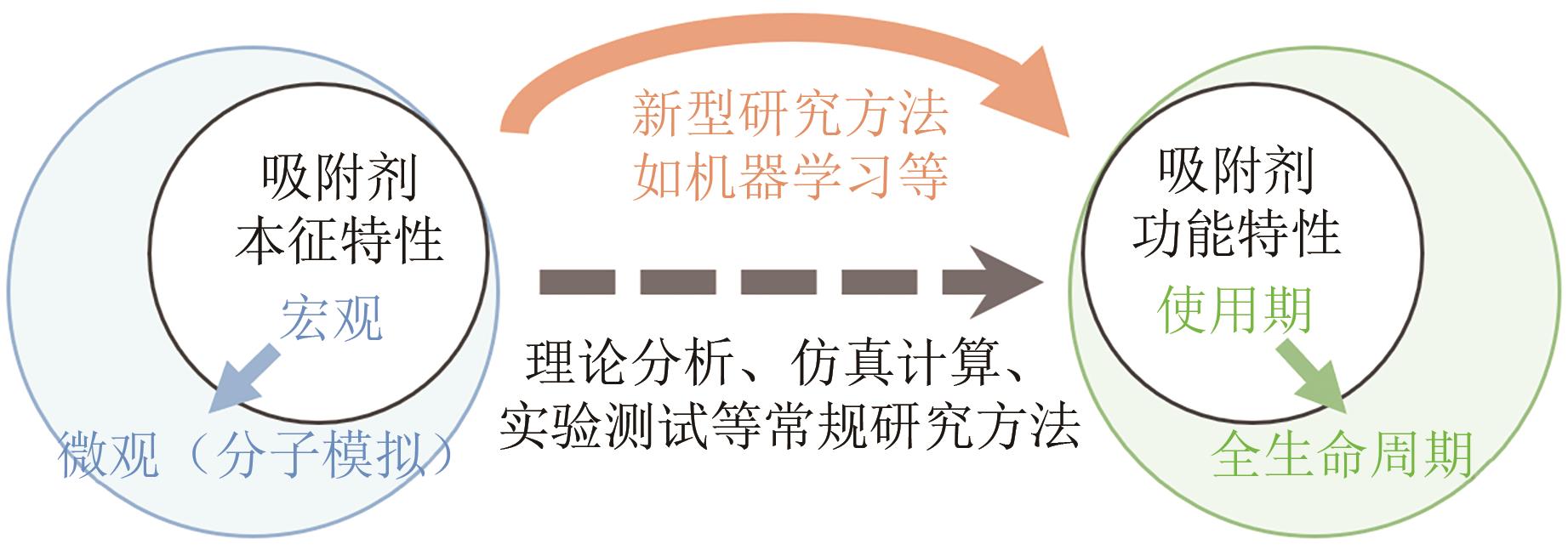
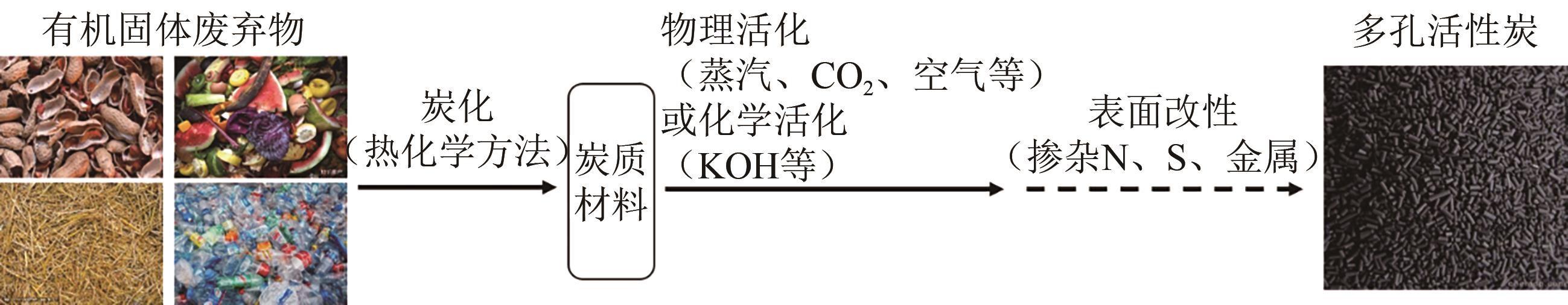
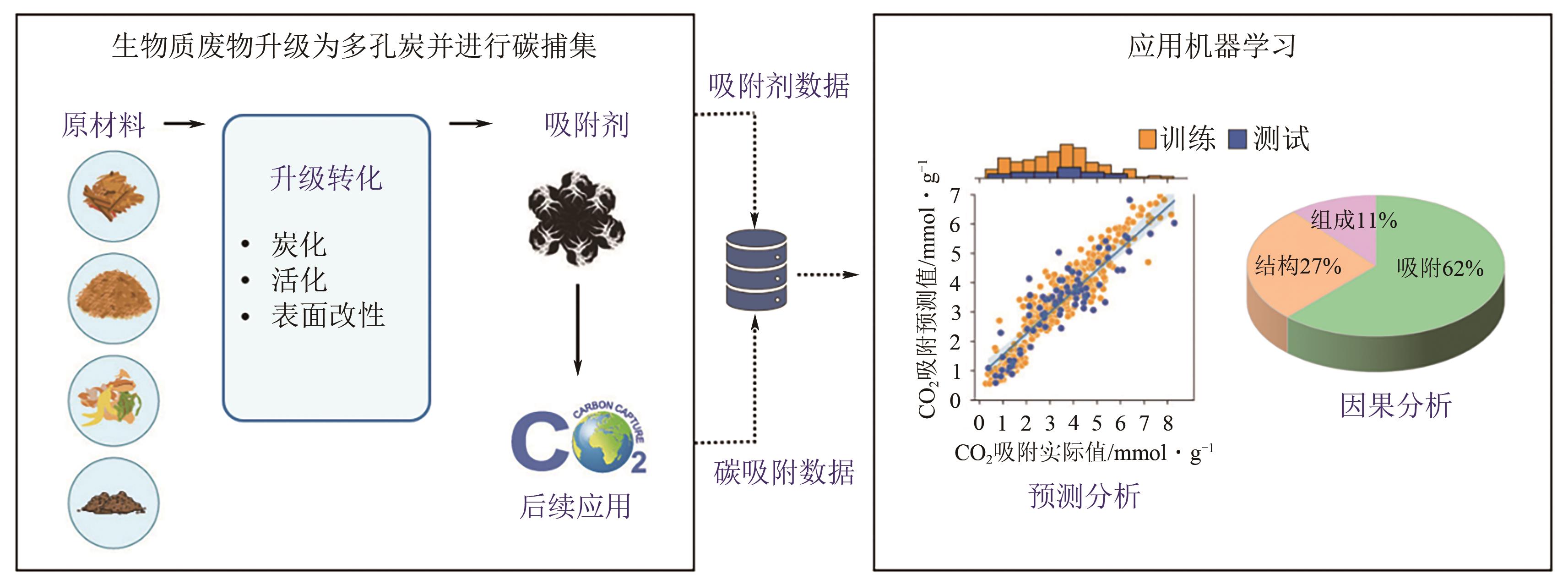
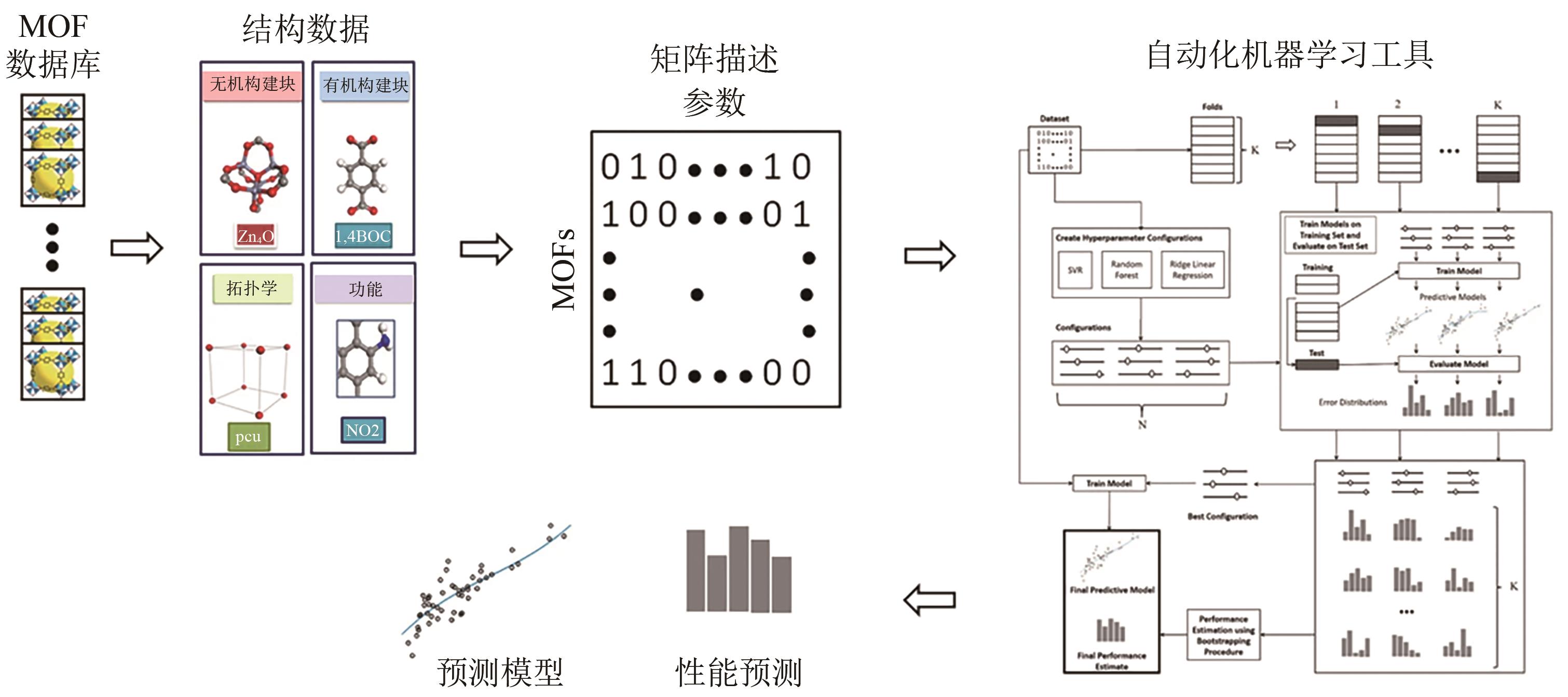
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (10): 5748-5764.DOI: 10.16085/j.issn.1000-6613.2023-1544
• Resources and environmental engineering • Previous Articles
Research advances on upcycling organic solid waste into CO2 adsorbents: A cross-research review
HUANG Zhixin1( ), WANG Junyao2, YUAN Xiangzhou3, DENG Shuai1(
), WANG Junyao2, YUAN Xiangzhou3, DENG Shuai1( ), ZHAO Jie4, ZHANG Xinyi1
), ZHAO Jie4, ZHANG Xinyi1
- 1.Key Laboratory of Efficient Utilization of Low and Medium Grade Energy,Tianjin 300350,China
2.School of Materials and Energy, Guangdong University of Technology,Guangzhou 510006,Guangdong, China
3.Ministry of Education of Key Laboratory of Energy Thermal Conversion and Control, School of Energy and Environment, Southeast University, Nanjing 210096, Jiangsu, China
4.Department of Chemical and Biomolecular Engineering,Faculty of Engineering,National University of Singapore,Kent Ridge Crescent 119260,Singapore
-
Received:2023-09-04Revised:2023-11-16Online:2024-10-29Published:2024-10-15 -
Contact:DENG Shuai
有机固废高值化为CO2吸附剂研究进展:交叉研究综述
黄致新1( ), 王珺瑶2, 袁湘洲3, 邓帅1(
), 王珺瑶2, 袁湘洲3, 邓帅1( ), 赵洁4, 张欣懿1
), 赵洁4, 张欣懿1
- 1.中低温热能高效利用教育部重点实验室,天津 300350
2.广东工业大学材料与能源学院,广东 广州 510006
3.东南大学能源与环境学院,能源热转换及其过程测控教育部重点实验室,江苏 南京 210096
4.新加坡国立大学工程学院化学与生物分子工程系,新加坡 肯特岗 119260
-
通讯作者:邓帅 -
作者简介:黄致新(2001—),男,硕士研究生,研究方向为生命周期评价。E-mail:zhixinhuang@tju.edu.cn。 -
基金资助:国家自然科学基金青年项目(72104257);天津市自然科学基金重点项目(22JCZDJC00540)
CLC Number:
Cite this article
HUANG Zhixin, WANG Junyao, YUAN Xiangzhou, DENG Shuai, ZHAO Jie, ZHANG Xinyi. Research advances on upcycling organic solid waste into CO2 adsorbents: A cross-research review[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5748-5764.
黄致新, 王珺瑶, 袁湘洲, 邓帅, 赵洁, 张欣懿. 有机固废高值化为CO2吸附剂研究进展:交叉研究综述[J]. 化工进展, 2024, 43(10): 5748-5764.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1544
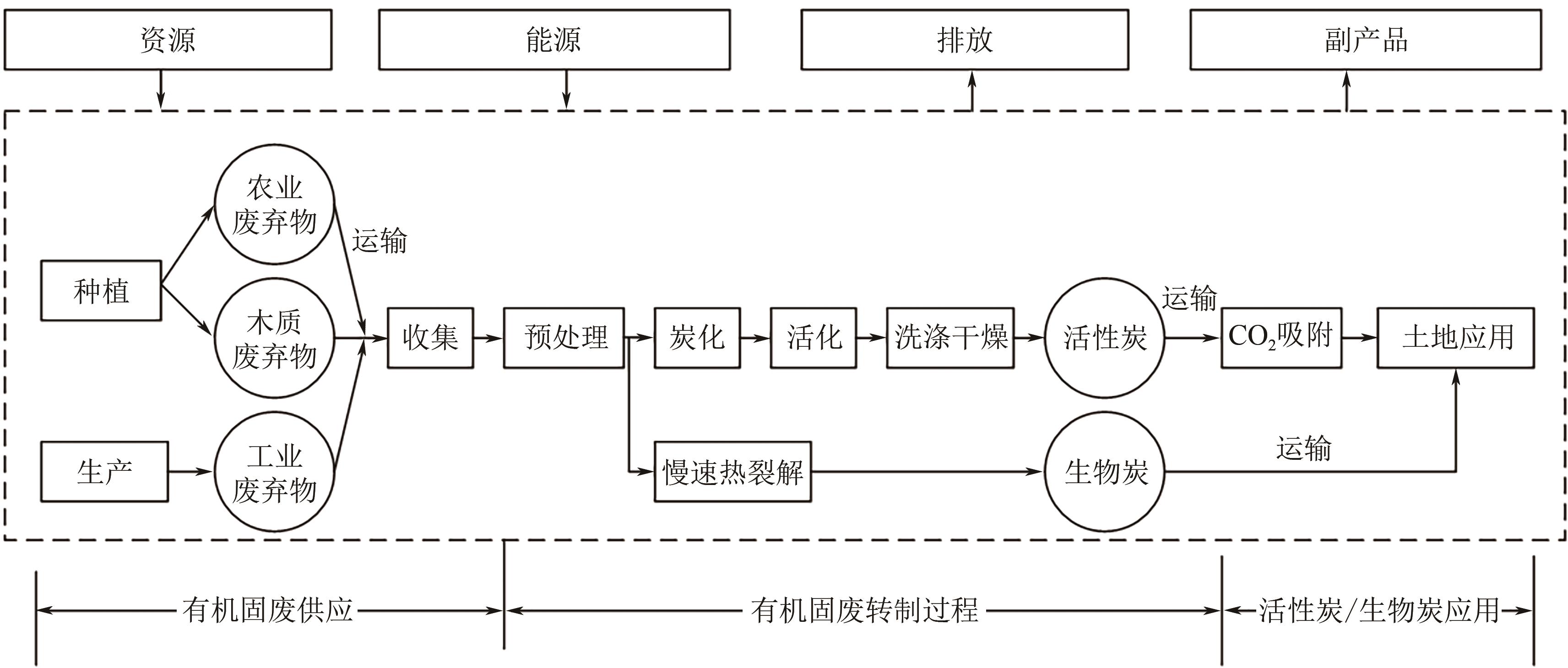
| 原料 | 炭化方法 | 炭化温度 /℃ | 活化剂 | 活化温度 /℃ | 表面改性剂 | 吸附容量(25℃,1bar) /mmol·g-1 | 动态吸附容量 /mmol·g-1 | 选择性 (CO2-N2)/% |
|---|---|---|---|---|---|---|---|---|
| 菠萝废弃物[ | 水热炭化 | 210 | 草酸锂、草酸钠、草酸钾 | 700 | — | 4.25 | 0.93 | 18.4~38.4 |
| 腐烂草莓[ | 水热炭化 | 180 | KOH | 650 | — | 4.49 | — | 20 |
| 菊君草[ | 水热炭化 | 250 | KOH | 700 | — | 4.90 | — | — |
| 山茶花[ | 水热炭化 | 250 | KOH | 700 | — | 5.00 | — | — |
| 中药固废[ | 水热炭化 | 270 | KOH | 600~800 | 尿素 | 4.02 | — | 21.26 |
| 虾壳[ | 热解 | 400 | KOH | 700 | — | 4.20 | — | 23 |
| 荷叶[ | 热解 | 500 | NaNH2 | 450~550 | NaNH2 | 3.5 | 0.82 | 21 |
| 榛子壳[ | 热解 | 500 | NaNH2 | 500~600 | NaNH2 | 4.23 | 1.00 | 17 |
| 菱角壳[ | 热解 | 500 | KOH | 550~650 | 硫脲 | 4.34 | 0.89 | 22 |
| 玉米芯[ | — | 700 | — | — | PEI | 4.75(20℃) | 2.6~2.7 | — |
| 莲杆[ | 热解 | 1000 | K2CO3/KHCO3 | 80 | — | — | — | — |
| 甘蔗渣[ | 热解 | 600 | KOH | 600 | 尿素 | 4.8 | — | 22 |
| 水热炭化 | 240 | KOH | 800 | 乙酸 | 4.47 | — | 21.5 | |
| 稻壳[ | — | 520 | KOH | 710 | — | 4.16 | — | — |
| — | 200 | KOH | 700 | PEI | 4.50 | — | — | |
| 藻类[ | 热解 | 400~800 | KOH | 400~800 | — | 0.37~1.05 | — | — |
| 热解 | 800 | KOH | 600 | 尿素 | 3.44 | — | — | |
| 热解 | 800 | KOH | 600 | 尿素 | 3.94 | — | — | |
| 椰子壳[ | 热解 | 500 | KOH | 600 | — | 4.23 | — | — |
| 热解 | 800 | CO2 | 800 | — | 3.90 | — | — | |
| 热解 | 500 | K2CO3 | 600 | 尿素 | 4.70 | — | 11 | |
| 热解 | 500 | KOH | 650 | 尿素 | 4.80 | — | 15 | |
| 热解 | 500 | KOH | 650 | 氨 | 4.26 | — | — | |
| PET废塑料[ | 热解 | 700 | KOH | 700 | — | — | 2.31 | — |
| 热解 | 600 | KOH | 700 | — | 4.42 | 3.31 | 14 | |
| 热解 | 600 | KOH | 700 | 尿素 | 4.58 | 3.51 | 19 | |
| 热解 | 600 | CO2 | 900 | — | 3.63 | 2.68 | — |
| 原料 | 炭化方法 | 炭化温度 /℃ | 活化剂 | 活化温度 /℃ | 表面改性剂 | 吸附容量(25℃,1bar) /mmol·g-1 | 动态吸附容量 /mmol·g-1 | 选择性 (CO2-N2)/% |
|---|---|---|---|---|---|---|---|---|
| 菠萝废弃物[ | 水热炭化 | 210 | 草酸锂、草酸钠、草酸钾 | 700 | — | 4.25 | 0.93 | 18.4~38.4 |
| 腐烂草莓[ | 水热炭化 | 180 | KOH | 650 | — | 4.49 | — | 20 |
| 菊君草[ | 水热炭化 | 250 | KOH | 700 | — | 4.90 | — | — |
| 山茶花[ | 水热炭化 | 250 | KOH | 700 | — | 5.00 | — | — |
| 中药固废[ | 水热炭化 | 270 | KOH | 600~800 | 尿素 | 4.02 | — | 21.26 |
| 虾壳[ | 热解 | 400 | KOH | 700 | — | 4.20 | — | 23 |
| 荷叶[ | 热解 | 500 | NaNH2 | 450~550 | NaNH2 | 3.5 | 0.82 | 21 |
| 榛子壳[ | 热解 | 500 | NaNH2 | 500~600 | NaNH2 | 4.23 | 1.00 | 17 |
| 菱角壳[ | 热解 | 500 | KOH | 550~650 | 硫脲 | 4.34 | 0.89 | 22 |
| 玉米芯[ | — | 700 | — | — | PEI | 4.75(20℃) | 2.6~2.7 | — |
| 莲杆[ | 热解 | 1000 | K2CO3/KHCO3 | 80 | — | — | — | — |
| 甘蔗渣[ | 热解 | 600 | KOH | 600 | 尿素 | 4.8 | — | 22 |
| 水热炭化 | 240 | KOH | 800 | 乙酸 | 4.47 | — | 21.5 | |
| 稻壳[ | — | 520 | KOH | 710 | — | 4.16 | — | — |
| — | 200 | KOH | 700 | PEI | 4.50 | — | — | |
| 藻类[ | 热解 | 400~800 | KOH | 400~800 | — | 0.37~1.05 | — | — |
| 热解 | 800 | KOH | 600 | 尿素 | 3.44 | — | — | |
| 热解 | 800 | KOH | 600 | 尿素 | 3.94 | — | — | |
| 椰子壳[ | 热解 | 500 | KOH | 600 | — | 4.23 | — | — |
| 热解 | 800 | CO2 | 800 | — | 3.90 | — | — | |
| 热解 | 500 | K2CO3 | 600 | 尿素 | 4.70 | — | 11 | |
| 热解 | 500 | KOH | 650 | 尿素 | 4.80 | — | 15 | |
| 热解 | 500 | KOH | 650 | 氨 | 4.26 | — | — | |
| PET废塑料[ | 热解 | 700 | KOH | 700 | — | — | 2.31 | — |
| 热解 | 600 | KOH | 700 | — | 4.42 | 3.31 | 14 | |
| 热解 | 600 | KOH | 700 | 尿素 | 4.58 | 3.51 | 19 | |
| 热解 | 600 | CO2 | 900 | — | 3.63 | 2.68 | — |
| 材料 | 孔隙形状 | 表面化学组成 | 模拟方法 | 相互作用势 | 模拟条件 | 参考文献 |
|---|---|---|---|---|---|---|
| 多孔炭 | 多孔材料 | 氢、羟基团、醚、酯 | GCMC | Compass | 298K | [ |
| 石墨层 | 狭缝型孔隙 | 吡啶N、羧基团 | GCMC | Lennard-Jones | 273~304K | [ |
| 多孔炭 | 纳米管 | 羧基团 | GCMC | Lennard-Jones | 119.8K | [ |
| 多孔炭 | 蜂窝状 | C-H group | GCMC | Lennard-Jones | 318.15K | [ |
| 多孔炭 | 纳米管 | — | GCMC | Lennard-Jones | 300K | [ |
| 煤炭 | 层状堆叠 | — | MD | GROMOS | 312~394K | [ |
| 多孔炭 | 多孔球 | — | GCMC | Lennard-Jones | — | [ |
| 多孔炭 | 片状 | 硫、氮掺杂 | MD | CVFF | 365K | [ |
| 石墨烯层 | 狭缝型纳米孔隙 | — | MD | Lennard-Jones | 353~413K | [ |
| 多孔炭 | 纳米管 | 羧、羰、羟基团 | GCMC | Lennard-Jones; Coulomb | 298K | [ |
| 煤炭 | 多孔材料 | 硫、氮掺杂 | MD | Lennard-Jones | 298K,313K,373K | [ |
| 多孔炭 | 纳米管 | 氢、羟、胺、羧基团 | GCMC | Lennard-Jones;Columbic | 298K,313K,373K | [ |
| 材料 | 孔隙形状 | 表面化学组成 | 模拟方法 | 相互作用势 | 模拟条件 | 参考文献 |
|---|---|---|---|---|---|---|
| 多孔炭 | 多孔材料 | 氢、羟基团、醚、酯 | GCMC | Compass | 298K | [ |
| 石墨层 | 狭缝型孔隙 | 吡啶N、羧基团 | GCMC | Lennard-Jones | 273~304K | [ |
| 多孔炭 | 纳米管 | 羧基团 | GCMC | Lennard-Jones | 119.8K | [ |
| 多孔炭 | 蜂窝状 | C-H group | GCMC | Lennard-Jones | 318.15K | [ |
| 多孔炭 | 纳米管 | — | GCMC | Lennard-Jones | 300K | [ |
| 煤炭 | 层状堆叠 | — | MD | GROMOS | 312~394K | [ |
| 多孔炭 | 多孔球 | — | GCMC | Lennard-Jones | — | [ |
| 多孔炭 | 片状 | 硫、氮掺杂 | MD | CVFF | 365K | [ |
| 石墨烯层 | 狭缝型纳米孔隙 | — | MD | Lennard-Jones | 353~413K | [ |
| 多孔炭 | 纳米管 | 羧、羰、羟基团 | GCMC | Lennard-Jones; Coulomb | 298K | [ |
| 煤炭 | 多孔材料 | 硫、氮掺杂 | MD | Lennard-Jones | 298K,313K,373K | [ |
| 多孔炭 | 纳米管 | 氢、羟、胺、羧基团 | GCMC | Lennard-Jones;Columbic | 298K,313K,373K | [ |
| 材料 | 表面化学组成 | 模拟方法 | CO2吸附能/kJ·mol-1 | 机制 | 参考文献 |
|---|---|---|---|---|---|
| 多孔炭 | N/O共掺杂 | DFT | -29.34 | 氮氧协同增加表面吸附的范德华作用 | [ |
| 多孔炭球 | N掺杂、O掺杂 | DFT | -21.5 | 氧掺杂表面亲和力比氮掺杂表面亲和力弱 | [ |
| 石墨层 | N、P、S、O掺杂 | DFT/GCMC | -34.42 | 表面极性官能团的氢键相互作用增强CO2吸附性能 | [ |
| C9N7 | N掺杂 | DFT/GCMC | -26.32 | 层间距与CO2吸附性能相关 | [ |
| 多孔炭 | Co掺杂、吡咯掺杂 | DFT | -21.4 | 表面与CO2没有明显电荷转移,阐明物理吸附机制 | [ |
| 多孔炭 | N/O共掺杂 | DFT/GCMC | -35.1 | 高氧含量表面掺杂氮增强静电相互作用 | [ |
| C3N | B掺杂、P掺杂 | DFT | -30.53 | 磷掺杂表面的异质电荷密度分布和C、P原子的p轨道杂化 | [ |
| 石墨炔 | Sc掺杂、Cr掺杂 | DFT | -23.23 | 多金属共修饰的石墨炔具有协同作用 | [ |
| 多孔炭 | N/O共掺杂 | GCMC | -39.4 | 孔径增大和氮氧掺杂增强CO2吸附性能 | [ |
| 石墨烯 | N掺杂 | DFT | -34.75 | 负电荷密度增强氮掺杂表面的相互作用 | [ |
| 材料 | 表面化学组成 | 模拟方法 | CO2吸附能/kJ·mol-1 | 机制 | 参考文献 |
|---|---|---|---|---|---|
| 多孔炭 | N/O共掺杂 | DFT | -29.34 | 氮氧协同增加表面吸附的范德华作用 | [ |
| 多孔炭球 | N掺杂、O掺杂 | DFT | -21.5 | 氧掺杂表面亲和力比氮掺杂表面亲和力弱 | [ |
| 石墨层 | N、P、S、O掺杂 | DFT/GCMC | -34.42 | 表面极性官能团的氢键相互作用增强CO2吸附性能 | [ |
| C9N7 | N掺杂 | DFT/GCMC | -26.32 | 层间距与CO2吸附性能相关 | [ |
| 多孔炭 | Co掺杂、吡咯掺杂 | DFT | -21.4 | 表面与CO2没有明显电荷转移,阐明物理吸附机制 | [ |
| 多孔炭 | N/O共掺杂 | DFT/GCMC | -35.1 | 高氧含量表面掺杂氮增强静电相互作用 | [ |
| C3N | B掺杂、P掺杂 | DFT | -30.53 | 磷掺杂表面的异质电荷密度分布和C、P原子的p轨道杂化 | [ |
| 石墨炔 | Sc掺杂、Cr掺杂 | DFT | -23.23 | 多金属共修饰的石墨炔具有协同作用 | [ |
| 多孔炭 | N/O共掺杂 | GCMC | -39.4 | 孔径增大和氮氧掺杂增强CO2吸附性能 | [ |
| 石墨烯 | N掺杂 | DFT | -34.75 | 负电荷密度增强氮掺杂表面的相互作用 | [ |
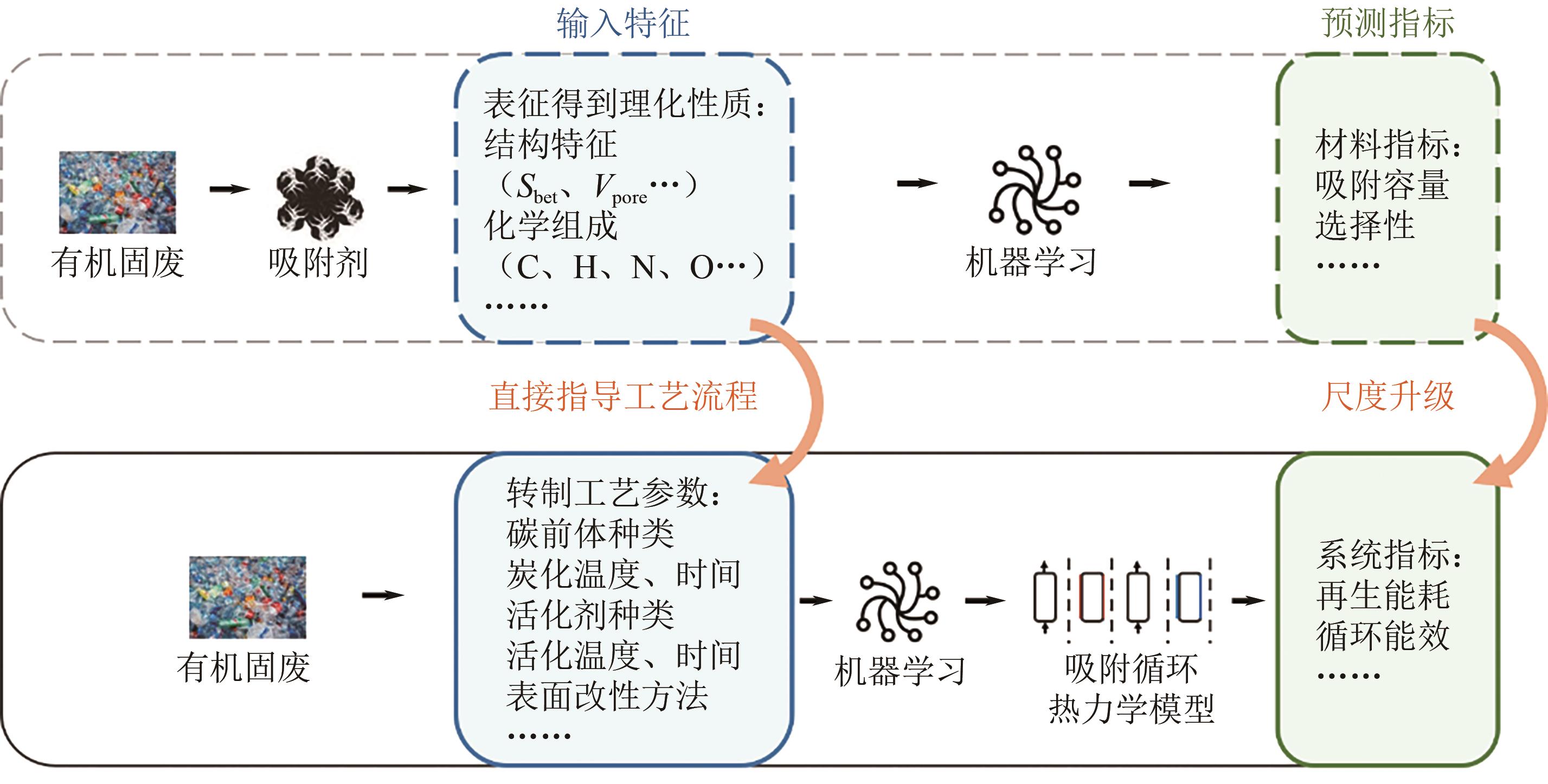
| 年份 | 吸附剂类型 | 输入特征 | 预测指标 | 算法 | 数据集大小 | 预测效果 |
|---|---|---|---|---|---|---|
| 2018[ | AC | T、P、比表面积、孔体积 | CO2吸附量 | LSSVM | 104 | R2>0.89 |
| 2019[ | PC | Sbet、Vmicro、Vmeso、Vtotal、T、P | CO2吸附量 | ANN | 1000 | R2>0.9 |
| 2019[ | PC | Sbet、Vmicro、Vmeso、T、P | CO2、N2吸附量 | ANN | 1138 | R2=0.96 |
| 2019[ | AC | 比表面积、Vmicro、T、P | CO2, CH4, N2及其混合物吸附量 | ANN | 40~417 | R2>0.99 |
| 2020[ | PC | N2等温线、T、P | CO2 、N2吸附量 | CNN | 679 | — |
| 2020[ | BWDPC | C、N、O、H、T、P、Sbet、Vmicro、Vmeso、Vultra | CO2吸附量 | RF | 6244 | 0.43(省略 k) |
| 2020[ | AC | P、T | CH4、CO2、N2吸附量 | ANN | — | R2=0.997~0.999 |
| 2020[ | 水炭、热解炭 | C、H、N、O、FC、A、V、tH、TH、CW、TP、PHR、tP | HHV、ER、ED | SVM | 248 | R2=0.90,0.94 |
| 2021[ | BWDPC | Sbet、Vtotal、Vmicro、C、O、H、N、T、P | CO2吸附量 | GBDT | 527 | R2=0.84 |
| 2021[ | BWDPC | 碳前体、活化剂、TP、孔体积、P、T | 比表面积、CO2吸附量 | ANN | 421 | R2>0.99 |
| 2022[ | AC、碳分子筛 | P、T、吸附剂类型(等温线数据) | N2、N2O、O2吸附量 | ANN | 1242 | R2=0.993~0.996 |
| 2022[ | AC、沸石 | P、(303K) | Ar, Xe, Kr, O2吸附量 | ANN | 1400 | R2=0.9978~0.9998 |
| 2022[ | BWDPC | Vmicro、Vmeso、Sbet、T、P | CO2吸附量 | ANN | 500 | 0.43(省略 k) |
| 2023[ | 生物炭 | TP、C、H、O、N、Sbet、Vtotal、Vmicro | CO2吸附量 | LGBM | 334 | R2=0.94 |
| 2023[ | PC | Vtotal、Vmicro、Vultra、Sbet、O、N、T、P | CO2吸附量 | RF | 1594 | R2>0.97 |
| 年份 | 吸附剂类型 | 输入特征 | 预测指标 | 算法 | 数据集大小 | 预测效果 |
|---|---|---|---|---|---|---|
| 2018[ | AC | T、P、比表面积、孔体积 | CO2吸附量 | LSSVM | 104 | R2>0.89 |
| 2019[ | PC | Sbet、Vmicro、Vmeso、Vtotal、T、P | CO2吸附量 | ANN | 1000 | R2>0.9 |
| 2019[ | PC | Sbet、Vmicro、Vmeso、T、P | CO2、N2吸附量 | ANN | 1138 | R2=0.96 |
| 2019[ | AC | 比表面积、Vmicro、T、P | CO2, CH4, N2及其混合物吸附量 | ANN | 40~417 | R2>0.99 |
| 2020[ | PC | N2等温线、T、P | CO2 、N2吸附量 | CNN | 679 | — |
| 2020[ | BWDPC | C、N、O、H、T、P、Sbet、Vmicro、Vmeso、Vultra | CO2吸附量 | RF | 6244 | 0.43(省略 k) |
| 2020[ | AC | P、T | CH4、CO2、N2吸附量 | ANN | — | R2=0.997~0.999 |
| 2020[ | 水炭、热解炭 | C、H、N、O、FC、A、V、tH、TH、CW、TP、PHR、tP | HHV、ER、ED | SVM | 248 | R2=0.90,0.94 |
| 2021[ | BWDPC | Sbet、Vtotal、Vmicro、C、O、H、N、T、P | CO2吸附量 | GBDT | 527 | R2=0.84 |
| 2021[ | BWDPC | 碳前体、活化剂、TP、孔体积、P、T | 比表面积、CO2吸附量 | ANN | 421 | R2>0.99 |
| 2022[ | AC、碳分子筛 | P、T、吸附剂类型(等温线数据) | N2、N2O、O2吸附量 | ANN | 1242 | R2=0.993~0.996 |
| 2022[ | AC、沸石 | P、(303K) | Ar, Xe, Kr, O2吸附量 | ANN | 1400 | R2=0.9978~0.9998 |
| 2022[ | BWDPC | Vmicro、Vmeso、Sbet、T、P | CO2吸附量 | ANN | 500 | 0.43(省略 k) |
| 2023[ | 生物炭 | TP、C、H、O、N、Sbet、Vtotal、Vmicro | CO2吸附量 | LGBM | 334 | R2=0.94 |
| 2023[ | PC | Vtotal、Vmicro、Vultra、Sbet、O、N、T、P | CO2吸附量 | RF | 1594 | R2>0.97 |
| 1 | CAROLINA COLL, GIBBINS JON, SIGURD HEIBERG, et al. Carbon carpture, use and storage (CCUS)[R]. New York: United Nations Economic Commission for Europe (UNECE), 2021. |
| 2 | Mara OLIVARES-MARÍN, Mercedes MAROTO-VALER M. Development of adsorbents for CO2 capture from waste materials: A review[J]. Greenhouse Gases: Science and Technology, 2012, 2(1): 20-35. |
| 3 | WANG Junya, HUANG Liang, YANG Ruoyan, et al. Recent advances in solid sorbents for CO2 capture and new development trends[J]. Energy & Environmental Science, 2014, 7(11): 3478-3518. |
| 4 | WANG Qiang, LUO Jizhong, ZHONG Ziyi, et al. CO2 capture by solid adsorbents and their applications: Current status and new trends[J]. Energy & Environmental Science, 2011, 4(1): 42-55. |
| 5 | YUAN Xiangzhou, LEE Jong Gyu, YUN Heesun, et al. Solving two environmental issues simultaneously: Waste polyethylene terephthalate plastic bottle-derived microporous carbons for capturing CO2 [J]. Chemical Engineering Journal, 2020, 397: 125350. |
| 6 | KAZA Silpa, YAO Lisa, Perinaz BHADA-TATA, et al. What a waste 2.0: A global snapshot of solid waste management to 2050[M]. Washington, DC: World Bank, 2018. |
| 7 | YOU Siming, SONNE Christian, Yong Sik OK. COVID-19’s unsustainable waste management[J]. Science, 2020, 368(6498): 1438. |
| 8 | SINGH Gurwinder, LAKHI Kripal S, Sanchita SIL, et al. Biomass derived porous carbon for CO2 capture[J]. Carbon, 2019, 148: 164-186. |
| 9 | LI Shuangjun, YUAN Xiangzhou, DENG Shuai, et al. A review on biomass-derived CO2 adsorption capture: Adsorbent, adsorber, adsorption, and advice[J]. Renewable and Sustainable Energy Reviews, 2021, 152: 111708. |
| 10 | YUAN Xiangzhou, WANG Junyao, DENG Shuai, et al. Recent advancements in sustainable upcycling of solid waste into porous carbons for carbon dioxide capture[J]. Renewable and Sustainable Energy Reviews, 2022, 162: 112413. |
| 11 | YUAN Xiangzhou, DISSANAYAKE Pavani Dulanja, GAO Bin, et al. Review on upgrading organic waste to value-added carbon materials for energy and environmental applications[J]. Journal of Environmental Management, 2021, 296: 113128. |
| 12 | DANISH Mohammed, AHMAD Tanweer. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application[J]. Renewable and Sustainable Energy Reviews, 2018, 87: 1-21. |
| 13 | Jin Sun CHA, PARK Sung Hoon, JUNG Sang-Chul, et al. Production and utilization of biochar: A review[J]. Journal of Industrial and Engineering Chemistry, 2016, 40: 1-15. |
| 14 | WEI Lu, SEVILLA Marta, FUERTES Antonio B, et al. Hydrothermal carbonization of abundant renewable natural organic chemicals for high-performance supercapacitor electrodes[J]. Advanced Energy Materials, 2011, 1(3): 356-361. |
| 15 | GONZÁLEZ-GARCÍA P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications[J]. Renewable and Sustainable Energy Reviews, 2018, 82: 1393-1414. |
| 16 | YUAN Xiangzhou, LI Shuangjun, JEON Sunbin, et al. Valorization of waste polyethylene terephthalate plastic into N-doped microporous carbon for CO2 capture through a one-pot synthesis[J]. Journal of Hazardous Materials, 2020, 399: 123010. |
| 17 | THINES K R, ABDULLAH E C, MUBARAK N M, et al. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review[J]. Renewable and Sustainable Energy Reviews, 2017, 67: 257-276. |
| 18 | YUAN Xiangzhou, SUVARNA Manu, Sean LOW, et al. Applied machine learning for prediction of CO2 adsorption on biomass waste-derived porous carbons[J]. Environmental Science & Technology, 2021, 55(17): 11925-11936. |
| 19 | NAZIR Ghazanfar, REHMAN Adeela, PARK Soo-Jin. Role of heteroatoms (nitrogen and sulfur)-dual doped corn-starch based porous carbons for selective CO2 adsorption and separation[J]. Journal of CO2 Utilization, 2021, 51: 101641. |
| 20 | REHMAN Adeela, NAZIR Ghazanfar, RHEE Kyong YOP, et al. A rational design of cellulose-based heteroatom-doped porous carbons: Promising contenders for CO2 adsorption and separation[J]. Chemical Engineering Journal, 2021, 420: 130421. |
| 21 | LAHIJANI Pooya, MOHAMMADI Maedeh, MOHAMED Abdul Rahman. Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition[J]. Journal of CO2 Utilization, 2018, 26: 281-293. |
| 22 | Pennline H W. Sorbent research for the capture of carbon dioxide[R]. Pittsburgh: National Energy Technology Laboratory (NETL), 2016. |
| 23 | YUAN Xiangzhou, KUMAR Nallapaneni Manoj, Boris BRIGLJEVIĆ, et al. Sustainability-inspired upcycling of waste polyethylene terephthalate plastic into porous carbon for CO2 capture[J]. Green Chemistry, 2022, 24(4): 1494-1504. |
| 24 | RAGANATI Federica, CHIRONE Riccardo, AMMENDOLA Paola. CO2 capture by temperature swing adsorption: Working capacity As affected by temperature and CO2 partial pressure[J]. Industrial & Engineering Chemistry Research, 2020, 59(8): 3593-3605. |
| 25 | MALLESH D, ANBARASAN J, Mahesh KUMAR P, et al. Synthesis, characterization of carbon adsorbents derived from waste biomass and its application to CO2 capture[J]. Applied Surface Science, 2020, 530: 147226. |
| 26 | PLAZA M G, GONZÁLEZ A S, PEVIDA C, et al. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications[J]. Applied Energy, 2012, 99: 272-279. |
| 27 | 朱梦媛. 菠萝废弃物基活性炭的制备及其低温CO2吸附性能[D]. 武汉: 武汉理工大学, 2019. |
| ZHU Mengyuan. Preparation of pineapple waste-based activated carbon and its low-temperature CO2 adsorption performance[D]. Wuhan: Wuhan University of Technology, 2019. | |
| 28 | YUE Limin, RAO Linli, WANG Liwei, et al. Efficient CO2 capture by nitrogen-doped biocarbons derived from rotten strawberries[J]. Industrial & Engineering Chemistry Research, 2017, 56(47): 14115-14122. |
| 29 | COROMINA Helena Matabosch, WALSH Darren A, MOKAYA Robert. Biomass-derived activated carbon with simultaneously enhanced CO2 uptake for both pre and post combustion capture applications[J]. Journal of Materials Chemistry A, 2016, 4(1): 280-289. |
| 30 | 俞泽涛, 曾光华, 周雅彬, 等. 中药固废制备多孔碳及其CO2吸附性能[J]. 洁净煤技术, 2022, 28(10): 203-211. |
| YU Zetao, ZENG Guanghua, ZHOU Yabin, et al. Preparation of porous carbon from solid waste of traditional Chinese medicine and its CO2 adsorption performance[J]. Clean Coal Technology, 2022, 28(10): 203-211. | |
| 31 | YANG Fangqi, WANG Jun, LIU Lu, et al. Synthesis of porous carbons with high N-content from shrimp shells for efficient CO2-capture and gas separation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 15550-15559. |
| 32 | 刘沈芳. 生物质基氮掺杂多孔炭用于CO2吸附和超级电容器电极的研究[D]. 金华: 浙江师范大学, 2021. |
| LIU Shenfang. Study on biomass-based nitrogen-doped porous carbon for CO2 adsorption and supercapacitor electrode[D]. Jinhua: Zhejiang Normal University, 2021. | |
| 33 | 刘清涛. PEI改性生物炭的制备及对CO2吸附性能的评价[J]. 环境科学学报, 2021, 41(3): 932-939. |
| LIU Qingtao. Preparation of PEI-modified biochar and evaluation of its CO2 adsorption performance[J]. Acta Scientiae Circumstantiae, 2021, 41(3): 932-939. | |
| 34 | 邓淋, 俞倩倩. 莲杆基生物炭吸附CO2的性能研究[J]. 化工技术与开发, 2021, 50(4): 42-45. |
| DENG Lin, YU Qianqian. Study on CO2 adsorption performance of sugarcane-based activated carbon[J]. Technology & Development of Chemical Industry, 2021, 50(4): 42-45. | |
| 35 | 张莉. 甘蔗渣活性炭的制备及其CO2吸附性能研究[D]. 武汉: 武汉科技大学, 2019. |
| ZHANG Li. Study on preparation of bagasse activated carbon and its CO2 adsorption performance[D].Wuhan: Wuhan University of Science and Technology, 2019. | |
| 36 | LI Dawei, MA Tengfei, ZHANG Ruliang, et al. Preparation of porous carbons with high low-pressure CO2 uptake by KOH activation of rice husk char[J]. Fuel, 2015, 139: 68-70. |
| 37 | LIU Xin, SUN Chenggong, LIU Hao, et al. Developing hierarchically ultra-micro/mesoporous biocarbons for highly selective carbon dioxide adsorption[J]. Chemical Engineering Journal, 2019, 361: 199-208. |
| 38 | 丁帅. 改性海藻焦吸附脱除烟气中二氧化碳的研究[D]. 镇江: 江苏大学, 2020. |
| DING Shuai. Study on adsorption and removal of carbon dioxide from flue gas by modified seaweed coke[D].Zhenjiang: Jiangsu University, 2020. | |
| 39 | 石硕. 藻基多孔生物炭的制备及其吸附烟气中CO2的研究[D]. 镇江: 江苏大学, 2021. |
| SHI Shuo. Study on preparation of algae-based porous biochar and its adsorption of CO2 in flue gas[D]. Zhenjiang: Jiangsu University, 2021. | |
| 40 | YANG Jie, YUE Limin, HU Xin, et al. Efficient CO2 capture by porous carbons derived from coconut shell[J]. Energy & Fuels, 2017, 31(4): 4287-4293. |
| 41 | ELLO Aimé Serge, DE SOUZA Luiz K C, TROKOUREY Albert, et al. Coconut shell-based microporous carbons for CO2 capture[J]. Microporous and Mesoporous Materials, 2013, 180: 280-283. |
| 42 | YUE Limin, XIA Qiongzhang, WANG Liwei, et al. CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell[J]. Journal of Colloid and Interface Science, 2018, 511: 259-267. |
| 43 | CHEN Jie, YANG Jie, HU Gengshen, et al. Enhanced CO2 capture capacity of nitrogen-doped biomass-derived porous carbons[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 1439-1445. |
| 44 | YANG Mingli, GUO Liping, HU Gengshen, et al. Highly cost-effective nitrogen-doped porous coconut shell-based CO2 sorbent synthesized by combining ammoxidation with KOH activation[J]. Environmental Science & Technology, 2015, 49(11): 7063-7070. |
| 45 | KAUR Balpreet, SINGH Jasminder, GUPTA Raj Kumar, et al. Porous carbons derived from polyethylene terephthalate (PET) waste for CO2 capture studies[J]. Journal of Environmental Management, 2019, 242: 68-80. |
| 46 | ZHAO Yunfeng, LIU Xin, HAN Yu. Microporous carbonaceous adsorbents for CO2 separation via selective adsorption[J]. RSC Advances, 2015, 5(38): 30310-30330. |
| 47 | LI Jiaxin, MICHALKIEWICZ Beata, MIN Jiakang, et al. Selective preparation of biomass-derived porous carbon with controllable pore sizes toward highly efficient CO2 capture[J]. Chemical Engineering Journal, 2019, 360: 250-259. |
| 48 | ZHAO Jie, DENG S, ZHAO Li, et al. Synergistic and competitive effect of H2O on CO2 adsorption capture: Mechanism explanations based on molecular dynamic simulation[J]. Journal of CO2 Utilization, 2021, 52: 101662. |
| 49 | HORSTMEIER J F, GOMEZ LOPEZ A, AGAR D W. Performance improvement of vacuum swing adsorption processes for CO2 removal with integrated phase change material[J]. International Journal of Greenhouse Gas Control, 2016, 47: 364-375. |
| 50 | BAHAMON Daniel, OGUNGBENRO Adetola E, KHALEEL Maryam, et al. Performance of activated carbons derived from date seeds in CO2 swing adsorption determined by combining experimental and molecular simulation data[J]. Industrial & Engineering Chemistry Research, 2020, 59(15): 7161-7173. |
| 51 | QUEREJETA N, GARCÍA S, ÁLVAREZ-GUTIÉRREZ N, et al. Measuring heat capacity of activated carbons for CO2 capture[J]. Journal of CO2 Utilization, 2019, 33: 148-156. |
| 52 | UDDIN Kutub, ISLAM Md Amirul, MITRA Sourav, et al. Specific heat capacities of carbon-based adsorbents for adsorption heat pump application[J]. Applied Thermal Engineering, 2018, 129: 117-126. |
| 53 | GUO Bo, CHANG Liping, XIE Kechang. Adsorption of carbon dioxide on activated carbon[J]. Journal of Natural Gas Chemistry, 2006, 15(3): 223-229. |
| 54 | 姚晨牧, 冯丽, 安亚雄, 等. 碳材料的分子模型及模拟方法在吸附中应用的研究进展[J]. 天然气化工(C1化学与化工), 2021, 46(S1): 1-9. |
| YAO Chenmu, FENG Li, AN Yaxiong, et al. Research progress of molecular model and simulation method of carbon materials in adsorption application[J]. Natural Gas Chemical Industry, 2021, 46(S1): 1-9. | |
| 55 | TENNEY C M, LASTOSKIE C M. Molecular simulation of carbon dioxide adsorption in chemically and structurally heterogeneous porous carbons[J]. Environmental Progress, 2006, 25(4): 343-354. |
| 56 | MADDOX M, ULBERG D, GUBBINS K E. Molecular simulation of simple fluids and water in porous carbons[J]. Fluid Phase Equilibria, 1995, 104: 145-158. |
| 57 | BILLEMONT Pierre, COASNE Benoit, DE WEIRELD Guy. Adsorption of carbon dioxide, methane, and their mixtures in porous carbons: Effect of surface chemistry, water content, and pore disorder[J]. Langmuir, 2013, 29(10): 3328-3338. |
| 58 | LIU Lang, NICHOLSON David, BHATIA Suresh K. Adsorption of CH4 and CH4/CO2 mixtures in carbon nanotubes and disordered carbons: A molecular simulation study[J]. Chemical Engineering Science, 2015, 121: 268-278. |
| 59 | ZHANG Junfang, LIU Keyu, CLENNELL M B, et al. Molecular simulation of CO2-CH4 competitive adsorption and induced coal swelling[J]. Fuel, 2015, 160: 309-317. |
| 60 | PALMER Jeremy C, MOORE Joshua D, ROUSSEL Thomas J, et al. Adsorptive behavior of CO2, CH4 and their mixtures in carbon nanospace: A molecular simulation study[J]. Physical Chemistry Chemical Physics, 2011, 13(9): 3985-3996. |
| 61 | MICHALEC Lukáš, Martin LÍSAL. Molecular simulation of shale gas adsorption onto overmature type Ⅱ model kerogen with control microporosity[J]. Molecular Physics, 2017, 115(9/10/11/12): 1086-1103. |
| 62 | WANG Sen, JAVADPOUR Farzam, FENG Qihong. Fast mass transport of oil and supercritical carbon dioxide through organic nanopores in shale[J]. Fuel, 2016, 181: 741-758. |
| 63 | SIZOVA Anastasia A, SIZOV Vladimir V, BRODSKAYA Elena N. Adsorption of CO2/CH4 and CO2/N2 mixtures in SBA-15 and CMK-5 in the presence of water: A computer simulation study[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015, 474: 76-84. |
| 64 | DANG Yong, ZHAO Lianming, LU Xiaoqing, et al. Molecular simulation of CO2/CH4 adsorption in brown coal: Effect of oxygen-, nitrogen-, and sulfur-containing functional groups[J]. Applied Surface Science, 2017, 423: 33-42. |
| 65 | LU Xiaoqing, JIN Dongliang, WEI Shuxian, et al. Competitive adsorption of a binary CO2-CH4 mixture in nanoporous carbons: Effects of edge-functionalization[J]. Nanoscale, 2015, 7(3): 1002-1012. |
| 66 | WANG Yanxia, HU Xiude, HAO Jian, et al. Nitrogen and oxygen codoped porous carbon with superior CO2 adsorption performance: A combined experimental and DFT calculation study[J]. Industrial & Engineering Chemistry Research, 2019, 58(29): 13390-13400. |
| 67 | WU Dawei, YANG Yingju, LIU Jing, et al. Plasma-modified N/O-doped porous carbon for CO2 capture: An experimental and theoretical study[J]. Energy & Fuels, 2020, 34(5): 6077-6084. |
| 68 | CHEN Hongyu, GUO Yang, DU Yankun, et al. The synergistic effects of surface functional groups and pore sizes on CO2 adsorption by GCMC and DFT simulations[J]. Chemical Engineering Journal, 2021, 415: 128824. |
| 69 | LIU Zilong, LI Xue, SHI Di, et al. Superior selective CO2 adsorption and separation over N2 and CH4 of porous carbon nitride nanosheets: Insights from GCMC and DFT simulations[J]. Langmuir, 2023, 39(18): 6613-6622. |
| 70 | WU Dawei, LIU Jing, YANG Yingju, et al. Nitrogen/oxygen co-doped porous carbon derived from biomass for low-pressure CO2 capture[J]. Industrial & Engineering Chemistry Research, 2020, 59(31): 14055-14063. |
| 71 | MA Xiancheng, LI Liqing, ZENG Zheng, et al. Experimental and theoretical demonstration of the relative effects of O-doping and N-doping in porous carbons for CO2 capture[J]. Applied Surface Science, 2019, 481: 1139-1147. |
| 72 | LI Xiaofang, YIN Yingying, CHANG Xiao, et al. Doping-induced enhancement of CO2 adsorption on negatively charged C3N nanosheet: Insights from DFT calculations[J]. Chemical Engineering Journal, 2020, 387: 123403. |
| 73 | DARVISHNEJAD Mohammad Hossein, Adel REISI-VANANI. Synergetic effects of metals in graphyne 2D carbon structure for high promotion of CO2 capturing[J]. Chemical Engineering Journal, 2021, 406: 126749. |
| 74 | MA Xiancheng, SU Changqing, LIU Baogen, et al. Heteroatom-doped porous carbons exhibit superior CO2 capture and CO2/N2 selectivity: Understanding the contribution of functional groups and pore structure[J]. Separation and Purification Technology, 2021, 259: 118065. |
| 75 | SATHISHKUMAR Nadaraj, WU Shiuan-Yau, CHEN Hsin-Tsung. Charge-modulated/electric-field controlled reversible CO2/H2 capture and storage on metal-free N-doped penta-graphene[J]. Chemical Engineering Journal, 2020, 391: 123577. |
| 76 | 周逸寰, 解强, 周红阳, 等. 基于分子模拟的多孔炭材料结构模型构建方法研究进展[J]. 化工进展, 2024, 43(3): 1535-1551. |
| ZHOU Yihuan, XIE Qiang, ZHOU Hongyang, et al. Modeling of porous carbon materials based on molecular simulation: State-of-the art[J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1535-1551. | |
| 77 | LI Jiali, Kaizhuo LIM, YANG Haitao, et al. AI applications through the whole life cycle of material discovery[J]. Matter, 2020, 3(2): 393-432. |
| 78 | 张子杭, 许丹, 胡艳军, 等. 机器学习在有机固废全链条处置中的应用进展[J]. 能源环境保护, 2023, 37(1): 184-193. |
| ZHANG Zihang, XU Dan, HU Yanjun, et al. Application progress of machine learning in whole chain disposal of organic solid waste[J]. Energy Environmental Protection, 2023, 37(1): 184-193. | |
| 79 | WANG Song, ZHANG Zihao, DAI Sheng, et al. Insights into CO2/N2 selectivity in porous carbons from deep learning[J]. ACS Materials Letters, 2019, 1(5): 558-563. |
| 80 | WANG Song, LI Yi, DAI Sheng, et al. Prediction by convolutional neural networks of CO2/N2 selectivity in porous carbons from N2 adsorption isotherm at 77K[J]. Angewandte Chemie International Edition, 2020, 59(44): 19645-19648. |
| 81 | ZHOU Liang. Prediction of CO2 adsorption on different activated carbons by hybrid group method of data-handling networks and LSSVM[J]. Energy Sources A: Recovery, Utilization, and Environmental Effects, 2019, 41(16): 1960-1971. |
| 82 | ZHANG Zihao, SCHOTT Jennifer A, LIU Miaomiao, et al. Prediction of carbon dioxide adsorption via deep learning[J]. Angewandte Chemie International Edition, 2019, 58(1): 259-263. |
| 83 | BARKI Hadjer, KHAOUANE Latifa, HANINI Salah. Modelling of adsorption of methane, nitrogen, carbon dioxide, their binary mixtures, and their ternary mixture on activated carbons using artificial neural network[J]. Kemija u Industriji, 2019, 68(7/8): 289-302. |
| 84 | ZHU Xinzhe, TSANG Daniel C W, WANG Lei, et al. Machine learning exploration of the critical factors for CO2 adsorption capacity on porous carbon materials at different pressures[J]. Journal of Cleaner Production, 2020, 273: 122915. |
| 85 | GHALANDARI Vahab, HASHEMIPOUR Hassan, BAGHERI Hamidreza. Experimental and modeling investigation of adsorption equilibrium of CH4, CO2, and N2 on activated carbon and prediction of multi-component adsorption equilibrium[J]. Fluid Phase Equilibria, 2020, 508: 112433. |
| 86 | LI Jie, PAN Lanjia, SUVARNA Manu, et al. Fuel properties of hydrochar and pyrochar: Prediction and exploration with machine learning[J]. Applied Energy, 2020, 269: 115166. |
| 87 | HOSSEIN Mashhadimoslem, MILAD Vafaeinia, MOBIN Safarzadeh, et al. Development of predictive models for activated carbon synthesis from different biomass for CO2 adsorption using artificial neural networks[J]. Industrial & Engineering Chemistry Research, 2021, 60(38): 13950-13966. |
| 88 | Mashhadimoslem Hossein, Ghaemi Ahad. Machine learning analysis and prediction of N2, N2O, and O2 adsorption on activated carbon and carbon molecular sieve[J]. Environmental Science and Pollution Research, 2023, 30(2): 4166-4186. |
| 89 | Somayeh Kolbadinejad, Hossein Mashhadimoslem, Ahad Ghaemi, et al. Deep learning analysis of Ar, Xe, Kr, and O2 adsorption on Activated Carbon and Zeolites using ANN approach[J]. Chemical Engineering and Processing:Process Intensification, 2022, 170: 108662. |
| 90 | PALLE Kishor, VUNGUTURI Shanthi, GAYATRI Sambhani Naga, et al. The prediction of CO2 adsorption on rice husk activated carbons via deep learning neural network[J]. MRS Communications, 2022, 12(4): 434-440. |
| 91 | 陈一飞, 张晓晴, 谭康豪, 等. 基于机器学习的多孔生物炭吸附CO2性能预测[J]. 土木与环境工程学报(中英文). DOI:10.11835/j.issn.2096-6717.2023.060 . |
| CHEN Yifei, ZHANG Xiaoqing, TAN Kanghao, et al. Prediction of CO2 adsorption performance in porous biochar based on machine learning[J]. Journal of Civil and Environmental Engineering. DOI:10.11835/j.issn.2096-6717.2023.060 . | |
| 92 | MA Xiancheng, XU Wenjun, SU Rongkui, et al. Insights into CO2 capture in porous carbons from machine learning, experiments and molecular simulation[J]. Separation and Purification Technology, 2023, 306: 122521. |
| 93 | 王璐, 张磊, 都健. 机器学习高效筛选用于CO2/N2选择性吸附分离的沸石材料[J]. 化工进展, 2023, 42(1): 148-158. |
| WANG Lu, ZHANG Lei, DU Jian. Efficient screening of zeolite materials for selective adsorption and separation of CO2/N2 by machine learning[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 148-158. | |
| 94 | 姜明星, 王斯坦, 许端平. 基于机器学习的金属有机框架吸附水中重金属性能预测[J]. 中国环境科学, 2023, 43(5): 2319-2327. |
| JIANG Mingxing, WANG Sitan, XU Duanping. Prediction of adsorption performance of MOFs for heavy metals in water based on machine learning[J]. China Environmental Science, 2023, 43(5): 2319-2327. | |
| 95 | 李炜, 梁添贵, 林元创, 等. 机器学习辅助高通量筛选金属有机骨架材料[J]. 化学进展, 2022, 34(12): 2619-2637. |
| LI Wei, LIANG Tiangui, LIN Yuanchuang, et al. Machine learning accelerated high-throughput computational screening of metal-organic frameworks[J]. Progress in Chemistry, 2022, 34(12): 2619-2637. | |
| 96 | 杨潇. 用于化学战剂捕获的MOF吸附剂高通量分子模拟研究[D]. 广州: 广州大学, 2023. |
| YANG Xiao. Study on high throughput molecular simulation of MOF adsorbent for chemical warfare agent capture[D]. Guangzhou: Guangzhou University, 2023. | |
| 97 | FANOURGAKIS George S, GKAGKAS Konstantinos, TYLIANAKIS Emmanuel, et al. A universal machine learning algorithm for large-scale screening of materials[J]. Journal of the American Chemical Society, 2020, 142(8): 3814-3822. |
| 98 | BORBOUDAKIS Giorgos, STERGIANNAKOS Taxiarchis, FRYSALI Maria, et al. Chemically intuited, large-scale screening of MOFs by machine learning techniques[J]. NPJ Computational Materials, 2017, 3: 40. |
| 99 | DU Zhenyu, DENG Shuai, ZHAO Li, et al. A high-throughput computational screening of potential adsorbents for a thermal compression CO2 Brayton cycle[J]. Sustainable Energy & Fuels, 2021, 5(5): 1415-1428. |
| 100 | LI Wei, XIA Xiaoxiao, LI Song. Screening of covalent-organic frameworks for adsorption heat pumps[J]. ACS Applied Materials & Interfaces, 2020, 12(2): 3265-3273. |
| 101 | 翟一杰, 张天祚, 申晓旭, 等. 生命周期评价方法研究进展[J]. 资源科学, 2021, 43(3): 446-455. |
| ZHAI Yijie, ZHANG Tianzuo, SHEN Xiaoxu, et al. Development of life cycle assessment method[J]. Resources Science, 2021, 43(3): 446-455. | |
| 102 | 刘蔚, 毛开伟, 张廷军, 等. 生命周期评价体系的开发及其在生物质资源化领域的应用进展[J]. 环境工程, 2019, 37: 384-388. |
| LIU Wei, MAO Kaiwei, ZHANG Tingjun, et al. Development of life cycle assessment and application in biomass resource recovery[J]. Environmental Engineering, 2019, 37:384-388. | |
| 103 | ARENA Noemi, LEE Jacquetta, CLIFT Roland. Life cycle assessment of activated carbon production from coconut shells[J]. Journal of Cleaner Production, 2016, 125: 68-77. |
| 104 | Darithsa LOYA-GONZÁLEZ, Margarita LOREDO-CANCINO, Eduardo SOTO-REGALADO, et al. Optimal activated carbon production from corn pericarp: A life cycle assessment approach[J]. Journal of Cleaner Production, 2019, 219: 316-325. |
| 105 | LEFEBVRE David, WILLIAMS Adrian, KIRK Guy J D, et al. An anticipatory life cycle assessment of the use of biochar from sugarcane residues as a greenhouse gas removal technology[J]. Journal of Cleaner Production, 2021, 312: 127764. |
| 106 | SEPÚLVEDA-CERVANTES Cynthia V, Eduardo SOTO-REGALADO, Pasiano RIVAS-GARCÍA, et al. Technical-environmental optimisation of the activated carbon production of an agroindustrial waste by means response surface and life cycle assessment[J]. Waste Management & Research: the Journal for a Sustainable Circular Economy, 2018, 36(2): 121-130. |
| 107 | 刘阳, 靳晨生, 张海亚, 等. 秸秆生物炭的固碳减排潜力及其环境影响[J]. 中国环境科学, 2024, 44(1): 396-403. |
| LIU Yang, JIN Chensheng, ZHANG Haiya, et al. Carbon sequestration and emission reduction potential of straw biochar and its environmental impacts[J]. China Environmental Science, 2024, 44(1): 396-403. | |
| 108 | STRUHS Ethan, MIRKOUEI Amin, YOU Yaqi, et al. Techno-economic and environmental assessments for nutrient-rich biochar production from cattle manure: A case study in Idaho, USA[J]. Applied Energy, 2020, 279: 115782. |
| 109 | ZHU Xiefei, LABIANCA Claudia, HE Mingjing, et al. Life-cycle assessment of pyrolysis processes for sustainable production of biochar from agro-residues[J]. Bioresource Technology, 2022, 360: 127601. |
| 110 | LI Xianyue, WANG Rongchen, SHAO Chenyang, et al. Biochar and hydrochar from agricultural residues for soil conditioning: Life cycle assessment and microbially mediated C and N cycles[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(11): 3574-3583. |
| 111 | HEIDARI Ava, KHAKI Eshagh, YOUNESI Habibollah, et al. Evaluation of fast and slow pyrolysis methods for bio-oil and activated carbon production from eucalyptus wastes using a life cycle assessment approach[J]. Journal of Cleaner Production, 2019, 241: 118394. |
| 112 | HERSH BENJAMIN, MIRKOUEI AMIN. Life cycle assessment of pyrolysis-derived biochar from organic wastes and advanced feedstocks[C]//Proceedings of ASME 2019 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, August 18-21, 2019, Anaheim, California, USA. 2019 |
| 113 | RAJABI HAMEDANI Sara, KUPPENS Tom, MALINA Robert, et al. Life cycle assessment and environmental valuation of biochar production: Two case studies in Belgium[J]. Energies, 2019, 12(11): 2166. |
| 114 | PIEROBON Francesca, EASTIN Ivan L, GANGULY Indroneil. Life cycle assessment of residual lignocellulosic biomass-based jet fuel with activated carbon and lignosulfonate as co-products[J]. Biotechnology for Biofuels, 2018, 11(1): 139. |
| 115 | YUAN Xiangzhou, WANG Junyao, DENG Shuai, et al. Sustainable food waste management: Synthesizing engineered biochar for CO2 capture[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(39): 13026-13036. |
| 116 | WANG Junyao, YUAN Xiangzhou, DENG Shuai, et al. Waste polyethylene terephthalate (PET) plastics-derived activated carbon for CO2 capture: A route to a closed carbon loop[J]. Green Chemistry, 2020, 22(20): 6836-6845. |
| 117 | DUTTA Baishali, RAGHAVAN Vijaya. A life cycle assessment of environmental and economic balance of biochar systems in Quebec[J]. International Journal of Energy and Environmental Engineering, 2014, 5(2): 106. |
| 118 | PUETTMANN Maureen, SAHOO Kamalakanta, WILSON Kelpie, et al. Life cycle assessment of biochar produced from forest residues using portable systems[J]. Journal of Cleaner Production, 2020, 250: 119564. |
| 119 | HAMMOND Jim, SHACKLEY Simon, SOHI Saran, et al. Prospective life cycle carbon abatement for pyrolysis biochar systems in the UK[J]. Energy Policy, 2011, 39(5): 2646-2655. |
| 120 | TADELE Debela, ROY Poritosh, DEFERSHA Fantahun, et al. Life Cycle Assessment of renewable filler material (biochar) produced from perennial grass (Miscanthus) [J]. Aims Energy, 2019, 7(4): 430-440. |
| 121 | ROBERTS Kelli G, GLOY Brent A, JOSEPH Stephen, et al. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential[J]. Environmental Science & Technology, 2010, 44(2): 827-833. |
| 122 | PAPAGEORGIOU Asterios, AZZI Elias S, ENELL Anja, et al. Biochar produced from wood waste for soil remediation in Sweden: Carbon sequestration and other environmental impacts[J]. The Science of the Total Environment, 2021, 776: 145953. |
| 123 | HJAILA K, BACCAR R, SARRÀ M, et al. Environmental impact associated with activated carbon preparation from olive-waste cake via life cycle assessment[J]. Journal of Environmental Management, 2013, 130: 242-247. |
| [1] | LI Meixuan, CHENG Jianfeng, HUANG Guoyong, XU Shengming, YU Fengshan, WENG Yaqing, CAO Caifang, WEN Jiawei, WANG Junlian, WANG Chunxia, GU Bintao, ZHANG Yuanhua, LIU Bin, WANG Caiping, PAN Jianming, XU Zeliang, WANG Chong, WANG Ke. Synthesis and electrochemical mechanism of high voltage lithium nickel manganate cathode materials [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5086-5094. |
| [2] | WANG Yucheng, GUO Xiong, LUAN Xinqi, ZHOU Jian, LI Xiang, XING Linguang, ZHOU Xueyun, LIU Ying, WANG Deyong, WU Xuejuan, PAN Qi, LIU Jianxin, ZHAO Zhenxia, ZHAO Zhongxing. Production process optimization of vitamin U and its thermal decomposition mechanism [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5157-5167. |
| [3] | HUAI Liye, ZHONG Zhaoping, YANG Yuxuan. Characteristics and mechanism of desulfurization gypsum to α-hemihydrate gypsum: Experiments and simulations [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4694-4703. |
| [4] | XIE Juan, HE Wen, ZHAO Xucheng, LI Shuaihui, LU Zhenzhen, DING Zheyu. Research progress on the application of molecular dynamics simulation in asphalt systems [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4432-4449. |
| [5] | SONG Xingfei, JIA Xin, AN Ping, HAN Zhennan, XU Guangwen. Development of science and technology in thermochemical reaction engineering [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3513-3533. |
| [6] | ZHANG Zihang, WANG Shurong. Research advances in biomass pyrolysis conversion and low-carbon utilization of products [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3692-3708. |
| [7] | SUN Yue, XING Baolin, ZHANG Yaojie, FENG Laihong, ZENG Huihui, JIANG Zhendong, XU Bing, JIA Jianbo, ZHANG Chuanxiang, CHEN Lunjian, ZHANG Yue, ZHANG Wenhao. Preparation of B-doped porous carbon nanosheets and their lithium storage performance [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3209-3220. |
| [8] | LI Jingying, MA Longfei, PAN Yibo, LU Shan, ZHANG Hongjuan, XU Long, MA Xiaoxun. Life cycle environmental analysis of coke oven gas to liquefied natural gas based on decarburization and methanation processes [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2872-2879. |
| [9] | LI Jingying, MA Longfei, ZHANG Hongjuan, PAN Yibo, LU Shan, XU Long, MA Xiaoxun. Current status and research progress of life cycle assessment method in pharmaceutical field [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2851-2861. |
| [10] | XIE Zhongkai, SHI Weidong. Research progress of charge polarized photocatalysts in photoconversion carbon dioxide into multi-carbon chemicals [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2714-2722. |
| [11] | LIU Siyu, YANG Juan, CHEN Pei, CHEN Zutian, YAN Bin, LIU Yuhong, QIU Jieshan. Tuning N-doped configurations of N-enriched porous carbon nanosheets for high-performance zinc ion storage [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2673-2683. |
| [12] | HAN Wei, HAN Hengwen, CHENG Wei, TANG Weijian. Research progress of biomass fuels technology driven by carbon neutrality [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2463-2474. |
| [13] | ZHOU Yihuan, XIE Qiang, ZHOU Hongyang, LIANG Dingcheng, LIU Jinchang. Modeling of porous carbon materials based on molecular simulation: State-of-the art [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1535-1551. |
| [14] | HUANG Sheng, YANG Zhenli, LI Zhenyu. Analysis of optimization path of developing China's hydrogen industry [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 882-893. |
| [15] | LOU Rui, NIU Taoyuan, CAO Qihang, ZHANG Yiyi, LEI Wenqi, LU Congmin, WANG Zhiwei. Preparation and electrochemical performances of in-situ growth of δ-MnO2 on hierarchical porous carbon derived from LNP [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 1013-1021. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||