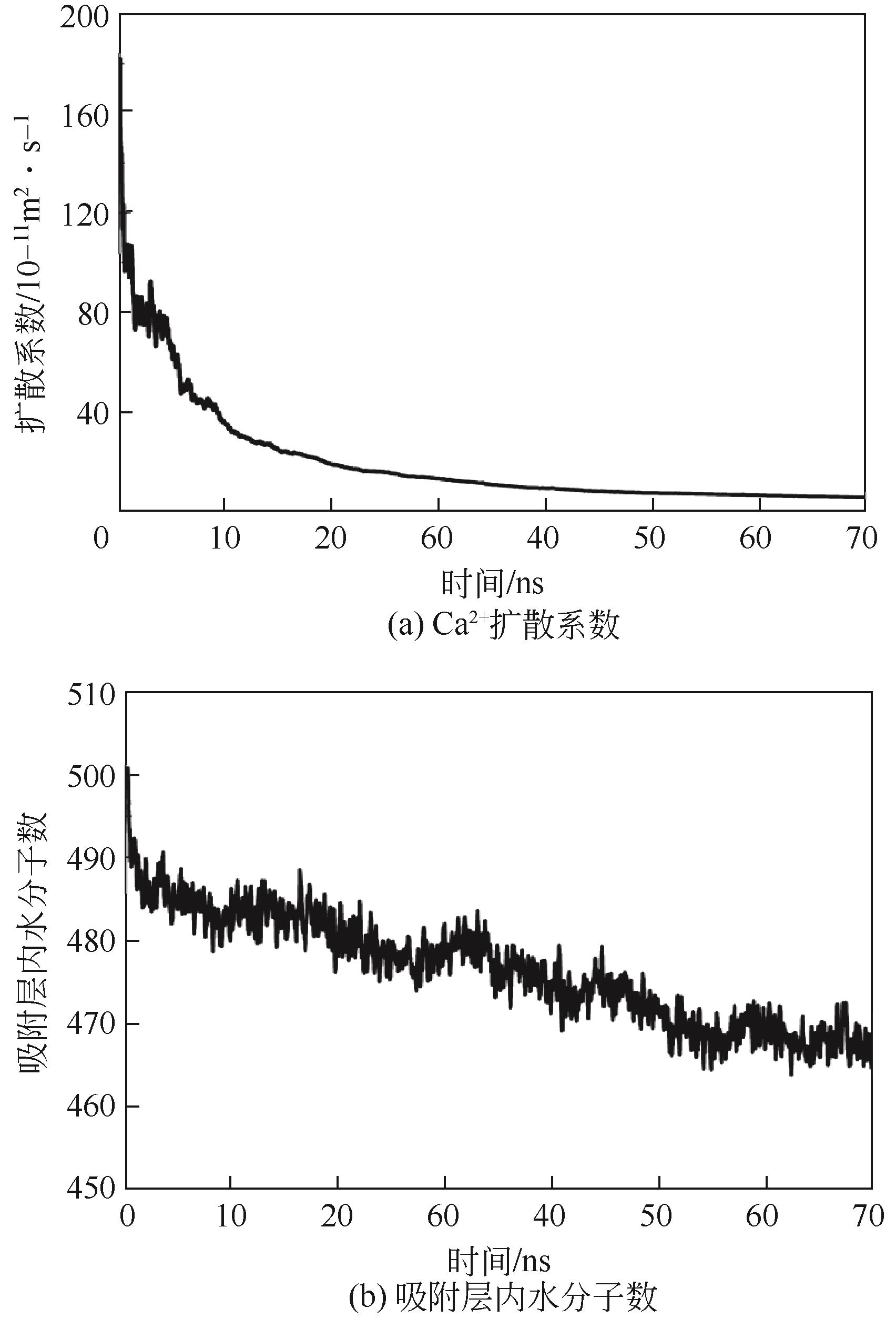
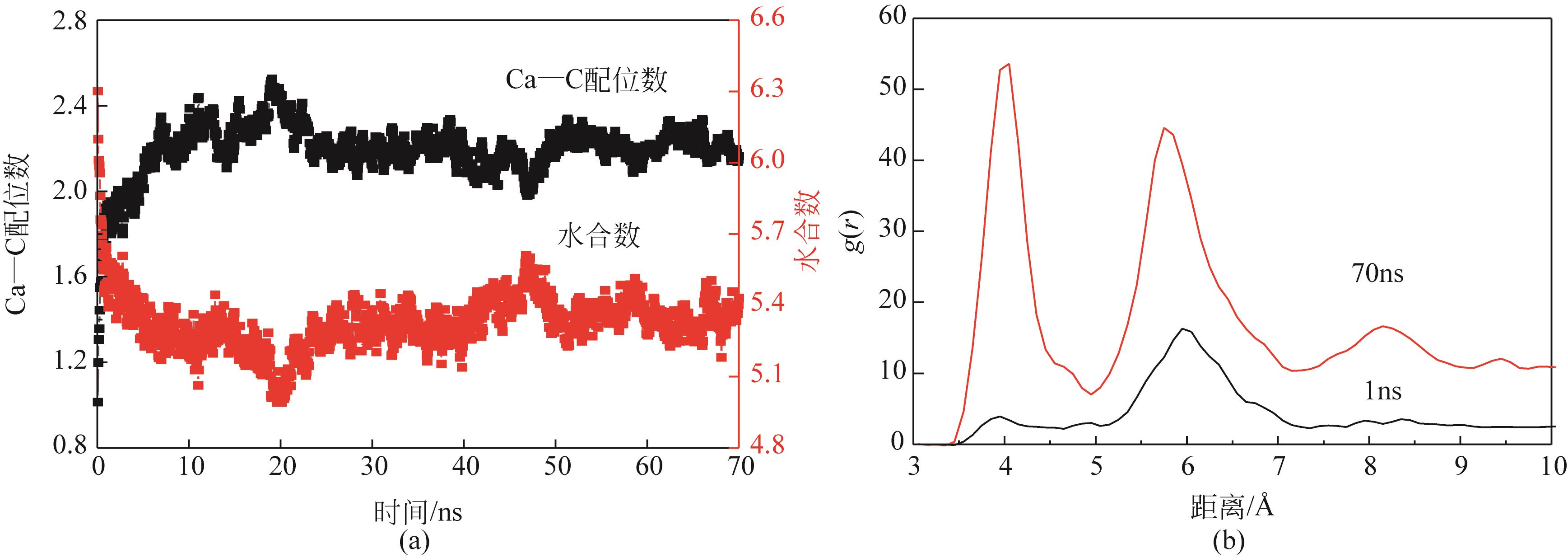
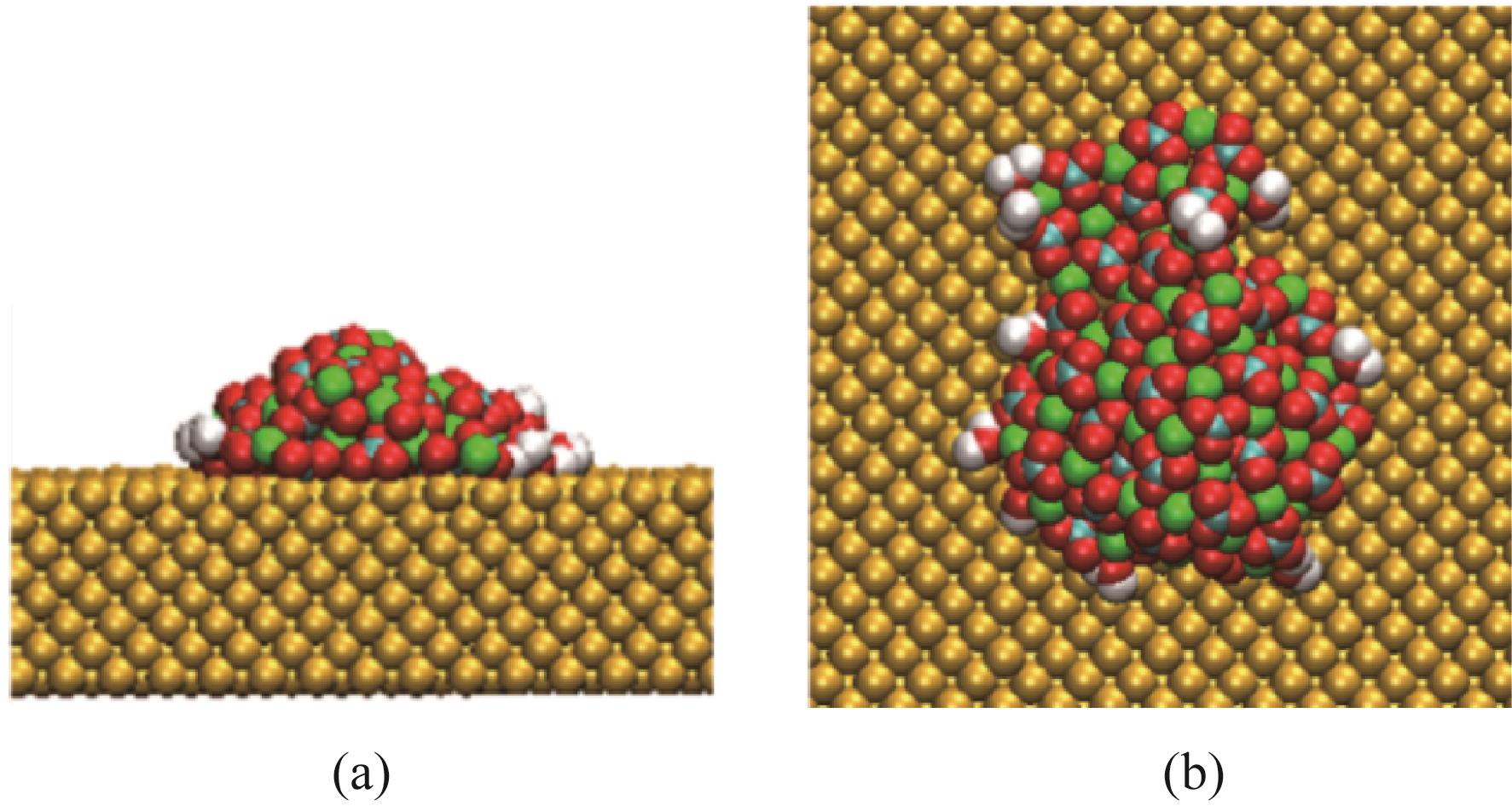
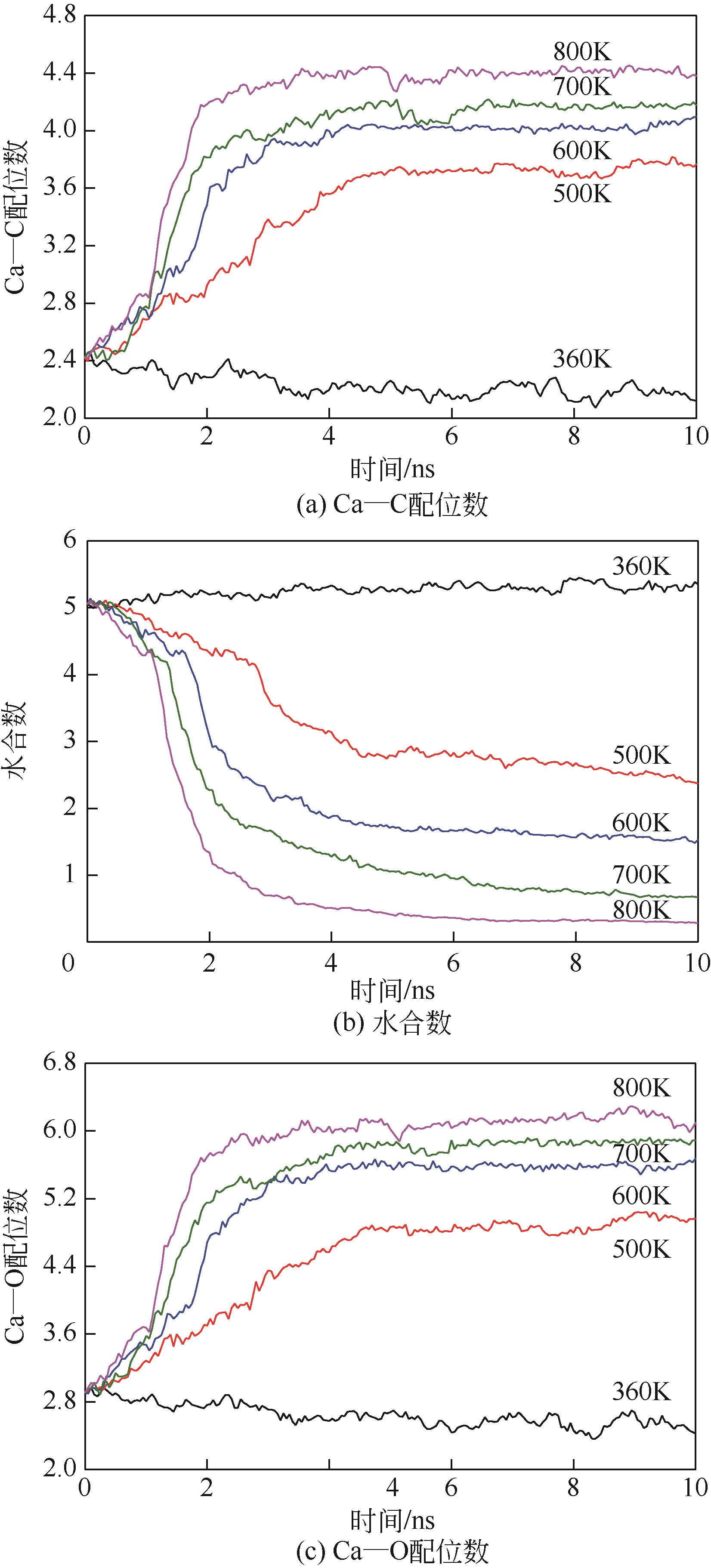
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (8): 4077-4085.DOI: 10.16085/j.issn.1000-6613.2021-1943
• Chemical processes and equipment • Previous Articles Next Articles
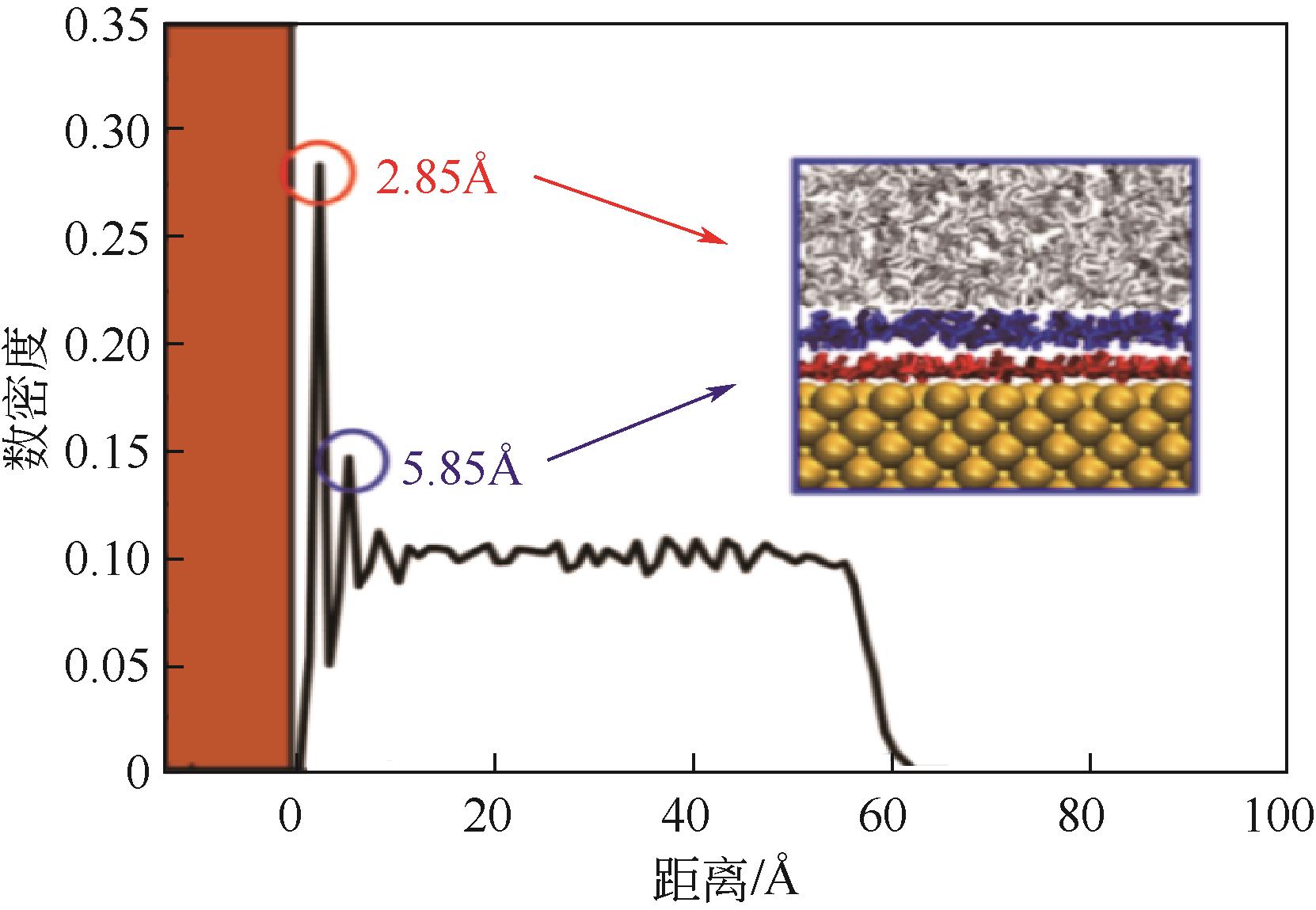
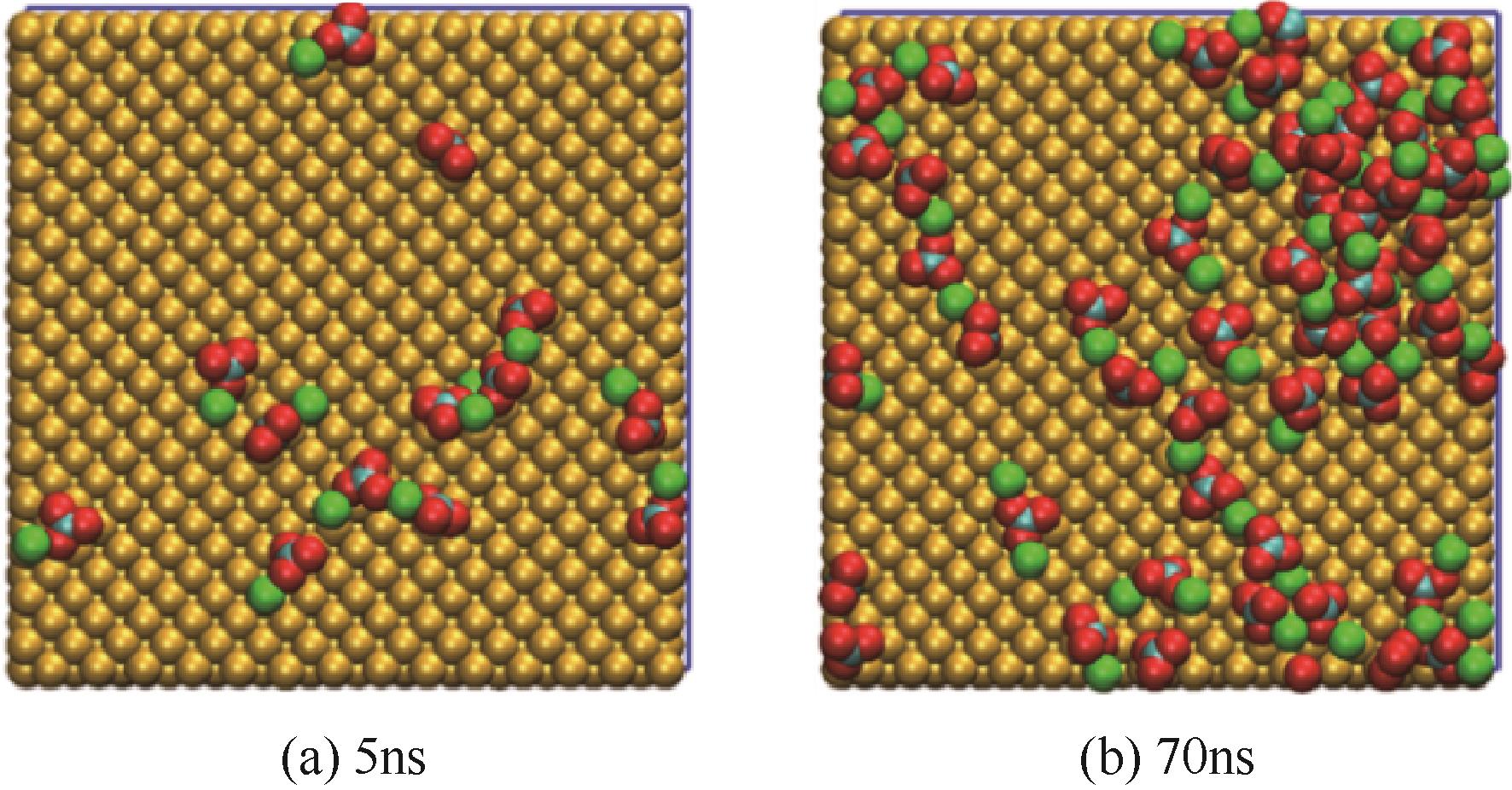
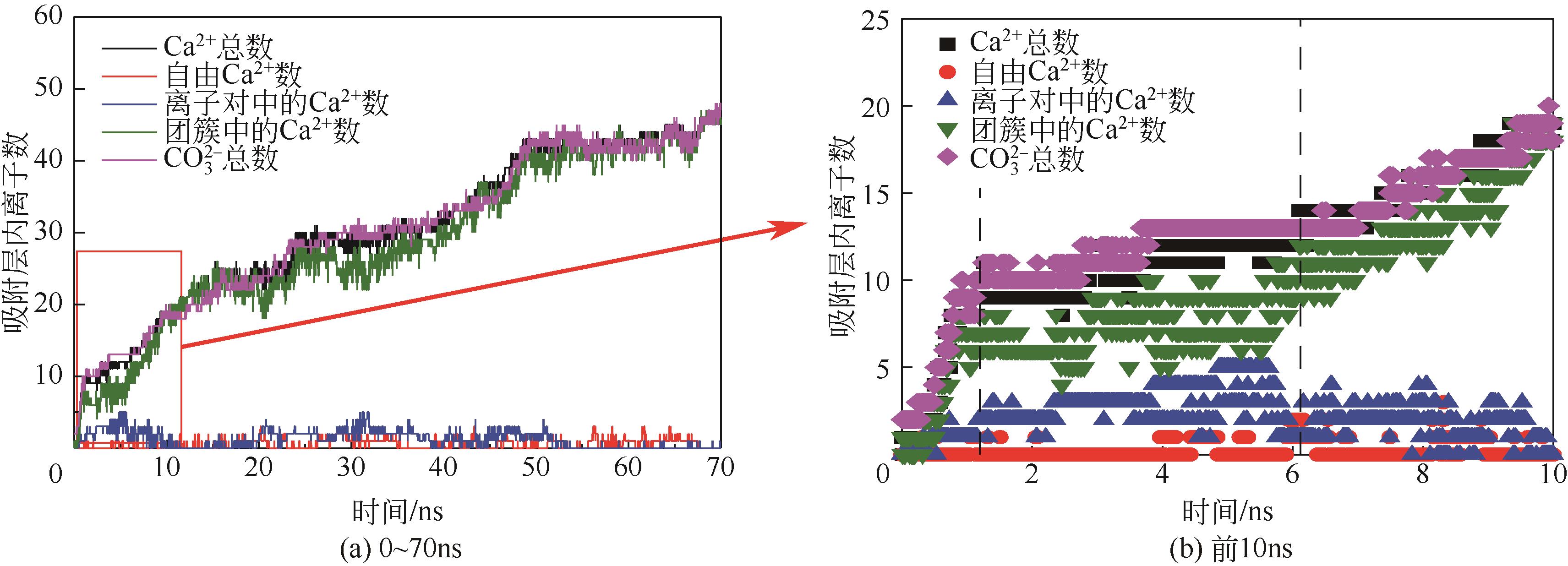
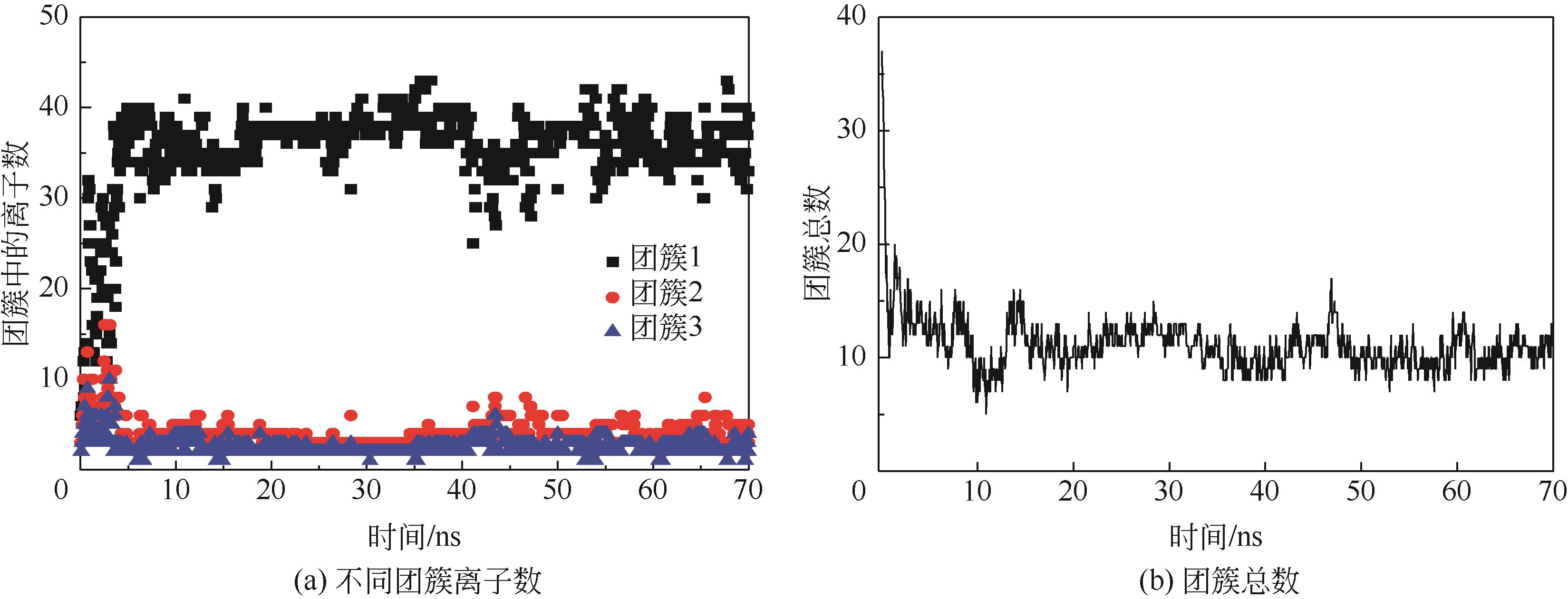
Molecular dynamics simulation on adsorption and dehydration behavior of calcium carbonate on heat exchange surface
XIAO Yi( ), WANG Bingbing(
), WANG Bingbing( ), YU Xuliang, WANG Xin, CAI Hanyou
), YU Xuliang, WANG Xin, CAI Hanyou
- School of Energy and Power Engineering, Northeast Electric Power University, Jilin 132012, Jilin, China
-
Received:2021-09-09Revised:2021-10-31Online:2022-08-22Published:2022-08-25 -
Contact:WANG Bingbing
换热壁面碳酸钙吸附与脱水行为的分子动力学
- 东北电力大学能源与动力工程学院,吉林 吉林 132012
-
通讯作者:王兵兵 -
作者简介:肖毅(1997—),男,硕士研究生,研究方向为换热表面污垢形成机理与抑制方法。E-mail:975152973@qq.com 。 -
基金资助:国家自然科学基金(51706038);吉林省科技厅优秀青年人才基金(20190103059JH)
CLC Number:
Cite this article
XIAO Yi, WANG Bingbing, YU Xuliang, WANG Xin, CAI Hanyou. Molecular dynamics simulation on adsorption and dehydration behavior of calcium carbonate on heat exchange surface[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4077-4085.
肖毅, 王兵兵, 于旭亮, 王鑫, 蔡汉友. 换热壁面碳酸钙吸附与脱水行为的分子动力学[J]. 化工进展, 2022, 41(8): 4077-4085.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-1943
| 项目 | 类型 | k/eV·?-2 | r0/? | kθ/eV·rad-2 | θ0/(°) |
|---|---|---|---|---|---|
| Hw—Ow | Harmonic | 45.93 | 1.012 | ||
| Hw—Ow—Hw | Harmonic | 3.291 | 113.24 |
| 项目 | 类型 | k/eV·?-2 | r0/? | kθ/eV·rad-2 | θ0/(°) |
|---|---|---|---|---|---|
| Hw—Ow | Harmonic | 45.93 | 1.012 | ||
| Hw—Ow—Hw | Harmonic | 3.291 | 113.24 |
| 项目 | 类型 | k/eV·?-2 | r0/? | ka/eV·rad-2 | kbb/eV·?-2 | kba/eV·?-1·rad-1 | k2/eV·?-2 | k4/eV·?-4 |
|---|---|---|---|---|---|---|---|---|
| C—O | Harmonic | 40.8493 | 1.3042 | |||||
| O—C—O | class2 | 13.234 | 12.818 | 1.53319 | ||||
| C—O/O/O | Distance | 13.647 | 360.0 |
| 项目 | 类型 | k/eV·?-2 | r0/? | ka/eV·rad-2 | kbb/eV·?-2 | kba/eV·?-1·rad-1 | k2/eV·?-2 | k4/eV·?-4 |
|---|---|---|---|---|---|---|---|---|
| C—O | Harmonic | 40.8493 | 1.3042 | |||||
| O—C—O | class2 | 13.234 | 12.818 | 1.53319 | ||||
| C—O/O/O | Distance | 13.647 | 360.0 |
| 原子 | 电荷量/e | 原子量 |
|---|---|---|
| Hw | 0.41 | 1.008 |
| Ow | -0.82 | 16.000 |
| Ca | 2 | 40.080 |
| C | 1.123285 | 12.010 |
| O | -1.041095 | 16.000 |
| Cu | 0 | 63.550 |
| 原子 | 电荷量/e | 原子量 |
|---|---|---|
| Hw | 0.41 | 1.008 |
| Ow | -0.82 | 16.000 |
| Ca | 2 | 40.080 |
| C | 1.123285 | 12.010 |
| O | -1.041095 | 16.000 |
| Cu | 0 | 63.550 |
| Lennard-Jones | ε/eV | σ/? | |
|---|---|---|---|
| Ow—Ow(lj/cut) | 0.00674 | 3.16549 | |
| Ow—Ca(lj/mdf) | 0.00095 | 3.35 | |
| Ow—Cu(lj/cut) | 0.05252 | 2.72029 | |
| Ca—Cu(lj/cut) | 0.09211 | 2.35409 | |
| C—Cu(lj/cut) | 0.03956 | 2.98960 | |
| O—Cu(lj/cut) | 0.04969 | 2.68805 | |
| Cu—Cu(lj/cut) | 0.4093 | 2.3377 | |
| Buckingham | A/eV | ρ/? | C/eV·?6 |
| Ca—O(buck/mdf) | 3161.63 | 0.27151 | 0 |
| Ca—C(buck/mdf) | 120000000 | 0.12 | 0 |
| O—O(buck/mdf) | 63840.20 | 0.19891 | 27.899 |
| O—Ow(buck/mdf) | 12534.46 | 0.2020 | 12.090 |
| O—Hw(buck/mdf) | 396.30 | 0.2170 | 0 |
| Lennard-Jones | ε/eV | σ/? | |
|---|---|---|---|
| Ow—Ow(lj/cut) | 0.00674 | 3.16549 | |
| Ow—Ca(lj/mdf) | 0.00095 | 3.35 | |
| Ow—Cu(lj/cut) | 0.05252 | 2.72029 | |
| Ca—Cu(lj/cut) | 0.09211 | 2.35409 | |
| C—Cu(lj/cut) | 0.03956 | 2.98960 | |
| O—Cu(lj/cut) | 0.04969 | 2.68805 | |
| Cu—Cu(lj/cut) | 0.4093 | 2.3377 | |
| Buckingham | A/eV | ρ/? | C/eV·?6 |
| Ca—O(buck/mdf) | 3161.63 | 0.27151 | 0 |
| Ca—C(buck/mdf) | 120000000 | 0.12 | 0 |
| O—O(buck/mdf) | 63840.20 | 0.19891 | 27.899 |
| O—Ow(buck/mdf) | 12534.46 | 0.2020 | 12.090 |
| O—Hw(buck/mdf) | 396.30 | 0.2170 | 0 |
| 1 | PÄÄKKÖNEN T M, RIIHIMÄKI M, SIMONSON C J, et al. Crystallization fouling of CaCO3-analysis of experimental thermal resistance and its uncertainty[J]. International Journal of Heat & Mass Transfer, 2012, 55(23-24): 6927-6937. |
| 2 | CHAUSSEMIER M, POURMOHTASHAM E, GELUS D, et al. State of art of natural inhibitors of calcium carbonate scaling[J]. Desalination, 2015, 356: 47-55. |
| 3 | 贺姗姗. 圆管内三角翼涡流发生器CaCO3污垢特性的数值模拟[D]. 吉林: 东北电力大学, 2018. |
| HE S S. Numerical simulation of CaCO3 fouling characteristics in tube with delta wing vortex generator[D]. Jilin: Northeast Electric Power University, 2018. | |
| 4 | 罗志强, 杨庆峰. 旋转磁场与水量耦合对CaCO3结晶的影响[J]. 化工学报, 2018, 69(7): 3029-3037. |
| LUO Z Q, YANG Q F. Effect of rotating magnetic field coupled with water volume on CaCO3 crystallization[J]. CIESC Journal, 2018, 69(7): 3029-3037. | |
| 5 | WANG J G, LIANG Y D. Anti-fouling effect of axial alternating electromagnetic field on calcium carbonate fouling in U-shaped circulating cooling water heat exchange tube[J]. International Journal of Heat & Mass Transfer, 2017, 115: 774-781. |
| 6 | 李海花, 刘振法, 高玉华, 等. 静电场对CaCO3结晶过程的影响及与绿色阻垢剂的协同阻垢性能[J]. 化工学报, 2013, 64(5): 1736-1742. |
| LI H H, LIU Z F, GAO Y H, et al. Influence of electrostatic water treatment on crystallization behavior of CaCO3 and synergistic scale inhibition with a green scale inhibitor[J]. CIESC Journal, 2013, 64(5): 1736-1742. | |
| 7 | ALIMI F, TLILI M, AMOR M B, et al. Influence of magnetic field on calcium carbonate precipitation[J]. Desalination, 2007, 206(1/2/3): 163-168. |
| 8 | TIJING L D, LEE D H, KIM D W, et al. Effect of high-frequency electric fields on calcium carbonate scaling[J]. Desalination, 2011, 279(1/2/3): 47-53. |
| 9 | 徐志明, 常宏亮, 王兵兵, 等. 电场作用下CaCO3污垢特性的实验研究[J]. 中国电机工程学报, 2018, 38(21): 6346-6352. |
| XU Z M, CHANG H L, WANG B B, et al. Experimental study on CaCO3 fouling characteristics under electric field[J]. Proceedings of the CSEE, 2018, 38(21): 6346-6352. | |
| 10 | SMEETS P, KANG R C, KEMPEN R, et al. Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy[J]. Nature Materials, 2015, 14(4): 394-399. |
| 11 | GEBAUER D, CÖLFEN H. Prenucleation clusters and non-classical nucleation[J]. Nano Today, 2011, 6(6): 564-584. |
| 12 | GEBAUER D, VÖLKEL A, CÖLFEN H. Stable prenucleation calcium carbonate clusters[J]. Science, 2008, 322(5909): 1819-1822. |
| 13 | POUGET E M, BOMANS P H H, GOOS J A C M, et al. The initial stages of template-controlled CaCO3 formation revealed by Cryo-TEM[J]. Science, 2009, 323(5920): 1455-1458. |
| 14 | GOODWIN A L, MICHEL F M, PHILLIPS B L, et al. Nanoporous structure and medium-range order in synthetic amorphous calcium carbonate[J]. Chemistry of Materials, 2010, 22(10): 3197-3205. |
| 15 | RAITERI P, GALE J D. Water is the key to nonclassical nucleation of amorphous calcium carbonate[J]. Journal of the American Chemical Society, 2010, 132(49): 17623–17634. |
| 16 | RODRIGUEZ-BLANCO J D, SHAW S, BENNING L G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite[J]. Nanoscale, 2010, 3(1): 265-271. |
| 17 | NIELSEN M H, ALONI S, YOREO J J D. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways[J]. Science, 2014, 345(6201): 1158-1162. |
| 18 | SAHARAY M, YAZAYDIN A O, KIRKPATRICK R J. Dehydration-induced amorphous phases of calcium carbonate[J]. Journal of Physical Chemistry B, 2013, 117(12): 3328-3336. |
| 19 | SAHARAY M, KIRKPATRICK R J. Water dynamics in hydrated amorphous materials: a molecular dynamics study of the effects of dehydration in amorphous calcium carbonate[J]. Physical Chemistry Chemical Physics, 2017, 19(43): 29594-29600. |
| 20 | PLIMPTON S. Fast parallel algorithms for short-range molecular dynamics[J]. Journal of Computational Physics, 1995, 117(1): 1-19. |
| 21 | MA M, WANG Y H, CAO X F, et al. Temperature and supersaturation as key parameters controlling the spontaneous precipitation of calcium carbonate with distinct physicochemical properties from pure aqueous solutions[J]. Crystal Growth and Design, 2019, 19(12): 6972-6988. |
| 22 | WU Y J, TEPPER H L, VOTH G A. Flexible simple point-charge water model with improved liquid-state properties[J]. The Journal of Chemical Physics, 2006, 124(2): 24503-24503. |
| 23 | DEMICHELIS R, RAITERI P, GALE J D, et al. Stable prenucleation mineral clusters are liquid-like ionic polymers[J]. Nature Communications, 2011, 2(6): 590. |
| 24 | XIAO S J, EDWARDS S A, GRÄTER F. A new transferable forcefield for simulating the mechanics of CaCO3 crystals[J]. Journal of Physical Chemistry C, 2011, 115(41): 20067-20075. |
| 25 | SWOPE W C, ANDERSEN H C, BERENS P H, et al. A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: application to small water clusters[J]. Journal of Chemical Physics, 1982, 76(1): 637-649. |
| 26 | BECKERS J V L, LOWE C P, LEEUW S W D. An iterative PPPM method for simulating coulombic systems on distributed memory parallel computers[J]. Molecular Simulation, 1998, 20(6): 369-383. |
| 27 | GEBAUER D, GUNAWIDJAJA P N, KO J Y P. Proto-calcite and proto-vaterite in amorphous calcium carbonates[J]. Angewandte Chemie International Edition, 2010, 49(47): 8889-8891. |
| 28 | SURFACEACE A F, HEDGES L O, FERNANDEZ-MARTINEZ A, et al. Microscopic evidence for liquid-liquid separation in supersaturated CaCO3 solutions[J]. Science, 2013, 341(6148): 885-889. |
| 29 | LHLI J, WONG W C, NOEL E H, et al. Dehydration and crystallization of amorphous calcium carbonate in solution and in air[J]. Nature Communications, 2014, 5(1): 3169. |
| 30 | ZOU Z Y, BERTINETTI L, POLITI Y, et al. Opposite particle size effect on amorphous calcium carbonate crystallization in water and during heating in air[J]. Chemistry of Materials, 2015, 27(12): 4237-4246. |
| [1] | LI Mengyuan, GUO Fan, LI Qunsheng. Simulation and optimization of the third and fourth distillation columns in the recovery section of polyvinyl alcohol production [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 113-123. |
| [2] | ZHANG Ruijie, LIU Zhilin, WANG Junwen, ZHANG Wei, HAN Deqiu, LI Ting, ZOU Xiong. On-line dynamic simulation and optimization of water-cooled cascade refrigeration system [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 124-132. |
| [3] | WANG Tai, SU Shuo, LI Shengrui, MA Xiaolong, LIU Chuntao. Dynamic behavior of single bubble attached to the solid wall in the AC electric field [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 133-141. |
| [4] | SUN Jipeng, HAN Jing, TANG Yangchao, YAN Bowen, ZHANG Jieyao, XIAO Ping, WU Feng. Numerical simulation and optimization of operating parameters of sulfur wet molding process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 189-196. |
| [5] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [6] | CHEN Kuangyin, LI Ruilan, TONG Yang, SHEN Jianhua. Structure design of gas diffusion layer in proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 246-259. |
| [7] | YANG Yudi, LI Wentao, QIAN Yongkang, HUI Junhong. Analysis of influencing factors of natural gas turbulent diffusion flame length in industrial combustion chamber [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 267-275. |
| [8] | ZHANG Jie, BAI Zhongbo, FENG Baoxin, PENG Xiaolin, REN Weiwei, ZHANG Jingli, LIU Eryong. Effect of PEG and its compound additives on post-treatment of electrolytic copper foils [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 374-381. |
| [9] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [10] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [11] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [12] | XU Ruosi, TAN Wei. Flow field simulation and fluid-structure coupling analysis of C-tube pool boiling two-phase flow model [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 47-55. |
| [13] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [14] | ZHANG Fengqi, CUI Chengdong, BAO Xuewei, ZHU Weixuan, DONG Hongguang. Design and evaluation of sweetening process with amine solution absorption and multiple desorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 518-528. |
| [15] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||