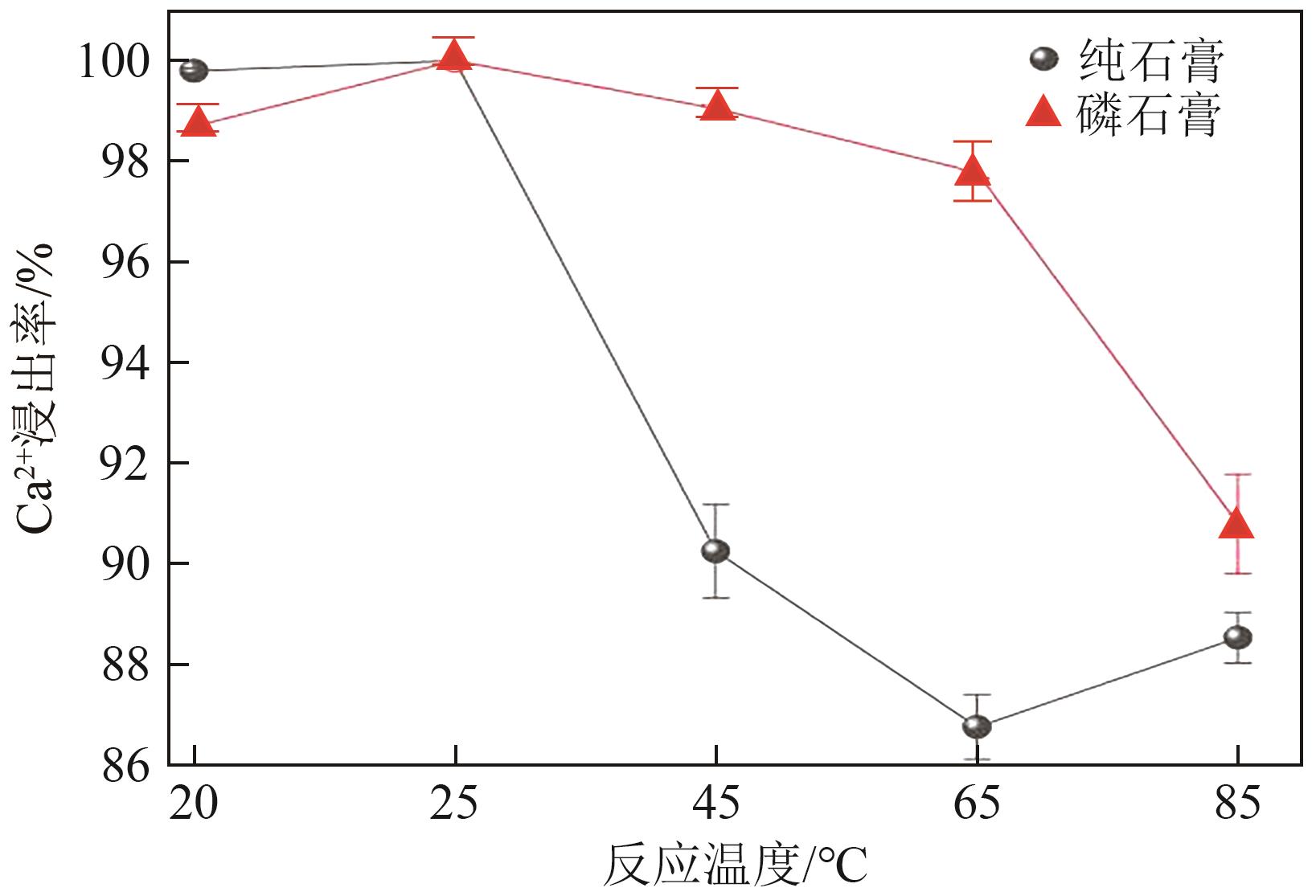
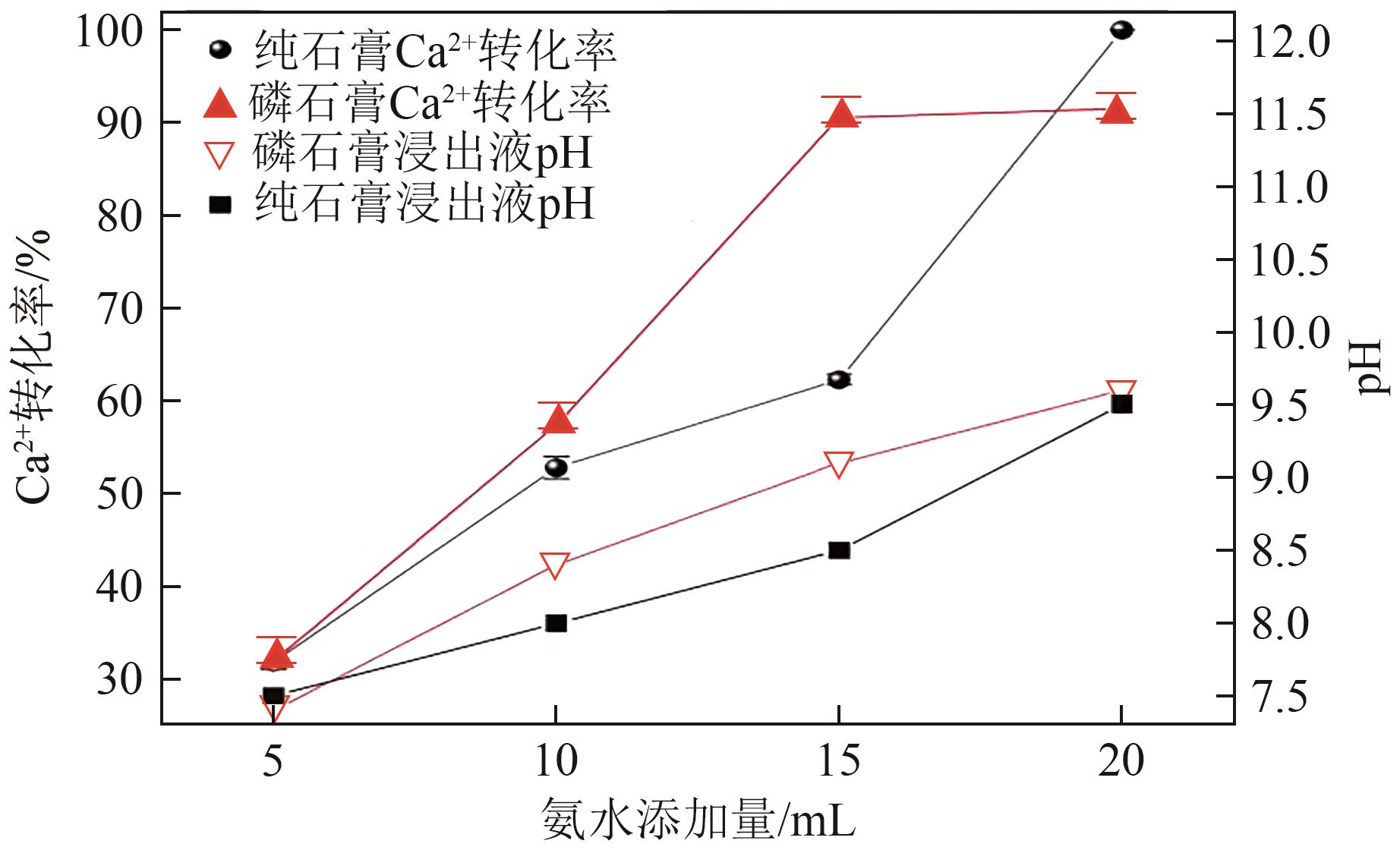
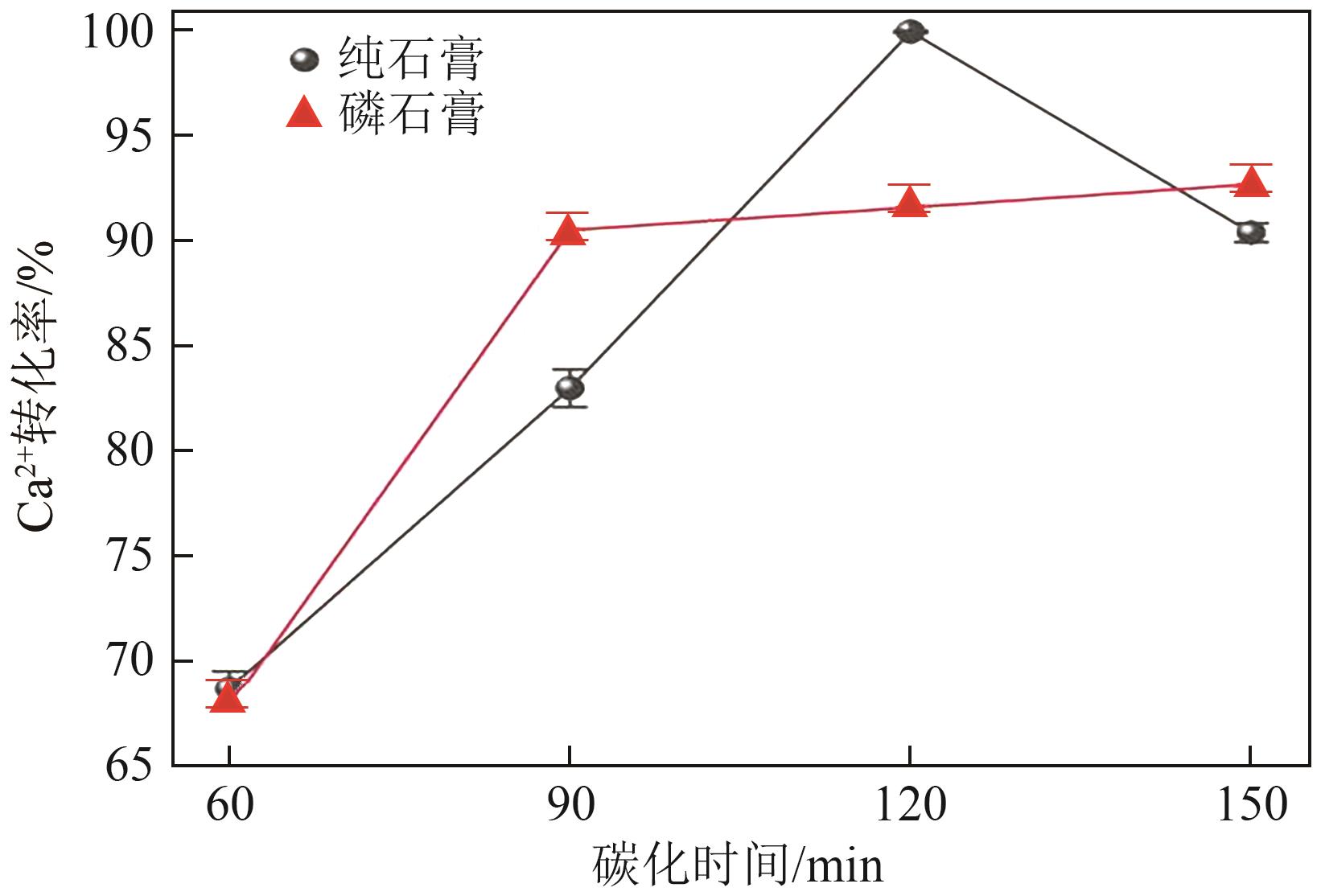
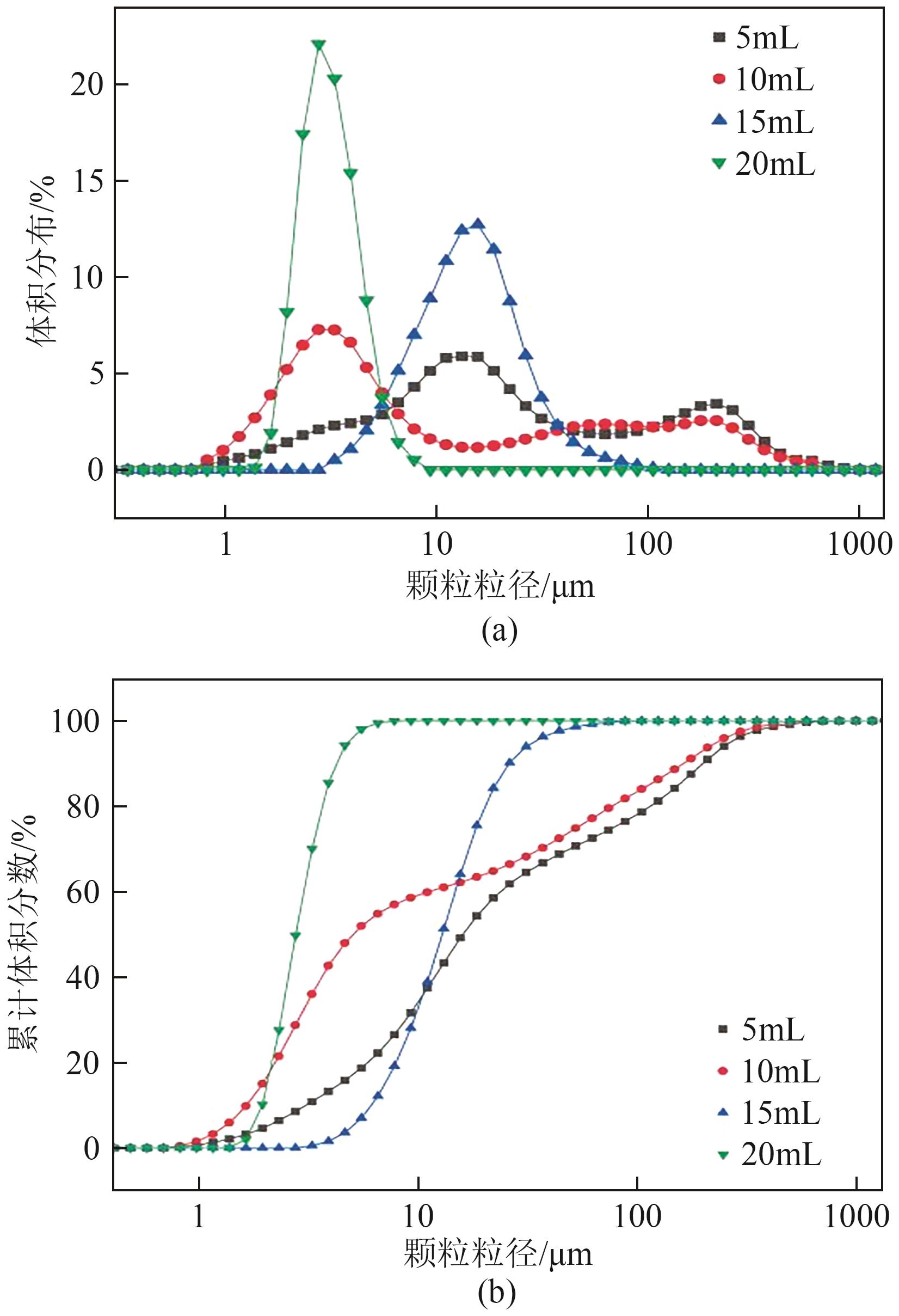
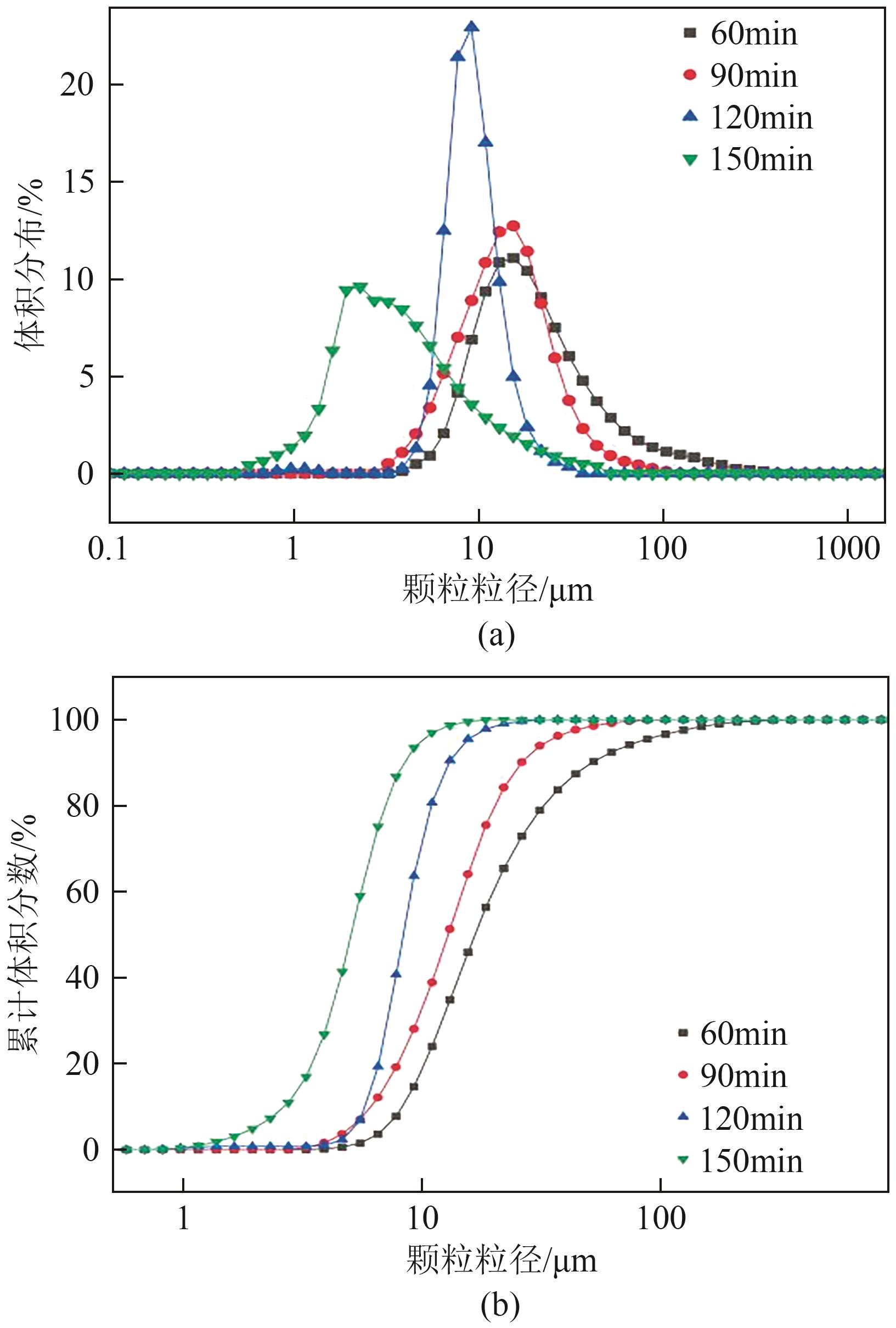
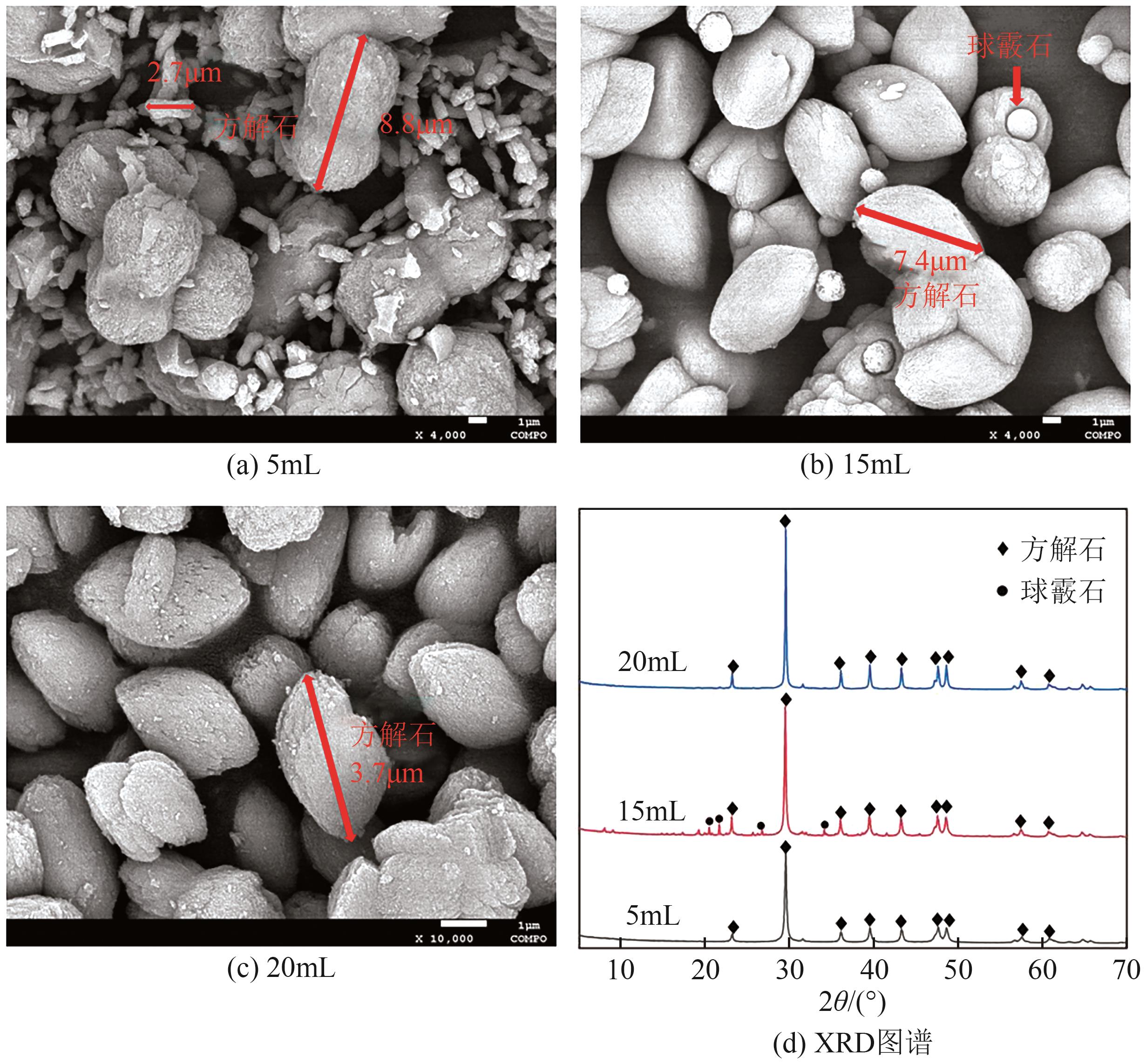
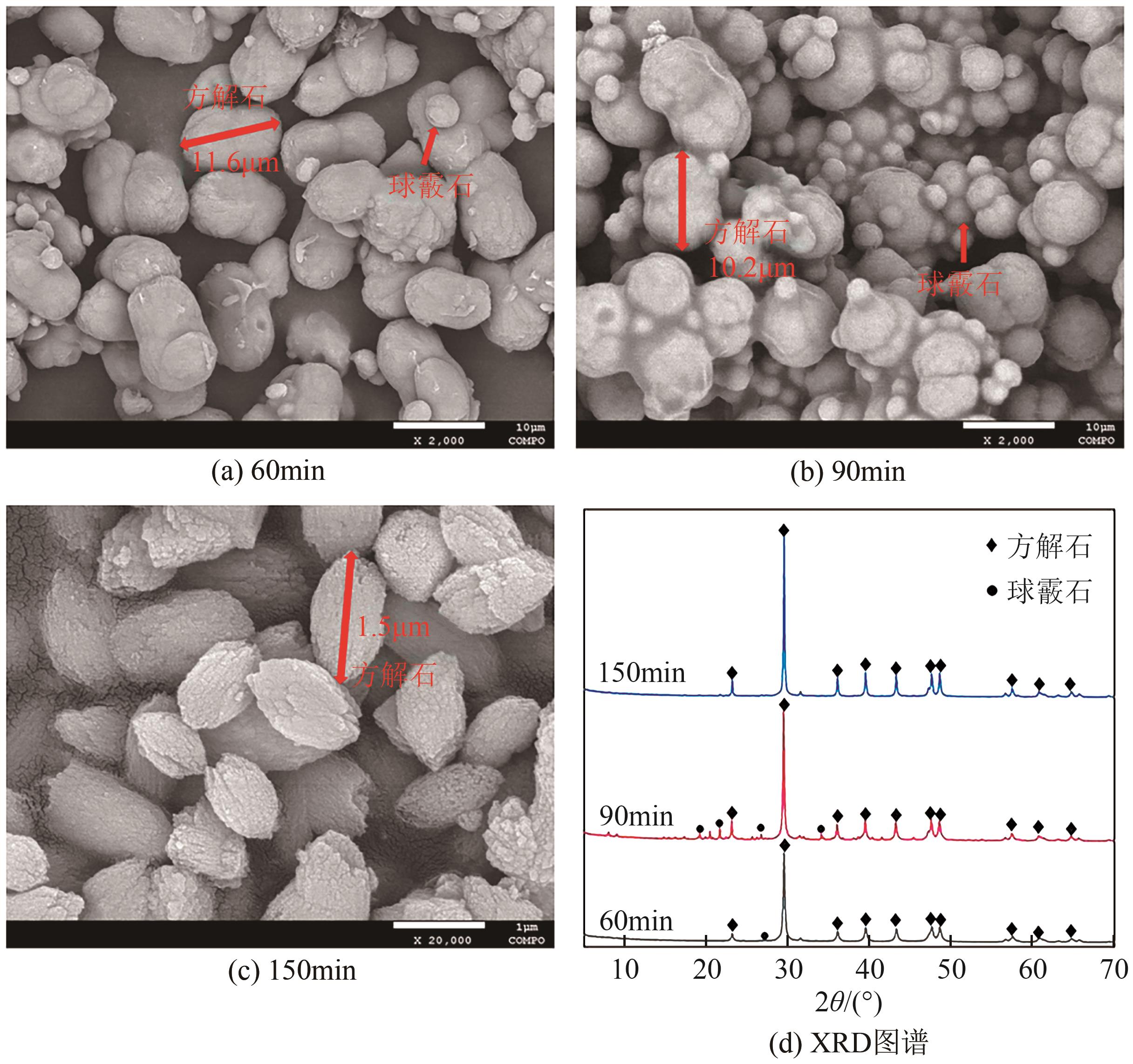
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (1): 66-74.DOI: 10.16085/j.issn.1000-6613.2023-2224
• Chemical processes and equipment • Previous Articles Next Articles
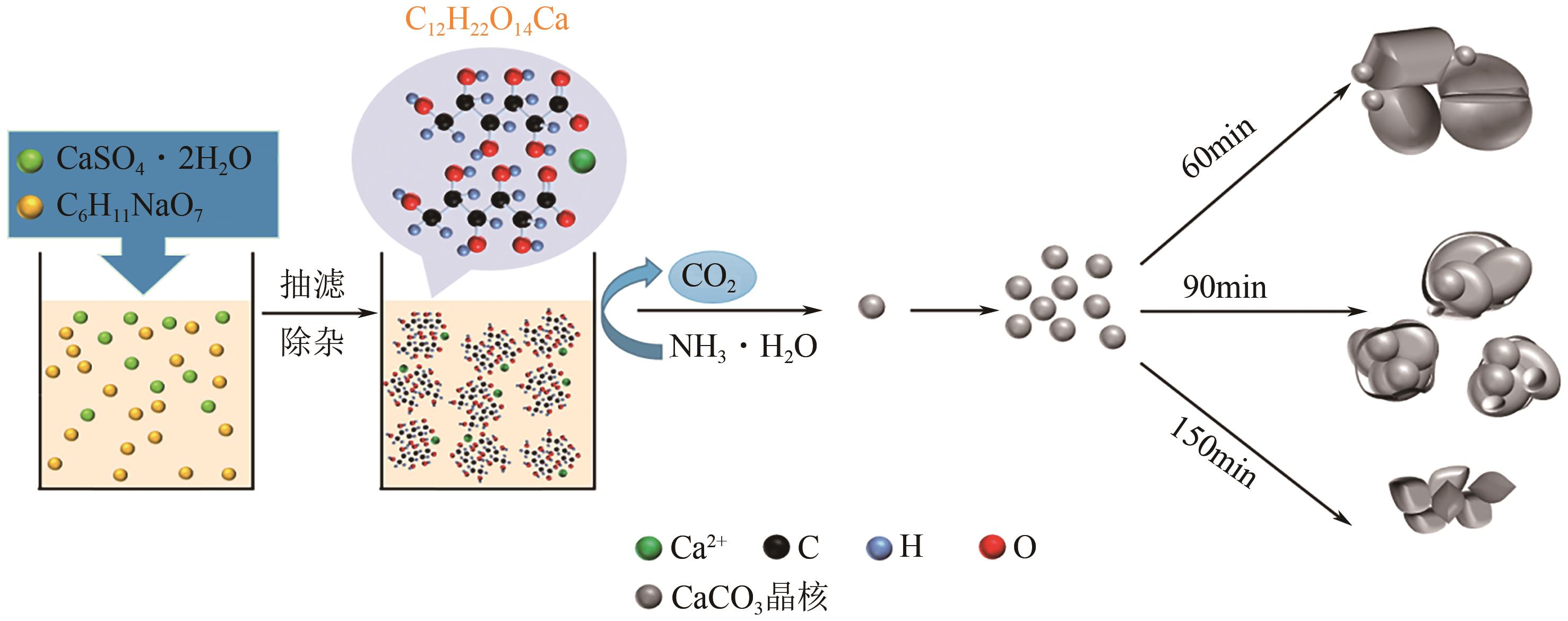
Preparation of calcium carbonate powder by phosphogypsum mineralization for CO2 capture
LIU Dongmei1,2( ), ZHUANG Zhaolin1,2, WANG Qing1,2(
), ZHUANG Zhaolin1,2, WANG Qing1,2( ), DIAO Huali1,2, XU Gang1,2, PENG Yanzhou1,2, BAO Hao1,2, LI Dongsheng3
), DIAO Huali1,2, XU Gang1,2, PENG Yanzhou1,2, BAO Hao1,2, LI Dongsheng3
- 1.Hubei Key Laboratory of Disaster Prevention and Mitigation, Yichang 443002, Hubei, China
2.College of Civil Engineering & Architecture, China Three Gorges University, Yichang 443002, Hubei, China
3.Hubei Three Gorges Laboratory, Yichang 443007, Hubei, China
-
Received:2023-12-19Revised:2024-05-07Online:2025-02-13Published:2025-01-15 -
Contact:WANG Qing
磷石膏矿化固定CO2制备碳酸钙微粉
刘冬梅1,2( ), 庄昭霖1,2, 王青1,2(
), 庄昭霖1,2, 王青1,2( ), 刁华利1,2, 徐港1,2, 彭艳周1,2, 鲍浩1,2, 李东升3
), 刁华利1,2, 徐港1,2, 彭艳周1,2, 鲍浩1,2, 李东升3
- 1.防灾减灾湖北省重点实验室,湖北 宜昌 443002
2.三峡大学土木与建筑学院,湖北 宜昌 443002
3.湖北三峡实验室,湖北 宜昌 443007
-
通讯作者:王青 -
作者简介:刘冬梅(1977—),女,副教授,硕士生导师,研究方向为工业固体废弃物在水泥混凝土中的应用。E-mail:58983701@qq.com。 -
基金资助:国家级地方高校能源和环境材料化学学科创新引智基地项目(D20015);土木工程防灾减灾湖北省引智创新示范基地项目(2021EJD026);国家自然科学基金(52308266);湖北三峡实验室开放/创新基金(SC211006)
CLC Number:
Cite this article
LIU Dongmei, ZHUANG Zhaolin, WANG Qing, DIAO Huali, XU Gang, PENG Yanzhou, BAO Hao, LI Dongsheng. Preparation of calcium carbonate powder by phosphogypsum mineralization for CO2 capture[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 66-74.
刘冬梅, 庄昭霖, 王青, 刁华利, 徐港, 彭艳周, 鲍浩, 李东升. 磷石膏矿化固定CO2制备碳酸钙微粉[J]. 化工进展, 2025, 44(1): 66-74.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-2224
| 化学成分 | 质量分数/% |
|---|---|
| SO3 | 54.683 |
| CaO | 34.334 |
| SiO2 | 8.841 |
| Fe2O3 | 0.614 |
| K2O | 0.539 |
| CuO | 0.015 |
| BaO | 0.171 |
| TiO2 | 0.112 |
| SrO | 0.371 |
| P2O5 | 0.32 |
| 化学成分 | 质量分数/% |
|---|---|
| SO3 | 54.683 |
| CaO | 34.334 |
| SiO2 | 8.841 |
| Fe2O3 | 0.614 |
| K2O | 0.539 |
| CuO | 0.015 |
| BaO | 0.171 |
| TiO2 | 0.112 |
| SrO | 0.371 |
| P2O5 | 0.32 |
| 1 | 崔荣政, 白海丹, 高永峰, 等. 磷石膏综合利用现状及“十四五”发展趋势[J]. 无机盐工业, 2022, 54(4): 1-4. |
| CUI Rongzheng, BAI Haidan, GAO Yongfeng, et al. Current situation of comprehensive utilization of phosphogypsum and its development trend of 14th Five-Year Plan[J]. Inorganic Chemicals Industry, 2022, 54(4): 1-4. | |
| 2 | 许金辉, 邵龙义, 侯海海, 等. 磷石膏综合利用背景下的环境影响研究现状[J]. 矿业科学学报, 2023, 8(1): 115-126. |
| XU Jinhui, SHAO Longyi, HOU Haihai, et al. Review of environmental impact of comprehensive utilization of phosphogypsum[J]. Journal of Mining Science and Technology, 2023, 8(1): 115-126. | |
| 3 | 张贤, 李凯, 马乔, 等. 碳中和目标下CCUS技术发展定位与展望[J]. 中国人口·资源与环境, 2021, 31(9): 29-33. |
| ZHANG Xian, LI Kai, MA Qiao, et al. Orientation and prospect of CCUS development under carbon neutrality target[J]. China Population, Resources and Environment, 2021, 31(9): 29-33. | |
| 4 | KOBELEVA A R, POILOV V Z. Technology for production of calcium carbonate with prescribed properties[J]. Russian Journal of Applied Chemistry, 2007, 80(9): 1447-1452. |
| 5 | FADIA Preksha, TYAGI Simona, BHAGAT Stuti, et al. Calcium carbonate nano- and microparticles: Synthesis methods and biological applications[J]. 3 Biotech, 2021, 11(11): 457. |
| 6 | JIMOH Onimisi A, ARIFFIN Kamar Shah, HUSSIN Hashim Bin, et al. Synthesis of precipitated calcium carbonate: A review[J]. Carbonates and Evaporites, 2018, 33(2): 331-346. |
| 7 | ZHAO Hongtao, LI Huiquan, BAO Weijun, et al. Experimental study of enhanced phosphogypsum carbonation with ammonia under increased CO2 pressure[J]. Journal of CO2 Utilization, 2015, 11: 10-19. |
| 8 | 贺翩翩, 刘晓静, 范勇, 等. 磷石膏固碳制备CaCO3的实验研究[J]. 非金属矿, 2015, 38(2): 28-30. |
| HE Pianpian, LIU Xiaojing, FAN Yong, et al. Study on the preparation of CaCO3 by phosphogypsum solid carbon through orthogonal experiment[J]. Non-Metallic Mines, 2015, 38(2): 28-30. | |
| 9 | BAO Weijun, ZHAO Hongtao, LI Huiquan, et al. Process simulation of mineral carbonation of phosphogypsum with ammonia under increased CO2 pressure[J]. Journal of CO2 Utilization, 2017, 17: 125-136. |
| 10 | LEE Myung gyu, JANG Young Nam, Kyung won RYU, et al. Mineral carbonation of flue gas desulfurization gypsum for CO2 sequestration[J]. Energy, 2012, 47(1): 370-377. |
| 11 | ZHANG Man, FAN Xing. Preparation of gypsum with high purity and whiteness from phosphogypsum for CO2 mineral sequestration[J]. Scientific Reports, 2023, 13(1): 4156. |
| 12 | WU Baizhi, WANG Haibin, LI Chunlei, et al. Progress in the preparation of calcium carbonate by indirect mineralization of industrial by-product gypsum[J]. Sustainability, 2023, 15(12): 9629. |
| 13 | 刘健, 解田, 朱云勤, 等. 硝酸浸取磷石膏钙渣制备高品质轻质碳酸钙[J]. 环境化学, 2010, 29(4): 772-773. |
| LIU Jian, XIE Tian, ZHU Yunqin, et al. Preparation of high quality light calcium carbonate by leaching phosphogypsum calcium slag with nitric acid[J]. Environmental Chemistry, 2010, 29(4): 772-773. | |
| 14 | RAHMANI Omeid, JUNIN Radzuan, TYRER Mark, et al. Mineral carbonation of red gypsum for CO2 sequestration[J]. Energy & Fuels, 2014, 28(9): 5953-5958. |
| 15 | ALTINER Mahmut. Effect of alkaline types on the production of calcium carbonate particles from gypsum waste for fixation of CO2 by mineral carbonation[J]. International Journal of Coal Preparation and Utilization, 2019, 39(3): 113-131. |
| 16 | LACHEHAB Adil, MERTAH Oumaima, KHERBECHE Abdelhak, et al. Utilization of phosphogypsum in CO2 mineral sequestration by producing potassium sulphate and calcium carbonate[J]. Materials Science for Energy Technologies, 2020, 3: 611-625. |
| 17 | ZDAH Ilham, ALAOUI-BELGHITI Hanan EL, CHERRAT Ayoub, et al. Temperature effect on phosphogypsum conversion into potassium fertilizer K2SO4 and portlandite[J]. Nanotechnology for Environmental Engineering, 2021, 6(2): 27. |
| 18 | 田萍, 宁朋歌, 曹宏斌, 等. 二水硫酸钙在铵盐溶液中溶解度测定及热力学计算[J]. 过程工程学报, 2012, 12(4): 625-630. |
| TIAN Ping, NING Pengge, CAO Hongbin, et al. Solubility measurement of calcium sulfate dihydrate in NH4Cl-(NH4)2SO4 solutions and thermodynamic calculation[J]. The Chinese Journal of Process Engineering, 2012, 12(4): 625-630. | |
| 19 | LIANG Ya qing, SUN Hong juan, PENG Tong Jiang. Effect of pH and concentration of Ca2+ on spherical calcium carbonate crystallization by continuous CO2 gas bubbling into phosphogypsum leaching solution[J]. Materials Science Forum, 2015, 814: 552-558. |
| 20 | 乔静怡, 陈秋菊, 刘卓齐, 等. 磷石膏矿化CO2制备球霰石型碳酸钙试验研究[J]. 化工矿物与加工, 2023, 52(9): 14-18, 25. |
| QIAO Jingyi, CHEN Qiuju, LIU Zhuoqi, et al. Experimental study on preparation of vaterite-based calcium carbonate by CO2 mineralization with phosphogypsum[J]. Industrial Minerals & Processing, 2023, 52(9): 14-18, 25. | |
| 21 | 梁亚琴, 孙红娟, 彭同江. 磷石膏固碳制备不同球形碳酸钙的实验研究[J]. 宁夏大学学报(自然科学版), 2015, 36(1): 51-55. |
| LIANG Yaqin, SUN Hongjuan, PENG Tongjiang. Experimental study on preparation different spherical calcium carbonate with ardealite carbon sequestration[J]. Journal of Ningxia University (Natural Science Edition), 2015, 36(1): 51-55. | |
| 22 | 时婷, 王新刚, 巫建锋, 等. 磷石膏脱硫钙渣制备轻质碳酸钙[J]. 化工进展, 2015, 34(1): 178-182. |
| SHI Ting, WANG Xingang, WU Jianfeng, et al. Preparation of light calcium carbonate from phosphorus gypsum desulfurization slag[J]. Chemical Industry and Engineering Progress, 2015, 34(1): 178-182. | |
| 23 | DING Wenjin, CHEN Qiuju, SUN Hongjuan, et al. Modified mineral carbonation of phosphogypsum for CO2 sequestration[J]. Journal of CO2 Utilization, 2019, 34: 507-515. |
| 24 | CHEN Qiuju, DING Wenjin, SUN Hongjuan, et al. Indirect mineral carbonation of phosphogypsum for CO2 sequestration[J]. Energy, 2020, 206: 118148. |
| 25 | 刘禄, 乔静怡, 刘卓齐, 等. 磷石膏制备高纯碳酸钙的试验研究[J]. 矿产保护与利用, 2022, 42(5): 126-131. |
| LIU Lu, QIAO Jingyi, LIU Zhuoqi, et al. Experimental study on preparation of high-purity CaCO3 from phosphogypsum[J]. Conservation and Utilization of Mineral Resources, 2022, 42(5): 126-131. | |
| 26 | QI Huahui, MA Baoguo, TAN Hongbo, et al. Effect of sodium gluconate on molecular conformation of polycarboxylate superplasticizer studied by the molecular dynamics simulation[J]. Journal of Molecular Modeling, 2020, 26(3): 45. |
| 27 | Xingdong LYU, LI Jiazheng, LU Chao, et al. The effect of sodium gluconate on pastes’ performance and hydration behavior of ordinary Portland cement[J]. Advances in Materials Science and Engineering, 2020(1): 9231504. |
| 28 | 史刘宾, 唐名德, 汤勇, 等. 高压碳化法可控制备微纳米分级结构中空棒状碳酸钙及其表征[J]. 化工进展, 2020, 39(11): 4742-4748. |
| SHI Liubin, TANG Mingde, TANG Yong, et al. Preparation and characterization of micro-nano hierarchical hollow rod-like calcium carbonate by high pressure carbonization[J]. Chemical Industry and Engineering Progress, 2020, 39(11): 4742-4748. | |
| 29 | 杨敏. 磷石膏的溶解度研究[J]. 广州化工, 2016, 44(15): 62-63, 72. |
| YANG Min. Study on solubility of phosphogypsum[J]. Guangzhou Chemical Industry, 2016, 44(15): 62-63, 72. | |
| 30 | 李美, 彭家惠, 张欢, 等. 共晶磷对石膏性能的影响及其作用机理[J]. 四川大学学报(工程科学版), 2012, 44(3): 200-204. |
| LI Mei, PENG Jiahui, ZHANG Huan, et al. Influence of P2O5 in crystal lattice on gypsum properties and its mechanisms[J]. Journal of Sichuan University (Engineering Science Edition), 2012, 44(3): 200-204. | |
| 31 | 梁玺, 赵改菊, 路春美, 等. 葡萄糖酸钙结晶的热力学特性[J]. 化学工程, 2021, 49(3): 22-27. |
| LIANG Xi, ZHAO Gaiju, LU Chunmei, et al. Thermodynamic properties of calcium gluconate crystallization[J]. Chemical Engineering (China), 2021, 49(3): 22-27. | |
| 32 | DE LUNA Mark Daniel G, SIOSON Arianne S, CHOI Angelo Earvin Sy, et al. Operating pH influences homogeneous calcium carbonate granulation in the frame of CO2 capture[J]. Journal of Cleaner Production, 2020, 272: 122325. |
| 33 | HU Yubin, WOLTHERS Mariëtte, WOLF-GLADROW Dieter A, et al. Effect of pH and phosphate on calcium carbonate polymorphs precipitated at near-freezing temperature[J]. Crystal Growth & Design, 2015, 15(4): 1596-1601. |
| 34 | 丁光月, 李岳, 樊彩梅, 等. 杂质对磷石膏与碳酸铵反应及产物碳酸钙结晶的影响[J]. 太原理工大学学报, 2011, 42(6): 593-597. |
| DING Guangyue, LI Yue, FAN Caimei, et al. Effect of impurities on conversion of gypsum and crystallization of calcium carbonate[J]. Journal of Taiyuan University of Technology, 2011, 42(6): 593-597. | |
| 35 | 钟汝永. 扑朔迷离的碳酸氢钙[J]. 化学教学, 2014(10): 78-79. |
| ZHONG Ruyong. The confusing calcium bicarbonate[J]. Education in Chemistry, 2014(10): 78-79. | |
| 36 | Donata KONOPACKA-ŁYSKAWA, CZAPLICKA Natalia, Barbara KOŚCIELSKA, et al. Influence of selected saccharides on the precipitation of calcium-vaterite mixtures by the CO2 bubbling method[J]. Crystals, 2019, 9(2): 117. |
| 37 | Romuald BABOU-KAMMOE, HAMOUDI Safia, LARACHI Faïçal, et al. Synthesis of CaCO3 nanoparticles by controlled precipitation of saturated carbonate and calcium nitrate aqueous solutions[J]. The Canadian Journal of Chemical Engineering, 2012, 90(1): 26-33. |
| 38 | 陈秋鸽, 王戬, 张志业, 等. 磷石膏脱硫钙渣浸取液中杂质对碳酸钙晶型的影响[J]. 化工进展, 2016, 35(11): 3714-3719. |
| CHEN Qiuge, WANG Jian, ZHANG Zhiye, et al. Effects of impurities in leaching liquid of phosphogypsum desulfurization slag on crystal form of calcium carbonate[J]. Chemical Industry and Engineering Progress, 2016, 35(11): 3714-3719. | |
| 39 | RODRIGUEZ-BLANCO Juan Diego, SHAW Samuel, BENNING Liane G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite[J]. Nanoscale, 2011, 3(1): 265-271. |
| 40 | Donata KONOPACKA-ŁYSKAWA. Synthesis methods and favorable conditions for spherical vaterite precipitation: A review[J]. Crystals, 2019, 9(4): 223. |
| 41 | Donata KONOPACKA-ŁYSKAWA, CZAPLICKA Natalia, Marcin ŁAPIŃSKI, et al. Precipitation and transformation of vaterite calcium carbonate in the presence of some organic solvents[J]. Materials, 2020, 13(12):2742. |
| [1] | MA Dong, XIE Guilin, TIAN Zhihua, WANG Qinhui, ZHANG Jianguo, SONG Huilin, ZHONG Jin. Analysis of high temperature reduction process of phosphogypsum by coal gasification fine slag in fluidized bed [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3479-3491. |
| [2] | LIU Yang, WANG Yungang, XIU Haoran, ZOU Li, BAI Yanyuan. Optimal carbonization process of walnut shell based on dynamic analysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 94-103. |
| [3] | DING Wenjin, LIU Zhuoqi, LU Haichen, SUN Hongjuan, PENG Tongjiang. Preparation of high-purity CaCO3 from phosphogypsum for CO2 mineralization in CH3COONa-NH4OH-H2O system [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3824-3833. |
| [4] | WANG Xue, XU Qiyong, ZHANG Chao. Hydrothermal carbonization of the lignocellulosic biomass and application of the hydro-char [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2536-2545. |
| [5] | WANG Hao, DI Lu, WANG Fang, ZHANG Deli, YI Weiming, LI Yongjun, SHEN Xiuli. Organic matter conversion and methane production characteristics during anaerobic co-digestion of corn stover and aqueous phase derived from cellulose hydrothermal carbonization [J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6666-6675. |
| [6] | FAN Xuyang, CHEN Yanxin, ZHAO Bo, ZHANG Leilei. Numerical simulation of pre-reduction for a new process of acid production from phosphogypsum by gas sulfur reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5414-5426. |
| [7] | BI Qiang, MEI Yi, XIA Jupei. Basic research on preparation and activity combined excitation of anhydrite-Ⅱ phosphogypsum [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5427-5435. |
| [8] | LI Yeqing, YANG Xingru, LIANG Zhuo, JIANG Hao, XU Quan, ZHOU Hongjun, FENG Lu. Impact of exogenous additives on hydrothermal dechlorination performance of polyvinyl chloride [J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2706-2712. |
| [9] | WANG Yujuan, TANG Jianfeng, HUA Yihuai, CHEN Jing, SANG Wei, LIU Yunfei. Influence of different start-up conditions on response characteristics of natural gas decarbonization device [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1770-1780. |
| [10] | ZHANG Yu, YANG Jiahao, LIU Yu, SONG Ziyu, HE Hanxiao, ZHAO Fengqing. Regulating technology of setting and hardening process of anhydrite-Ⅱphosphogypsum [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5637-5644. |
| [11] | TIAN Xiaohua, ZHANG Yu, ZHAO Fengqing. Mechanism of steel slag-phosphoric acid system regulating the setting performance of phosphogypsum based building gypsum [J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4438-4444. |
| [12] | XU Jie, HUANG Qunxing, MENG Xiangdong, GAO Huaping. Effect of calcium-based additive on phosphorus form and bioavailability during hydrothermal carbonization of sewage sludge [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3507-3514. |
| [13] | Jianfeng TANG, Yujuan WANG, Yue WANG, Yihuai HUA, Jie CHU, Wei SANG, Jing CHEN. Applicability of Aspen HYSYS for simulation of amine decarbonization regeneration process [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 747-754. |
| [14] | Xiaoyuan ZHENG, Zhengwei JIANG, Wei CHEN, Yutong YE, Zhi YING, Shasha JI, Bo WANG. Migration and transformation of phosphorus in sewage sludge during hydrothermal carbonization process [J]. Chemical Industry and Engineering Progress, 2020, 39(5): 2017-2025. |
| [15] | Zheng TANG,Song ZHAO,Yajie QIAN,Gang XUE,Hanzhong JIA,Pin GAO. Formation mechanisms and environmental applications of persistent free radicals in biochar: a review [J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1521-1527. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||