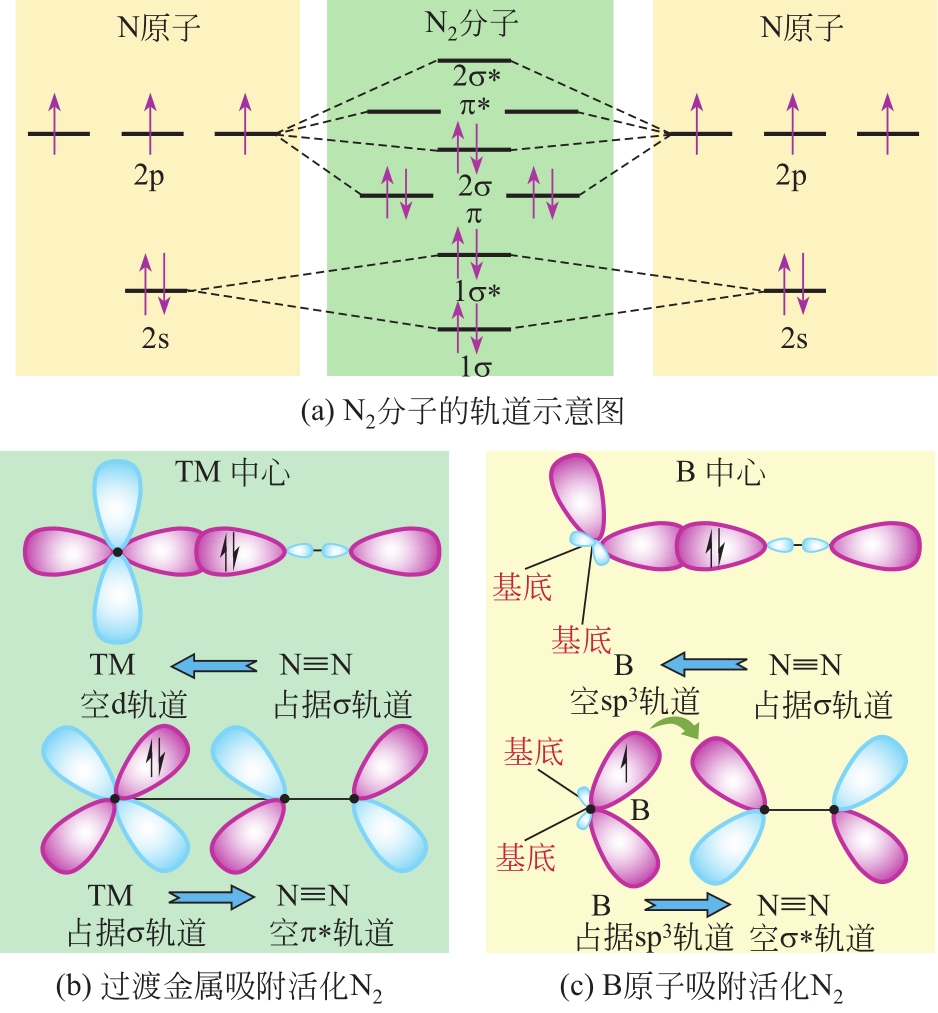
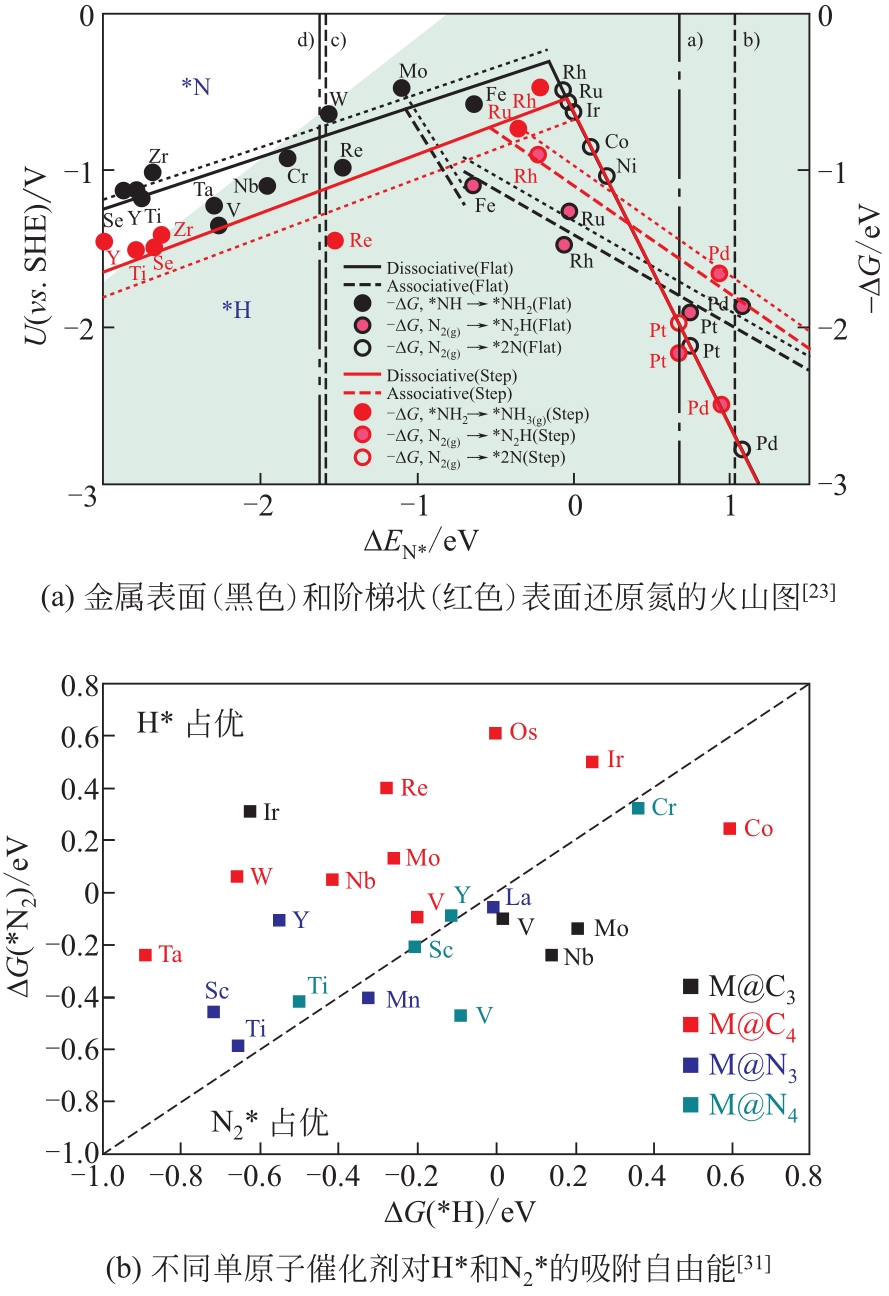
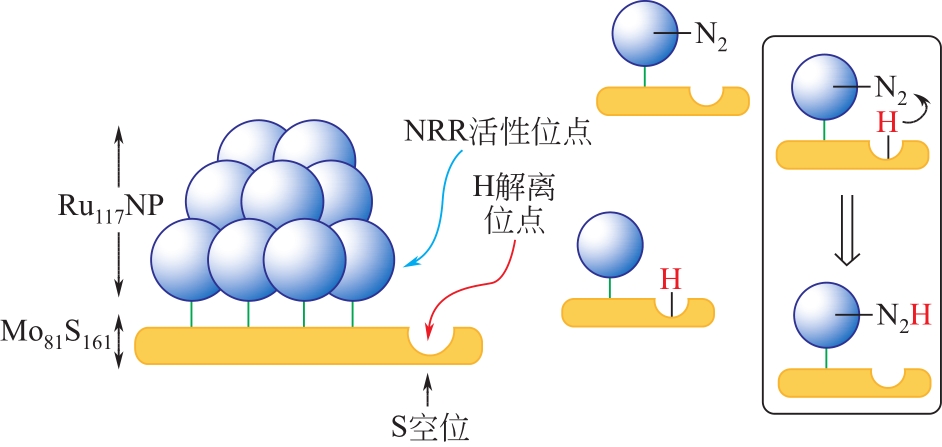
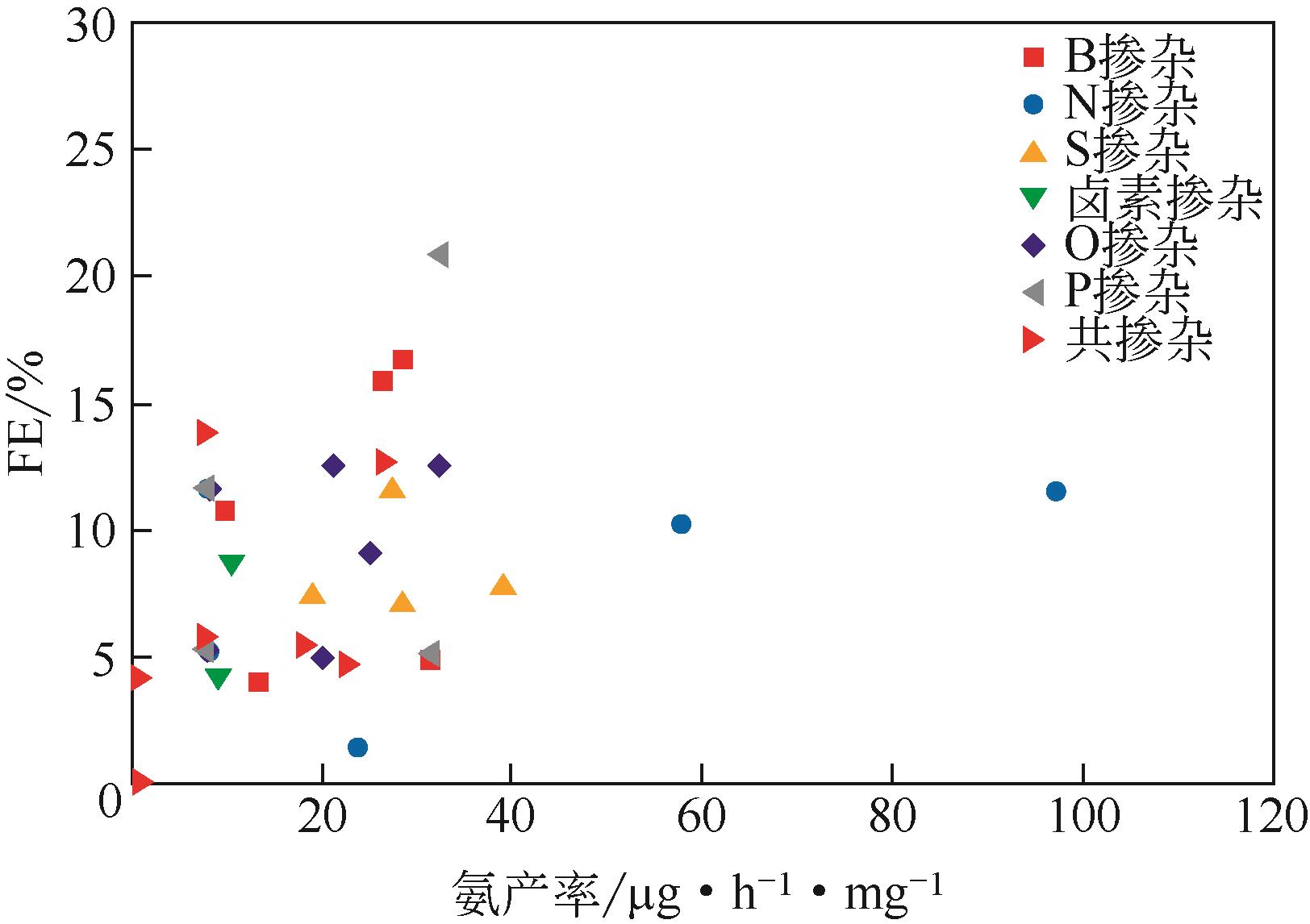
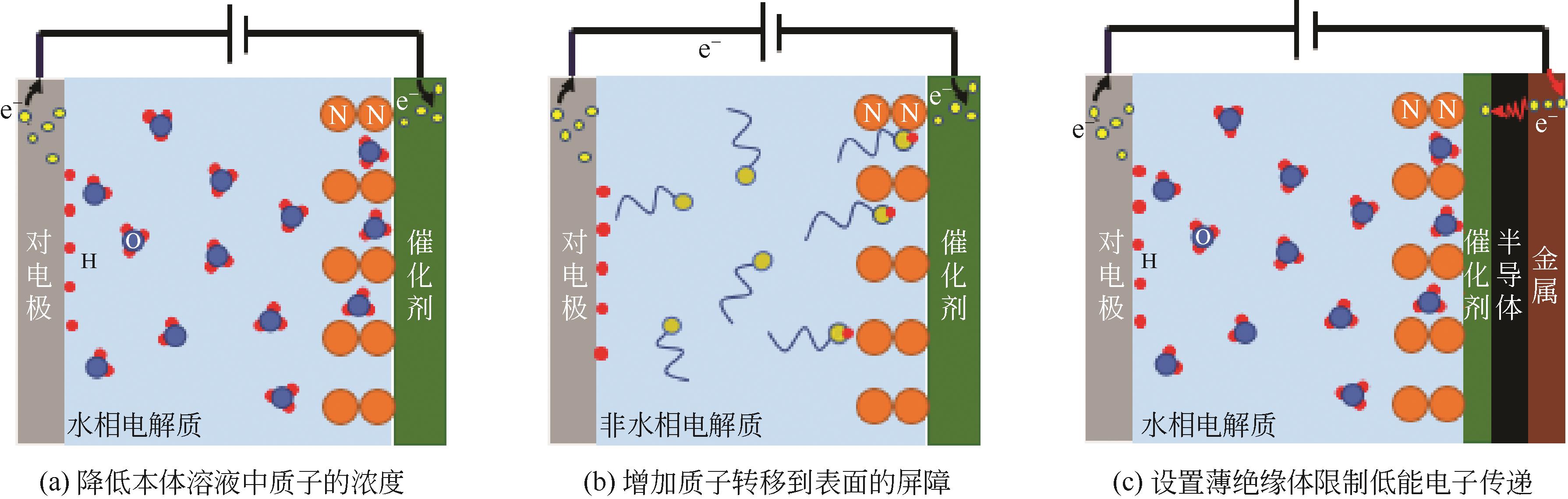
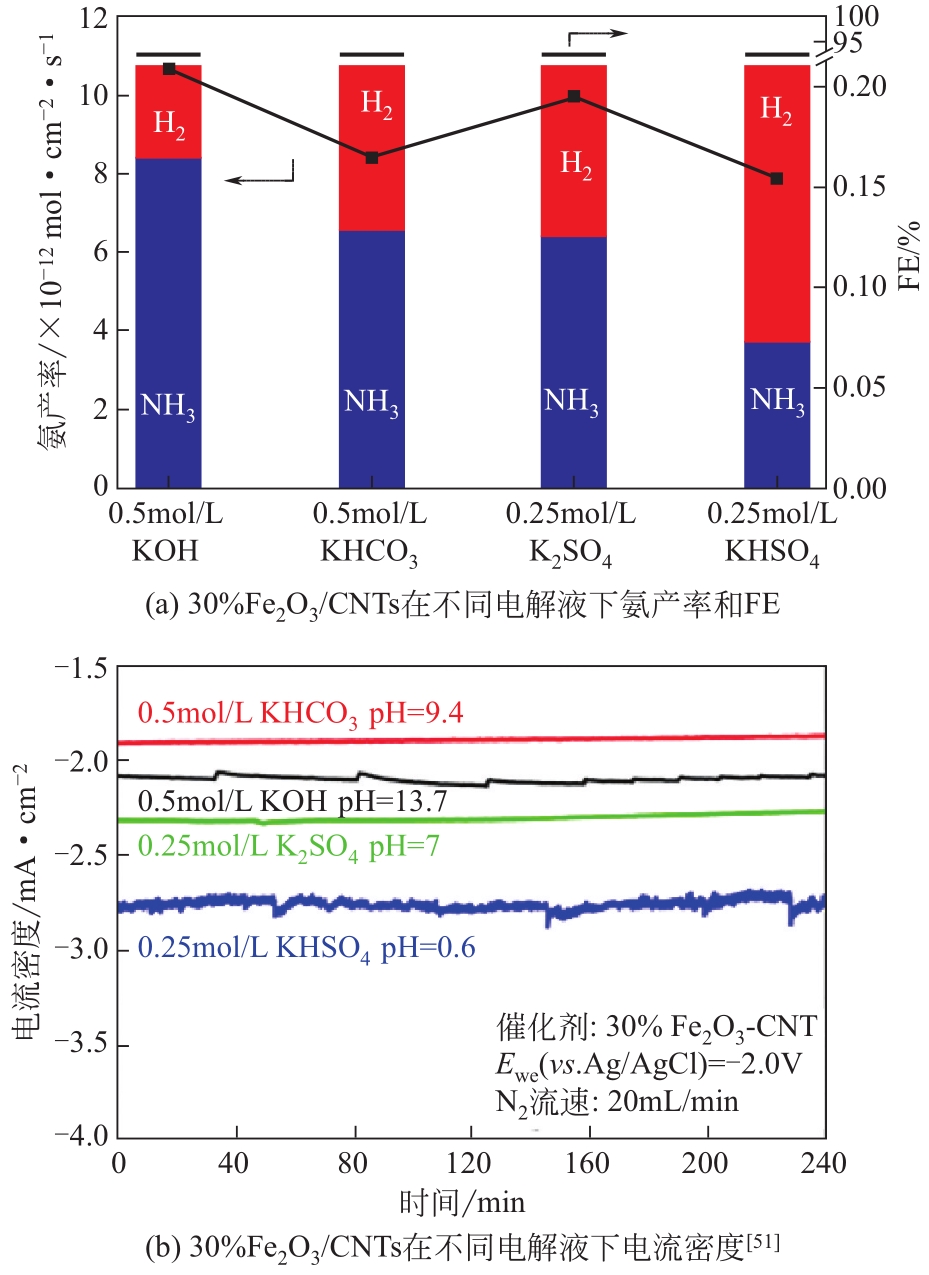
化工进展 ›› 2021, Vol. 40 ›› Issue (12): 6670-6687.DOI: 10.16085/j.issn.1000-6613.2020-2520
电催化氮气还原合成氨反应中抑制水解析氢竞争的研究进展
- 南开大学环境科学与工程学院,天津 300350
-
收稿日期:2020-12-17修回日期:2021-03-08出版日期:2021-12-05发布日期:2021-12-21 -
通讯作者:董恒 -
作者简介:张婷(1995—),女,硕士研究生,研究方向为电化学。E-mail:zhangt@nankai.edu.cn 。 -
基金资助:国家自然科学基金(51708300)
Research progress of inhibiting hydrogen evolution in electro-catalytic ammonia synthesis
ZHANG Ting( ), SUN Xiaohong, YU Hongbing, DONG Heng(
), SUN Xiaohong, YU Hongbing, DONG Heng( )
)
- College of Environmental Science and Engineering, Nankai University, TianJin 300350, China
-
Received:2020-12-17Revised:2021-03-08Online:2021-12-05Published:2021-12-21 -
Contact:DONG Heng
摘要:
传统工业合成氨Haber-Bosch工艺条件要求严苛,并且存在高能耗以及高CO2排放问题。电催化氮气还原(nitrogen reduction reaction, NRR)是一种在常温常压下利用氮气合成氨的新工艺,具有成本低、反应条件温和、环境友好等优势。但该反应所需过电位较高,水解析氢反应(hydrogen evolution reaction, HER)竞争明显,导致电流密度和选择性较低,无法达到工业应用水平。本文在介绍电催化NRR合成氨的反应机理的基础上,主要从氮气分子的吸附活化和电还原阶段反应过程出发,综述了电催化氮气还原合成氨反应中HER与NRR的竞争机制。重点梳理了通过设计催化剂和反应体系抑制HER的国内外最新研究成果,最后对电催化NRR合成氨面临的挑战和机遇进行了展望。
中图分类号:
引用本文
张婷, 孙晓红, 于宏兵, 董恒. 电催化氮气还原合成氨反应中抑制水解析氢竞争的研究进展[J]. 化工进展, 2021, 40(12): 6670-6687.
ZHANG Ting, SUN Xiaohong, YU Hongbing, DONG Heng. Research progress of inhibiting hydrogen evolution in electro-catalytic ammonia synthesis[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6670-6687.
| 项目 | 反应方程式 | E0(vs. SHE)/V |
|---|---|---|
| R1 | N2+e- | -3.37(pH=0) |
| R2 | N2+H++e- | -3.20(pH=0) |
| R3 | N2+2H++2e- | -1.10(pH=0) |
| R4 | N2+4H++4e- | -0.36(pH=0) |
| R5 | N2+6H++6e- | 0.148(pH=0) |
| R6 | 2H++2e- | 0(pH=0) |
| R7 | N2+6H2O+6e- | -0.763(pH=14) |
| R8 | 2H2O+2e- | -0.828(pH=14) |
表1 涉及电催化NRR合成氨反应中的标准电极电位
| 项目 | 反应方程式 | E0(vs. SHE)/V |
|---|---|---|
| R1 | N2+e- | -3.37(pH=0) |
| R2 | N2+H++e- | -3.20(pH=0) |
| R3 | N2+2H++2e- | -1.10(pH=0) |
| R4 | N2+4H++4e- | -0.36(pH=0) |
| R5 | N2+6H++6e- | 0.148(pH=0) |
| R6 | 2H++2e- | 0(pH=0) |
| R7 | N2+6H2O+6e- | -0.763(pH=14) |
| R8 | 2H2O+2e- | -0.828(pH=14) |
| 催化剂 | 电解液 | 过电位/V | FE/% | 氨产率 | 参考文献 |
|---|---|---|---|---|---|
| 贵金属 | |||||
| Au纳米笼 | 0.5mol/L LiClO4 | -0.4 | 30.2 | 3.9μg?h-1?cm-2 | [ |
| Au纳米棒 | 0.1mol/L KOH | -0.2 | 4 | 1.648μg?h-1?cm-2 | [ |
| Ru纳米粒子 | 0.01mol/L HCl | 0.01 | 5.4 | 约0.2μg?h-1?cm-2 | [ |
| Rh纳米片 | 0.1mol/L KOH | -0.2 | 21.7 | 23.88μg?h-1?cm-2 | [ |
| Pd/C | 0.1mol/L PBS | -0.1 | 8.2 | 4.5μg?h-1?mg-1 | [ |
| Au/TiO2 | 0.1mol/L HCl | -0.2 | 8.11 | 21.4μg?h-1?mg-1 | [ |
| Pt/C | H+/Li+/NH4+ | -1.2 | 0.83 | 47.2μg?h-1?cm-2 | [ |
| 过渡金属及其杂化物 | |||||
| Bi纳米片 | 0.1mol/L Na2SO4 | -0.8 | 10.46 | (2.54±0.16)μg?cm-2?h-1 | [ |
| Bi4V2O11/CeO2 | 0.1mol/L HCl | -0.2 | 10.16 | 23.21μg??h-1?mg-1 | [ |
| Fe3S4纳米片 | 0.1mol/L HCl | -0.4 | 6.45 | 75.4μg?h-1?mg-1 | [ |
| Fe/Fe3O4 | 0.1mol/L PBS | -0.3 | 8.29 | 0.19μg?h-1?cm-2 | [ |
| 30% Fe2O3-CNT | 0.5mol/L KOH | -0.2 | 0.16 | 0.65μg?h-1?cm-2 | [ |
| β-FeOOH | 0.5mol/L LiClO4 | -0.7 | 6.7 | 23.32μg?h-1?mg-1 | [ |
| MoO3纳米片 | 0.1mol/L HCl | -0.3 | 1.9 | 29.43μg?h-1?mg-1 | [ |
| Mo2C纳米棒 | 0.1mol/L HCl | -0.3 | 8.13 | 95.1μg?h-1?mg-1 | [ |
| MoS2 | 0.1mol/L Na2SO4 | -0.5 | 1.17 | 8.08×10-11mol?s-1?cm-2 | [ |
| Mo2N | 0.1mol/L HCl | -0.3 | 4.5 | 78.4μg?h-1?mg-1 | [ |
| MoN | 0.1mol/L HCl | -0.3 | 1.15 | 3.01×10-10mol?s-1?cm-2 | [ |
| MoS2纳米花 | 0.1mol/L Na2SO4 | -0.4 | 8.34 | 29.28μg?h-1?mg-1 | [ |
| Mo2C | 0.5mol/L Li2SO4 | -0.3 | 1.1 | 11.3μg?h-1?mg-1 | [ |
| MoSe2纳米球 | 0.1mol/L Na2SO4 | -0.6 | 14.2 | 11.2μg?h-1?mg-1 | [ |
| SnO2 | 0.1mol/L Na2SO4 | -0.7 | 2.17 | 0.53μmmol?cm-2?h-1 | [ |
| Nb2O5 | 0.1mol/L HCl | -0.55 | 9.26 | 43.6μg?h-1?mg-1 | [ |
| NbO2纳米颗粒 | 0.05mol/L H2SO4 | -0.65 | 32 | 11.6μg?h-1?mg-1 | [ |
| TiO2 | 0.1mol/L HCl | 0.15 | 9.17 | 1.24×10-10mol?s-1?cm-2 | [ |
| Fe-TiO2 | 0.5mol/L LiClO4 | -0.4 | 25.6 | 25.47μg?h-1?mg-1 | [ |
| TiO2/Ti | 0.1mol/L Na2SO4 | -0.7 | 2.5 | 5.60μg?h-1?mg-1 | [ |
| Cr-CeO2 | 0.1mol/L Na2SO4 | -0.4 | 3.7 | 16.4μg?h-1?mg-1 | [ |
| Cu-CeO2 | 0.1mol/L Na2SO4 | -0.45 | 19.1 | 5.3×10-10mol?s-1?cm-2 | [ |
| MnO | 0.1mol/L Na2SO4 | -0.39 | 8.02 | 1.11×10-10mol?s-1?cm-2 | [ |
| WO3纳米片 | 0.1mol/L HCl | -0.3 | 7 | 17.28μg?h-1?mg-1 | [ |
| Cr2O3 | 0.1mol/L Na2SO4 | -0.9 | 6.78 | 25.3μg?h-1?mg-1 | [ |
| W2N3纳米片 | 0.1mol/L KOH | -0.2 | 11.67 | (11.66±0.98)μg?h-1?mg-1 | [ |
| NiN3 | 0.5mol/L LiClO4 | -0.8 | — | 115μg?cm-2?h-1 | [ |
| LaF3 | 0.5mol/L LiClO4 | -0.45 | 16 | 55.9μg?h-1?mg-1 | [ |
| VN/Ti | 0.1mol/L HCl | -0.5 | 2.25 | 5.14μg?h-1?cm-2 | [ |
| VN | 0.05mol/L H2SO4 | -0.1 | 6 | 20.2μg?h-1?cm-2 | [ |
| 单金属原子催化剂(SACs) | |||||
| ISAS-Fe/NC | 0.5mol/L LiSO4 | -0.4 | 18.6 | (62.9±2.7)μg?h-1?mg-1 | [ |
| Cu SAC[N] | 0.1mol/L KOH | -0.35 | 13.8 | 53.3μg?h-1?mg-1 | [ |
| Au/C3N4 | 5mmol/L H2SO4 | -0.1 | 11.1 | 1.3mg?h-1?mg-1 | [ |
| Au-TiO2 | 0.1mol/L HCl | -0.2 | 8.11 | 21.4μg?mg-1?h-1 | [ |
| Ru/NC | 0.1mol/L HCl | -0.21 | 8 | 3.665mg?h-1?mg-1 | [ |
| Ru-ZrO2/NC | 0.1mol/L HCl | -0.1 | 21 | 约1mg?h-1?mg-1 | [ |
| 碳基催化剂 | |||||
| B4C | 0.1mol/L HCl | -0.2 | 10.1 | 26.57μg?h-1?mg-1 | [ |
| B掺杂石墨烯 | 0.05mol/L H2SO4 | -0.75 | 4.83 | 9.8mg?h-1?cm-2 | [ |
| N掺杂多孔碳 | 0.1mol/L KOH | -0.85 | 3.52 | 57.8μg?h-1?cm-2 | [ |
| N掺杂的碳纳米片 | 0.1mol/L HCl | -0.5 | 7.1 | (97.18±7.13)mg?h-1?cm-2 | [ |
| S掺杂三维石墨烯 | 0.1mol/L HCl | -0.85 | 7.07 | 38.81μg?h-1?mg-1 | [ |
| S掺杂碳纳米球 | 0.1mol/L Na2SO4 | -0.5 | 6.9 | 19.07μg?h-1?mg-1 | [ |
| S掺杂石墨烯 | 0.1mol/L HCl | -0.5 | 5.89 | 27.3μg?h-1?mg-1 | [ |
| Cl掺杂石墨 | 0.1mol/L HCl | -0.45 | 8.7 | 10.7μg?h-1cm-2 | [ |
| F掺杂石墨烯 | 0.1mol/L KOH | -0.7 | 4.2 | 9.3μg?h-1?mg-1 | [ |
| F掺杂多孔碳 | 0.1mol/L HCl | -0.2 | 54.8 | 197.7μg?mg-1?h-1 | [ |
| O掺杂石墨烯 | 0.1mol/L HCl | -0.45 | 12.6 | 21.3μg?h-1?mg-1 | [ |
| 黑磷纳米片 | 0.01mol/L HCl | -0.6 | 5.07 | 31.37μg?h-1?mg-1 | [ |
| P掺杂石墨烯 | 0.1mol/L KOH | -0.65 | 20.82 | 32.33μg?h-1?mg-1 | [ |
| N,S-石墨烯 | 0.5mol/L LiClO4 | -0.6 | 5.8 | 7.7μg?h-1?mg-1 | [ |
| 金属+碳基 | |||||
| Au/CeOx-RGO | 0.1mol/L KOH | -0.2 | 10.1 | 24.7μg?h-1?mg-1 | [ |
| FeP2-rGO | 0.1mol/L HCl | -0.4 | 21.99 | 22.13μg?h-1?mg-1 | [ |
| TA-rGO | 0.1mol/L Na2SO4 | -0.75 | 4.83 | 17.02μg?h-1?mg-1 | [ |
| MoS2/石墨烯 | 0.1mol/L LiClO4 | -0.45 | 4.58 | 24.82μg?h-1?mg-1 | [ |
| TiO2-rGO | 0.1mol/L HCl | -0.9 | 3.3 | 15.13μg?h-1?mg-1 | [ |
| Mn3O4-rGO | 0.1mol/L Na2SO4 | -0.85 | 3.52 | 17.4μg?h-1?mg-1 | [ |
| Cr2O3-rGO | 0.1mol/L Na2SO4 | -0.6 | 7.33 | 33.3μg?h-1?mg-1 | [ |
| SnO2/RGO | 0.1mol/L HCl | -0.5 | 7.1 | 25.6μg?h-1?mg-1 | [ |
表2 不同电催化NRR合成氨催化剂的总结
| 催化剂 | 电解液 | 过电位/V | FE/% | 氨产率 | 参考文献 |
|---|---|---|---|---|---|
| 贵金属 | |||||
| Au纳米笼 | 0.5mol/L LiClO4 | -0.4 | 30.2 | 3.9μg?h-1?cm-2 | [ |
| Au纳米棒 | 0.1mol/L KOH | -0.2 | 4 | 1.648μg?h-1?cm-2 | [ |
| Ru纳米粒子 | 0.01mol/L HCl | 0.01 | 5.4 | 约0.2μg?h-1?cm-2 | [ |
| Rh纳米片 | 0.1mol/L KOH | -0.2 | 21.7 | 23.88μg?h-1?cm-2 | [ |
| Pd/C | 0.1mol/L PBS | -0.1 | 8.2 | 4.5μg?h-1?mg-1 | [ |
| Au/TiO2 | 0.1mol/L HCl | -0.2 | 8.11 | 21.4μg?h-1?mg-1 | [ |
| Pt/C | H+/Li+/NH4+ | -1.2 | 0.83 | 47.2μg?h-1?cm-2 | [ |
| 过渡金属及其杂化物 | |||||
| Bi纳米片 | 0.1mol/L Na2SO4 | -0.8 | 10.46 | (2.54±0.16)μg?cm-2?h-1 | [ |
| Bi4V2O11/CeO2 | 0.1mol/L HCl | -0.2 | 10.16 | 23.21μg??h-1?mg-1 | [ |
| Fe3S4纳米片 | 0.1mol/L HCl | -0.4 | 6.45 | 75.4μg?h-1?mg-1 | [ |
| Fe/Fe3O4 | 0.1mol/L PBS | -0.3 | 8.29 | 0.19μg?h-1?cm-2 | [ |
| 30% Fe2O3-CNT | 0.5mol/L KOH | -0.2 | 0.16 | 0.65μg?h-1?cm-2 | [ |
| β-FeOOH | 0.5mol/L LiClO4 | -0.7 | 6.7 | 23.32μg?h-1?mg-1 | [ |
| MoO3纳米片 | 0.1mol/L HCl | -0.3 | 1.9 | 29.43μg?h-1?mg-1 | [ |
| Mo2C纳米棒 | 0.1mol/L HCl | -0.3 | 8.13 | 95.1μg?h-1?mg-1 | [ |
| MoS2 | 0.1mol/L Na2SO4 | -0.5 | 1.17 | 8.08×10-11mol?s-1?cm-2 | [ |
| Mo2N | 0.1mol/L HCl | -0.3 | 4.5 | 78.4μg?h-1?mg-1 | [ |
| MoN | 0.1mol/L HCl | -0.3 | 1.15 | 3.01×10-10mol?s-1?cm-2 | [ |
| MoS2纳米花 | 0.1mol/L Na2SO4 | -0.4 | 8.34 | 29.28μg?h-1?mg-1 | [ |
| Mo2C | 0.5mol/L Li2SO4 | -0.3 | 1.1 | 11.3μg?h-1?mg-1 | [ |
| MoSe2纳米球 | 0.1mol/L Na2SO4 | -0.6 | 14.2 | 11.2μg?h-1?mg-1 | [ |
| SnO2 | 0.1mol/L Na2SO4 | -0.7 | 2.17 | 0.53μmmol?cm-2?h-1 | [ |
| Nb2O5 | 0.1mol/L HCl | -0.55 | 9.26 | 43.6μg?h-1?mg-1 | [ |
| NbO2纳米颗粒 | 0.05mol/L H2SO4 | -0.65 | 32 | 11.6μg?h-1?mg-1 | [ |
| TiO2 | 0.1mol/L HCl | 0.15 | 9.17 | 1.24×10-10mol?s-1?cm-2 | [ |
| Fe-TiO2 | 0.5mol/L LiClO4 | -0.4 | 25.6 | 25.47μg?h-1?mg-1 | [ |
| TiO2/Ti | 0.1mol/L Na2SO4 | -0.7 | 2.5 | 5.60μg?h-1?mg-1 | [ |
| Cr-CeO2 | 0.1mol/L Na2SO4 | -0.4 | 3.7 | 16.4μg?h-1?mg-1 | [ |
| Cu-CeO2 | 0.1mol/L Na2SO4 | -0.45 | 19.1 | 5.3×10-10mol?s-1?cm-2 | [ |
| MnO | 0.1mol/L Na2SO4 | -0.39 | 8.02 | 1.11×10-10mol?s-1?cm-2 | [ |
| WO3纳米片 | 0.1mol/L HCl | -0.3 | 7 | 17.28μg?h-1?mg-1 | [ |
| Cr2O3 | 0.1mol/L Na2SO4 | -0.9 | 6.78 | 25.3μg?h-1?mg-1 | [ |
| W2N3纳米片 | 0.1mol/L KOH | -0.2 | 11.67 | (11.66±0.98)μg?h-1?mg-1 | [ |
| NiN3 | 0.5mol/L LiClO4 | -0.8 | — | 115μg?cm-2?h-1 | [ |
| LaF3 | 0.5mol/L LiClO4 | -0.45 | 16 | 55.9μg?h-1?mg-1 | [ |
| VN/Ti | 0.1mol/L HCl | -0.5 | 2.25 | 5.14μg?h-1?cm-2 | [ |
| VN | 0.05mol/L H2SO4 | -0.1 | 6 | 20.2μg?h-1?cm-2 | [ |
| 单金属原子催化剂(SACs) | |||||
| ISAS-Fe/NC | 0.5mol/L LiSO4 | -0.4 | 18.6 | (62.9±2.7)μg?h-1?mg-1 | [ |
| Cu SAC[N] | 0.1mol/L KOH | -0.35 | 13.8 | 53.3μg?h-1?mg-1 | [ |
| Au/C3N4 | 5mmol/L H2SO4 | -0.1 | 11.1 | 1.3mg?h-1?mg-1 | [ |
| Au-TiO2 | 0.1mol/L HCl | -0.2 | 8.11 | 21.4μg?mg-1?h-1 | [ |
| Ru/NC | 0.1mol/L HCl | -0.21 | 8 | 3.665mg?h-1?mg-1 | [ |
| Ru-ZrO2/NC | 0.1mol/L HCl | -0.1 | 21 | 约1mg?h-1?mg-1 | [ |
| 碳基催化剂 | |||||
| B4C | 0.1mol/L HCl | -0.2 | 10.1 | 26.57μg?h-1?mg-1 | [ |
| B掺杂石墨烯 | 0.05mol/L H2SO4 | -0.75 | 4.83 | 9.8mg?h-1?cm-2 | [ |
| N掺杂多孔碳 | 0.1mol/L KOH | -0.85 | 3.52 | 57.8μg?h-1?cm-2 | [ |
| N掺杂的碳纳米片 | 0.1mol/L HCl | -0.5 | 7.1 | (97.18±7.13)mg?h-1?cm-2 | [ |
| S掺杂三维石墨烯 | 0.1mol/L HCl | -0.85 | 7.07 | 38.81μg?h-1?mg-1 | [ |
| S掺杂碳纳米球 | 0.1mol/L Na2SO4 | -0.5 | 6.9 | 19.07μg?h-1?mg-1 | [ |
| S掺杂石墨烯 | 0.1mol/L HCl | -0.5 | 5.89 | 27.3μg?h-1?mg-1 | [ |
| Cl掺杂石墨 | 0.1mol/L HCl | -0.45 | 8.7 | 10.7μg?h-1cm-2 | [ |
| F掺杂石墨烯 | 0.1mol/L KOH | -0.7 | 4.2 | 9.3μg?h-1?mg-1 | [ |
| F掺杂多孔碳 | 0.1mol/L HCl | -0.2 | 54.8 | 197.7μg?mg-1?h-1 | [ |
| O掺杂石墨烯 | 0.1mol/L HCl | -0.45 | 12.6 | 21.3μg?h-1?mg-1 | [ |
| 黑磷纳米片 | 0.01mol/L HCl | -0.6 | 5.07 | 31.37μg?h-1?mg-1 | [ |
| P掺杂石墨烯 | 0.1mol/L KOH | -0.65 | 20.82 | 32.33μg?h-1?mg-1 | [ |
| N,S-石墨烯 | 0.5mol/L LiClO4 | -0.6 | 5.8 | 7.7μg?h-1?mg-1 | [ |
| 金属+碳基 | |||||
| Au/CeOx-RGO | 0.1mol/L KOH | -0.2 | 10.1 | 24.7μg?h-1?mg-1 | [ |
| FeP2-rGO | 0.1mol/L HCl | -0.4 | 21.99 | 22.13μg?h-1?mg-1 | [ |
| TA-rGO | 0.1mol/L Na2SO4 | -0.75 | 4.83 | 17.02μg?h-1?mg-1 | [ |
| MoS2/石墨烯 | 0.1mol/L LiClO4 | -0.45 | 4.58 | 24.82μg?h-1?mg-1 | [ |
| TiO2-rGO | 0.1mol/L HCl | -0.9 | 3.3 | 15.13μg?h-1?mg-1 | [ |
| Mn3O4-rGO | 0.1mol/L Na2SO4 | -0.85 | 3.52 | 17.4μg?h-1?mg-1 | [ |
| Cr2O3-rGO | 0.1mol/L Na2SO4 | -0.6 | 7.33 | 33.3μg?h-1?mg-1 | [ |
| SnO2/RGO | 0.1mol/L HCl | -0.5 | 7.1 | 25.6μg?h-1?mg-1 | [ |
| 1 | SHIPMAN M A, SYMES M D. Recent progress towards the electrosynthesis of ammonia from sustainable resources[J]. Catalysis Today, 2017, 286(1): 57-68. |
| 2 | Xianwei LYU, WENG Chenchen, YUAN Zhongyong. Ambient ammonia electrosynthesis: current status, challenges, and perspectives[J]. ChemSusChem, 2020, 13(12): 3061-3078. |
| 3 | PICKETT C J, TALARMIN J. Electrosynthesis of ammonia[J]. Nature, 1985, 317(6038): 652-653. |
| 4 | FURUYA N, YOSHIBA H. Electroreduction of nitrogen to ammonia on gas-diffusion electrodes loaded with inorganic catalyst[J]. Journal of Electroanalytical Chemistry & Interfacial Electrochemistry, 1990, 291(1/2): 269-272. |
| 5 | BAO Di, ZHANG Qi, MENG Fanlu, et al. Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle[J]. Advanced Materials, 2017, 29(3): 1604799-1604803. |
| 6 | WANG Ziqiang, LI Yinghao, YU Hongjie, et al. Ambient electrochemical synthesis of ammonia from nitrogen and water catalyzed by flower-like gold microstructures[J]. ChemSusChem, 2018, 17(32): 3480-3485. |
| 7 | WANG D B, AZOFRA L M, HARB M, et al. Energy efficient nitrogen reduction to ammonia at low overpotential in aqueous electrolyte under ambient conditions[J]. ChemSusChem, 2018, 11(24): 3416-3422. |
| 8 | KORDALI V, KYRIACOU G, LAMBROU C. Electrochemical synthesis of ammonia at atmospheric pressure and low temperature in a solid polymer electrolyte cell[J]. Chemical Communications, 2000, 17: 1673-1684. |
| 9 | MANJUNATHA R, SCHECHTER A. Electrochemical synthesis of ammonia using ruthenium-platinum alloy at ambient pressure and low temperature[J]. Electrochemistry Communications, 2018, 90(2): 96-100. |
| 10 | LIU Huimin, HAN Shuhe, ZHAO Yue, et al. Surfactant-free atomically ultrathin rhodium nanosheet nanoassemblies for efficient nitrogen electroreduction[J]. Journal of Materials Chemistry A, 2018, 6(7): 3211-3217. |
| 11 | WANG Jun, YU Liang, HU Lin, et al. Ambient ammonia synthesis via palladium-catalyzed electrohydrogenation of dinitrogen at low overpotential[J]. Nature Communications, 2018, 9(1): 1795-1806. |
| 12 | WANG Hong, WANG Lu, WANG Qiang, et al. Ambient electrosynthesis of ammonia: electrode porosity and composition engineering[J]. Angew. Chem. Int. Ed., 2018, 57(38): 12360-12364. |
| 13 | ZHANG Shengbo, ZHAO Cuijiao, LIU Yanyan, et al. Cu doping in CeO2 to form multiple oxygen vacancies for dramatically enhanced ambient N2 reduction performance[J]. Chem. Commun., 2019, 55(20): 2952-2955. |
| 14 | XU Bo, XIA Li, ZHOU Fuling, et al. Enhancing electrocatalytic N2 reduction to NH3 by CeO2 nanorod with oxygen vacancies[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(3): 2889-2893. |
| 15 | HAN Jingrui, JI Xuqiang, REN Xiang, et al. MoO3 nanosheets for efficient electrocatalytic N2 fixation to NH3[J]. Journal of Materials Chemistry A, 2018, 6(27): 12974-12977. |
| 16 | KONG Wenhan, ZHANG Rong, ZHANG Xiaoxue, et al. WO3 nanosheets rich in oxygen vacancies for enhanced electrocatalytic N2 reduction to NH3[J]. Nanoscale, 2019, 11(41): 19274-19277. |
| 17 | Fang LYU, ZHAO Shunzheng, GUO Ruijie, et al. Nitrogen-coordinated single Fe sites for efficient electrocatalytic N2 fixation in neutral media[J]. Nano Energy, 2019, 61(13): 420-427. |
| 18 | ZANG Wenjie, YANG Tong, ZOU Haiyuan, et al. Copper single atoms anchored in porous nitrogen-doped carbon as efficient pH-universal catalysts for the nitrogen reduction reaction[J]. ACS Catalysis, 2019, 2019(38): 2944-2956. |
| 19 | WANG Xiaoqian, WANG Wenyu, QIAO Man, et al. Atomically dispersed Au1 catalyst towards efficient electrochemical synthesis of ammonia[J]. Science Bulletin, 2018, 63(19): 1246-1253. |
| 20 | ZHAO Jingxiang, CHEN Zhongfang. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: a computational study[J]. J. Am. Chem. Soc., 2017, 139(36): 12480-12487. |
| 21 | LING Chongyi, BAI Xiaowan, YANG Yixin. et al. Single molybdenum atom anchored on N-doped carbon as a promising electrocatalyst for nitrogen reduction into ammonia at ambient conditions[J]. The Journal of Physical Chemistry C, 2018, 122(29): 16842-16847. |
| 22 | TANG Cheng, QIAO Shizhang. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully[J]. Chemical Society Reviews, 2019, 48(23): 2366-2380. |
| 23 | SKULASON E, BLIGAARD T, GUDMUNDSDOTTIR S, et al. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction[J]. Physical Chemistry Chemical Physics, 2012, 14(3): 1235-1245. |
| 24 | SHI Li, LI Qiang, LING Chongyi, et al. Metal-free electrocatalyst for reducing nitrogen to ammonia using a Lewis acid pair[J]. Journal of Materials Chemistry A, 2019, 7(9): 4865-4871. |
| 25 | LÉGARÉ M A, BÉLANGER-CHABOT G, DEWHURST R D, et al. Nitrogen fixation and reduction at boron[J]. Science, 2018, 359(12): 896-900. |
| 26 | LING Chongyi, NIU Xianghong, LI Qiang, et al. Metal-free single atom catalyst for N2 fixation driven by visible light[J]. Journal of the American Chemical Society, 2018, 140(43): 14161-14168. |
| 27 | GUO Wenhan, ZHANG Kexin, LIANG Zibin, et al. Electrochemical nitrogen fixation and utilization: theories, advanced catalyst materials and system design[J]. Chem. Soc. Rev., 2019, 48(24): 5658-5716. |
| 28 | YANG Xuan, NASH Jared, ANIBAL Jacob, et al. Mechanistic insights into electrochemical nitrogen reduction reaction on vanadium nitride nanoparticles[J]. Journal of the American Chemical Society, 2018, 140(41): 13387-13391. |
| 29 | ABGHOUI Y, SKÚLASON E. Onset potentials for different reaction mechanisms of nitrogen activation to ammonia on transition metal nitride electro-catalysts[J]. Catalysis Today, 2017, 286(2): 69-77. |
| 30 | SINGH A R, ROHR B A, SCHWALBE J A, et al. Electrochemical ammonia synthesis—the selectivity challenge[J]. ACS Catalysis, 2016, 7(1): 706-709. |
| 31 | CHOI Changhyeok, BACK S, KIM N, et al. Suppression of hydrogen evolution reaction in electrochemical N2 reduction using single-atom catalysts: a computational guideline[J]. ACS Catalysis, 2018, 8(8): 7517-7525. |
| 32 | MARTÍN A J, SHINAGAWA T, PÉREZ-RAMÍREZ J. Electrocatalytic reduction of nitrogen: from haber-bosch to ammonia artificial leaf[J]. Chem, 2019, 5(2): 263-283. |
| 33 | NASH J, YANG Xuan, ANIBAL Jaced, et al. Electrochemical nitrogen reduction reaction on noble metal catalysts in proton and hydroxide exchange membrane electrolyzers[J]. Journal of the Electrochemical Society, 2017, 164(14): F1712-F1716. |
| 34 | HOWALT J G, VEGGE T. Electrochemical ammonia production on molybdenum nitride nanoclusters[J]. Physical Chemistry Chemical Physics, 2013, 15(48): 20957-20965. |
| 35 | CHU Ke, LIU Yaping, LI Yubiao, et al. Electronically coupled SnO2 quantum dots and graphene for efficient nitrogen reduction reaction[J]. ACS Applied Materials & Interfaces, 2019, 11(35): 758-763. |
| 36 | ZHANG Ling, REN Xiang, LUO Yonglan, et al. Ambient NH3 synthesis via electrochemical reduction of N2 over cubic sub-micron SnO2 particles[J]. Chemical Communications, 2018, 54(92): 12966-12969. |
| 37 | CHU Ke, LIU Yaping, LI Yubiao, et al. Efficient electrocatalytic N2 reduction on CoO quantum dots[J]. Journal of Materials Chemistry A, 2019, 7(9): 4389-4394. |
| 38 | HAN Jingrui, LIU Zaichun, MA Yongjun, et al. Ambient N2 fixation to NH3 at ambient conditions: using Nb2O5 nanofiber as a high-performance electrocatalyst[J]. Nano Energy, 2018, 52(15): 264-270. |
| 39 | ZHU Xiaojuan, LIU Zaichun, LIU Qin, et al. Efficient and durable N2 reduction electrocatalysis under ambient conditions: beta-FeOOH nanorods as a non-noble-metal catalyst[J]. Chemical Communications, 2018, 54(80): 11332-11335. |
| 40 | LI Laiquan, TANG Cheng, XIA Bingquan, et al. Two-dimensional mosaic bismuth nanosheets for highly selective ambient electrocatalytic nitrogen reduction[J]. ACS Catalysis, 2019, 9(4): 2902-2908. |
| 41 | QIN Qing, ZHAO Yun, SCHMALLEGGER Max, et al. Enhanced electrocatalytic N2 reduction via partial anion substitutionin titanium oxide-carbon composites[J]. Angewandte Chemie, 2019, 131(37): 13235-13240. |
| 42 | ZHAO Lu, KUANG Xuan, CHEN Cheng, et al. Boosting electrocatalytic nitrogen fixation via energy-efficient anodic oxidation of sodium gluconate[J]. Chemical Communications, 2019, 55(68): 10170-10173. |
| 43 | SONG Zhongxin, ZHANG Lei, Kieran DOYLE-DAVIS, et al. Recent advances in MOF-derived single atom catalysts for electrochemical applications[J]. Advanced Energy Materials, 2020, 10(38): 2001561-2001567. |
| 44 | ZHANG Ya, QIU Weibin, MA Yongjun, et al. High-performance electrohydrogenation of N2 to NH3 catalyzed by multishelled hollow Cr2O3 microspheres under ambient conditions[J]. ACS Catalysis, 2018, 8(9): 8540-8544. |
| 45 | NAZEMI M, PANIKKANVALAPPIL S R, EL-SAYED M A. Enhancing the rate of electrochemical nitrogen reduction reaction for ammonia synthesis under ambient conditions using hollow gold nanocages[J]. Nano Energy, 2018, 49(39): 316-323. |
| 46 | YANG Liuxin, WANG Hui, WANG Xin, et al. Flower-like hollow MoSe2 nanospheres as efficient earth-abundant electrocatalysts for nitrogen reduction reaction under ambient conditions[J]. Inorg. Chem., 2020, 59(17): 12941-12946. |
| 47 | WAN Yuchi, XU Jichu, Rutiao LYU. Heterogeneous electrocatalysts design for nitrogen reduction reaction under ambient conditions[J]. Materials Today, 2019, 27(1): 69-90. |
| 48 | ZHANG Rong, REN Xiang, SHI Xifeng, et al. Enabling effective electrocatalytic N2 conversion to NH3 by the TiO2 nanosheets array under ambient conditions[J]. ACS Appl. Mater. Interfaces, 2018, 10(34): 28251-28255. |
| 49 | ZHANG Ling, XIE Xiaoying, WANG Huanbo, et al. Boosting electrocatalytic N2 reduction by MnO2 with oxygen vacancies[J]. Chemical Communications, 2019, 55(32): 4627-4630. |
| 50 | LI Lei, WANG Xingyong, GUO Haoran, et al. Theoretical screening of single transition metal atoms embedded in MXene defects as superior electrocatalyst of nitrogen reduction reaction[J]. Small Methods, 2019, 3(11): 1900337-1900342. |
| 51 | CHEN Shiming, PERATHONER Siglinda, AMPELLI Claudio, et al. Room-temperature electrocatalytic synthesis of NH3 from H2O and N2 in a gas-liquid-solid three-phase reactor[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(8): 7393-7400. |
| 52 | LYU C, YAN Chunshuang, CHEN Gang, et al. An amorphous noble-metal-free electrocatalyst that enables nitrogen fixation under ambient conditions[J]. Angewandte Chemie International Edition, 2018, 57(21): 6073-6076. |
| 53 | LI Hong, TSAI Charlie, Ai Leen KOH, et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies[J]. Nature Materials, 2016, 15(1): 48-53. |
| 54 | SURYANTO B H R, WANG Dabin, AZOFRA L M, et al. MoS2 polymorphic engineering enhances selectivity in the electrochemical reduction of nitrogen to ammonia[J]. ACS Energy Letters, 2018, 4(2): 430-435. |
| 55 | LYU C, QIAN Yumin, YAN Chunshuang, et al. Defect engineering metal-free polymeric carbon nitride electrocatalyst for effective nitrogen fixation under ambient conditions[J]. Angewandte Chemie International Edition, 2018, 57(32): 10246-10250. |
| 56 | JIN Huanyu, LI Laiquan, LIU Xin, et al. Nitrogen vacancies on 2D layered W2N3: a stable and efficient active site for nitrogen reduction reaction[J]. Adv. Mater., 2019, 31(32): 1902709-1902716. |
| 57 | ZHAO Shenlong, LU Xunyu, WANG Lianzhou, et al. Carbon-based metal-free catalysts for electrocatalytic reduction of nitrogen for synthesis of ammonia at ambient conditions[J]. Adv. Mater., 2019, 31(13): 1805367-1805374. |
| 58 | ZHANG Xiaoxue, WU Tongwei, WANG Huanbo, et al. Boron nanosheet: an elemental two-dimensional (2D) material for ambient electrocatalytic N2-to-NH3 fixation in neutral media[J]. ACS Catalysis, 2019, 9(5): 4609-4615. |
| 59 | CHEN Chen, YAN Dafang, WANG Yu, et al. B-N pairs enriched defective carbon nanosheets for ammonia synthesis with high efficiency[J]. Small, 2019, 15(7): e1805029. |
| 60 | YU Xiaomin, HAN Peng, WEI Zengxi, et al. Boron-doped graphene for electrocatalytic N2 reduction[J]. Joule, 2018, 2(8): 1610-1622. |
| 61 | LIU Yanming, SU Yan, QUAN Xie, et al. Facile ammonia synthesis from electrocatalytic N2 reduction under ambient conditions on N-doped porous carbon[J]. ACS Catalysis, 2018, 8(2): 1186-1191. |
| 62 | CHEN Hongyu, ZHU Xiaojuan, HUANG Hong, et al. Sulfur dots-graphene nanohybrid: a metal-free electrocatalyst for efficient N2-to-NH3 fixation under ambient conditions[J]. Chemical Communications, 2019, 55(21): 3152-3155. |
| 63 | XIA Li, YANG Jiajia, WANG Huanbo, et al. Sulfur-doped graphene for efficient electrocatalytic N2-to-NH3 fixation[J]. Chemical Communications, 2019, 55(23): 3371-3374. |
| 64 | WU Tongwei, LI Xinyi, ZHU Xiaojuan, et al. P-doped graphene toward enhanced electrocatalytic N2 reduction[J]. Chemical Communications, 2020, 56(12): 1831-1834. |
| 65 | ZHANG Rong, JI Ling, KONG Wang, et al. Electrocatalytic N2-to-NH3 conversion with high faradaic efficiency enabled using a Bi nanosheet array[J]. Chemical Communications, 2019, 55(36): 5263-5266. |
| 66 | WANG Ting, XIA Li, YANG Jiajia, et al. Electrocatalytic N2-to-NH3 conversion using oxygen-doped graphene: experimental and theoretical studies[J]. Chemical Communications, 2019, 55(52): 7502-7505. |
| 67 | LIU Yan, LI Qiuyao, GUO Xu, et al. A highly efficient metal-free electrocatalyst of F-doped porous carbon toward N2 electroreduction[J]. Adv. Mater., 2020, 32(24): 1907690-1907695. |
| 68 | ZOU Haiyuan, RONG Weifeng, LONG Baihua, et al. Corrosion-induced Cl-doped ultrathin graphdiyne toward electrocatalytic nitrogen reduction at ambient conditions[J]. ACS Catalysis, 2019, 9(12): 10649-10655. |
| 69 | SHI Miaomiao, BAO Di, WULAN B, et al. Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions[J]. Advanced Materials, 2017, 29(17): 1606550-1606555. |
| 70 | LAN Rong, IRVINE J T S, TAO Shanwen. Synthesis of ammonia directly from air and water at ambient temperature and pressure[J]. Sci. Rep., 2013, 3(1): 1-7. |
| 71 | ZHAO Xinhui, LAN Xue, YU Dongkun, et al. Deep eutectic-solvothermal synthesis of nanostructured Fe3S4 for electrochemical N2 fixation under ambient conditions[J]. Chemical Communications, 2018, 54(92): 13010-13013. |
| 72 | HU Lin, KHANIYA Asim, WANG Jun, et al. Ambient electrochemical ammonia synthesis with high selectivity on Fe/Fe oxide catalyst[J]. ACS Catalysis, 2018, 8(10): 9312-9319. |
| 73 | REN Xiang, ZHAO Jinxiu, WEI Qin, et al. High-performance N2-to-NH3 conversion electrocatalyzed by Mo2C nanorod[J]. ACS Cent. Sci., 2019, 5(1): 116-121. |
| 74 | ZHANG Ling, JI Xuqiang, REN Xiang, et al. Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 catalyst: theoretical and experimental studies[J]. Adv. Mater., 2018, 30(28): e1800191. |
| 75 | REN Xiang, CUI Guanwei, CHEN Liang, et al. Electrochemical N2 fixation to NH3 under ambient conditions: Mo2N nanorod as a highly efficient and selective catalyst[J]. Chemical Communications, 2018, 54(61): 8474-8477. |
| 76 | ZHANG Ling, JI Xuqiang, REN Xiang, et al. Efficient electrochemical N2 reduction to NH3 on MoN nanosheets array under ambient conditions[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 9550-9554. |
| 77 | CHENG Hui, DING Liangxin, CHEN Gaofeng, et al. Molybdenum carbide nanodots enable efficient electrocatalytic nitrogen fixation under ambient conditions[J]. Adv. Mater., 2018, 30(46): 1803694-1803697. |
| 78 | HUANG Linsong, WU Jiawen, HAN Peng,et al. NbO2 electrocatalyst toward 32% faradaic efficiency for N2 fixation[J]. Small Methods, 2019, 3(6): 1800386. |
| 79 | YANG Li, WU Tongwei, ZHANG Rong, et al. Insights into defective TiO2 in electrocatalytic N2 reduction: combining theoretical and experimental studies[J]. Nanoscale, 2019, 11(4): 1555-1562. |
| 80 | WU Tongwei, ZHU Xiaojuan, XING Zhe, et al. Greatly improving electrochemical N2 reduction over TiO2 nanoparticles by iron doping[J]. Angewandte Chemie, 2019, 58(51): 18449-18453. |
| 81 | WANG Zao, GONG Feng, ZHANG Ling, et al. Electrocatalytic hydrogenation of N2 to NH3 by MnO: experimental and theoretical investigations[J]. Adv. Sci., 2019, 6(1): 1801182-1801190. |
| 82 | HAN Lili, LIU Xijun, CHEN Jinping, et al. Atomically dispersed molybdenum catalysts for efficient ambient nitrogen fixation[J]. Angewandte Chemie International Edition, 2019, 58(8): 2321-2325. |
| 83 | LI Peipei, LIU Zaichun, WU Tongwei, et al. Ambient electrocatalytic N2 reduction to NH3 by metal fluorides[J]. Journal of Materials Chemistry A, 2019, 7(30): 17761-17765. |
| 84 | TAO Hengcong, CHOI Changhyeok, DING Liangxin, et al. Nitrogen fixation by Ru single-atom electrocatalytic reduction[J]. Chem, 2019, 5(1): 204-214. |
| 85 | TAO Huan, LI Na, ZHANG Zhao, et al. Erlotinib protects LPS-induced acute lung injury in mice by inhibiting EGFR/TLR4 signaling pathway[J]. Shock, 2019, 51(1): 131-138. |
| 86 | ZHANG Ya, QIU Weibin, Ma Yongjun, et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst[J]. Nature Communications, 2018, 9(2): 3485. |
| 87 | MUKHERJEE S, CULLEN D A, KARAKALOS Stavros, et al. Metal-organic framework-derived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N2 and H2O in alkaline electrolytes[J]. Nano Energy, 2018, 48(5): 217-226. |
| 88 | SONG Yang, JOHNSON D, PENG Rui, et al. A physical catalyst for the electrolysis of nitrogen to ammonia[J]. Science Advances, 2018, 4(4): e1700336. |
| 89 | ZHANG Lipeng, NIU Jianbing, LI Mingtao, et al. Catalytic mechanisms of sulfur-doped graphene as efficient oxygen reduction reaction catalysts for fuel cells[J]. The Journal of Physical Chemistry C, 2014, 118(7): 3545-3553. |
| 90 | LIU Lin, LIU Yuhong, LIU Chaoxiang, et al. Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions[J]. Ecological Engineering, 2013, 53(1):138-143. |
| 91 | ZHAO Jinxiu, YANG Jiajia, LEI Ji, et al. Defect-rich fluorographene nanosheets for artificial N2 fixation under ambient conditions[J]. Chemical Communications, 2019, 55(29): 4266-4269. |
| 92 | LIU Yan, LI Qiuyao, GUO Xu, et al. A highly efficient metal free electrocatalyst of F doped porous carbon toward N2 electroreduction[J]. Advanced Materials, 2020, 32(24): 1907690. |
| 93 | ZHANG Lili, DING LiangXin, CHEN Gaofeng, et al. Ammonia synthesis under ambient conditions: selective electroreduction of dinitrogen to ammonia on black phosphorus nanosheets[J]. Angewandte Chemie International Edition, 2019, 58(9): 2612-2616. |
| 94 | TIAN Ye, XU Dazhong, CHU Ke, et al. Metal-free N, S co-doped graphene for efficient and durable nitrogen reduction reaction[J]. Journal of Materials Science, 2019, 54(12): 9088-9097. |
| 95 | LI Sijia, BAO Di, SHI Miaomiao, et al. Amorphizing of Au nanoparticles by CeOx-RGO hybrid support towards highly efficient electrocatalyst for N2 reduction under ambient conditions[J]. Adv. Mater., 2017, 29(33): 1700001-1700006. |
| 96 | ZHU Xiaojuan, WU Tongwei, JI Lei, et al. Unusual electrochemical N2 reduction activity in an earth-abundant iron catalyst via phosphorous modulation[J]. Chemical Communications, 2020, 56(5): 731-734. |
| 97 | SONG Yanyan, WANG Ting, SUN Junwei, et al. Enhanced electrochemical N2 reduction to NH3 on reduced graphene oxide by tannic acid modification[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(17): 14368-14372. |
| 98 | LI Xianghong, REN Xiang, LIU Xuejing, et al. A MoS2 nanosheet-reduced graphene oxide hybrid: an efficient electrocatalyst for electrocatalytic N2 reduction to NH3 under ambient conditions[J]. Journal of Materials Chemistry A, 2019, 7(6): 2524-2528. |
| 99 | HUANG Hong, GONG Feng, WANG Yuan, et al. Mn3O4 nanoparticles reduced graphene oxide composite: an efficient electrocatalyst for artificial N2 fixation to NH3 at ambient conditions[J]. Nano Research, 2019, 12(5): 1093-1098. |
| 100 | XIA Li, LI Baihai, ZHANG Ya, et al. Cr2O3 nanoparticle-reduced graphene oxide hybrid: a highly active electrocatalyst for N2 reduction at ambient conditions[J]. Inorg. Chem., 2019, 58(4): 2257-2260. |
| 101 | WANG Weikang, ZHANG Haimin, ZHANG Shengbo, et al. Potassium-ion-assisted regeneration of active cyano groups in carbon nitride nanoribbons: visible-light-driven photocatalytic nitrogen reduction[J]. Angewandte Chemie International Edition, 2019, 58(46): 16644-16650. |
| 102 | LI Yan, KONG Yan, HOU Yang, et al. In situ growth of nitrogen-doped carbon-coated γ-Fe2O3 nanoparticles on carbon fabric for electrochemical N2 fixation[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(9): 8853-8859. |
| 103 | YANDULOV Dmitry V, SCHROCK Richard R. et al. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center[J]. Science, 2003, 301(5629): 76-78. |
| 104 | ZHOU Fengling, AZOFRA Luis Miguel, Muata ALI, et al. Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids[J]. Energy & Environmental Science, 2017, 10(12): 2516-2520. |
| 105 | LICHT S, CUI Baochen, WANG Baohui, et al. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3[J]. Science, 2014, 345(6197): 637-640. |
| 106 | LIU Ruiquan, XIE Yahong, WANG Jide, et al. Synthesis of ammonia at atmospheric pressure with Ce0.8M0.2O2-δ (M = La, Y, Gd, Sm) and their proton conduction at intermediate temperature[J]. Solid State Ionics, 2006, 177(1/2): 73-76. |
| 107 | PANG Fangjie, WANG Fei, YANG Liting, et al. Hierarchical nanoporous Pd1Ag1 alloy enables efficient electrocatalytic nitrogen reduction under ambient conditions[J]. Chemical Communications, 2019, 55(68): 10108-10111. |
| 108 | CHEN Shiming, PERATHONER Siglinda, AMPELLI Claudio, et al. Electrocatalytic synthesis of ammonia at room temperature and atmospheric pressure from water and nitrogen on a carbon-nanotube-based electrocatalyst[J]. Angewandte Chemie International Edition, 2017, 56(10): 2699-2703. |
| 109 | THOMSEN L B, NIELSEN G, VENDELBO S B, et al. Ultralarge area MoS tunnel devices for electron emission[J]. Physical Review B, 2007, 76(15): 155315. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [6] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [7] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [8] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 刘炫麟, 王驿凯, 戴苏洲, 殷勇高. 热泵中氨基甲酸铵分解反应特性及反应器结构优化[J]. 化工进展, 2023, 42(9): 4522-4530. |
| [11] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [15] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||