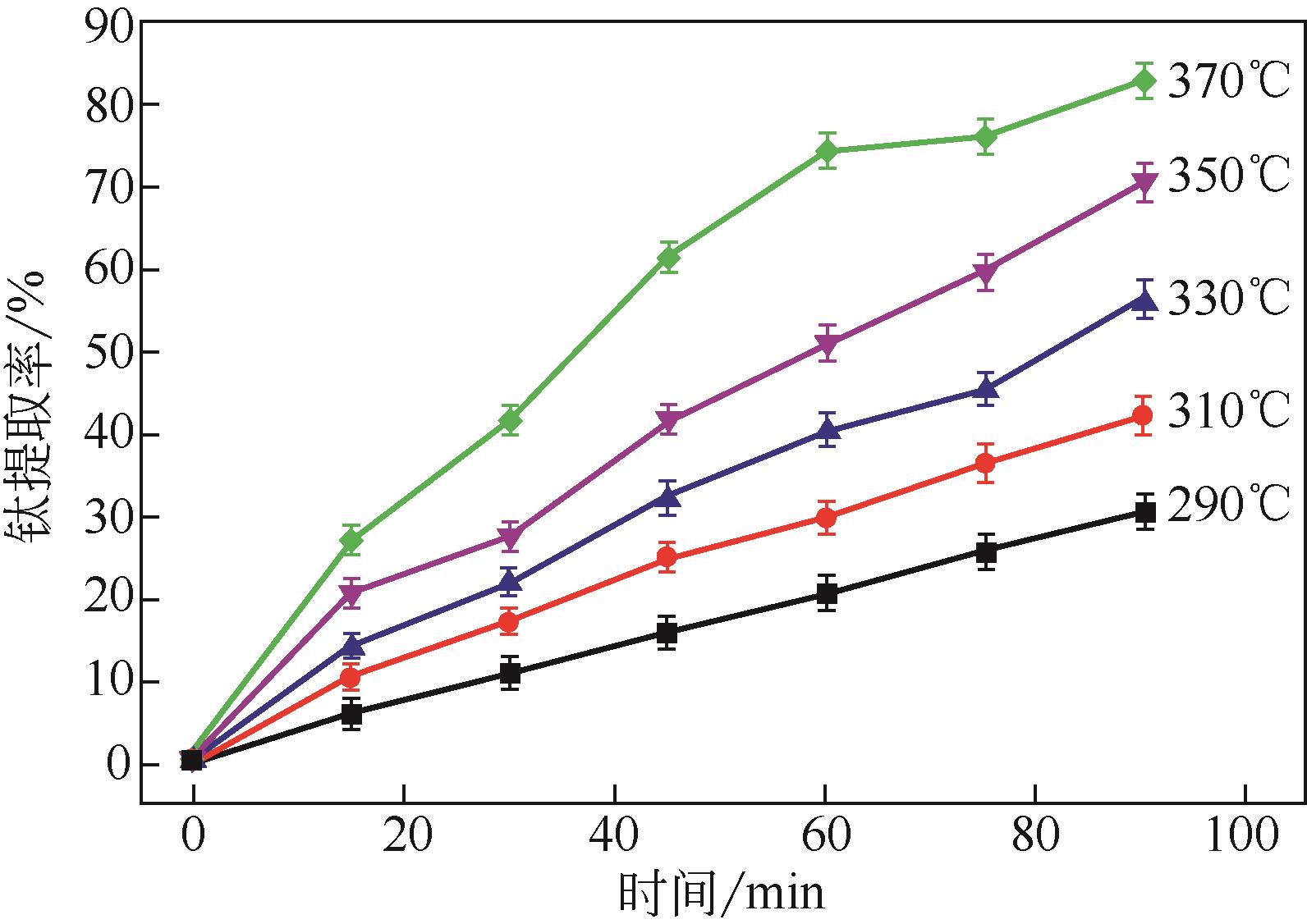
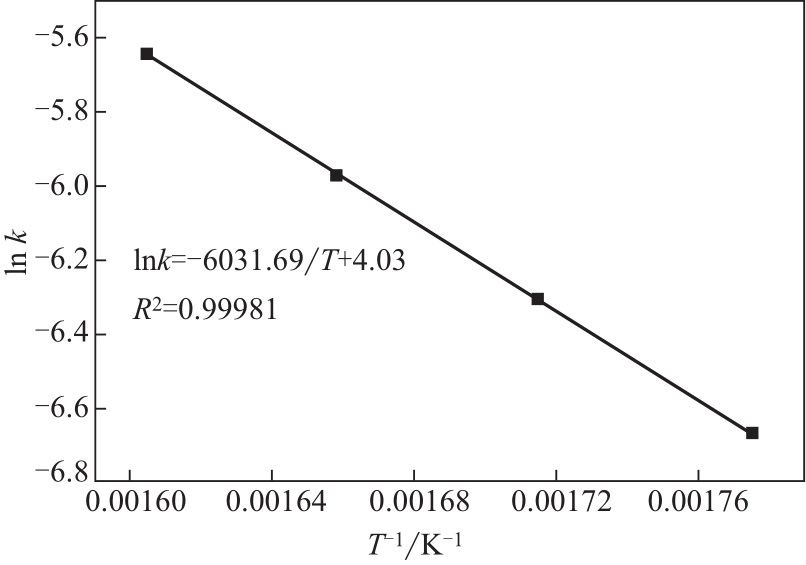
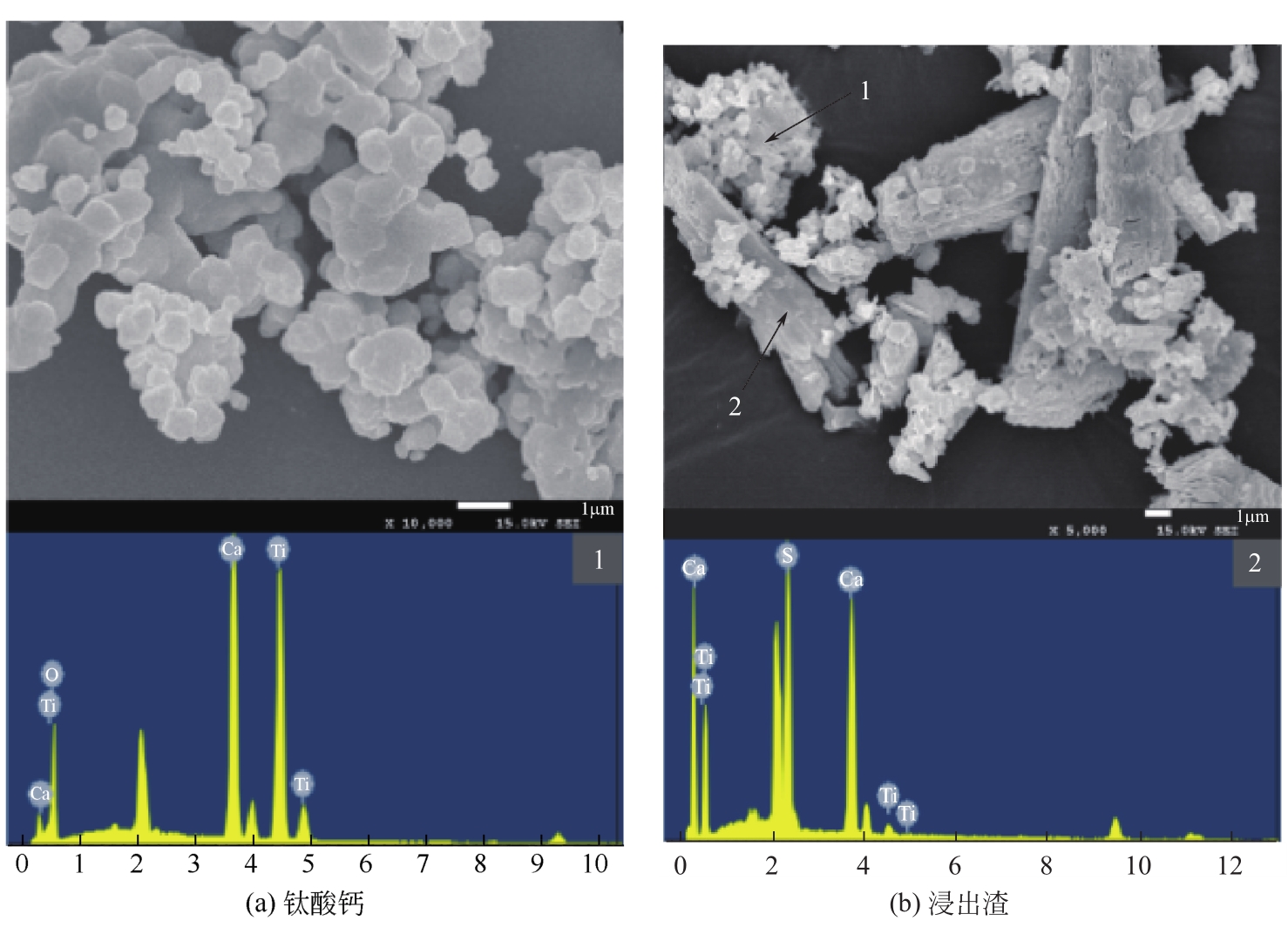
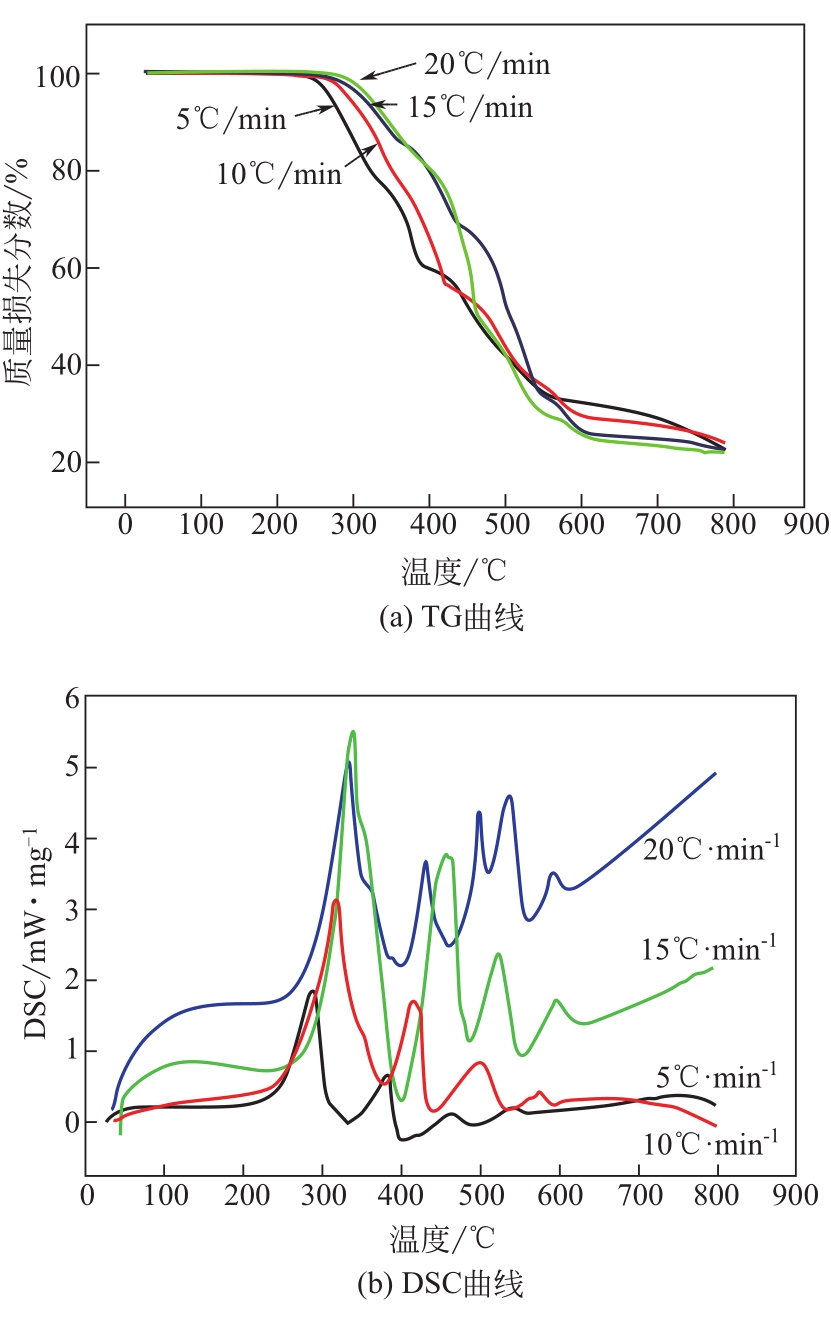
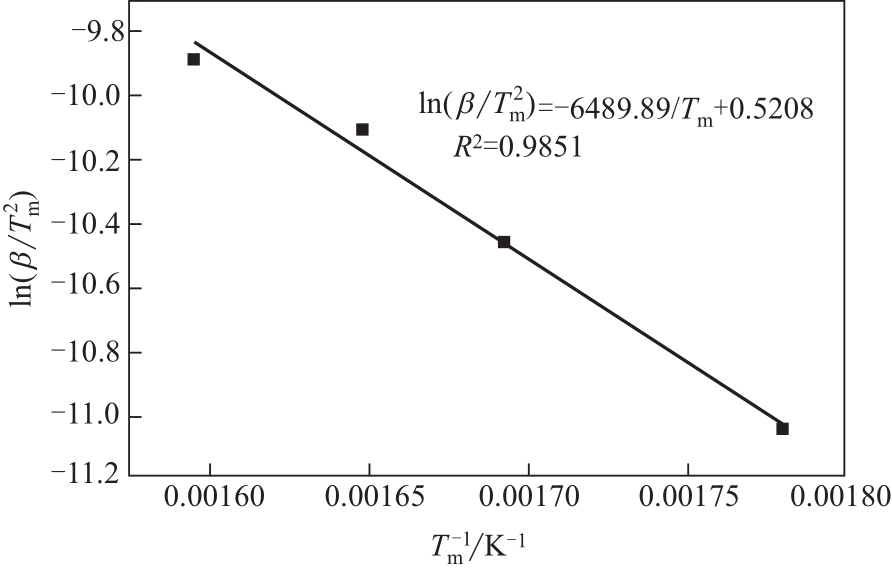
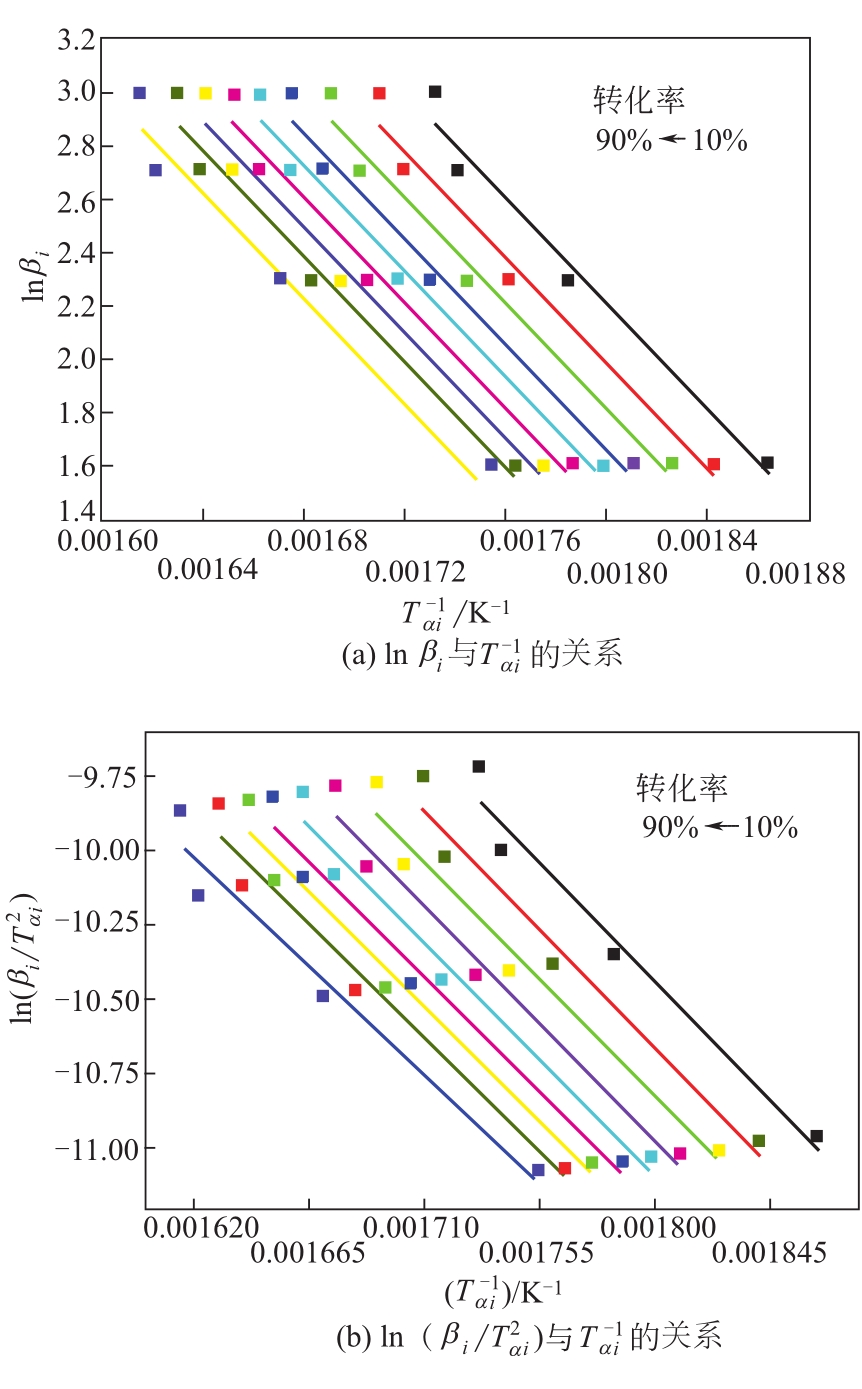
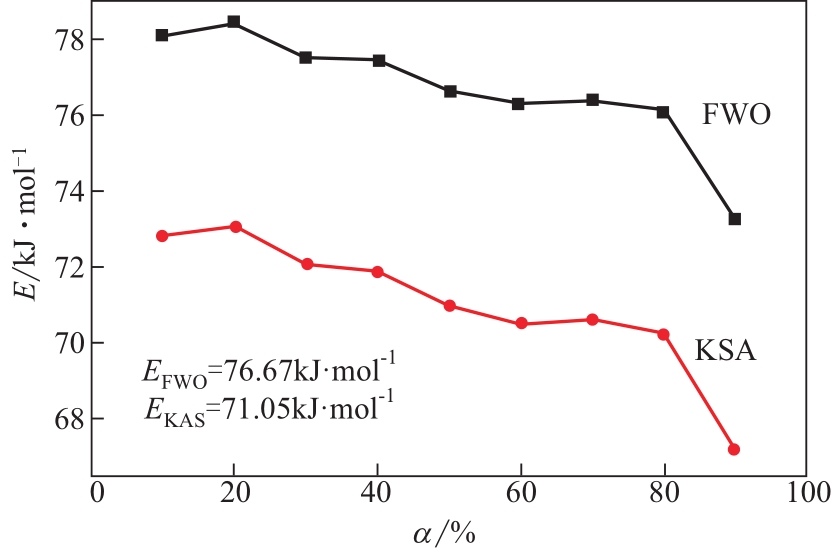
| 1 |
YAN J. Carbon capture and storage (CCS)[J]. Applied Energy, 2015, 148(1): 1-6.
|
| 2 |
LEE S, KIM J W, CHAE S, et al. CO2 sequestration technology through mineral carbonation: an extraction and carbonation of blast slag[J]. Journal of CO2 Utilization, 2016, 16: 336-345.
|
| 3 |
HU J, LIU W, WANG L, et al. Indirect mineral carbonation of blast furnace slag with (NH4)2SO4 as a recyclable extractant[J]. Journal of Energy Chemistry, 2017, 26(5): 927-935.
|
| 4 |
LIU Q, LIU W, HU J, et al. Energy-efficient mineral carbonation of blast furnace slag with high value-added products[J]. Journal of Cleaner Production, 2018, 197: 242-252
|
| 5 |
CHU G, LI C, LIU W, et al. Facile and cost-efficient indirect carbonation of blast furnace slag with multiple high value-added products through a completely wet process[J]. Energy, 2019, 166: 1314-1322.
|
| 6 |
LIU W, YIN S, LUO D, et al. Optimising the recovery of high-value-added ammonium alum during mineral carbonation of blast furnace slag[J]. Journal of Alloys and Compounds, 2019, 774: 1151-1159.
|
| 7 |
ÖZBAY E, ERDEMIR M, DURMUS H İ. Utilization and efficiency of ground granulated blast furnace slag on concrete properties: a review[J]. Construction and Building Materials, 2016, 105: 423-434.
|
| 8 |
Ober JOYCE A. Mineral commodity summaries 2018[R]. Reston, VA: U.S. Geological Survey, 2018.
|
| 9 |
李亮, 罗建林. 攀枝花地区某钒钛磁铁矿工艺矿物学研究[J]. 金属矿山, 2010(4): 89-92.
|
|
LI L, LUO J L. Process mineralogy studies on V-Ti magnetite in Panzhihua region[J]. Metal Mine, 2010(4): 89-92.
|
| 10 |
熊瑶, 李春, 梁斌, 等. 盐酸浸出自然冷却含钛高炉渣[J]. 中国有色金属学报, 2008, 18(3): 557-563.
|
|
XIONG Y, LI C, LIANG B, et al. Leaching behavior of air cooled Ti-bearing blast-furnace slag in hydrochloric acid[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(3): 557-563.
|
| 11 |
严芳, 李春, 梁斌. 水淬含钛高炉渣二段酸解工艺[J]. 过程工程学报, 2006, 6(3): 413-417.
|
|
YAN F, LI C, LIANG B. A two-step sulfuric acid leaching process of Ti-bearing blast furnace slag[J]. The Chinese Journal of Process Engineering, 2006, 6(3): 413-417.
|
| 12 |
陈启福, 张燕秋, 方民宪, 等. 攀钢高炉渣提取二氧化钛及三氧化二钪的研究[J]. 钢铁钒钛, 1991(3): 30.
|
|
CHEN Q F, ZHANG Y Q, FANG M X, et al. Study on extraction of titanium dioxide and scandium trioxide from Pangang blast furnace slag[J]. Iron Steel Vanadium Titanium, 1991(3): 30.
|
| 13 |
WANG L, LIU W Z, HU J P, et al. Indirect mineral carbonation of titanium-bearing blast furnace slag coupled with recovery of TiO2 and Al2O3[J]. Chinese Journal of Chemical Engineering, 2017, 26(3): 583-592.
|
| 14 |
XIONG Y J, ALDAHRI T, LIU W Z, et al. Simultaneous preparation of TiO2 and ammonium alum, and microporous SiO2 during the mineral carbonation of titanium-bearing blast furnace slag[J]. Chinese Journal of Chemical Engineering, 2020, 28(9): 2256-2266.
|
| 15 |
RAISAKU K, Mechanism KOHEI U., kinetics, and equilibrium of thermal decomposition of ammonium sulfate[J]. Industrial & Engineering Chemistry Process Design and Development, 1970, 9(4): 489-494.
|
| 16 |
刘科伟, 陈天朗. 硫酸铵的热分解[J]. 化学研究与应用, 2002, 14(6): 737-738.
|
|
LIU K W, CHEN T L. Studies on the thermal decomposition of ammonium sulfate[J]. Chemical Research and Application, 2002, 14(6): 737-738.
|
| 17 |
范芸珠, 曹发海. 硫酸铵热分解反应动力学研究[J]. 高校化学工程学报, 2011, 25(2): 341-346.
|
|
FAN Y Z, CAO F H. Thermal decomposition kinetics of ammonium sulfate[J]. Journal of Chemical Engineering of Chinese Universities, 2011, 25(2): 341-346.
|
| 18 |
潘腾, 成有为, 王丽军, 等. 多重扫描速率法的硫酸铵热分解动力学[J]. 高校化学工程学报, 2019, 33(5): 1086-1091.
|
|
PAN T, CHENG Y W, WANG L J, et al. Thermal decomposition kinetics of ammonium sulfate studied with multiple scanning methods[J]. Journal of Chemical Engineering of Chinese Universities, 2019, 33(5): 1086-1091.
|
| 19 |
唐定兴, 冯文. 用热重法研究硫酸铵及硫酸铵与氯化钾体系的热分解行为[J]. 安徽机电学院学报, 1999, 14(1): 55-58.
|
|
TANG D X, FENG W. Studies on the thermal decomposition of (NH4)2SO4 and KCl + (NH4)2SO4 by thermogravimetric method[J]. Journal of Anhui Institute of Mechanical & Electrical Engineering, 1999, 14(1): 55-58.
|
| 20 |
SHEN X Y, SHAO H M, DING J W, et al. Roasting and water leaching behavior of zinc in zinc oxidized ore using (NH4)2SO4[J]. International Journal of Minerals, Metallurgy and Materials, 2020, 27(11): 1471-1481.
|
| 21 |
YIN S, ALDAHRI T, ROHANI S, et al. Insights into the roasting kinetics and mechanism of blast furnace slag with ammonium sulfate for CO2 mineralization[J]. Industrial & Engineering Chemistry Research, 2019, 58(31): 14026-14036.
|
 ), HU Jinpeng2, LIU Qingcai1, LI Chun2
), HU Jinpeng2, LIU Qingcai1, LI Chun2