Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (11): 5764-5775.DOI: 10.16085/j.issn.1000-6613.2022-2267
• Materials science and technology • Previous Articles Next Articles
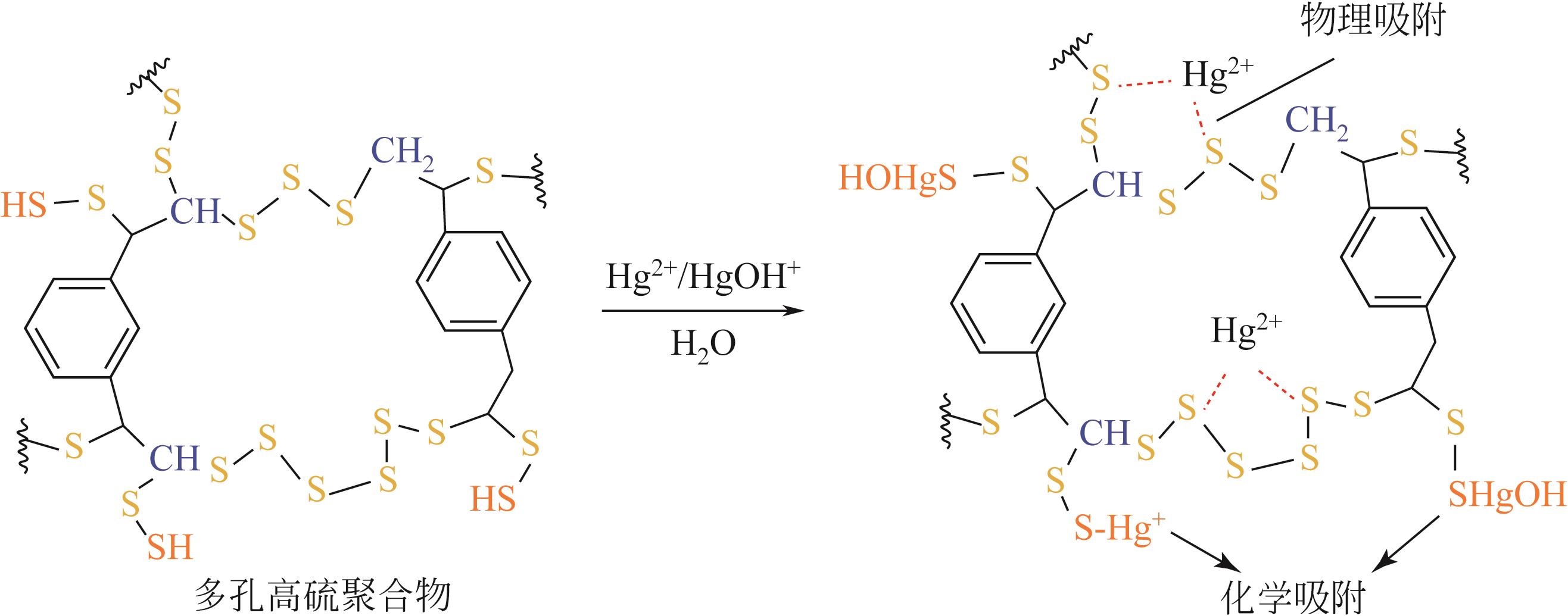
Preparation of the porous high sulfur polymers and its application in mercury adsorption
GUO Lizhen( ), LIN Xiangyu, DONG Fuhao, WANG Zhuomin, LIU He(
), LIN Xiangyu, DONG Fuhao, WANG Zhuomin, LIU He( )
)
- National Engineering Laboratory for Biomass Chemical Utilization, Key Laboratory of Biomass Energy and Materials, Institute of Chemical Industry of Forest Products CAF, Nanjing 210042, Jiangsu, China
-
Received:2022-12-06Revised:2023-01-20Online:2023-12-15Published:2023-11-20 -
Contact:LIU He
多孔高硫聚合物的制备及其在汞吸附中的应用
- 中国林业科学研究院林产化学工业研究所,生物质化学利用国家工程实验室,江苏省生物质能源与材料重点实验室,江苏 南京 210042
-
通讯作者:刘鹤 -
作者简介:郭丽珍(1994—),女,硕士,实习研究员,研究方向为林业生物质资源的化学利用。E-mail:guolizhen2014@163.com。 -
基金资助:国家自然科学基金(32071708)
CLC Number:
Cite this article
GUO Lizhen, LIN Xiangyu, DONG Fuhao, WANG Zhuomin, LIU He. Preparation of the porous high sulfur polymers and its application in mercury adsorption[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5764-5775.
郭丽珍, 林详宇, 董阜豪, 王倬敏, 刘鹤. 多孔高硫聚合物的制备及其在汞吸附中的应用[J]. 化工进展, 2023, 42(11): 5764-5775.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-2267
制备 方法 | 孔径 | 比表面积 | 吸附 等温线 | 金属吸附量 | 参考 文献 |
|---|---|---|---|---|---|
| 致孔剂 | 100~200μm | — | Ⅰ型 | 119~470μg/g | [ |
二氧化碳 发泡 | 0.2~20μm | — | — | (52±1.8)mg/g | [ |
| 静电纺丝 | <1μm | 2.35m2/g | Ⅱ型 | — | [ |
| 炭化 | <2nm和 2~50nm | >110m2/g | Ⅰ和Ⅱ型 | 1.1~187mg/g | [ |
制备 方法 | 孔径 | 比表面积 | 吸附 等温线 | 金属吸附量 | 参考 文献 |
|---|---|---|---|---|---|
| 致孔剂 | 100~200μm | — | Ⅰ型 | 119~470μg/g | [ |
二氧化碳 发泡 | 0.2~20μm | — | — | (52±1.8)mg/g | [ |
| 静电纺丝 | <1μm | 2.35m2/g | Ⅱ型 | — | [ |
| 炭化 | <2nm和 2~50nm | >110m2/g | Ⅰ和Ⅱ型 | 1.1~187mg/g | [ |
| 1 | WU Y, WANG S X, STREETS D G, et al. Trends in anthropogenic mercury emissions in China from 1995 to 2003[J]. Environmental Science & Technology, 2006, 40(17): 5312-5318. |
| 2 | CLARKSON T W, MAGOS L. The toxicology of mercury and its chemical compounds[J]. Critical Reviews in Toxicology, 2006, 36(8): 609-662. |
| 3 | ULLRICH S M, TANTON T W, ABDRASHITOVA S A. Mercury in the aquatic environment: A review of factors affecting methylation[J]. Critical Reviews in Environmental Science and Technology, 2001, 31(3): 241-293. |
| 4 | ZAHIR F, RIZWI S J, HAQ S K, et al. Low dose mercury toxicity and human health[J]. Environmental Toxicology and Pharmacology, 2005, 20(2): 351-360. |
| 5 | BECKERS Felix, RINKLEBE Joerg. Cycling of mercury in the environment: Sources, fate, and human health implications: A review[J]. Critical Reviews in Environmental Science and Technology, 2017, 47(9): 693-794. |
| 6 | O'CONNOR D, HOU D, OK Y S, et al. Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review[J]. Environment International, 2019, 126: 747-761. |
| 7 | ARIYA P A, AMYOT M, DASTOOR A, et al. Mercury physicochemical and biogeochemical transformation in the atmosphere and at atmospheric interfaces: A review and future directions[J]. Chemical Reviews, 2015, 115(10): 3760-3802. |
| 8 | SELIN N E. Global change and mercury cycling: Challenges for implementing a global mercury treaty[J]. Environmental Toxicology and Chemistry, 2014, 33(6): 1202-1210. |
| 9 | LIN C J, PEHKONEN S O. The chemistry of atmospheric mercury: A review[J]. Atmospheric Environment, 1999, 33(13): 2067-2079. |
| 10 | WU C Y, MOURI H, CHEN S S, et al. Removal of trace-amount mercury from wastewater by forward osmosis[J]. Journal of Water Process Engineering, 2016, 14: 108-116. |
| 11 | CAI Chong, WANG Ran, LIU Shufeng, et al. Synthesis of self-assembled phytic acid-MXene nanocomposites via a facile hydrothermal approach with elevated dye adsorption capacities[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 589: 124468. |
| 12 | FENG Wenguo, KWON Seokjoon, FENG Xue, et al. Sulfur impregnation on activated carbon fibers through H2S oxidation for vapor phase mercury removal[J]. Journal of Environmental Engineering, 2006, 132(3): 292-300. |
| 13 | HSI H C, ROOD M J, ROSTAM-ABADI M, et al. Effects of sulfur impregnation temperature on the properties and mercury adsorption capacities of activated carbon fibers (ACFs)[J]. Environmental Science & Technology, 2001, 35(13): 2785-2791. |
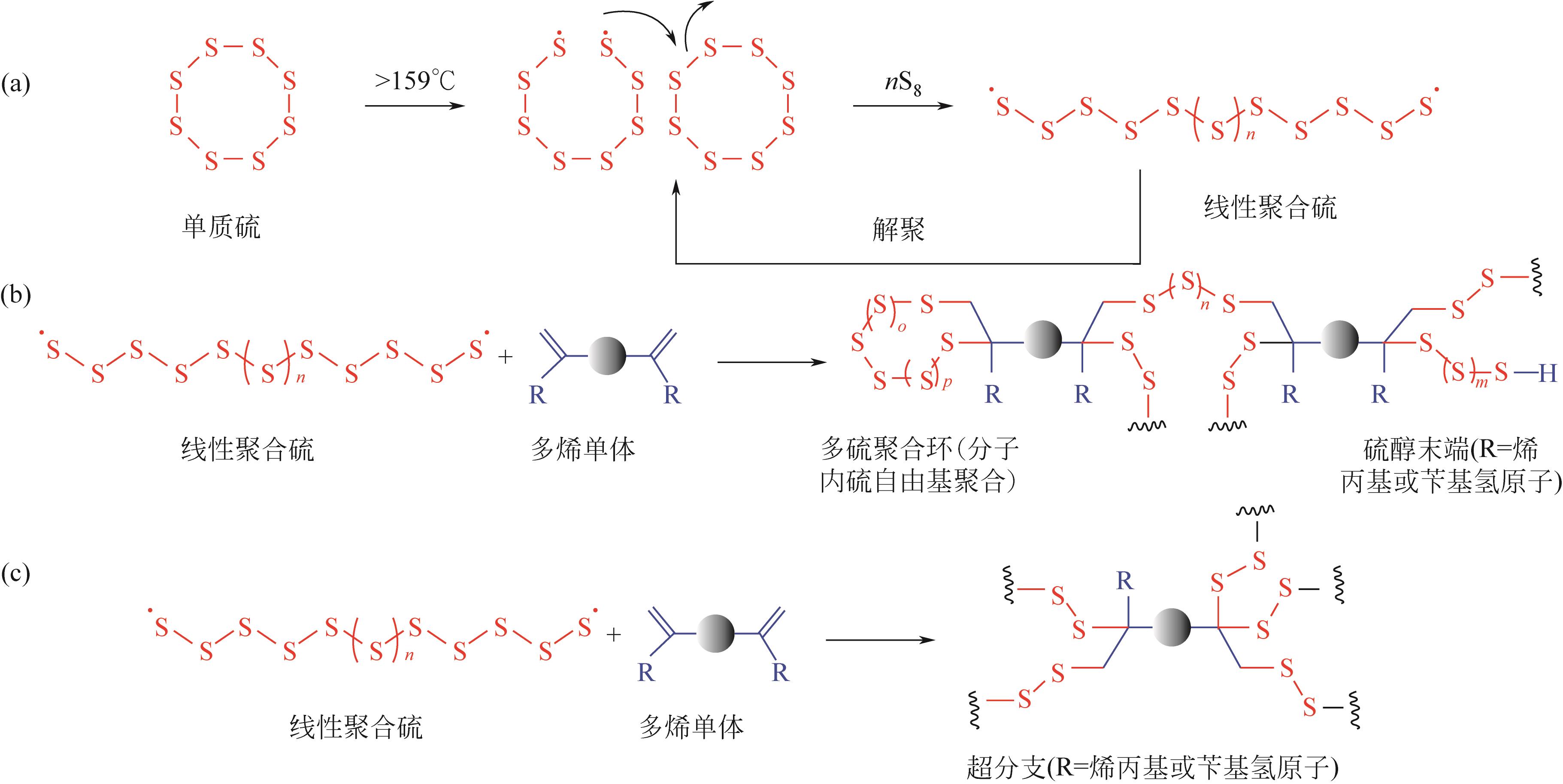
| 14 | HASELL T, PARKER D J, JONES H A, et al. Porous inverse vulcanised polymers for mercury capture[J]. Chemical Communications, 2016, 52(31): 5383-5386. |
| 15 | BEAR J C, PEVELER W J, MCNAUGHTER P D, et al. Nanoparticle-sulphur “inverse vulcanisation” polymer composites[J]. Chemical Communications, 2015, 51(52): 10467-10470. |
| 16 | HOEFLING A, NGUYEN D T, LEE Y J, et al. A sulfur-eugenol allyl ether copolymer: A material synthesized via inverse vulcanization from renewable resources and its application in Li-S batteries[J]. Materials Chemistry Frontiers, 2017, 1(9): 1818-1822. |
| 17 | HOEFLING A, LEE Y J, THEATO P. Sulfur-based polymer composites from vegetable oils and elemental sulfur: A sustainable active material for Li-S batteries[J]. Macromolecular Chemistry and Physics, 2017, 218(1): 1600303. |
| 18 | MANN M, KRUGER J E, ANDARI F, et al. Sulfur polymer composites as controlled-release fertilisers[J]. Organic & Biomolecular Chemistry, 2019, 17(7): 1929-1936. |
| 19 | DIRLAM P T, SIMMONDS A G, KLEINE T S, et al. Inverse vulcanization of elemental sulfur with 1,4-diphenylbutadiyne for cathode materials in Li-S batteries[J]. RSC Advances, 2015, 5(31): 24718-24722. |
| 20 | SMITH J A, WU X F, BERRY N G, et al. High sulfur content polymers: The effect of crosslinker structure on inverse vulcanization[J]. Journal of Polymer Science A: Polymer Chemistry, 2018, 56(16): 1777-1781. |
| 21 | WESTERMAN C R, JENKINS C L. Dynamic sulfur bonds initiate polymerization of vinyl and allyl ethers at mild temperatures[J]. Macromolecules, 2018, 51(18): 7233-7238. |
| 22 | KWON Minho, LEE Hongchan, LEE Seohui, et al. Dynamic covalent polymerization of chalcogenide hybrid inorganic/organic polymer resins with norbornenyl comonomers[J]. Macromolecular Research, 2020, 28(11): 1003-1009. |
| 23 | LIU Yanxia, CHEN Yidan, ZHANG Yagang, et al. Density-adjustable bio-based polysulfide composite prepared by inverse vulcanization and bio-based fillers[J]. Polymers, 2020, 12(9): 2127. |
| 24 | SMITH J A, GREEN S J, PETCHER S, et al. Crosslinker copolymerization for property control in inverse vulcanization[J]. Chemistry: A European Journal, 2019, 25(44): 10433-10440. |
| 25 | ZENG Shuaibo, LI Ligui, XIE Lihong, et al. Conducting polymers crosslinked with sulfur as cathode materials for high-rate, ultralong-life lithium-sulfur batteries[J]. ChemSusChem, 2017, 10(17): 3378-3386. |
| 26 | PARKER D J, JONES H A, PETCHER S, et al. Low cost and renewable sulfur-polymers by inverse vulcanisation, and their potential for mercury capture[J]. Journal of Materials Chemistry A, 2017, 5(23): 11682-11692. |
| 27 | NGUYEN T B. Elemental sulfur and molecular iodine as efficient tools for carbon-nitrogen bond formation through redox reactions[J]. Asian Journal of Organic Chemistry, 2017, 6(5): 477-491. |
| 28 | WRECZYCKI J, BIELIŃSKI D M, ANYSZKA R. Sulfur/organic copolymers as curing agents for rubber[J]. Polymers, 2018, 10(8): 870. |
| 29 | KUTNEY Gerald. Sulfur: History, technology, applications & industry[M]. Toronto: Chem. Tec. Publishing, 2007. |
| 30 | HU Jinghong, LEI Zhengdong, CHEN Zhangxin, et al. Effect of sulphur deposition on well performance in a sour gas reservoir[J]. The Canadian Journal of Chemical Engineering, 2018, 96(4): 886-894. |
| 31 | MILLER G W. Thermal analysis of polymers. VIII. Dilatometric and thermal optical behavior of sulfur[J]. Journal of Applied Polymer Science, 1971, 15(8): 1985-1994. |
| 32 | BOYD D A. Sulfur and its role in modern materials science[J]. Angewandte Chemie-International Edition, 2016, 55(50): 15486-15502. |
| 33 | NGUYEN T B. Recent advances in organic reactions involving elemental sulfur[J]. Advanced Synthesis & Catalysis, 2017, 359(7): 1066-1130. |
| 34 | GRIEBEL J J, GLASS R S, CHAR K, et al. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense[J]. Progress in Polymer Science, 2016, 58: 90-125. |
| 35 | WORTHINGTON M J, KUCERA R L, CHALKER J M. Green chemistry and polymers made from sulfur[J]. Green Chemistry, 2017, 19(12): 2748-2761. |
| 36 | DIEZ Sergei, HOEFLING Alexande, THEATO Patrick, et al. Mechanical and electrical properties of sulfur-containing polymeric materials prepared via inverse vulcanization[J]. Polymers, 2017, 9(2): 59. |
| 37 | CHUNG W J, GRIEBEL J J, KIM E T, et al. The use of elemental sulfur as an alternative feedstock for polymeric materials[J]. Nature Chemistry, 2013, 5(6): 518-524. |
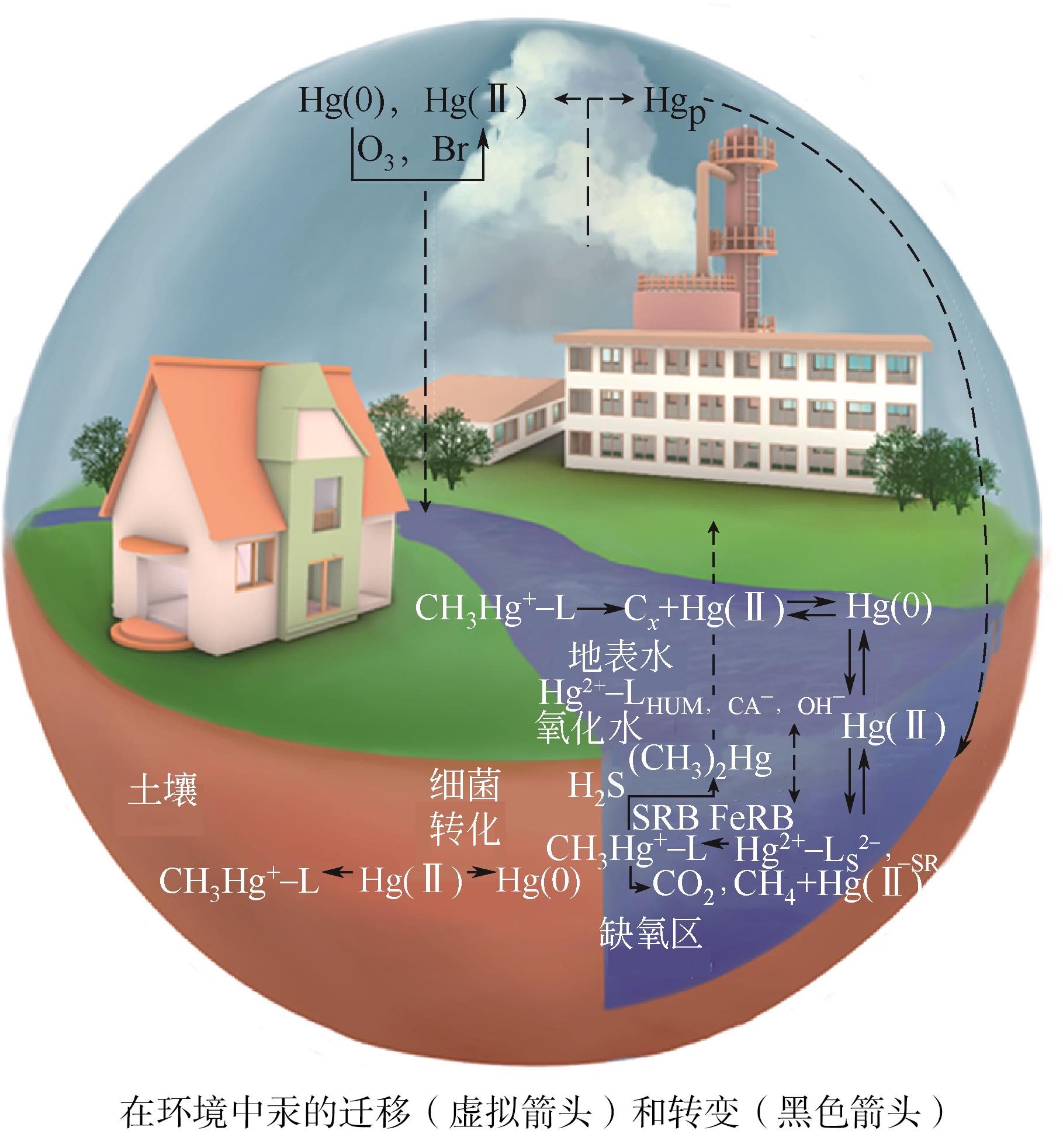
| 38 | BOYD D A, BAKER C C, MYERS J D, et al. ORMOCHALCs: Organically modified chalcogenide polymers for infrared optics[J]. Chemical Communications, 2016, 53(1): 259-262. |
| 39 | LUNDQUIST N A, TIKOALU A D, WORTHINGTON M J, et al. Reactive compression molding post-inverse vulcanization: A method to assemble, recycle, and repurpose sulfur polymers and composites[J]. Chemistry: A European Journal, 2020, 26(44): 10035-10044. |
| 40 | HERRERA C, YSINGA K J, JENKINS C L. Polysulfides synthesized from renewable garlic components and repurposed sulfur form environmentally friendly adhesives[J]. ACS Applied Materials & Interfaces, 2019, 11(38): 35312-35318. |
| 41 | PETCHER Samuel, ZHANG Bowen, HASELL Tom. Mesoporous knitted inverse vulcanised polymers[J]. Chemical Communications, 2021, 57(41): 5059-5062. |
| 42 | TIKOALU A D, LUNDQUIST N A, CHALKER J M. Mercury sorbents made by inverse vulcanization of sustainable triglycerides: The plant oil structure influences the rate of mercury removal from water[J]. Advanced Sustainable Systems, 2020, 4(3): 1900111. |
| 43 | GOMEZ I, LEONET O, BLAZQUEZ J A, et al. Inverse vulcanization of sulfur using natural dienes as sustainable materials for lithium-sulfur batteries[J]. ChemSusChem, 2016, 9(24): 3419-3425. |
| 44 | CHALKER J M, WORTHINGTON M J, LUNDQUIST N A, et al. Synthesis and applications of polymers made by inverse vulcanization[J]. Topics in Current Chemistry, 2019, 377(3): 16. |
| 45 | WU X F, SMITH J A, PETCHER S, et al. Catalytic inverse vulcanization[J]. Nature Communications, 2019, 10: 647. |
| 46 | KLEINE T S, NGUYEN N A, ANDERSON L E, et al. High refractive index copolymers with improved thermomechanical properties via the inverse vulcanization of sulfur and 1,3,5-triisopropenylbenzene[J]. ACS Macro Letters, 2016, 5(10): 1152-1156. |
| 47 | SING K S. Assessment of surface area by gas adsorption[M]. France: Adsorption by Powders and Porous Solids, 2013: 237-268. |
| 48 | TIEN Chi. Introduction to adsorption: Chapter 3-Adsorption equilibrium relationships, isotherm expressions, their determinations, and predictions[M]. USA: Elsevier, 2019: 23-85. |
| 49 | BARTON T J, BULL LUCY M, KLEMPERER W G, et al. Tailored porous materials[J]. Chemistry of Materials, 1999, 11(10): 2633-2656. |
| 50 | SING K S W, EVERETT D H, HAUL R A W, et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984)[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 51 | WORTHINGTON M J, KUCERA R L, ALBUQUERQUE I S, et al. Laying waste to mercury: Inexpensive sorbents made from sulfur and recycled cooking oils[J]. Chemistry—A European Journal, 2017, 23(64): 16219-16230. |
| 52 | LUNDQUIST N A, WORTHINGTON M J, ADAMSON N, et al. Polysulfides made from re-purposed waste are sustainable materials for removing iron from water[J]. RSC Advances, 2018, 8(3): 1232-1236. |
| 53 | ABRAHAM A M, KUMAR S V, ALHASSAN S M. Porous sulphur copolymer for gas-phase mercury removal and thermal insulation[J]. Chemical Engineering Journal, 2018, 332: 1-7. |
| 54 | REN Zixuan, JIANG Xue, LIU Lingli, et al. Modification of high-sulfur polymer using a mixture porogen and its application as advanced adsorbents for Au( Ⅲ ) from wastewater[J]. Journal of Molecular Liquids, 2021, 328: 115437. |
| 55 | KOEBEL M, STRUTZ E O. Thermal and hydrolytic decomposition of urea for automotive selective catalytic reduction systems: thermochemical and practical aspects[J]. Industrial & Engineering Chemistry Research, 2003, 42(10): 2093-2100. |
| 56 | JACOBS L J, KEMMERE M F, KEURENTJES J T. Sustainable polymer foaming using high pressure carbon dioxide: A review on fundamentals, processes and applications[J]. Green Chemistry, 2008, 10(7): 731-738. |
| 57 | COOPER A I. Polymer synthesis and processing using supercritical carbon dioxide[J]. Journal of Materials Chemistry, 2000, 10(2): 207-234. |
| 58 | NALAWADE S P, PICCHIONI F, JANSSEN L P. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications[J]. Progress in Polymer Science, 2006, 31(1): 19-43. |
| 59 | PICCHIONI Francesco. Supercritical carbon dioxide and polymers: An interplay of science and technology[J]. Polymer International, 2014, 63(8): 1394-1399. |
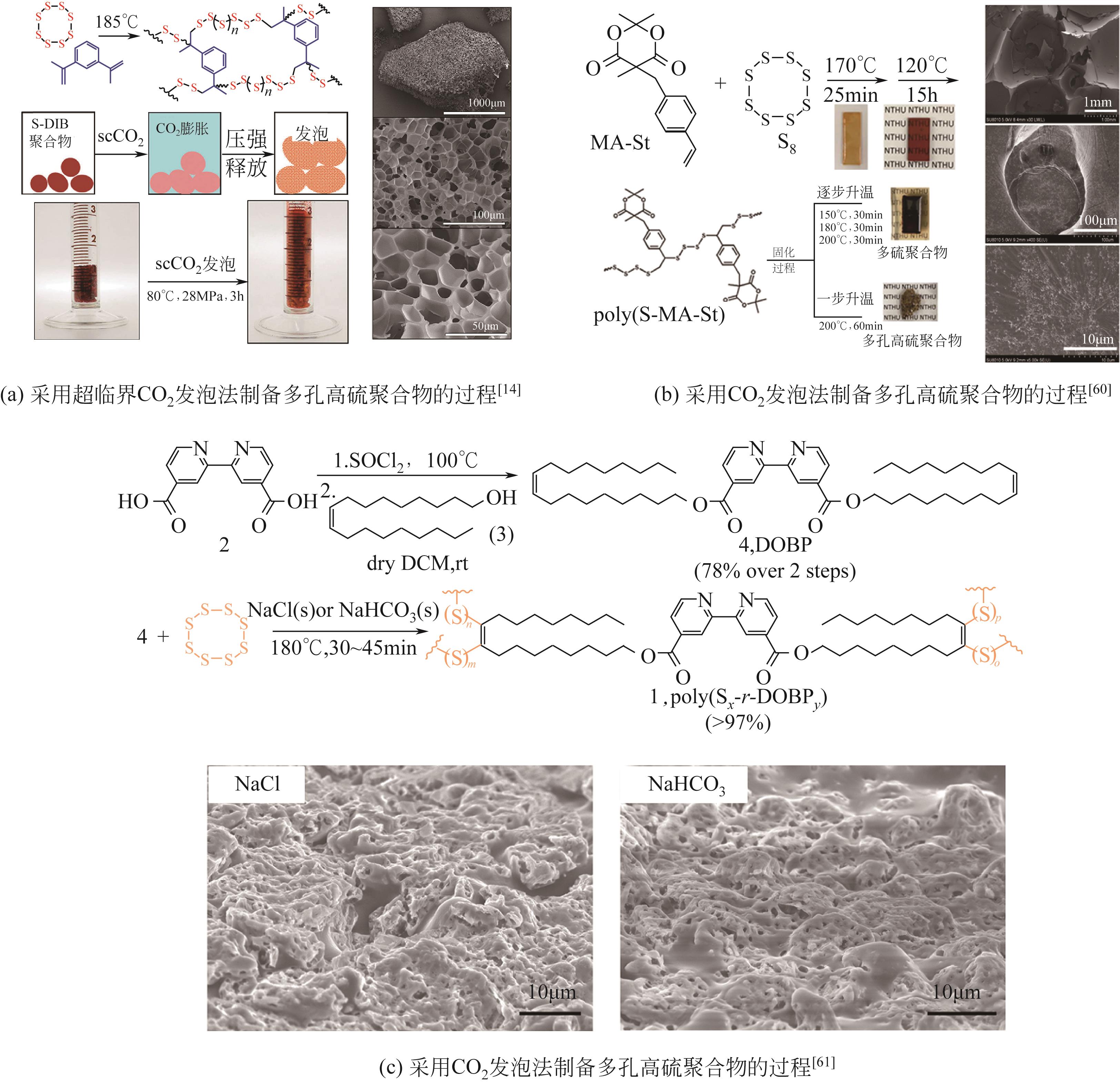
| 60 | LIN H K, LAI Y S, LIU Y L. Cross-linkable and self-foaming polysulfide materials for repairable and mercury capture applications[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(4): 4515-4522. |
| 61 | KO L A, HUANG Y S, LIN Y A. Bipyridine-containing polysulfide materials for broad-spectrum removal of heavy metals from water[J]. ACS Applied Polymer Materials, 2021, 3(7): 3363-3372. |
| 62 | XUE Jiajia, WU Tong, DAI Yunqian, et al. Electrospinning and electrospun nanofibers: Methods, materials, and applications[J]. Chemical Reviews, 2019, 119(8): 5298-5415. |
| 63 | HUANG Yunpeng, MIAO Yue'e, LIU Tianxi. Electrospun fibrous membranes for efficient heavy metal removal[J]. Journal of Applied Polymer Science, 2014, 131(19): 40864. |
| 64 | WANJALE Santosh, BIRAJDAR Mallinath, Jyoti JOG, et al. Surface tailored PS/TiO2 composite nanofiber membrane for copper removal from water[J]. Journal of Colloid and Interface Science, 2016, 469: 31-37. |
| 65 | SANG Yimin, LI Fasheng, GU Qingbao, et al. Heavy metal-contaminated groundwater treatment by a novel nanofiber membrane[J]. Desalination, 2008, 223(1/2/3): 349-360. |
| 66 | THIELKE M W, BULTEMA L A, BRAUER D D, et al. Rapid mercury(Ⅱ) removal by electrospun sulfur copolymers[J]. Polymers, 2016, 8(7): 266. |
| 67 | LIMJUCO L A, NISOLA G M, PAROHINOG K J, et al. Water-insoluble hydrophilic polysulfides as microfibrous composites towards highly effective and practical Hg2+ capture[J]. Chemical Engineering Journal, 2019, 378: 122216. |
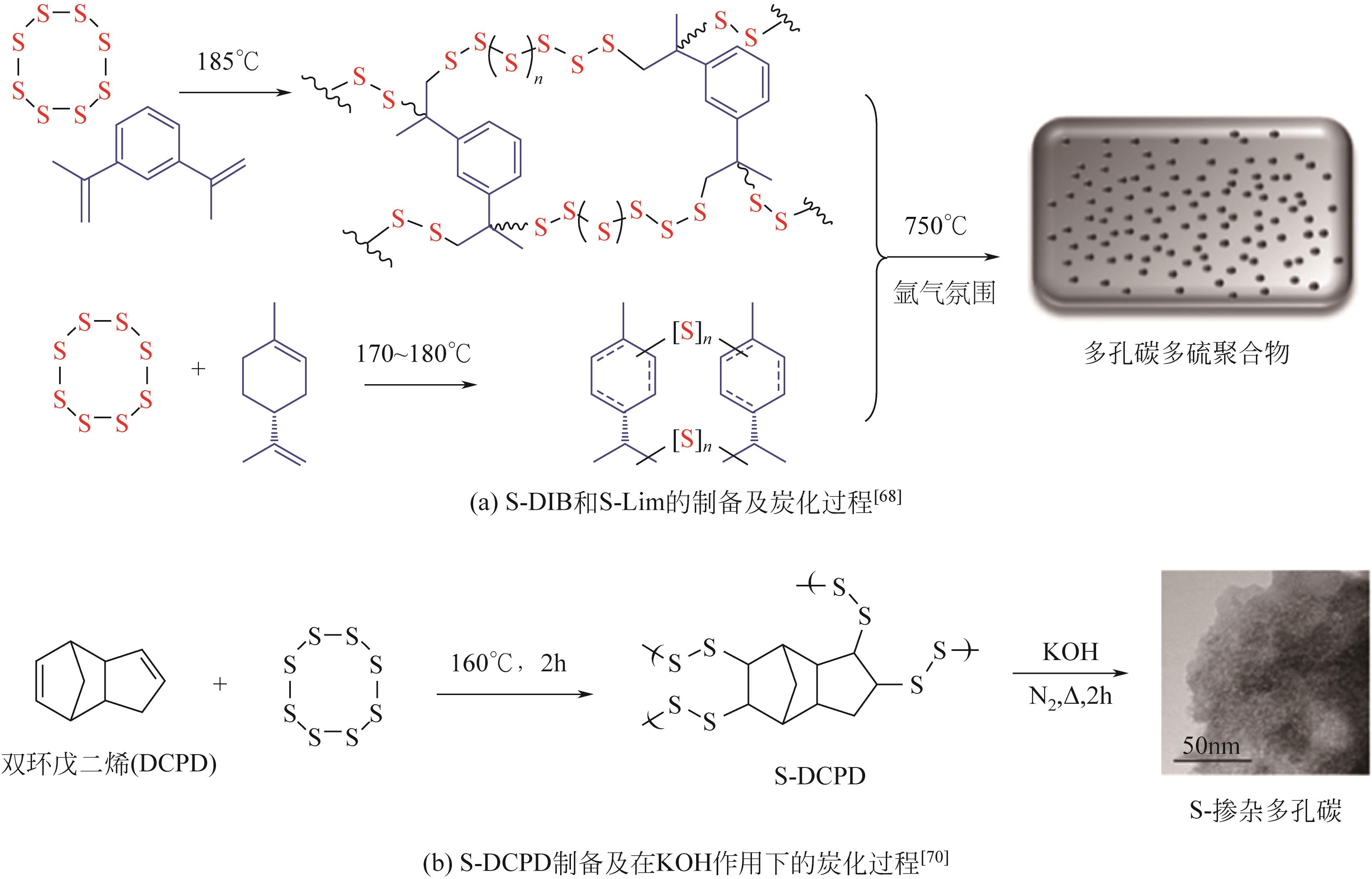
| 68 | BEAR J C, MCGETTRICK J D, PARKIN I P, et al. Porous carbons from inverse vulcanised polymers[J]. Microporous and Mesoporous Materials, 2016, 232: 189-195. |
| 69 | MANN M, LUO X, TIKOALU A D, et al. Carbonisation of a polymer made from sulfur and canola oil[J]. Chemical Communications, 2021, 57(51): 6296-6299. |
| 70 | LEE J M, PARKER D J, COOPER A I, et al. High surface area sulfur-doped microporous carbons from inverse vulcanised polymers[J]. Journal of Materials Chemistry A, 2017, 5(35): 18603-18609. |
| 71 | ZHANG Bowen, PETCHER Samuel, GAO Hui, et al. Magnetic sulfur-doped carbons for mercury adsorption[J]. Journal of Colloid and Interface Science, 2021, 603: 728-737. |
| 72 | 冯新斌, 陈玖斌, 付学吾, 等. 汞的环境地球化学研究进展[J]. 矿物岩石地球化学通报, 2013, 32(5): 503-530. |
| FENG Xinbin, CHEN Jiubin, FU Xuewu, et al. Progresses on environmental geochemistry of mercury[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2013, 32(5): 503-530. | |
| 73 | 孙阳昭, 陈扬, 蓝虹, 等. 中国汞污染的来源、成因及控制技术路径分析[J]. 环境化学, 2013, 32(6): 937-942. |
| SUN Yangzhao, CHEN Yang, LAN Hong, et al. Study on pollution sources, cause of mercury pollution and its control technical roadmap in China[J]. Environmental Chemistry, 2013, 32(6): 937-942. | |
| 74 | 张霞忠, 袁烈梅. 汞吸附研究进展[J]. 环境污染与防治, 2017, 39(5): 563-568. |
| ZHANG Xiazhong, YUAN Liemei. Research progress of mercury adsorption[J]. Environmental Pollution & Control, 2017, 39(5): 563-568. | |
| 75 | AKAY Sema, KAYAN Berkant, KALDERIS Dimitrios, et al. Poly(benzoxazine-co-sulfur): An efficient sorbent for mercury removal from aqueous solution[J]. Journal of Applied Polymer Science, 2017, 134(38): 45306. |
| 76 | RICE K M, WALKER E M, WU M Z, et al. Environmental mercury and its toxic effects[J]. Journal of Preventive Medicine and Public Health, 2014, 47(2): 74-83. |
| 77 | WADI V S, MITTAL H, FOSSO-KANKEU E, et al. Mercury removal by porous sulfur copolymers: Adsorption isotherm and kinetics studies[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 606: 125333. |
| 78 | WADI V K, JENA K K, KHAWAJA S Z, et al. NMR and EPR structural analysis and stability study of inverse vulcanized sulfur copolymers[J]. ACS Omega, 2018, 3(3): 3330-3339. |
| 79 | XU Yang, WANG Tianqi, HE Zidong, et al. A polymerization-cutting strategy: Self-protection synthesis of thiol-based nanoporous adsorbents for efficient mercury removal[J]. Chemistry: A European Journal, 2018, 24(54): 14436-14441. |
| 80 | WANG Qingfeng, LIU Yue, YANG Zhenmei, et al. Study of mercury re-emission in a simulated WFGD solution containing thiocyanate and sulfide ions[J]. Fuel, 2014, 134: 588-594. |
| 81 | DIRINGER S E, FEINGOLD B J, ORTIZ E J, et al. River transport of mercury from artisanal and small-scale gold mining and risks for dietary mercury exposure in Madre de Dios, Peru[J]. Environmental Science Processes & Impacts, 2015, 17(2): 478-487. |
| 82 | TCHOUNWOU P B, AYENSU W K, NINASHVILI N, et al. Environmental exposure to mercury and its toxicopathologic implications for public health[J]. Environmental Toxicology, 2003, 18(3): 149-175. |
| 83 | MASON R P, ROLFHUS K R, FITZGERALD W F. Methylated and elemental mercury cycling in surface and deep ocean waters of the North Atlantic[J]. Water, Air, and Soil Pollution, 1995, 80(1): 665-677. |
| 84 | CROCKETT M P, EVANS A M, WORTHINGTON M J, et al. Sulfur-limonene polysulfide: A material synthesized entirely from industrial by-products and its use in removing toxic metals from water and soil[J]. Angewandte Chemie-International Edition, 2016, 55(5): 1714-1718. |
| [1] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [2] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [3] | ZHANG Tingting, PAN Dawei, JU Xiaojie, LIU Zhuang, XIE Rui, WANG Wei, CHU Liangyin. Fabrication and performance of Hg2+-responsive smart hydrogel grating detector [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4143-4152. |
| [4] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [5] | CHEN Sen, YIN Pengyuan, YANG Zhenglu, MO Yiming, CUI Xili, SUO Xian, XING Huabin. Advances in the intelligent synthesis of functional solid materials [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3340-3348. |
| [6] | MAO Menglei, MENG Lingding, GAO Rui, MENG Zihui, LIU Wenfang. Research progress on enzyme immobilization on porous framework materials [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2516-2535. |
| [7] | KONG Xiangru, ZHANG Xiaoyang, SUN Pengxiang, CUI Lin, DONG Yong. Research progress of solid porous materials for direct CO2 capture from air [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1471-1483. |
| [8] | FAN Yunpei, JIN Jing, LIU Dunyu, WANG Jingjie, LIU Qiuqi, XU Kailong. Mercury removal by CaSO4 oxygen carrier during in-situ gasification and chemical-looping combustion of coal [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1638-1648. |
| [9] | JIN Xin, LI Yushan, XIE Qingqing, WANG Mengyu, XIA Xingfan, YANG Chaohe. Progress on solketal synthesis catalyzed by porous materials [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 731-743. |
| [10] | ZENG Junjian, ZHAO Jigang. Research progress of gold based mercury-free catalysts for acetylene hydrochlorination [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3589-3596. |
| [11] | DI Guancheng, ZHOU Qiang, TAO Xin, SHANG Yu, SONG Tao, LU Ping, XU Guiling. Preparation of sulfur-doped mesoporous carbon and its mercury removal [J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2761-2769. |
| [12] | GAO Tian, ZHANG Yili, XIONG Zhuo, ZHAO Yongchun, ZHANG Junying. Research progress of modified titanium oxide photocatalytic oxidation of elemental mercury and its influencing factors [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 690-700. |
| [13] | WANG Wenxia, LIU Xiaofeng, CHEN Xi, XU Yanhong, MENG Zhenbang, ZHENG Junxia, AN Taicheng. Research advances of synthesis and applications of porous g-C3N4-based photocatalyst [J]. Chemical Industry and Engineering Progress, 2022, 41(1): 300-309. |
| [14] | GUO Jinrong, CHENG Peng, JIA Li, GUO Jingnan, WANG Yanlin, ZHANG Yongqiang, QIAO Xiaolei, FAN Baoguo. Combustion characteristics and mercury emission characteristics of high sulfur coal slime [J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 411-420. |
| [15] | Lu DONG, Yaji HUANG, Shouyi DING, Haoqiang CHENG, Sheng WANG, Yufeng DUAN. Experimental on simultaneous NO and mercury removal over manganese modified Ti-Al composite catalyst [J]. Chemical Industry and Engineering Progress, 2021, 40(1): 234-241. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||