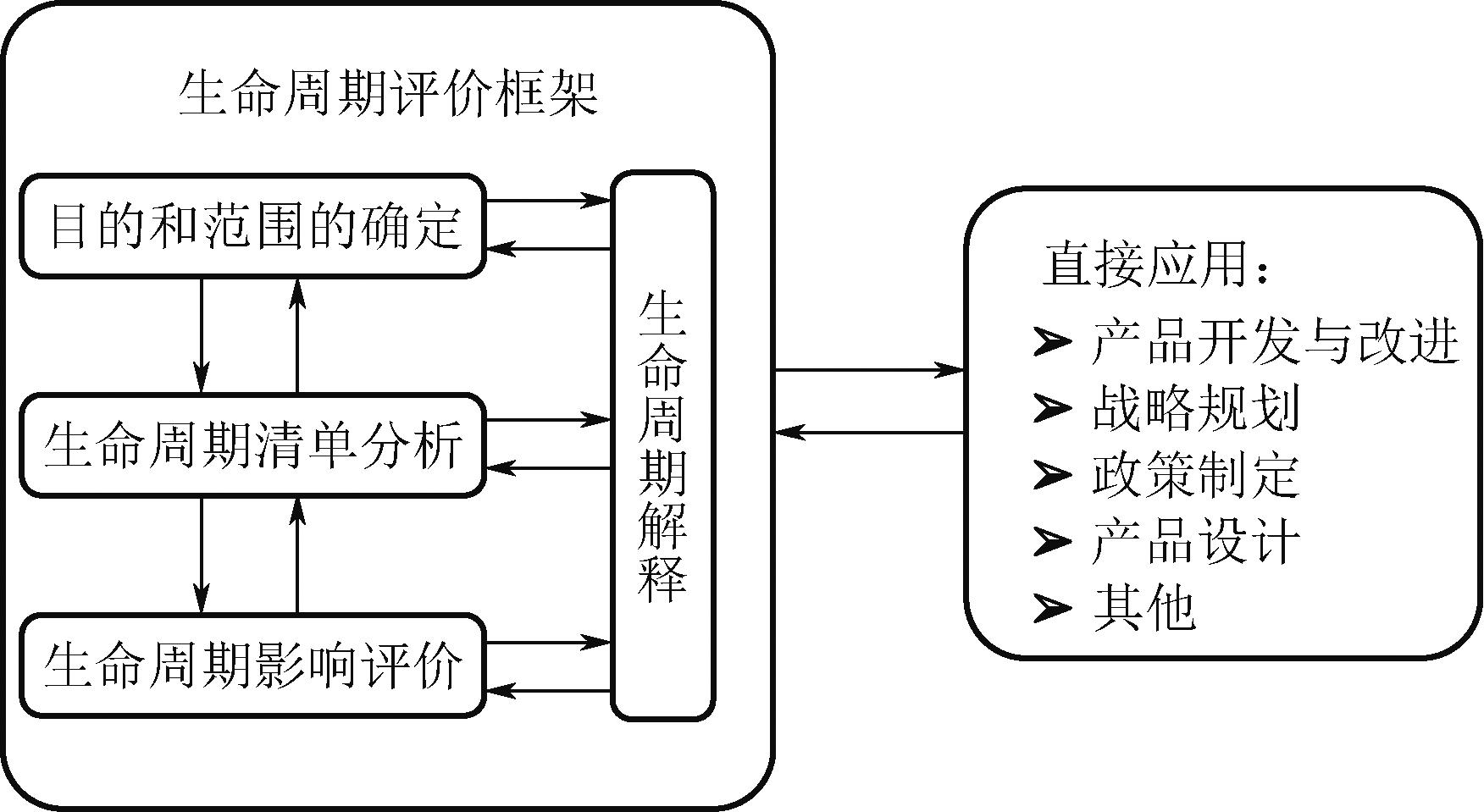
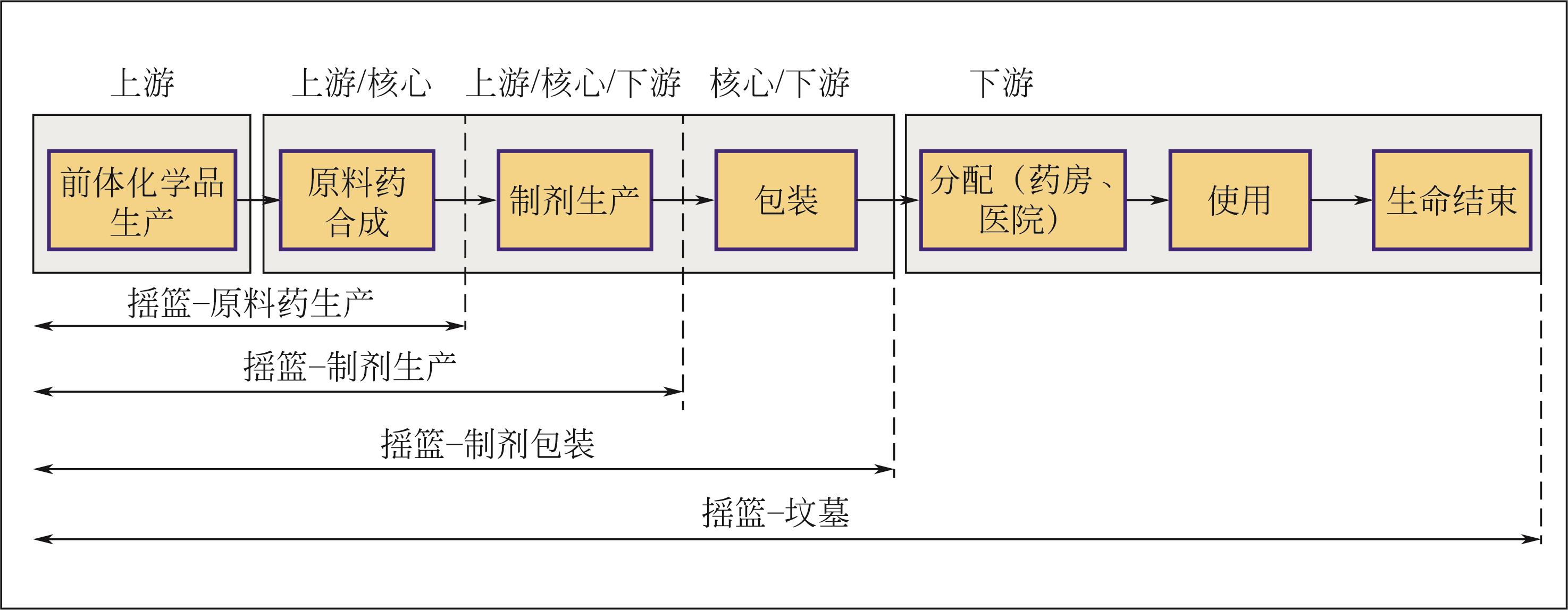
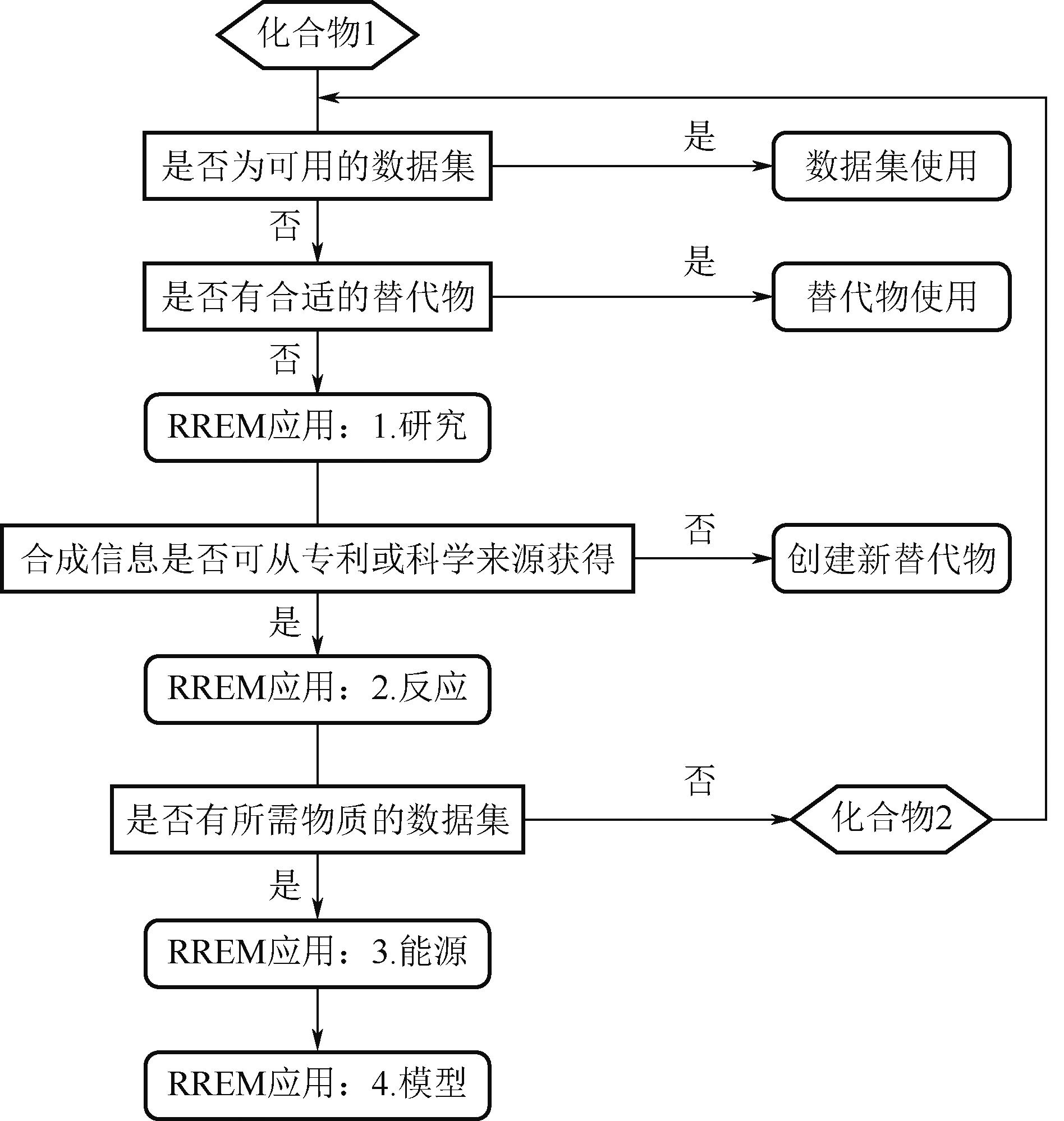
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (5): 2851-2861.DOI: 10.16085/j.issn.1000-6613.2023-2048
• Chemical processes integration and optimization • Previous Articles
Current status and research progress of life cycle assessment method in pharmaceutical field
LI Jingying1,2( ), MA Longfei1, ZHANG Hongjuan1, PAN Yibo1, LU Shan1, XU Long1,2, MA Xiaoxun1,2(
), MA Longfei1, ZHANG Hongjuan1, PAN Yibo1, LU Shan1, XU Long1,2, MA Xiaoxun1,2( )
)
- 1.School of Chemical Engineering, Northwest University, Xi’an 710127, Shaanxi, China
2.International Science & Technology Cooperation Base of MOST for Clean Utilization of Hydrocarbon Resources, Xi’an 710127, Shaanxi, China
-
Received:2023-11-23Revised:2023-12-19Online:2024-06-15Published:2024-05-15 -
Contact:MA Xiaoxun
生命周期评价方法在医药领域的应用现状与研究进展
李晶莹1,2( ), 马龙飞1, 张红娟1, 潘一搏1, 卢山1, 徐龙1,2, 马晓迅1,2(
), 马龙飞1, 张红娟1, 潘一搏1, 卢山1, 徐龙1,2, 马晓迅1,2( )
)
- 1.西北大学化工学院,陕西 西安 710127
2.碳氢资源清洁利用国际科技合作基地,陕西 西安 710127
-
通讯作者:马晓迅 -
作者简介:李晶莹(1990—),女,博士,副教授,硕士生导师,研究方向为生命周期可持续性评价与多目标集成优化。E-mail:lijingying99@nwu.edu.cn。 -
基金资助:国家自然科学基金(22008198);陕西省科协青年人才托举计划(20220602);陕西省教育厅高校青年创新团队科研项目(22JP090);陕西省博士后科研项目(2023BSHEDZZ231)
CLC Number:
Cite this article
LI Jingying, MA Longfei, ZHANG Hongjuan, PAN Yibo, LU Shan, XU Long, MA Xiaoxun. Current status and research progress of life cycle assessment method in pharmaceutical field[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2851-2861.
李晶莹, 马龙飞, 张红娟, 潘一搏, 卢山, 徐龙, 马晓迅. 生命周期评价方法在医药领域的应用现状与研究进展[J]. 化工进展, 2024, 43(5): 2851-2861.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-2048
| 数据库名称 | 国家/地区 | 简介 |
|---|---|---|
| Ecoinvent | 瑞士 | Ecoinvent数据库涵盖了欧洲及世界各国19000多种产品的单元过程和汇总过程数据集(3.8版),包含各种常见物质的LCA清单数据,是国际LCA领域使用最广泛的数据库之一,也是许多机构指定的基础数据库之一 |
| ELCD | 欧盟 | ELCD数据库涵盖了欧盟440多种大宗能源、原材料、运输的汇总LCI数据集,是欧盟环境总署和成员国政府机构指定的基础数据库之一 |
| GaBi | 德国 | GaBi数据库(2022年更新后)包括世界各国和各行业的17000个汇总过程数据集,涵盖了建筑与施工、化学品和材料、消费品等16个行业 |
| U.S.LCI | 美国 | U.S.LCI数据库包含了950多个单元过程数据集及390个汇总过程数据集,涵盖常用的材料生产、能源生产、运输等过程 |
| KCLCD | 韩国 | KCLCD数据库包含了393个汇总过程数据,涵盖物质及配件的制造、加工、运输、废物处置等过程 |
| IDEA | 日本 | IDEA数据库包含了4700条非制造业、制造业及其他部门的LCI数据集,涵盖了日本标准商品分类范围内的所有产品 |
| CLCD | 中国 | CLCD数据库由亿科环境自主开发,是目前国内唯一达到自身生命周期完整的基础数据库,包含数百种大宗能源、原材料、化学品的上千个生产过程数据,数据均来自中国本国行业统计、相关标准、企业公开报告等。CLCD数据库发布公开透明的CLCD数据库文档,并将发布可在线追溯的原始模型[ |
| CPCD | 中国 | CPCD为中国产品全生命周期温室气体排放系数库,涵盖了1081条能源产品、工业产品、生活产品、交通服务、废弃物处理和碳汇的数据集。数据集包括产品上游排放、下游排放、排放环节、温室气体占比、不确定性、数据时间、参考文献或数据来源等信息 |
| SinoCenter | 中国 | SinoCenter数据库由北京工业大学开发,涵盖了材料生命周期分析基础数据十余万条,包含公用系统、典型材料(建筑材料、钢铁、有色金属、高分子材料、连接材料)等70多个我国材料LCA数据集产品,被国内外广泛应用 |
| 数据库名称 | 国家/地区 | 简介 |
|---|---|---|
| Ecoinvent | 瑞士 | Ecoinvent数据库涵盖了欧洲及世界各国19000多种产品的单元过程和汇总过程数据集(3.8版),包含各种常见物质的LCA清单数据,是国际LCA领域使用最广泛的数据库之一,也是许多机构指定的基础数据库之一 |
| ELCD | 欧盟 | ELCD数据库涵盖了欧盟440多种大宗能源、原材料、运输的汇总LCI数据集,是欧盟环境总署和成员国政府机构指定的基础数据库之一 |
| GaBi | 德国 | GaBi数据库(2022年更新后)包括世界各国和各行业的17000个汇总过程数据集,涵盖了建筑与施工、化学品和材料、消费品等16个行业 |
| U.S.LCI | 美国 | U.S.LCI数据库包含了950多个单元过程数据集及390个汇总过程数据集,涵盖常用的材料生产、能源生产、运输等过程 |
| KCLCD | 韩国 | KCLCD数据库包含了393个汇总过程数据,涵盖物质及配件的制造、加工、运输、废物处置等过程 |
| IDEA | 日本 | IDEA数据库包含了4700条非制造业、制造业及其他部门的LCI数据集,涵盖了日本标准商品分类范围内的所有产品 |
| CLCD | 中国 | CLCD数据库由亿科环境自主开发,是目前国内唯一达到自身生命周期完整的基础数据库,包含数百种大宗能源、原材料、化学品的上千个生产过程数据,数据均来自中国本国行业统计、相关标准、企业公开报告等。CLCD数据库发布公开透明的CLCD数据库文档,并将发布可在线追溯的原始模型[ |
| CPCD | 中国 | CPCD为中国产品全生命周期温室气体排放系数库,涵盖了1081条能源产品、工业产品、生活产品、交通服务、废弃物处理和碳汇的数据集。数据集包括产品上游排放、下游排放、排放环节、温室气体占比、不确定性、数据时间、参考文献或数据来源等信息 |
| SinoCenter | 中国 | SinoCenter数据库由北京工业大学开发,涵盖了材料生命周期分析基础数据十余万条,包含公用系统、典型材料(建筑材料、钢铁、有色金属、高分子材料、连接材料)等70多个我国材料LCA数据集产品,被国内外广泛应用 |
| 年份/年 | 评价目的 | 功能单位 | 研究结果 | 参考文献 |
|---|---|---|---|---|
| 2015 | 分析废弃温度计中汞的不同处理方式下的环境影响 | 温度计中1kg汞废弃物 | 消除露天倾倒废弃温度计中的汞和提高汞的回收率,可以大幅度减少汞对环境的影响,并且在进行汞回收时,减压蒸馏工艺优于手工蒸馏工艺 | [ |
| 2016 | 分析一家医院七种医疗器械不同程度处理方式的环境影响 | 一家综合医疗外科医院中的七种医疗设备(深静脉血栓形成压缩套管、脉搏血氧仪、韧带、电脑反馈控制双极切割刀片、腹腔镜套管针、关节镜和剪刀) | 在全球变暖潜值(GWP 100 years)影响方面,加压套管和韧带对环境影响最大,剪刀对环境影响最小。对于这些器械,环氧乙烷、电力和水的优化将进一步降低对环境影响 | [ |
| 2017 | 比较计量吸入器和电动雾化器的全球变暖潜值 | 一剂硫酸沙丁胺醇 | 在全球变暖潜值(GWP 100 years)影响方面,计量吸入器(0.0972kg CO2)明显高于电动雾化器(0.0294kg CO2) | [ |
| 2021 | 评价医用口罩生产和消费的环境热点 | 2020年(2020年2月1日—2020年12月31日)在中国生产的医用外科口罩和N95口罩的总和 | 在COVID-19病毒发生期间,医用外科口罩大幅消耗导致聚丙烯消费量剧增,人类健康、环境质量、气候变化和资源类别的损害加深;同时,处理不当的废弃口罩进一步加深了环境危害 | [ |
| 2021 | 分析可重复使用电生理导管的环境影响 | 生产一根一次性电生理导管和一根可重复使用电生理导管 | 在全球变暖潜值(GWP 100 years)影响方面,可重复使用电生理导管相较于一次性电生理导管可减少50.4%,化石资源消耗减少28.8% | [ |
| 2022 | 分析可重复使用电生理导管的环境排放 | 生产一根一次性电生理导管和一根可重复使用电生理导管(与上述型号不同) | 可重复使用电生理导管相比于一次性电生理导管可减少60%的温室气体排放;且通过长期生产再循环电生理导管,总排放减少依然可达48% | [ |
| 2022 | 分析两种不同类型的医用吻合器对环境的影响 | 生产一个旋转头式医用吻合器和一个一次性医用吻合器 | 相比于没有加钢的医用吻合器,加钢的医用吻合器在富营养化、化石资源消耗和生态毒性方面明显高于一次性医用吻合器,且环境影响取决于加入的塑料质量 | [ |
| 2023 | 对比分析一次性防护服 和可重复使用防护服的环境足迹 | 分别生产1000kg一次性防护服和可重复使用防护服 | 全球变暖效应与陆地生态毒性在生命周期阶段中占主要地位,且一次性防护服环境各影响类别远大于可重复使用防护服 | [ |
| 年份/年 | 评价目的 | 功能单位 | 研究结果 | 参考文献 |
|---|---|---|---|---|
| 2015 | 分析废弃温度计中汞的不同处理方式下的环境影响 | 温度计中1kg汞废弃物 | 消除露天倾倒废弃温度计中的汞和提高汞的回收率,可以大幅度减少汞对环境的影响,并且在进行汞回收时,减压蒸馏工艺优于手工蒸馏工艺 | [ |
| 2016 | 分析一家医院七种医疗器械不同程度处理方式的环境影响 | 一家综合医疗外科医院中的七种医疗设备(深静脉血栓形成压缩套管、脉搏血氧仪、韧带、电脑反馈控制双极切割刀片、腹腔镜套管针、关节镜和剪刀) | 在全球变暖潜值(GWP 100 years)影响方面,加压套管和韧带对环境影响最大,剪刀对环境影响最小。对于这些器械,环氧乙烷、电力和水的优化将进一步降低对环境影响 | [ |
| 2017 | 比较计量吸入器和电动雾化器的全球变暖潜值 | 一剂硫酸沙丁胺醇 | 在全球变暖潜值(GWP 100 years)影响方面,计量吸入器(0.0972kg CO2)明显高于电动雾化器(0.0294kg CO2) | [ |
| 2021 | 评价医用口罩生产和消费的环境热点 | 2020年(2020年2月1日—2020年12月31日)在中国生产的医用外科口罩和N95口罩的总和 | 在COVID-19病毒发生期间,医用外科口罩大幅消耗导致聚丙烯消费量剧增,人类健康、环境质量、气候变化和资源类别的损害加深;同时,处理不当的废弃口罩进一步加深了环境危害 | [ |
| 2021 | 分析可重复使用电生理导管的环境影响 | 生产一根一次性电生理导管和一根可重复使用电生理导管 | 在全球变暖潜值(GWP 100 years)影响方面,可重复使用电生理导管相较于一次性电生理导管可减少50.4%,化石资源消耗减少28.8% | [ |
| 2022 | 分析可重复使用电生理导管的环境排放 | 生产一根一次性电生理导管和一根可重复使用电生理导管(与上述型号不同) | 可重复使用电生理导管相比于一次性电生理导管可减少60%的温室气体排放;且通过长期生产再循环电生理导管,总排放减少依然可达48% | [ |
| 2022 | 分析两种不同类型的医用吻合器对环境的影响 | 生产一个旋转头式医用吻合器和一个一次性医用吻合器 | 相比于没有加钢的医用吻合器,加钢的医用吻合器在富营养化、化石资源消耗和生态毒性方面明显高于一次性医用吻合器,且环境影响取决于加入的塑料质量 | [ |
| 2023 | 对比分析一次性防护服 和可重复使用防护服的环境足迹 | 分别生产1000kg一次性防护服和可重复使用防护服 | 全球变暖效应与陆地生态毒性在生命周期阶段中占主要地位,且一次性防护服环境各影响类别远大于可重复使用防护服 | [ |
| 1 | International Organization for Standardization. Environmental management life cycle assessment principles and framework: [S]. British: International Standard, 2006. |
| 2 | 刘蔚, 毛开伟, 张廷军, 等. 生命周期评价体系的开发及其在生物质资源化领域的应用进展[J]. 环境工程, 2019, 37: 384-388. |
| LIU Wei, MAO Kaiwei, ZHANG Tingjun, et al. Development of life cycle assessment and application in biomass resource recovery[J]. Environmental Engineering, 2019, 37: 384-388. | |
| 3 | International Organization for Standardization. Environmental management life cycle assessment requirements and guidelines: [S]. British: International Standard, 2006. |
| 4 | 翟一杰, 张天祚, 申晓旭, 等. 生命周期评价方法研究进展[J]. 资源科学, 2021, 43(3): 446-455. |
| ZHAI Yijie, ZHANG Tianzuo, SHEN Xiaoxu, et al. Development of life cycle assessment method[J]. Resources Science, 2021, 43(3): 446-455. | |
| 5 | 王长波, 张力小, 庞明月. 生命周期评价方法研究综述——兼论混合生命周期评价的发展与应用[J]. 自然资源学报, 2015, 30(7): 1232-1242. |
| WANG Changbo, ZHANG Lixiao, PANG Mingyue. A review on hybrid life cycle assessment: Development and application[J]. Journal of Natural Resources, 2015, 30(7): 1232-1242. | |
| 6 | 亿科环境科技. 中国生命周期评价基础数据库CLCD[EB/OL]. . |
| Integrated Knowledge for our Environment(IKE).China life cycle assessment basic database(CLCD)[EB/OL]. . | |
| 7 | 邹伦贵. 碳足迹认证LCA数据库应用现状研究[J]. 中国质量, 2023(8): 92-95. |
| ZOU Lungui. Research on application status of carbon footprint certification LCA database[J]. China Quality, 2023(8): 92-95. | |
| 8 | DREYER Louise Camilla, NIEMANN Anne Louise, HAUSCHILD Michael Z. Comparison of three different LCIA methods: EDIP97, CML2001 and Eco-indicator 99[J]. The International Journal of Life Cycle Assessment, 2003, 8(4): 191-200. |
| 9 | 谢明辉, 满贺诚, 段华波, 等. 生命周期影响评价方法及本地化研究进展[J]. 环境工程技术学报, 2022, 12(6): 2148-2156. |
| XIE Minghui, MAN Hecheng, DUAN Huabo, et al. Research progress on the life cycle impact assessment methods and their localization in China[J]. Journal of Environmental Engineering Technology, 2022, 12(6): 2148-2156. | |
| 10 | TOFFOLETTO Laurence, BULLE Cécile, GODIN Julie, et al. LUCAS—A new LCIA method used for a Canadian-specific context[J]. The International Journal of Life Cycle Assessment, 2007, 12(2): 93-102. |
| 11 | BARE Jane. TRACI 2.0: The tool for the reduction and assessment of chemical and other environmental impacts 2.0[J]. Clean Technologies and Environmental Policy, 2011, 13(5): 687-696. |
| 12 | AUDENAERT Amaryllis, DE CLEYN Sven H, BUYLE Matthias. LCA of low-energy flats using the Eco-indicator 99 method: Impact of insulation materials[J]. Energy Buildings, 2012, 4768-4773. |
| 13 | JOLLIET Olivier, MARGNI Manuele, CHARLES Raphaël, et al. IMPACT 2002+: A new life cycle impact assessment methodology[J]. The International Journal of Life Cycle Assessment, 2003, 8(6): 324-330. |
| 14 | OWSIANIAK Mikołaj, LAURENT Alexis, Anders BJØRN, et al. IMPACT 2002+, ReCiPe 2008 and ILCD’s recommended practice for characterization modelling in life cycle impact assessment: A case study-based comparison[J]. The International Journal of Life Cycle Assessment, 2014, 19(5): 1007-1021. |
| 15 | 杨建新, 王如松, 刘晶茹. 中国产品生命周期影响评价方法研究[J]. 环境科学学报, 2001, 21(2): 234-237. |
| YANG Jianxin, WANG Rusong, LIU Jingru. Methodology of life cycle impact assessment for Chinese products[J]. Acta Scientiae Circumstantiae, 2001, 21(2): 234-237. | |
| 16 | 王洪涛. 通往节能减排目标的新途径——生命周期节能减排评价方法[J]. 高科技与产业化, 2011(8): 49-53. |
| WANG Hongtao. A new approach to the target of energy saving and emission reduction—Life cycle assessment method for energy saving and emission reduction[J]. High-Technology & Industrialization, 2011(8): 49-53. | |
| 17 | 李晶莹. 焦化多联产系统的生命周期评价与系统分析[D]. 西安: 西北大学, 2018. |
| LI Jingying. Life cycle assessment and system analysis of coking poly-generation system[D]. Xi’an: Northwest University, 2018. | |
| 18 | SIEGERT Marc-William, LEHMANN Annekatrin, EMARA Yasmine, et al. Harmonized rules for future LCAs on pharmaceutical products and processes[J]. The International Journal of Life Cycle Assessment, 2019, 24(6): 1040-1057. |
| 19 | ROSCHANGAR Frank, LI Jun, ZHOU Yanyan, et al. Improved iGAL 2.0 metric empowers pharmaceutical scientists to make meaningful contributions to united nations sustainable development goal 12[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(16): 5148-5162. |
| 20 | RAYMOND Michael J, Stewart SLATER C, SAVELSKI Mariano J. LCA approach to the analysis of solvent waste issues in the pharmaceutical industry[J]. Green Chemistry, 2010, 12(10): 1826-1834. |
| 21 | Denise OTT, BORUKHOVA Svetlana, HESSEL Volker. Life cycle assessment of multi-step rufinamide synthesis—From isolated reactions in batch to continuous microreactor networks[J]. Green Chemistry, 2016, 18(4): 1096-1116. |
| 22 | KONG Weixin, Bihong LYU, YANG Siqi, et al. Case study on environmental safety and sustainability of pharmaceutical production based on life cycle assessment of enrofloxacin[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105734. |
| 23 | 杨珂宣. 药品生产工艺环境绩效评价模型及LCA优化研究——以乳酸环丙沙星为例[D]. 泉州: 华侨大学, 2021. |
| YANG Kexuan. Research on environmental performance evaluation model and LCA optimization of pharmaceutical production process[D]. Quanzhou: Huaqiao University, 2021. | |
| 24 | AMADO ALVIZ Patricia Lucía, ALVAREZ Alejandro J. Comparative life cycle assessment of the use of an ionic liquid ([Bmim]Br) versus a volatile organic solvent in the production of acetylsalicylic acid[J]. Journal of Cleaner Production, 2017, 168: 1614-1624. |
| 25 | HADINOTO Kunn, TRAN The-Thien, CHEOW Wean Sin. Beyond Tablets’ physical characteristics: Incorporating environmental sustainability metrics into the selection of lubricants for pharmaceutical tableting[J]. Journal of Cleaner Production, 2022, 362: 132336. |
| 26 | SHARMA Rachit Kumar, RAJU Geo, SARKAR Prabir, et al. Comparing the environmental impacts of paracetamol dosage forms using life cycle assessment[J]. Environment, Development and Sustainability, 2022, 24(10): 12446-12466. |
| 27 | BASSANI Fabiana, RODRIGUES Carla, MARQUES Pedro, et al. Ecodesign approach for pharmaceutical packaging based on life cycle assessment[J]. The Science of the Total Environment, 2022, 816: 151565. |
| 28 | BASSANI Fabiana, RODRIGUES Carla, MARQUES Pedro, et al. Life cycle assessment of pharmaceutical packaging[J]. The International Journal of Life Cycle Assessment, 2022, 27(7): 978-992. |
| 29 | SIEGERT Marc-William, SALING Peter, MIELKE Pascal, et al. Cradle-to-grave life cycle assessment of an ibuprofen analgesic[J]. Sustainable Chemistry and Pharmacy, 2020, 18: 100329. |
| 30 | ARGOUD Sarah, BUDZINSKI Kristi, Daniel D'AQUILA, et al. Green metrics for biologics[J]. Current Opinion in Green and Sustainable Chemistry, 2022, 35: 100614. |
| 31 | RENTERIA GAMIZ Ana Gabriela, DEWULF Jo, DE SOETE Wouter, et al. Freeze drying in the biopharmaceutical industry: An environmental sustainability assessment[J]. Food and Bioproducts Processing, 2019, 117: 213-223. |
| 32 | 尹阳阳. 基于LCA&LCC的生物质能源气化循环利用系统的综合评价[D]. 天津: 天津大学, 2018. |
| YIN Yangyang. Comprehensive evaluation of biomass gasification and recycling system based on LCA&LCC[D]. Tianjin: Tianjin University, 2018. | |
| 33 | LIU Yigang, LI Guoxuan, CHEN Zhengrun, et al. Comprehensive analysis of environmental impacts and energy consumption of biomass-to-methanol and coal-to-methanol via life cycle assessment[J]. Energy, 2020, 204: 117961. |
| 34 | 刘梦佳, 杨茂华, 刘新育, 等. 中药渣处理及其生命周期分析的研究进展[J]. 时珍国医国药, 2021, 32(7): 1714-1717. |
| LIU Mengjia, YANG Maohua, LIU Xinyu, et al. Research progress on treatment and life cycle analysis of traditional Chinese medicine residue[J]. Lishizhen Medicine and Materia Medica Research, 2021, 32(7): 1714-1717. | |
| 35 | SOUSA Ana Catarina, VEIGA Anabela, MAURÍCIO Ana Colette, et al. Assessment of the environmental impacts of medical devices: A review[J]. Environment Development and Sustainability, 2021, 23(7): 9641-9666. |
| 36 | YAMANOOR Srihari. Do medical device designers need to care about life cycle assessment?[C]// Materials and Processes for Medical Devices Conference and Exposition. Minneapolis, USA: Materials and Processes for Medical Devices American Society for Metals, 2011. |
| 37 | MOULTRIE James, SUTCLIFFE Laura, MAIER Anja. A maturity grid assessment tool for environmentally conscious design in the medical device industry[J]. Journal of Cleaner Production, 2016, 122: 252-265. |
| 38 | GAVILÁN-GARCÍA Irma C, Georgina FERNÁNDEZ-VILLAGOMEZ, Arturo GAVILÁN-GARCÍA, et al. Alternatives of management and disposal for mercury thermometers at the end of their life from Mexican health care institutions[J]. Journal of Cleaner Production, 2015, 86: 118-124. |
| 39 | UNGER Scott, LANDIS Amy. Assessing the environmental, human health, and economic impacts of reprocessed medical devices in a Phoenix hospital’s supply chain[J]. Journal of Cleaner Production, 2016, 112: 1995-2003. |
| 40 | GOULET Brandon, OLSON Lars, MAYER Brooke. A comparative life cycle assessment between a metered dose inhaler and electric nebulizer[J]. Sustainability, 2017, 9(10): 1725. |
| 41 | TABATABAEI Meisam, Homa HOSSEINZADEH-BANDBAFHA, YANG Yi, et al. Exergy intensity and environmental consequences of the medical face masks curtailing the COVID-19 pandemic: Malign bodyguard?[J]. Journal of Cleaner Production, 2021, 313: 127880. |
| 42 | VAN STRATEN Bart, LIGTELIJN S, DROOG L, et al. A life cycle assessment of reprocessing face masks during the COVID-19 pandemic[J]. Scientific Reports, 2021, 11(1): 17680. |
| 43 | SCHULTE Anna, MAGA Daniel, THONEMANN Nils. Combining life cycle assessment and circularity assessment to analyze environmental impacts of the medical remanufacturing of electrophysiology catheters[J]. Sustainability, 2021, 13(2): 898. |
| 44 | MEISTER Julia A, SHARP Jack, WANG Yan, et al. Assessing long-term medical remanufacturing emissions with life cycle analysis[J]. Processes, 2022, 11(1): 36. |
| 45 | FREUND Julissa, GAST Katherine, ZUEGGE Karin, et al. Environmental considerations in the selection of medical staplers: A comparative life cycle assessment[J]. Journal of Cleaner Production, 2022, 371: 133490. |
| 46 | HILOIDHARI Moonmoon, BANDYOPADHYAY Somnath. Environmental footprints of disposable and reusable personal protective equipment—A product life cycle approach for body coveralls[J]. Journal of Cleaner Production, 2023, 394: 136166. |
| 47 | ECKELMAN Matthew J, SHERMAN Jodi D. Estimated global disease burden from US health care sector greenhouse gas emissions[J]. American Journal of Public Health, 2018, 108(S2): S120-S122. |
| 48 | GORDON Ilyssa O, SHERMAN Jodi D, LEAPMAN Michael, et al. Life cycle greenhouse gas emissions of gastrointestinal biopsies in a surgical pathology laboratory[J]. American Journal of Clinical Pathology, 2021, 156(4): 540-549. |
| 49 | THIEL Cassandra L, ECKELMAN Matthew, GUIDO Richard, et al. Environmental impacts of surgical procedures: Life cycle assessment of hysterectomy in the United States[J]. Environmental Science & Technology, 2015, 49(3): 1779-1786. |
| 50 | LICHTER Katie E, CHARBONNEAU Kiley, SABBAGH Ali, et al. Evaluating the environmental impact of radiation therapy using life cycle assessments: A critical review[J]. International Journal of Radiation Oncology‘Biology’Physics, 2023, 117(3): 554-567. |
| 51 | THIEL Cassandra L, Andy CASSELS-BROWN, GOEL Hena, et al. Utilizing off-the-shelf LCA methods to develop a ‘triple bottom line’ auditing tool for global cataract surgical services[J]. Resources, Conservation and Recycling, 2020, 158: 104805. |
| 52 | HERNÁNDEZ-DE-ANDA Marco T, Paul TABOADA-GONZÁLEZ, Quetzalli AGUILAR-VIRGEN, et al. Environmental impacts of a Mexican hemodialysis unit through LCA[J]. Journal of Cleaner Production, 2023, 384: 135480. |
| 53 | PRASAD Purnima Aishwarya, JOSHI Dhruvi, LIGHTER Jennifer, et al. Environmental footprint of regular and intensive inpatient care in a large US hospital[J]. The International Journal of Life Cycle Assessment, 2022, 27(1): 38-49. |
| 54 | CIMPRICH Alexander, YOUNG Steven B. Environmental footprinting of hospitals: Organizational life cycle assessment of a Canadian hospital[J]. Journal of Industrial Ecology, 2023, 27(5): 1335-1353. |
| 55 | Denise OTT, KRALISCH Dana, Ivana DENČIĆ, et al. Life cycle analysis within pharmaceutical process optimization and intensification: Case study of active pharmaceutical ingredient production[J]. ChemSusChem, 2014, 7(12): 3521-3533. |
| 56 | HUBER Elena, BACH Vanessa, HOLZAPFEL Peter, et al. An approach to determine missing life cycle inventory data for chemicals (RREM)[J]. Sustainability, 2022, 14(6): 3161. |
| 57 | PARVATKER Abhijeet G, TUNCEROGLU Huseyin, SHERMAN Jodi D, et al. Cradle-to-gate greenhouse gas emissions for twenty anesthetic active pharmaceutical ingredients based on process scale-up and process design calculations[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(7): 6580-6591. |
| 58 | CREADORE Lauren T, CASTALDI Marco J. Quantitative comparison of life cycle assessments of advanced recycling technologies for end-of-life plastics[J]. Journal of Energy Resources Technology, 2023, 145(4): 042201. |
| [1] | GE Mingliang, TANG Wei. Progress in preparation of drug carriers based on Pickering emulsion [J]. Chemical Industry and Engineering Progress, 2017, 36(12): 4586-4591. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||