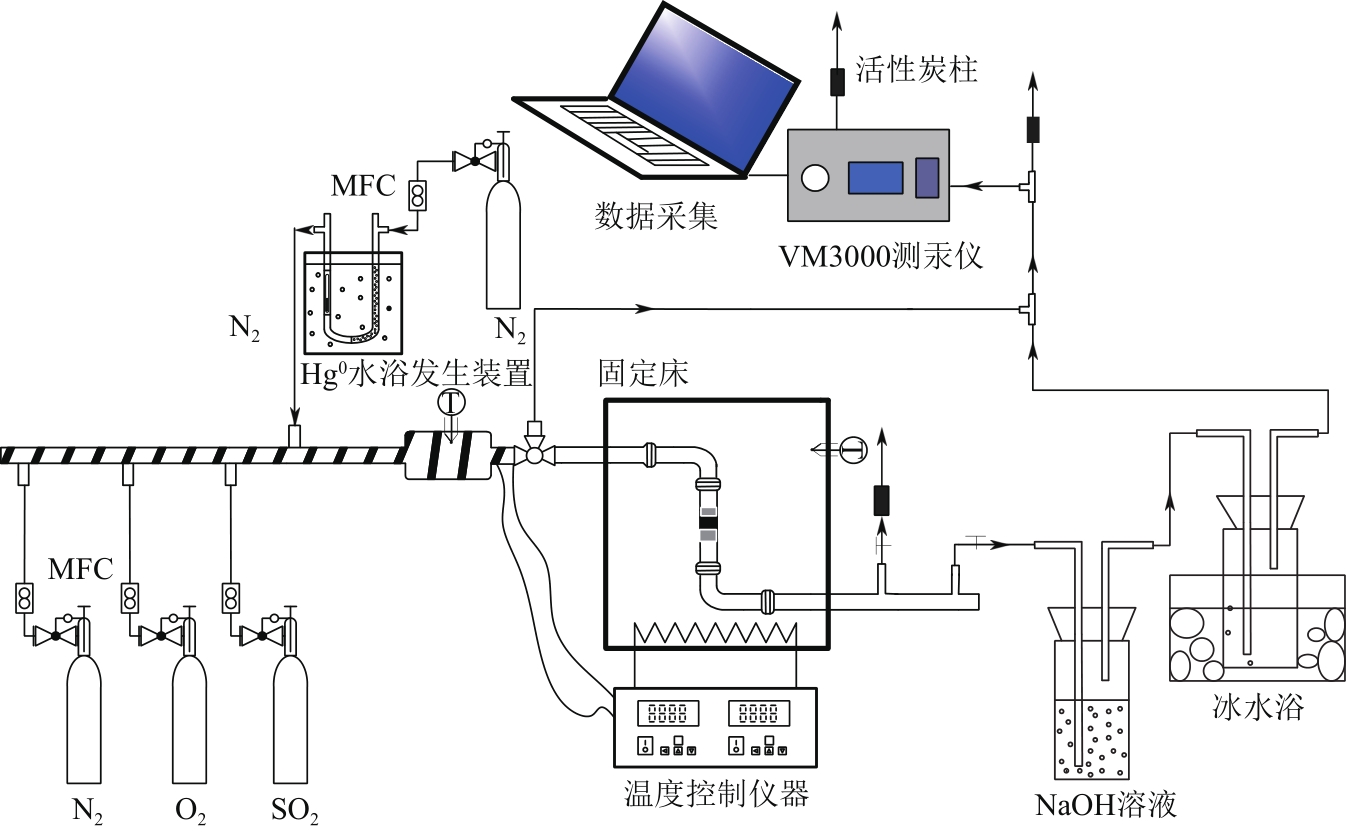
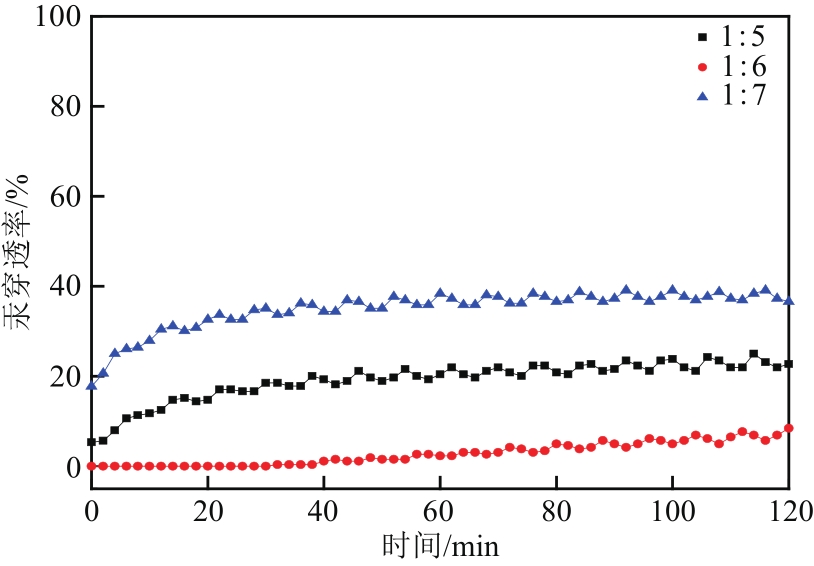
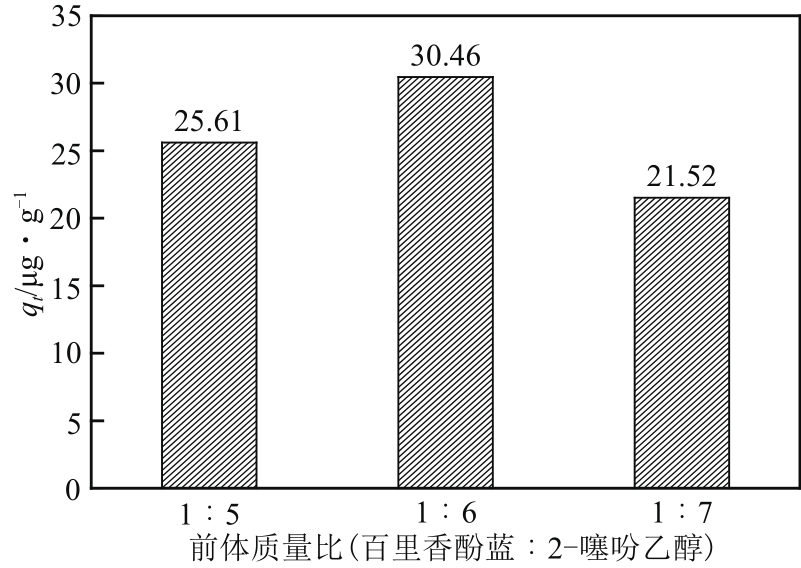
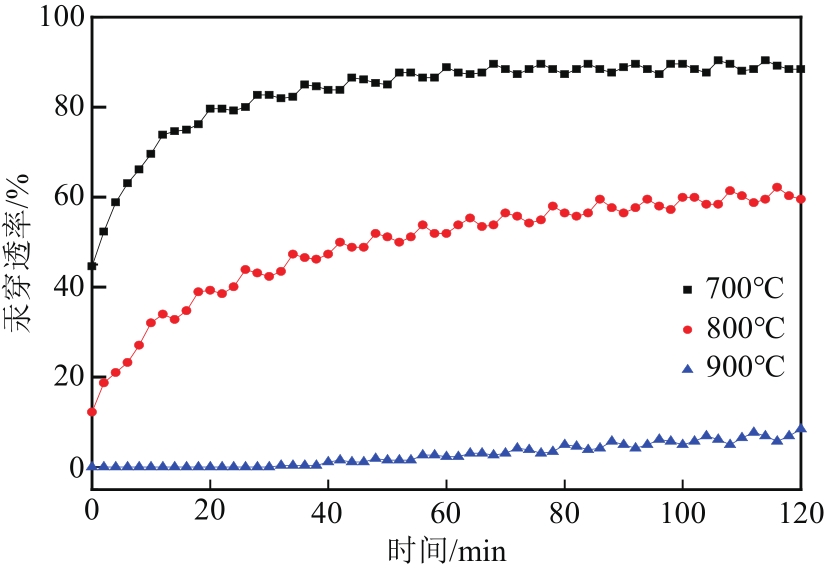
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (5): 2761-2769.DOI: 10.16085/j.issn.1000-6613.2021-1086
• Resources and environmental engineering • Previous Articles Next Articles
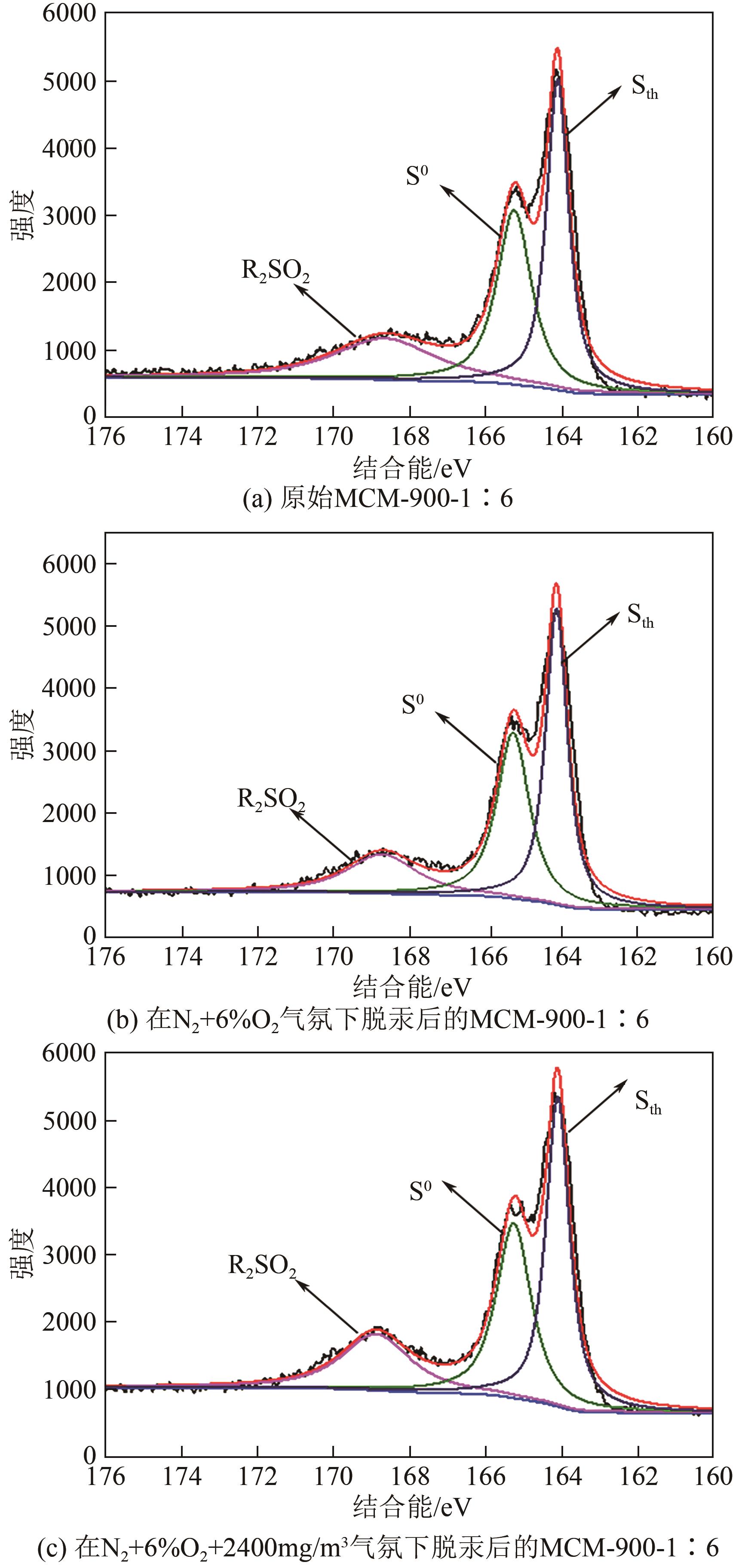
Preparation of sulfur-doped mesoporous carbon and its mercury removal
DI Guancheng( ), ZHOU Qiang(
), ZHOU Qiang( ), TAO Xin, SHANG Yu, SONG Tao, LU Ping, XU Guiling
), TAO Xin, SHANG Yu, SONG Tao, LU Ping, XU Guiling
- Engineering Laboratory for Energy System Process Conversion & Emission Control Technology of Jiangsu Province, School of Energy and Mechanical Engineering, Nanjing Normal University, Nanjing 210023, Jiangsu, China
-
Received:2021-05-21Revised:2021-07-19Online:2022-05-24Published:2022-05-05 -
Contact:ZHOU Qiang
掺硫介孔炭的制备及其汞脱除特性
狄冠丞( ), 周强(
), 周强( ), 陶信, 尚瑜, 宋涛, 卢平, 徐贵玲
), 陶信, 尚瑜, 宋涛, 卢平, 徐贵玲
- 南京师范大学能源与机械工程学院,江苏省能源系统过程转化与减排技术工程实验室,江苏 南京 210023
-
通讯作者:周强 -
作者简介:狄冠丞(1998—),男,硕士研究生,研究方向为燃煤汞污染物排放控制。E-mail:2455231773@qq.com 。 -
基金资助:国家自然科学基金(51906115);江苏省自然科学基金(BK20190709);中国博士后科学基金(2017M621780)
CLC Number:
Cite this article
DI Guancheng, ZHOU Qiang, TAO Xin, SHANG Yu, SONG Tao, LU Ping, XU Guiling. Preparation of sulfur-doped mesoporous carbon and its mercury removal[J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2761-2769.
狄冠丞, 周强, 陶信, 尚瑜, 宋涛, 卢平, 徐贵玲. 掺硫介孔炭的制备及其汞脱除特性[J]. 化工进展, 2022, 41(5): 2761-2769.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-1086
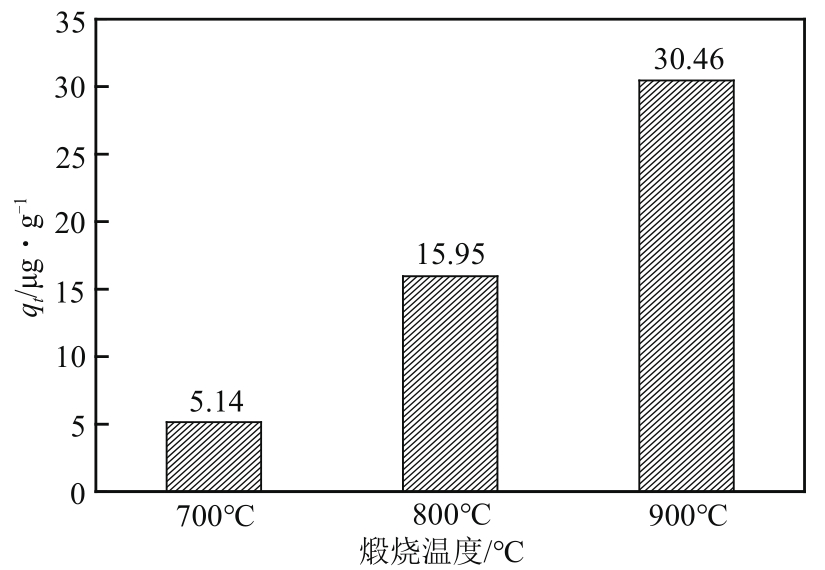
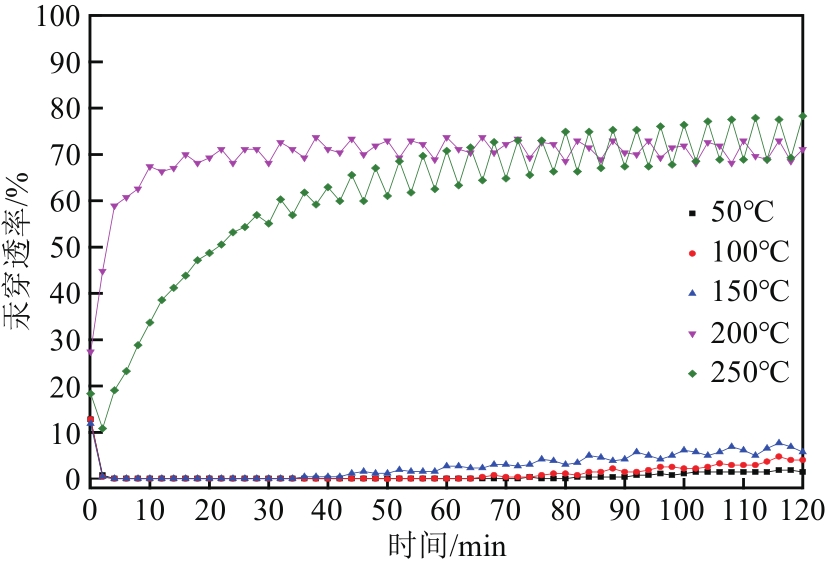
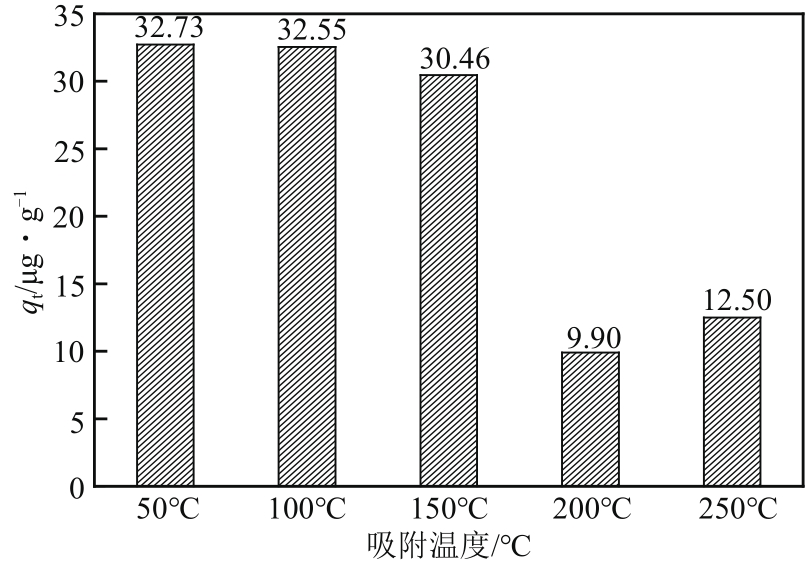
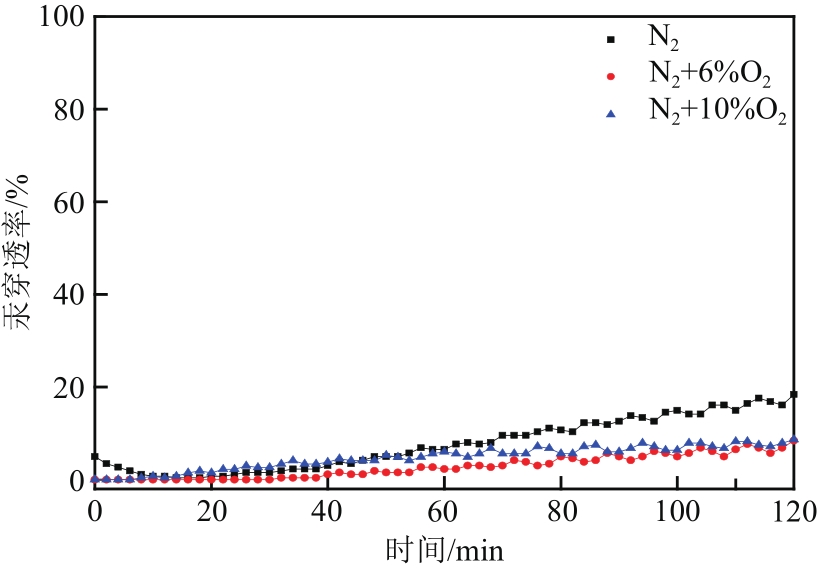
| 样品 | 比表面积/m2·g-1 | 平均孔径/nm | 总孔容积/cm3·g-1 | C质量分数/% | S质量分数/% |
|---|---|---|---|---|---|
| MCM-700-1∶6 | 934.351 | 3.517 | 0.821 | 77.94 | 12.24 |
| MCM-800-1∶6 | 944.888 | 3.161 | 0.747 | 80.50 | 11.23 |
| MCM-900-1∶6 | 932.998 | 3.013 | 0.703 | 81.12 | 10.24 |
| MCM-900-1∶5 | 1066.315 | 3.458 | 0.922 | 70.68 | 8.51 |
| MCM-900-1∶7 | 1009.406 | 3.441 | 0.868 | 67.69 | 8.49 |
| 样品 | 比表面积/m2·g-1 | 平均孔径/nm | 总孔容积/cm3·g-1 | C质量分数/% | S质量分数/% |
|---|---|---|---|---|---|
| MCM-700-1∶6 | 934.351 | 3.517 | 0.821 | 77.94 | 12.24 |
| MCM-800-1∶6 | 944.888 | 3.161 | 0.747 | 80.50 | 11.23 |
| MCM-900-1∶6 | 932.998 | 3.013 | 0.703 | 81.12 | 10.24 |
| MCM-900-1∶5 | 1066.315 | 3.458 | 0.922 | 70.68 | 8.51 |
| MCM-900-1∶7 | 1009.406 | 3.441 | 0.868 | 67.69 | 8.49 |
| 1 | NIKSA S, FUJIWARA N. Estimating Hg emissions from coal-fired power stations in China[J]. Fuel, 2009, 88(1): 214-217. |
| 2 | GUFFEY F D, BLAND A E. Thermal pretreatment of low-ranked coal for control of mercury emissions[J]. Fuel Processing Technology, 2004, 85(6/7): 521-531. |
| 3 | WANG H, SHEN C, DUAN Y F, et al. Synergistic effect between H2O and SO2 on mercury removal by activated carbon in O2/CO2 conditions[J]. Journal of Chemical Technology and Biotechnology, 2019, 94(4): 1195-1201. |
| 4 | TANG H J, YOU W Q, WANG Z W, et al. Detrimental effects of SO2 on gaseous mercury(Ⅱ) adsorption and retention by CaO-based sorbent traps: competition and heterogeneous reduction[J]. Journal of Hazardous Materials, 2020, 387: 121679. |
| 5 | ZHOU Q, DUAN Y F, CHEN M M, et al. Effect of flue gas component and ash composition on elemental mercury oxidation/adsorption by NH4Br modified fly ash[J]. Chemical Engineering Journal, 2018, 345: 578-585. |
| 6 | PEARSON R G. Hard and soft acids and bases[J]. Journal of the American Chemical Society, 1973, 85(22): 3533-3539. |
| 7 | PEARSON R G. Acids and bases[J]. Science, 1966, 151(3707): 172-177. |
| 8 | LIU W, VIDIC R D, BROWN T D. Optimization of high temperature sulfur impregnation on activated carbon for permanent sequestration of elemental mercury vapors[J]. Environmental Science & Technology, 2000, 34(3): 483-488. |
| 9 | 沈畅, 王卉, 沈昊天, 等. 烟气抗硫脱汞材料的研究进展[J]. 中南大学学报(自然科学版), 2021, 52(1): 133-143. |
| SHEN Chang, WANG Hui, SHEN Haotian, et al. Research progress on flue gas mercury removal materials with SO2 resistance[J]. Journal of Central South University (Science and Technology), 2021, 52(1): 133-143. | |
| 10 | HUO Q H, WANG Y H, CHEN H J, et al. ZnS/AC sorbent derived from the high sulfur petroleum coke for mercury removal[J]. Fuel Processing Technology, 2019, 191: 36-43. |
| 11 | YAO Y X, VELPARI V, ECONOMY J. Design of sulfur treated activated carbon fibers for gas phase elemental mercury removal[J]. Fuel, 2014, 116: 560-565. |
| 12 | RUMAYOR M, DIAZ-SOMOANO M, LOPEZ-ANTON M A, et al. Mercury compounds characterization by thermal desorption[J]. Talanta, 2013, 114(3): 318-322. |
| 13 | JIA C Q. Production of sulphur and activated carbon: US 6932956[P]. 2005. |
| 14 | LI Chaomin, HOU Jian, YU Yong, et al. Ozone decomposition under the irradiation of 253.7nm in the presence of methyl bromide[J]. Journal of Environmental Sciences, 2000, 12(3): 266-269. |
| 15 | ZHUANG X, WAN Y, FENG C M, et al. Highly efficient adsorption of bulky dye molecules in wastewater on ordered mesoporous carbons[J]. Chemistry of Materials, 2009, 21(4): 706-716. |
| 16 | WANG H, LAM F L Y, HU X J, et al. Ordered mesoporous carbon as an efficient and reversible adsorbent for the adsorption of fullerenes[J]. Langmuir, 2006, 22(10): 4583-4588. |
| 17 | HARTMANN M, VINU A, CHANDRASEKAR G. Adsorption of vitamin E on mesoporous carbon molecular sieves[J]. Chemistry of Materials, 2005, 17(4): 829-833. |
| 18 | VINU A, STREB C, MURUGESAN V, et al. Adsorption of cytochrome C on new mesoporous carbon molecular sieves[J]. The Journal of Physical Chemistry B, 2003, 107(33): 8297-8299. |
| 19 | 祝建中, 杨嘉, DENG Baolin. 有序介孔炭合成、改性及其对汞离子的吸附性能[J]. 新型炭材料, 2008, 23(3): 221-227. |
| ZHU Jianzhong, YANG Jia, DENG Baolin. Synthesis, modification, and characterization of ordered mesoporous carbons for aqueous mercury ion removal[J]. New Carbon Materials, 2008, 23(3): 221-227. | |
| 20 | YE J Q, ZHAO H Q, SONG W, et al. Enhanced electronic conductivity and sodium-ion adsorption in N/S co-doped ordered mesoporous carbon for high-performance sodium-ion battery anode[J]. Journal of Power Sources, 2019, 412: 606-614. |
| 21 | GU Z M, DENG B L, YANG J. Synthesis and evaluation of iron-containing ordered mesoporous carbon (FeOMC) for arsenic adsorption[J]. Microporous and Mesoporous Materials, 2007, 102(1/2/3): 265-273. |
| 22 | WU R P, YE Q, WU K, et al. Adsorption performance of CMK-3 and C-FDU-15 in NO removal at low temperature[J]. Journal of Environmental Sciences, 2020, 87(1): 289-298. |
| 23 | SHIN Y, FRYXELL G, UM W, et al. Sulfur-functionalized mesoporous carbon[J]. Advanced Functional Materials, 2007, 17(15): 2897-2901. |
| 24 | PENG C Y, HE M, CHEN B B, et al. Magnetic sulfur-doped porous carbon for preconcentration of trace mercury in environmental water prior to ICP-MS detection[J]. Analyst, 2017, 142(23): 4570-4579. |
| 25 | 周强.改性吸附剂喷射脱汞的实验及机理研究[D]. 南京: 东南大学,2016. |
| ZHOU Qiang. Experimental and mechanism researches of in-duct mercury removal by modified sorbent injection[D]. Nanjing: Southeast University, 2016. | |
| 26 | 王晨平. SO2活化改性高硫石油焦吸附剂的脱汞特性研究[D]. 南京: 东南大学, 2017. |
| WANG Chenping. Study on the mercury removal characteristics of SO2 activated modified high-sulfur petroleum coke adsorbent[D]. Nanjing: Southeast University, 2017. | |
| 27 | STAVROPOULOS G G, SAMARAS P, SAKELLAROPOULOS G P. Effect of activated carbons modification on porosity, surface structure and phenol adsorption[J]. Journal of Hazardous Materials, 2008, 151(2/3): 414-421. |
| 28 | WEI Y Y, YU D Q, TONG S T, et al. Effects of H2SO4 and O2 on Hg0 uptake capacity and reversibility of sulfur-impregnated activated carbon under dynamic conditions[J]. Environmental Science and Technology, 2015, 49(3): 1706-1712. |
| 29 | 李娜. 载硫活性炭的汞吸附与再生特性研究[D]. 南京: 东南大学, 2019. |
| LI Na. Study on the mercury adsorption and regeneration characteristics of sulfur-loaded activated carbon[D]. Nanjing: Southeast University, 2019. | |
| 30 | SUN P, ZHANG B, ZENG X B, et al. Deep study on effects of activated carbon’s oxygen functional groups for elemental mercury adsorption using temperature programmed desorption method[J]. Fuel, 2017, 200: 100-106. |
| 31 | SHAO H Z, LIU X W, ZHOU Z J, et al. Elemental mercury removal using a novel KI modified bentonite supported by starch sorbent[J]. Chemical Engineering Journal, 2016, 291: 306-316. |
| 32 | XIA Y D, ZHU Y Q, TANG Y. Preparation of sulfur-doped microporous carbons for the storage of hydrogen and carbon dioxide[J]. Carbon, 2012, 50(15): 5543-5553. |
| 33 | 吕维阳, 刘盛余, 能子礼超, 等. 载硫活性炭脱除天然气中单质汞的研究[J]. 中国环境科学, 2016, 36(2): 382-389. |
| Weiyang LYU, LIU Shengyu, NENGZI Lichao, et al. Remove elemental mercury by sulfur-impregnated activated carbon in natural gas[J]. China Environmental Science, 2016, 36(2): 382-389. | |
| 34 | 洪亚光, 段钰锋, 朱纯, 等. 硫改性椰壳活性炭管道喷射脱汞实验研究[J]. 东南大学学报(自然科学版), 2015, 45(3): 521-525. |
| HONG Yaguang, DUAN Yufeng, ZHU Chun, et al. Experimental study on mercury adsorption of S-impregnated coconut shell activated carbon by duct injection[J]. Journal of Southeast University (Natural Science Edition), 2015, 45(3): 521-525. |
| [1] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [2] | WANG Yungang, JIAO Jian, DENG Shifeng, ZHAO Qinxin, SHAO Huaishuang. Experimental analysis of condensation heat transfer and synergistic desulfurization [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4230-4237. |
| [3] | XIU Haoran, WANG Yungang, BAI Yanyuan, ZOU Li, LIU Yang. Combustion characteristics and ash melting behavior of Zhundong coal/municipal sludge blended combustion [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3242-3252. |
| [4] | WANG Baowen, LIU Tongqing, ZHANG Gang, LI Weiguang, LIN Deshun, WANG Mengjia, MA Jingjing. Reaction characteristics of CuFe2O4 modified desulfurization slag oxygen carrier with lignite [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2884-2894. |
| [5] | HE Chuan, WU Guoxun, LI Ang, ZHANG Fajie, BIAN Zijun, LU Chengzheng, WANG Lipeng, ZHAO Min. Characteristics of calcium and magnesium deactivation and regeneration of waste incineration SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2413-2420. |
| [6] | ZHANG Chenguang, FENG Shuo, XING Yuye, SHEN Boxiong, SU Lichao. Research progress of isolated Cu2+ in copper based zeolite NH3-SCR catalyst for diesel vehicles [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1321-1331. |
| [7] | FAN Yunpei, JIN Jing, LIU Dunyu, WANG Jingjie, LIU Qiuqi, XU Kailong. Mercury removal by CaSO4 oxygen carrier during in-situ gasification and chemical-looping combustion of coal [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1638-1648. |
| [8] | ZHANG Qunli, HUANG Haotian, ZHANG Lin, ZHAO Wenqiang, ZHANG Qiuyue. Analysis of condensation waste heat recovery system of spray flue gas source heat pump [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 650-657. |
| [9] | CHEN Shuhui, WU Yue, ZHANG Wenxiang, WANG Shanshan, MA Heping. Preparation of ionic organic porous polymer and its coupled desulfurization and decarbonization properties in flue gas [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1028-1038. |
| [10] | LI Xing, HUANG Hongyu, OSAKA Yugo, HUHE Taoli, XIAO Linfa, LI Jun. Study on the influencing factors of the adsorption performance of carbon materials for the sulfur dioxide removal [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4963-4972. |
| [11] | LU Shijian, LIU Ling, LIU Ziwu, GUO Bowen, YU Xulin, LIANG Yan, ZHAO Dongya, ZHU Quanmin. Study of CO2 absorption stability of AEP-DPA-CuO phase change nanofluids [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4555-4561. |
| [12] | LONG Hongming, DING Long, QIAN Lixin, CHUN Tiejun, ZHANG Hongliang, YU Zhengwei. Current situation and development trend of NO x and dioxins emission reduction in sintering flue gas [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3865-3876. |
| [13] | WU Chuanpeng, LI Chuankun, YANG Zhe, GOU Chengdong, GAO Xinjiang. Research progress of SO2 removal by solid adsorbents [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3840-3854. |
| [14] | MO Qianci, YE Haibo, LIN Xingsu, LI Guohua, CHEN Weichong, LU Wei. Energy analysis and energy efficiency improvement strategies of bagasse boiler based on test data [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3350-3359. |
| [15] | KONG Xiangyu, XIE Liang, WANG Yanmin, ZHAI Shangpeng, WANG Jianguo. CO2 capture and resource utilization [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1187-1198. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||