化工进展 ›› 2021, Vol. 40 ›› Issue (10): 5302-5312.DOI: 10.16085/j.issn.1000-6613.2021-0654
废加氢催化剂的回收现状与研究进展
- 1.北京科技大学新材料技术研究院,北京 100083
2.北京科技大学顺德研究生院,广东 佛山 528399
-
收稿日期:2021-03-30修回日期:2021-07-22出版日期:2021-10-10发布日期:2021-10-25 -
通讯作者:丁云集,张深根 -
作者简介:史志胜(1992—),男,博士研究生,研究方向为废催化剂资源化利用。E-mail:shizhisheng1992@163.com 。 -
基金资助:国家自然科学基金重点项目(U2002212);广东省基础与应用基础研究基金(2020A1515110408);北京科技大学顺德研究生院博士后科研经费项目(2020BH004);佛山市人民政府科技创新专项项目(BK21BE002);中央高校基本科研业务费项目(FRF-TP-20-031A1)
Status and research progress on recovery of spent hydrogenation catalysts
SHI Zhisheng1( ), DING Yunji1,2(
), DING Yunji1,2( ), ZHANG Shengen1(
), ZHANG Shengen1( )
)
- 1.Institute for Advanced Materials and Technology, University of Science and Technology Beijing, Beijing 100083, China
2.Shunde Graduate School, University of Science and Technology Beijing, Foshan 528399, Guangdong, China
-
Received:2021-03-30Revised:2021-07-22Online:2021-10-10Published:2021-10-25 -
Contact:DING Yunji,ZHANG Shengen
摘要:
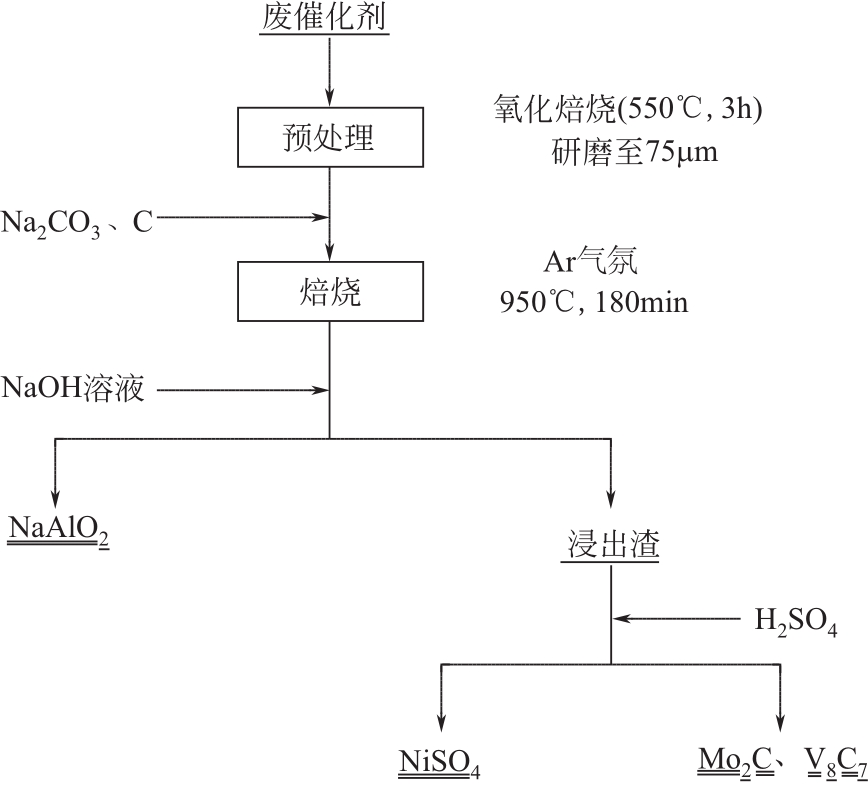
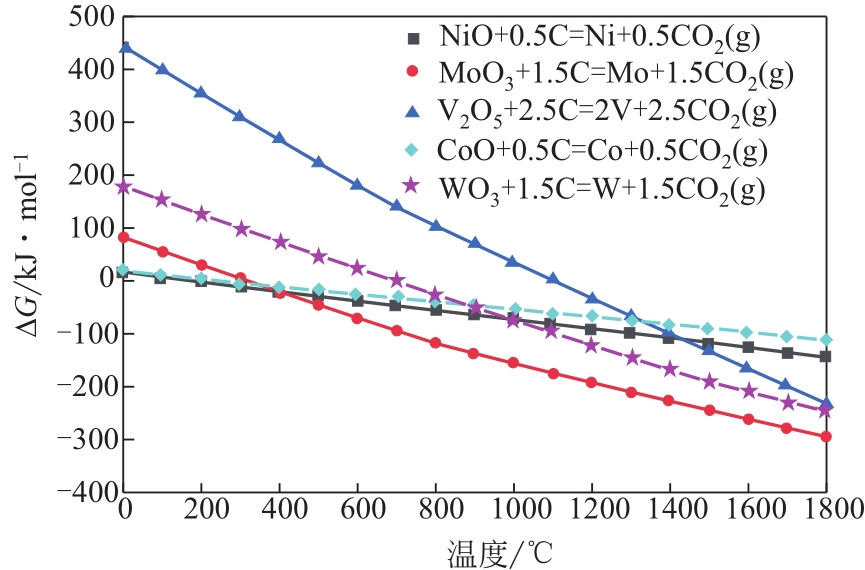
废加氢催化剂因含难降解有机物和Mo、W、Ni、Co、V等战略金属,是危险废弃物和重要的二次资源,资源化利用具有显著的经济、社会和环境效益。本文介绍了加氢催化剂概况,综述了废加氢催化剂的回收现状,包括酸浸出、碱浸出、焙烧-浸出、火法富集。文章指出,回收前需采用溶剂洗涤法、机械法或焙烧法进行有机物脱除。酸法浸出酸浓度较高,对设备腐蚀性大;碱法浸出对Ni和Co的回收率低,采用碱法、酸法两步浸出可实现多金属高效回收;但湿法回收存在废水量大、污染严重等问题。焙烧-浸出是目前主流回收方法,已产业化应用,但存在回收流程长、后续浸出废水量大等问题。针对现有技术废水量大、污染严重等问题,本文提出了碳热还原富集回收有价金属、尾渣用于绿色建材的方法。
中图分类号:
引用本文
史志胜, 丁云集, 张深根. 废加氢催化剂的回收现状与研究进展[J]. 化工进展, 2021, 40(10): 5302-5312.
SHI Zhisheng, DING Yunji, ZHANG Shengen. Status and research progress on recovery of spent hydrogenation catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5302-5312.
| 加氢过程 | 催化活性对比 |
|---|---|
| 加氢脱硫 | Mo-Co>Mo-Ni>W-Ni |
| 加氢脱氮 | Mo-Ni=W-Ni>Mo-Co |
| 加氢脱氧 | Mo-Ni>Mo-Co>W-Ni |
| 加氢饱和 | W-Ni>Mo-Ni>Mo-Co |
表1 不同体系加氢催化剂催化加氢活性对比[5]
| 加氢过程 | 催化活性对比 |
|---|---|
| 加氢脱硫 | Mo-Co>Mo-Ni>W-Ni |
| 加氢脱氮 | Mo-Ni=W-Ni>Mo-Co |
| 加氢脱氧 | Mo-Ni>Mo-Co>W-Ni |
| 加氢饱和 | W-Ni>Mo-Ni>Mo-Co |
| 废催化剂 组成 | 浸出方法 | 主要工艺参数 | 浸出率 | 参考文献 |
|---|---|---|---|---|
| Mo,Co | HCl | 3mol/L,固体添加量5%(质量分数),90℃,60min | Mo 97%;Co 94% | [ |
| Mo,Ni | H2SO4 | 9mol/L,固液比1/2.54(g/mL),90℃,90min | Mo 99%;Ni 99% | [ |
| Mo,Co | H2SO4 | 20%(体积分数),固体添加量5%(质量分数),90℃,2h | Mo 91%;Co 78% | [ |
| Ni | HNO3 | 5mol/L,固液比1/10(g/mL),90℃,120min | Ni 99% | [ |
| Mo,Ni,V | HNO3∶H2SO4∶HCl=2∶1∶1(体积比) | 固液比为70g/L,70℃,60min | Mo 90%;Ni 99%;V 99% | [ |
| Mo,Ni,Co | HCOOH | 0.6mol/L,固液比1/10(g/mL),80℃,90min | Mo 76%、Ni 93%、Co 97% | [ |
| Mo,Ni | H2C2O4 | 1mol/L,固液比1/10(g/mL),40℃,3h | Mo 92%;Ni 19% | [ |
| Mo,Ni,Co | EDTA | 0.2mol/L,固液比1/15(g/mL),60℃,60min | Mo 90%;Ni 95%;Co 97% | [ |
| Mo,Co | H2SO4+H2O2(pH=1.3) | H2O2浓度3.75mol/L,固液比1/7.5(g/mL),60℃,1h | Mo 90%;Co 83% | [ |
| Mo,Ni,V | H2C2O4+H2O2 | H2C2O4浓度0.5mol/L,H2O2浓度3mol/L,60℃,1h | Mo 90%;Ni 65%;V 94% | [ |
| Mo,V | NaOH加压 | NaOH 30%(g/mL),250℃ | Mo 98%;V 95% | [ |
| W,V | NaOH+Na2CO3加压 | NaOH浓度2mol/L,Na2CO3浓度0.2mol/L,固液比1/20(g/mL),300℃,2h | W>90%;V>90% | [ |
| Mo,V | NH3·H2O+H2O2 | NH3·H2O浓度4.5mol/L,H2O2浓度为1.0mol/L,固液比1/20(g/mL),140℃,2h | Mo 95%;V 46% | [ |
| Mo | Na2CO3+H2O2 | Na2CO3浓度85g/L,H2O2 10%(体积分数),废催化剂添加量20%(质量分数),25℃,1h | Mo 84% | [ |
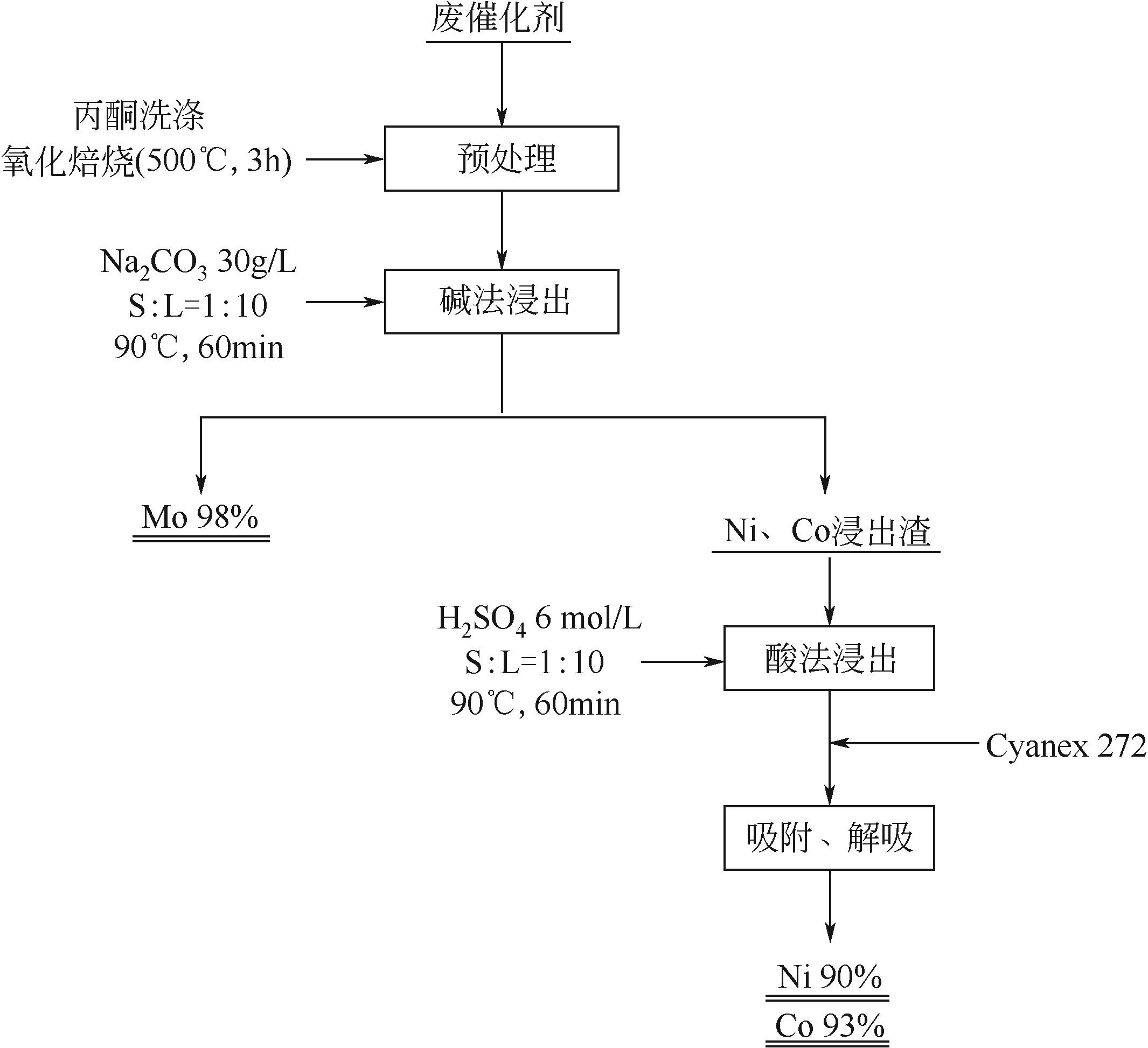
| Mo,Ni,Co | Na2CO3+H2SO4两步浸出 | Na2CO3浓度为30g/L,固液比1/10(g/mL),90℃,60min;H2SO4浓度为6mol/L,固液比1/10(g/mL),90℃,60min | Mo 98%;Ni 90%;Co 93% | [ |
| Mo,V | NaOH微波辅助 | 微波功率600W,NaOH浓度2mol/L,固液比1/5(g/mL),90℃,10min | Mo 96%;V 94% | [ |
表2 废加氢催化剂湿法浸出有价金属技术
| 废催化剂 组成 | 浸出方法 | 主要工艺参数 | 浸出率 | 参考文献 |
|---|---|---|---|---|
| Mo,Co | HCl | 3mol/L,固体添加量5%(质量分数),90℃,60min | Mo 97%;Co 94% | [ |
| Mo,Ni | H2SO4 | 9mol/L,固液比1/2.54(g/mL),90℃,90min | Mo 99%;Ni 99% | [ |
| Mo,Co | H2SO4 | 20%(体积分数),固体添加量5%(质量分数),90℃,2h | Mo 91%;Co 78% | [ |
| Ni | HNO3 | 5mol/L,固液比1/10(g/mL),90℃,120min | Ni 99% | [ |
| Mo,Ni,V | HNO3∶H2SO4∶HCl=2∶1∶1(体积比) | 固液比为70g/L,70℃,60min | Mo 90%;Ni 99%;V 99% | [ |
| Mo,Ni,Co | HCOOH | 0.6mol/L,固液比1/10(g/mL),80℃,90min | Mo 76%、Ni 93%、Co 97% | [ |
| Mo,Ni | H2C2O4 | 1mol/L,固液比1/10(g/mL),40℃,3h | Mo 92%;Ni 19% | [ |
| Mo,Ni,Co | EDTA | 0.2mol/L,固液比1/15(g/mL),60℃,60min | Mo 90%;Ni 95%;Co 97% | [ |
| Mo,Co | H2SO4+H2O2(pH=1.3) | H2O2浓度3.75mol/L,固液比1/7.5(g/mL),60℃,1h | Mo 90%;Co 83% | [ |
| Mo,Ni,V | H2C2O4+H2O2 | H2C2O4浓度0.5mol/L,H2O2浓度3mol/L,60℃,1h | Mo 90%;Ni 65%;V 94% | [ |
| Mo,V | NaOH加压 | NaOH 30%(g/mL),250℃ | Mo 98%;V 95% | [ |
| W,V | NaOH+Na2CO3加压 | NaOH浓度2mol/L,Na2CO3浓度0.2mol/L,固液比1/20(g/mL),300℃,2h | W>90%;V>90% | [ |
| Mo,V | NH3·H2O+H2O2 | NH3·H2O浓度4.5mol/L,H2O2浓度为1.0mol/L,固液比1/20(g/mL),140℃,2h | Mo 95%;V 46% | [ |
| Mo | Na2CO3+H2O2 | Na2CO3浓度85g/L,H2O2 10%(体积分数),废催化剂添加量20%(质量分数),25℃,1h | Mo 84% | [ |
| Mo,Ni,Co | Na2CO3+H2SO4两步浸出 | Na2CO3浓度为30g/L,固液比1/10(g/mL),90℃,60min;H2SO4浓度为6mol/L,固液比1/10(g/mL),90℃,60min | Mo 98%;Ni 90%;Co 93% | [ |
| Mo,V | NaOH微波辅助 | 微波功率600W,NaOH浓度2mol/L,固液比1/5(g/mL),90℃,10min | Mo 96%;V 94% | [ |
| 废催化剂组成 | 回收方法 | 主要工艺参数 | 回收率 | 参考文献 |
|---|---|---|---|---|
| Mo,Co | H2SO4焙烧+H2SO4浸出 | 焙烧条件:300℃,2h; 浸出条件:H2SO4体积分数2%,95℃,60min | Mo>90%;Co>90% | [ |
| Mo,Ni,Co | KHSO4焙烧-水浸 | 焙烧条件:500℃,4h;90℃水浸4h | Mo>90%;Ni>90%;Co>90% | [ |
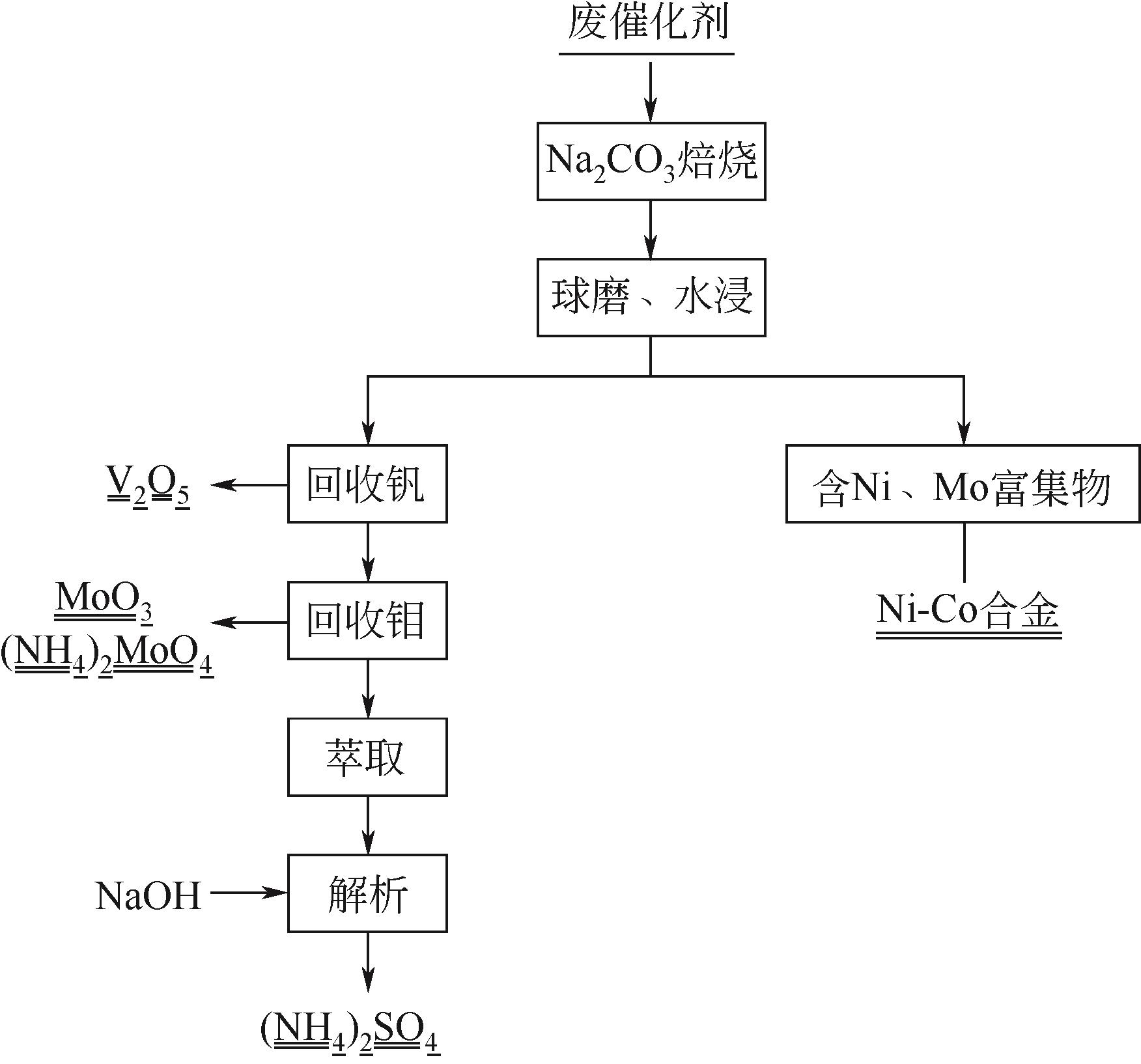
| Mo,V | Na2CO3焙烧-水浸 | 焙烧条件:750℃,45min;90℃水浸15min | Mo 90%;V 90% | [ |
| Mo | Na2CO3焙烧-水浸 | 焙烧条件:600℃,45min;60℃水浸 | Mo 99% | [ |
表3 废加氢催化剂焙烧-浸出回收有价金属技术
| 废催化剂组成 | 回收方法 | 主要工艺参数 | 回收率 | 参考文献 |
|---|---|---|---|---|
| Mo,Co | H2SO4焙烧+H2SO4浸出 | 焙烧条件:300℃,2h; 浸出条件:H2SO4体积分数2%,95℃,60min | Mo>90%;Co>90% | [ |
| Mo,Ni,Co | KHSO4焙烧-水浸 | 焙烧条件:500℃,4h;90℃水浸4h | Mo>90%;Ni>90%;Co>90% | [ |
| Mo,V | Na2CO3焙烧-水浸 | 焙烧条件:750℃,45min;90℃水浸15min | Mo 90%;V 90% | [ |
| Mo | Na2CO3焙烧-水浸 | 焙烧条件:600℃,45min;60℃水浸 | Mo 99% | [ |
| 1 | AKCIL A, VEGLIÒ F, FERELLA F, et al. A review of metal recovery from spent petroleum catalysts and ash[J]. Waste Management, 2015, 45: 420-433. |
| 2 | MARAFI M, RANA M S. Refinery waste: the spent hydroprocessing catalyst and its recycling options[J]. WIT Transactions on Ecology and the Environment, 2016, 202: 219-230. |
| 3 | 任春晓, 吴培, 李振昊, 等. 加氢催化剂预硫化技术现状[J]. 化工进展, 2013, 32(5): 1060-1064. |
| REN Chunxiao, WU Pei, LI Zhenhao, et al. The status of presulfurization technology for hydrogenation catalyst[J]. Chemical Industry and Engineering Progress, 2013, 32(5): 1060-1064. | |
| 4 | 张利波, 王璐, 曲雯雯, 等. Al2O3基石油加氢脱硫催化剂研究现状与进展[J]. 材料导报, 2018, 32(5): 772-779, 795. |
| ZHANG Libo, WANG Lu, QU Wenwen, et al. Research and development of petroleum hydrodesulfurization catalysts with Al2O3-based supports[J]. Materials Review, 2018, 32(5): 772-779, 795. | |
| 5 | GRANGE P, VANHAEREN X. Hydrotreating catalysts, an old story with new challenges[J]. Catalysis Today, 1997, 36(4): 375-391. |
| 6 | DUFRESNE P. Hydroprocessing catalysts regeneration and recycling[J]. Applied Catalysis A: General, 2007, 322: 67-75. |
| 7 | MARAFI M, STANISLAUS A. Spent catalyst waste management: a review: Part Ⅰ—Developments in hydroprocessing catalyst waste reduction and use[J]. Resources, Conservation and Recycling, 2008, 52(6): 859-873. |
| 8 | 刘勇军, 刘晨光. 炼厂加氢废催化剂的综合利用[J]. 化工进展, 2010, 29(6): 1066-1070. |
| LIU Yongjun, LIU Chenguang. Comprehensive utilization of spent hydrotreating catalysts in refinery[J]. Chemical Industry and Engineering Progress, 2010, 29(6): 1066-1070. | |
| 9 | 孙晓雪, 刘仲能, 杨为民. 废弃负载型加氢处理催化剂金属回收技术进展[J]. 化工进展, 2016, 35(6): 1894-1904. |
| SUN Xiaoxue, LIU Zhongneng, YANG Weimin. Research progress of metal recovery in spent supported hydroprocessing catalyst[J]. Chemical Industry and Engineering Progress, 2016, 35(6): 1894-1904. | |
| 10 | PATHAK A, VINOBA M, KOTHARI R. Emerging role of organic acids in leaching of valuable metals from refinery-spent hydroprocessing catalysts, and potential techno-economic challenges: a review[J]. Critical Reviews in Environmental Science and Technology, 2021, 51(1): 1-43. |
| 11 | EIJSBOUTS S, BATTISTON A A, LEERDAM G C VAN. Life cycle of hydroprocessing catalysts and total catalyst management[J]. Catalysis Today, 2008, 130(2/3/4): 361-373. |
| 12 | BARIK S P, PARK K H, PARHI P K, et al. Direct leaching of molybdenum and cobalt from spent hydrodesulphurization catalyst with sulphuric acid[J]. Hydrometallurgy, 2012, 111/112: 46-51. |
| 13 | 王曰杰, 李玲玲, 何春宏. 炼油废催化剂生物淋滤脱金属研究进展[J]. 化工学报, 2021, 72(2): 901-912. |
| WANG Yuejie, LI Lingling, HE Chunhong. Review on the bioleaching of spent refinery catalysts for metals removal[J]. CIESC Journal, 2021, 72(2): 901-912. | |
| 14 | LE M N, LEE M S. A review on hydrometallurgical processes for the recovery of valuable metals from spent catalysts and life cycle analysis perspective[J]. Mineral Processing and Extractive Metallurgy Review, 2021, 42(5): 335-354. |
| 15 | AL-SALEM S M, CONSTANTINOU A, LEEKE G A, et al. A review of the valorization and management of industrial spent catalyst waste in the context of sustainable practice: the case of the State of Kuwait in parallel to European industry[J]. Waste Management & Research, 2019, 37(11): 1127-1141. |
| 16 | WANG Hongjun, FENG Yali, LI Hailong, et al. Recovery of vanadium from acid leaching solutions of spent oil hydrotreating catalyst using solvent extraction with D2EHPA (P204)[J]. Hydrometallurgy, 2020, 195: 105404. |
| 17 | LE M N, LEE M S. Separation of Al(Ⅲ), Mo(Ⅵ), Ni(Ⅱ), and V(Ⅴ) from model hydrochloric acid leach solutions of spent petroleum catalyst by solvent extraction[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(11): 2886-2897. |
| 18 | WANG Hongjun, FENG Yali, LI Haoran, et al. The kinetics of vanadium extraction from spent hydroprocessing catalyst by leaching with sulfuric acid at atmospheric pressure[J]. Metallurgical Research & Technology, 2019, 116(2): 214. |
| 19 | SHEIK A R, GHOSH M K, SANJAY K, et al. Dissolution kinetics of nickel from spent catalyst in nitric acid medium[J]. Journal of the Taiwan Institute of Chemical Engineers, 2013, 44(1): 34-39. |
| 20 | VUYYURU K R, PANT K K, KRISHNAN V V, et al. Recovery of nickel from spent industrial catalysts using chelating agents[J]. Industrial & Engineering Chemistry Research, 2010, 49(5): 2014-2024. |
| 21 | CHAUHAN G, PANT K K, NIGAM K D P. Metal recovery from hydroprocessing spent catalyst: a green chemical engineering approach[J]. Industrial & Engineering Chemistry Research, 2013, 52(47): 16724-16736. |
| 22 | IMAM D M, EL-NADI Y A. Recovery of molybdenum from alkaline leach solution of spent hydrotreating catalyst by solvent extraction using methyl tricaprylammonium hydroxide[J]. Hydrometallurgy, 2018, 180: 172-179. |
| 23 | AL-SHEEHA H, MARAFI M, RAGHAVAN V, et al. Recycling and recovery routes for spent hydroprocessing catalyst waste[J]. Industrial & Engineering Chemistry Research, 2013, 52(36): 12794-12801. |
| 24 | SZYMCZYCHA-MADEJA A. Kinetics of Mo, Ni, V and Al leaching from a spent hydrodesulphurization catalyst in a solution containing oxalic acid and hydrogen peroxide[J]. Journal of Hazardous Materials, 2011, 186(2/3): 2157-2161. |
| 25 | MA Zhiyuan, LIU Yong, ZHOU Jikui, et al. Recovery of vanadium and molybdenum from spent petrochemical catalyst by microwave-assisted leaching[J]. International Journal of Minerals, Metallurgy, and Materials, 2019, 26(1): 33-40. |
| 26 | BANDA R, NGUYEN T H, SOHN S H, et al. Recovery of valuable metals and regeneration of acid from the leaching solution of spent HDS catalysts by solvent extraction[J]. Hydrometallurgy, 2013, 133: 161-167. |
| 27 | VALVERDE I M, PAULINO J F, AFONSO J C. Hydrometallurgical route to recover molybdenum, nickel, cobalt and aluminum from spent hydrotreating catalysts in sulphuric acid medium[J]. Journal of Hazardous Materials, 2008, 160(2/3): 310-317. |
| 28 | KIM H I, PARK K H, MISHRA D. Sulfuric acid baking and leaching of spent Co-Mo/Al2O3 catalyst[J]. Journal of Hazardous Materials, 2009, 166(2/3): 1540-1544. |
| 29 | LAI Y C, LEE W J, HUANG Kuolin, et al. Metal recovery from spent hydrodesulfurization catalysts using a combined acid-leaching and electrolysis process[J]. Journal of Hazardous Materials, 2008, 154(1/2/3): 588-594. |
| 30 | ARSLANOĞLU H, YARAŞ A. Recovery of precious metals from spent Mo-Co-Ni/Al2O3 catalyst in organic acid medium: process optimization and kinetic studies[J]. Petroleum Science and Technology, 2019, 37(19): 2081-2093. |
| 31 | ILHAN S. Extraction of molybdenum, nickel and aluminium from spent Ni-Mo hydrodesulphurization (HDS) catalyst in oxalic acid solutions[J]. Canadian Metallurgical Quarterly, 2020, 59(1): 26-35. |
| 32 | ALPASLAN O, YARAS A, ARSLANOĞLU H. A kinetic model for chelating extraction of metals from spent hydrodesulphurization catalyst by complexing agent[J]. Transactions of the Indian Institute of Metals, 2020, 73(7): 1925-1937. |
| 33 | RUIZ V, MEUX E, SCHNEIDER M, et al. Hydrometallurgical treatment for valuable metals recovery from spent CoMo/Al2O3Catalyst. 2. Oxidative leaching of an unroasted catalyst using H2O2[J]. Industrial & Engineering Chemistry Research, 2011, 50(9): 5307-5315. |
| 34 | MULAK W, SZYMCZYCHA A, LESNIEWICZ A, et al. Preliminary results of metals leaching from a spent hydrodesulphurization (HDS) catalyst[J]. Physicochemical Problems of Mineral Processing, 2006, 40: 69-76. |
| 35 | KIM J W, LEE W G, HWANG I S, et al. Recovery of tungsten from spent selective catalytic reduction catalysts by pressure leaching[J]. Journal of Industrial and Engineering Chemistry, 2015, 28: 73-77. |
| 36 | ZHAO Zhipeng, GUO Min, ZHANG Mei. Extraction of molybdenum and vanadium from the spent diesel exhaust catalyst by ammonia leaching method[J]. Journal of Hazardous Materials, 2015, 286: 402-409. |
| 37 | PARK K H, MOHAPATRA D, REDDY B R. Selective recovery of molybdenum from spent HDS catalyst using oxidative soda ash leach/carbon adsorption method[J]. Journal of Hazardous Materials, 2006, 138(2): 311-316. |
| 38 | PARK K H, MOHAPATRA D, NAM C W. Two stage leaching of activated spent HDS catalyst and solvent extraction of aluminium using organo-phosphinic extractant, Cyanex 272[J]. Journal of Hazardous Materials, 2007, 148(1/2): 287-295. |
| 39 | MARAFI M, STANISLAUS A. Spent hydroprocessing catalyst management: a review: Part Ⅱ. Advances in metal recovery and safe disposal methods[J]. Resources, Conservation and Recycling, 2008, 53(1/2): 1-26. |
| 40 | SÁMANO V, RANA M S, ANCHEYTA J. An easy approach based on textural properties to evaluate catalyst deactivation during heavy oil hydrotreating[J]. Catalysis Communications, 2020, 133: 105823. |
| 41 | LE M N, LEE M S. Selective dissolution of vanadium(Ⅴ) from spent petroleum catalysts by oxalic acid solution[J]. Journal of Mining and Metallurgy, Section B: Metallurgy, 2020, 56(1): 127-133. |
| 42 | YANG Yue, XU Shengming, LI Zhen, et al. Oil removal of spent hydrotreating catalyst CoMo/Al2O3via a facile method with enhanced metal recovery[J]. Journal of Hazardous Materials, 2016, 318: 723-731. |
| 43 | FU Pengbo, WANG Hualin, LI Jianping, et al. Cyclonic gas stripping deoiling and gas flow acceleration classification for the resource utilization of spent catalysts in residue hydrotreating process[J]. Journal of Cleaner Production, 2018, 190: 689-702. |
| 44 | DE SOUZA PEREIRA A L, SILVA C N D, AFONSO J C, et al. The importance of pre-treatment of spent hydrotreating catalysts on metals recovery[J]. Química Nova, 2011, 34(1): 145-150. |
| 45 | MENOUFY M F, AHMED H S. Treatment and reuse of spent hydrotreating catalyst[J]. Energy Sources A: Recovery, Utilization, and Environmental Effects, 2008, 30(13): 1213-1222. |
| 46 | ZHANG Di, LIU Yunqing, HU Qizhao, et al. Sustainable recovery of nickel, molybdenum, and vanadium from spent hydroprocessing catalysts by an integrated selective route[J]. Journal of Cleaner Production, 2020, 252: 119763. |
| 47 | FERELLA F, OGNYANOVA A, DE MICHELIS I, et al. Extraction of metals from spent hydrotreating catalysts: physico-mechanical pre-treatments and leaching stage[J]. Journal of Hazardous Materials, 2011, 192(1): 176-185. |
| 48 | HUANG Shaobo, ZHAO Zhongwei, CHEN Xingyu, et al. Alkali extraction of valuable metals from spent Mo-Ni/Al2O3 catalyst[J]. International Journal of Refractory Metals and Hard Materials, 2014, 46: 109-116. |
| 49 | DELIA ROJAS-RODRÍGUEZ A, FLORES-FAJARDO O, SELENE ALCÁNTAR GONZÁLEZ F, et al. Chemical treatment to recover molybdenum and vanadium from spent heavy gasoil hydrodesulfurization catalyst[J]. Advances in Chemical Engineering and Science, 2012, 2(3): 408-412. |
| 50 | PARK K H, REDDY B R, MOHAPATRA D, et al. Hydrometallurgical processing and recovery of molybdenum trioxide from spent catalyst[J]. International Journal of Mineral Processing, 2006, 80(2/3/4): 261-265. |
| 51 | PINTO I S S, SOARES H M V M. Selective leaching of molybdenum from spent hydrodesulphurisation catalysts using ultrasound and microwave methods[J]. Hydrometallurgy, 2012, 129/130: 19-25. |
| 52 | WANG Wenqiang, ZHANG Lei, HAN Yu, et al. Cleaner recycling of spent Ni-Mo/γ-Al2O3 catalyst based on mineral phase reconstruction[J]. Journal of Cleaner Production, 2019, 232: 266-273. |
| 53 | ZHANG Jialiang, YANG Cheng, CHEN Yongqiang, et al. Efficient phase transformation of γ-Al2O3 to α-Al2O3 in spent hydrodesulphurization catalyst by microwave roasting method[J]. Industrial & Engineering Chemistry Research, 2019, 58(4): 1495-1501. |
| 54 | LIU Qi, WANG Wenqiang, YANG Yue, et al. Recovery and regeneration of Al2O3 with a high specific surface area from spent hydrodesulfurization catalyst CoMo/Al2O3[J]. Rare Metals, 2019, 38(1): 1-13. |
| 55 | BUSNARDO R G, BUSNARDO N G, SALVATO G N, et al. Processing of spent NiMo and CoMo/Al2O3 catalysts via fusion with KHSO4[J]. Journal of Hazardous Materials, 2007, 139(2): 391-398. |
| 56 | CHEN Yun, FENG Qiming, SHAO Yanhai, et al. Investigations on the extraction of molybdenum and vanadium from ammonia leaching residue of spent catalyst[J]. International Journal of Mineral Processing, 2006, 79(1): 42-48. |
| 57 | YE Xiaolei, GUO Shenghui, QU Wenwen, et al. Microwave sodium roasting (MWSR) spent HDS catalysts for recovery Mo and in situ sulfur fixation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 97: 146-157. |
| 58 | KIM H I, PARK K H, MISHRA D. Influence of sulfuric acid baking on leaching of spent Ni-Mo/Al2O3 hydro-processing catalyst[J]. Hydrometallurgy, 2009, 98(1/2): 192-195. |
| 59 | YE Xiaolei, GUO Shenghui, QU Wenwen, et al. Microwave field: high temperature dielectric properties and heating characteristics of waste hydrodesulfurization catalysts[J]. Journal of Hazardous Materials, 2019, 366: 432-438. |
| 60 | 张邦胜, 刘贵清, 王芳, 等. 一种从废镍钼催化剂中回收镍、钼的方法: CN108467939A[P]. 2018-08-31. |
| ZHANG Bangsheng, LIU Guiqing, WANG Fang, et al. Method for recovering nickel and molybdenum from waste nickel-molybdenum catalyst: CN108467939A[P]. 2018-08-31. | |
| 61 | 朱兆鹏, 杨夫清, 梁宗跃, 等. 用等离子炉处理含钼废催化剂回收有价金属的研究[J]. 中国钼业, 2003, 27(3): 14-16. |
| ZHU Zhaopeng, YANG Fuqing, LIANG Zongyue, et al. Study on recovering valuable metals by treating molybdenum-containing waste catalysts using plasma furnace[J]. China Molybdenum Industry, 2003, 27(3): 14-16. | |
| 62 | 王成彦, 杨成, 张家靓, 等. 废加氢催化剂还原熔炼回收有价金属试验[J]. 有色金属(冶炼部分), 2019(9): 12-17. |
| WANG Chengyan, YANG Cheng, ZHANG Jialiang, et al. Study on recovery of valuable metals from spent hydrodesulphurization catalysts by reduction smelting[J]. Nonferrous Metals (Extractive Metallurgy), 2019(9): 12-17. | |
| 63 | YAO Zan, MA Xiaodong, Sha LYU. Phase equilibria of the Al2O3-CaO-SiO2-(0%, 5%, 10%)MgO slag system for non-metallic inclusions control[J]. Calphad, 2021, 72: 102227. |
| [1] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [2] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [3] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [4] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [5] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [6] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [7] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [11] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [12] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [13] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [14] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [15] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||