化工进展 ›› 2021, Vol. 40 ›› Issue (2): 722-735.DOI: 10.16085/j.issn.1000-6613.2020-0614
水合物利用技术应用进展
- 中国石油化工股份有限公司大连石油化工研究院,辽宁 大连 116045
-
收稿日期:2020-04-20修回日期:2020-05-10出版日期:2021-02-05发布日期:2021-02-09 -
通讯作者:薛倩 -
作者简介:薛倩(1986—),女,工程师,研究方向水合物气体分离。E-mail:xueqian.fshy@sinopec.com 。 -
基金资助:国家自然科学基金重点项目(21736005)
Research progresses in hydrate based technologies and processes
Qian XUE( ), Xiaolin WANG, Zunzhao LI, Mingrui LIU, Wei ZHAO
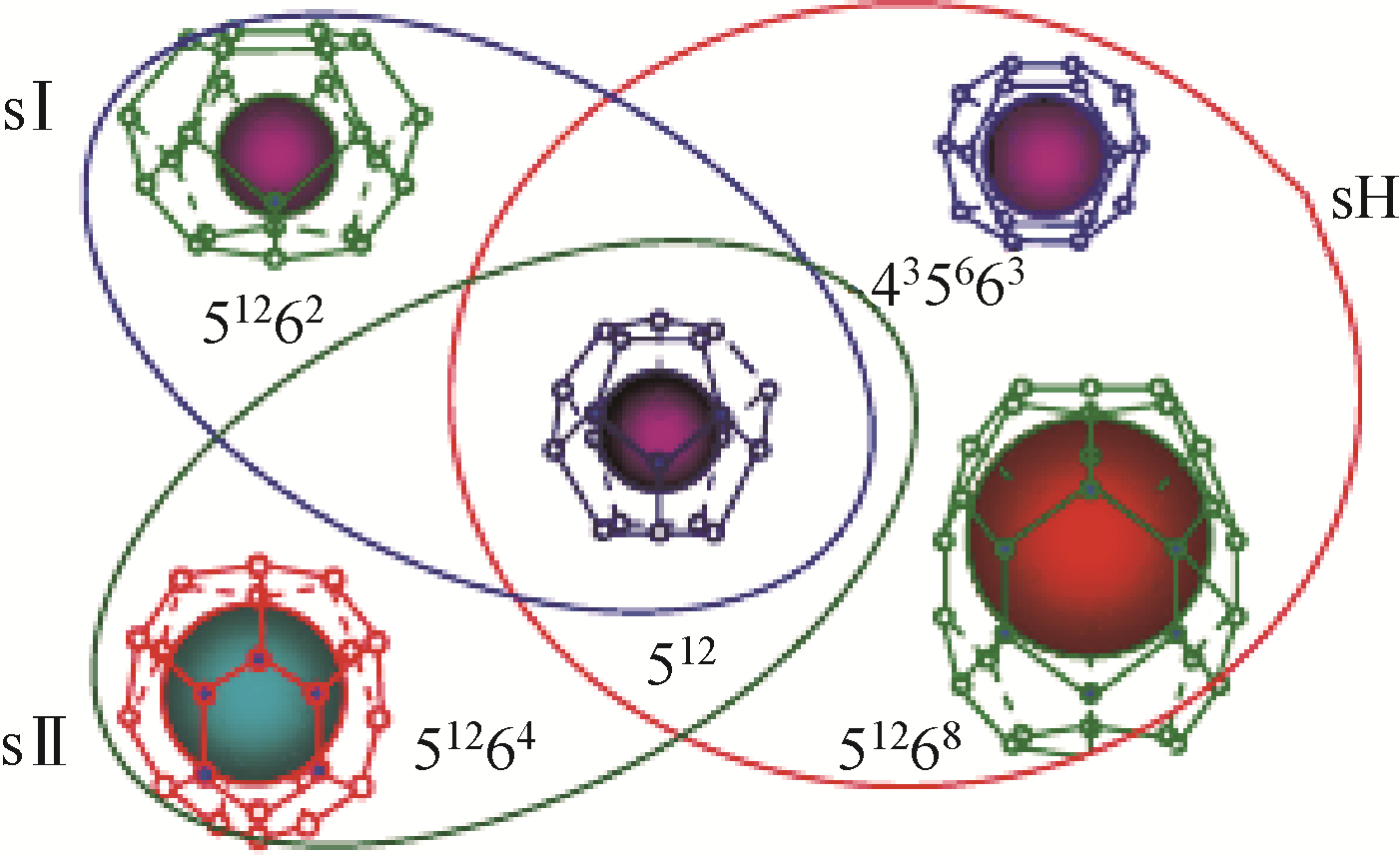
), Xiaolin WANG, Zunzhao LI, Mingrui LIU, Wei ZHAO
- Dalian Research Institute of Petroleum and Petrochemicals, SINOPEC, Dalian 116045, Liaoning, China
-
Received:2020-04-20Revised:2020-05-10Online:2021-02-05Published:2021-02-09 -
Contact:Qian XUE
摘要:
过去的几十年中,对于水合物的研究不单单集中在抑制天然气水合物的生成上,基于水合物的生成利用技术也得到了广泛的研究。基于水合物的生成利用技术是环保和可持续的新技术,利用不同气体生成水合物相平衡条件的差异,可用于气体分离、置换开采。由于水合物具有较高的气体浓度,可用于气体的存储。利用水合物较高的化解潜热,可将其用于蓄冷。本文综述了国内外水合物技术的研究应用现状,分析了水合物技术在气体分离与存储、溶液浓缩分离、蓄冷、二氧化碳(CO2)置换开采等领域有前景的研究方向。但是其水合反应速率慢、生成压力高、后期分离困难,极大地限制了水合物利用技术的工业应用。展望了水合物技术未来的研究发展方向,开发安全、高效和环保的水合物促进剂,开发高效水合物反应设备,开发连续水合物工艺,以便早日实现工业应用。
中图分类号:
引用本文
薛倩, 王晓霖, 李遵照, 刘名瑞, 赵巍. 水合物利用技术应用进展[J]. 化工进展, 2021, 40(2): 722-735.
Qian XUE, Xiaolin WANG, Zunzhao LI, Mingrui LIU, Wei ZHAO. Research progresses in hydrate based technologies and processes[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 722-735.
| 气体 | 生成压力/MPa |
|---|---|
| H2 | 200 |
| N2 | 16.3 |
| O2 | 11.1 |
| CO2 | 1.22 |
| C2H4 | 0.55 |
| C2H6 | 0.53 |
| C3H8 | 0.17 |
| H2S | 0.09 |
表1 不同气体在0℃时生成水合物所需压力
| 气体 | 生成压力/MPa |
|---|---|
| H2 | 200 |
| N2 | 16.3 |
| O2 | 11.1 |
| CO2 | 1.22 |
| C2H4 | 0.55 |
| C2H6 | 0.53 |
| C3H8 | 0.17 |
| H2S | 0.09 |
| 水合剂 | 特点 |
|---|---|
| 气体 | |
| CH4 | 易于分离,无毒,但需要较高的生成压力 |
| CO2 | 易于分离,无毒,生成压力较大 |
| C3H8 | 易于分离,操作条件适中,易燃 |
| 液体 | |
| HCFC-R141b | 在高温下可生成水合物,对臭氧层有破坏作用 |
| CP | 溶解度低,与水不互溶,常压下生成水合物 |
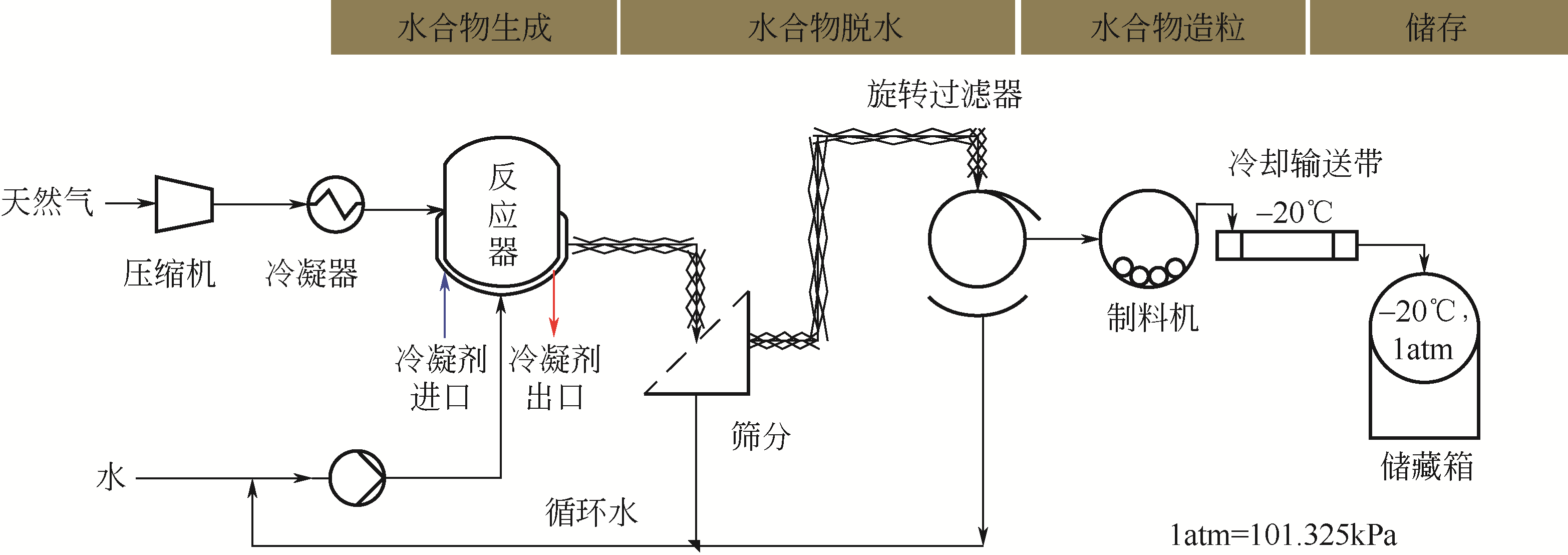
| 二元水合物 | |
| CP+CO2 | 提高了相平衡温度、生成速率,盐的去除率较高,但需要进一步分离 |
| CP+CH4 | 生成压力较低,溶解度小,水合物固体密度小于海水 |
| CO2+R141b | 价格便宜,脱盐率高,但需要进一步分离 |
| CO2+C3H8 | 生成压力较低 |
表2 海水淡化常用的水合剂
| 水合剂 | 特点 |
|---|---|
| 气体 | |
| CH4 | 易于分离,无毒,但需要较高的生成压力 |
| CO2 | 易于分离,无毒,生成压力较大 |
| C3H8 | 易于分离,操作条件适中,易燃 |
| 液体 | |
| HCFC-R141b | 在高温下可生成水合物,对臭氧层有破坏作用 |
| CP | 溶解度低,与水不互溶,常压下生成水合物 |
| 二元水合物 | |
| CP+CO2 | 提高了相平衡温度、生成速率,盐的去除率较高,但需要进一步分离 |
| CP+CH4 | 生成压力较低,溶解度小,水合物固体密度小于海水 |
| CO2+R141b | 价格便宜,脱盐率高,但需要进一步分离 |
| CO2+C3H8 | 生成压力较低 |
| 方法 | 能耗/ |
|---|---|
| 蒸发技术 | 180~2160 |
| 冷冻浓缩 | 936~1800 |
| 水合物浓缩技术 | 252~360 |
表3 几种浓缩技术的对比分析
| 方法 | 能耗/ |
|---|---|
| 蒸发技术 | 180~2160 |
| 冷冻浓缩 | 936~1800 |
| 水合物浓缩技术 | 252~360 |
| 储氢方法 | 优缺点 | 促进剂 | 反应条件 | 储氢量(质量分数) /% |
|---|---|---|---|---|
| 纯氢水合物 | ||||
| sⅡ型 | 储能密度大,生成条件苛刻 | 无 | 300MPa,249K | 5.3 |
| 液相促进剂 | ||||
| sⅡ型 | 生成条件温和储氢量低 | THF、CP、环己酮、呋喃、四氢噻吩 | 10~70MPa,255~280K | 0.1~4 |
| sⅥ型 | 储氢量大稳定性高 | 叔丁胺 | 13.8MPa,250K | 0.7 |
| sH型 | 储氢量比sⅡ型的储氢量高,生成条件较纯氢气水合物的生成条件温和 | 甲基叔丁基醚、2,2,3-三甲基丁烷、 甲基环己烷、1,1-二甲基环己烷 | 60~100MPa,269~275K | 0.85~1.05 |
| 半笼型 | 生成条件更加温和,储氢量更低 | TBAB、TBAF、TBAC和四丁基溴化磷、 四丁基氢硼化铵 | 13~70MPa,253~294K | 0.009~1.35 |
| 气体促进剂水合物 | ||||
| sⅡ型 | 生成条件温和,提升了总热值和储能密度 | CH4、C2H6和C3H8等 | 0.4~70MPa,263~281K | 0.31~0.33 |
| sⅠ型 | 氢气可以占据多个笼,不稳定 | CO2、CH4等 | 8~60MPa,253~263K | 0.02~0.31 |
表4 不同促进剂的比较
| 储氢方法 | 优缺点 | 促进剂 | 反应条件 | 储氢量(质量分数) /% |
|---|---|---|---|---|
| 纯氢水合物 | ||||
| sⅡ型 | 储能密度大,生成条件苛刻 | 无 | 300MPa,249K | 5.3 |
| 液相促进剂 | ||||
| sⅡ型 | 生成条件温和储氢量低 | THF、CP、环己酮、呋喃、四氢噻吩 | 10~70MPa,255~280K | 0.1~4 |
| sⅥ型 | 储氢量大稳定性高 | 叔丁胺 | 13.8MPa,250K | 0.7 |
| sH型 | 储氢量比sⅡ型的储氢量高,生成条件较纯氢气水合物的生成条件温和 | 甲基叔丁基醚、2,2,3-三甲基丁烷、 甲基环己烷、1,1-二甲基环己烷 | 60~100MPa,269~275K | 0.85~1.05 |
| 半笼型 | 生成条件更加温和,储氢量更低 | TBAB、TBAF、TBAC和四丁基溴化磷、 四丁基氢硼化铵 | 13~70MPa,253~294K | 0.009~1.35 |
| 气体促进剂水合物 | ||||
| sⅡ型 | 生成条件温和,提升了总热值和储能密度 | CH4、C2H6和C3H8等 | 0.4~70MPa,263~281K | 0.31~0.33 |
| sⅠ型 | 氢气可以占据多个笼,不稳定 | CO2、CH4等 | 8~60MPa,253~263K | 0.02~0.31 |
| 冷能储存介质 | 相变温度/℃ | 相变潜热/kJ·kg-1 | 换热性能 | 投资 | COP(制冷系数) | 蓄冷温度/℃ | 供冷温度/℃ |
|---|---|---|---|---|---|---|---|
| 水 | 0~10 | 好 | <0.6 | 1 | 4~6 | 1~4 | |
| 冰 | 0 | 334 | 较好 | 1① | 0.6~0.7 | -6~-3 | 1~3 |
| 共晶盐 | 8~12 | 153~253 | 一般 | 1.3~2 | 0.92~0.95 | -2~4 | 9~10 |
| 气体水合物 | 5~12 | 200~500 | 好 | 1.2~1.5 | 0.89~1 | 0~8 | 9~12 |
表5 不同蓄冷方式的比较[77-79]
| 冷能储存介质 | 相变温度/℃ | 相变潜热/kJ·kg-1 | 换热性能 | 投资 | COP(制冷系数) | 蓄冷温度/℃ | 供冷温度/℃ |
|---|---|---|---|---|---|---|---|
| 水 | 0~10 | 好 | <0.6 | 1 | 4~6 | 1~4 | |
| 冰 | 0 | 334 | 较好 | 1① | 0.6~0.7 | -6~-3 | 1~3 |
| 共晶盐 | 8~12 | 153~253 | 一般 | 1.3~2 | 0.92~0.95 | -2~4 | 9~10 |
| 气体水合物 | 5~12 | 200~500 | 好 | 1.2~1.5 | 0.89~1 | 0~8 | 9~12 |
| 1 | DAVY H. On some of the combinations of oxymuriatic gas and oxygene, and on the chemical relations of these principles, to inflammable bodies[J]. Philosophical Transactions of the Royal Society of London, 1811, 101: 1-35. |
| 2 | HAMMERSCHMIDT E G. Formation of gas hydrates in natural gas transmission lines[J]. Industrial & Engineering Chemistry1934, 26: 851-855. |
| 3 | KONDO W, OGAWA H, OHMURA R, et al. Clathrate hydrate formation from a hydrocarbon gas mixture: evolution of gas-phase composition in a hydrate-forming reactor[J]. Energy Fuels, 2010, 24: 6375-6383. |
| 4 | LIU Y, CHEN B, CHEN Y, et al. Methane storage in a hydrated form as promoted by leucines for possible application to natural gas transportation and storage[J]. Energy Technology, 2015, 3: 815-819. |
| 5 | ZHANG S X, CHEN G J, MA C F, et al. Hydrate formation of hydrogen plus hydrocarbon gas mixtures[J]. Journal of Chemical and Engineering Data, 2000, 45: 908-911. |
| 6 | SUN Q B, KANG Y T. Experimental correlation for the formation rate of CO2 hydrate with THF (tetrahydrofuran) for cooling application[J]. Energy, 2015, 91: 712-719. |
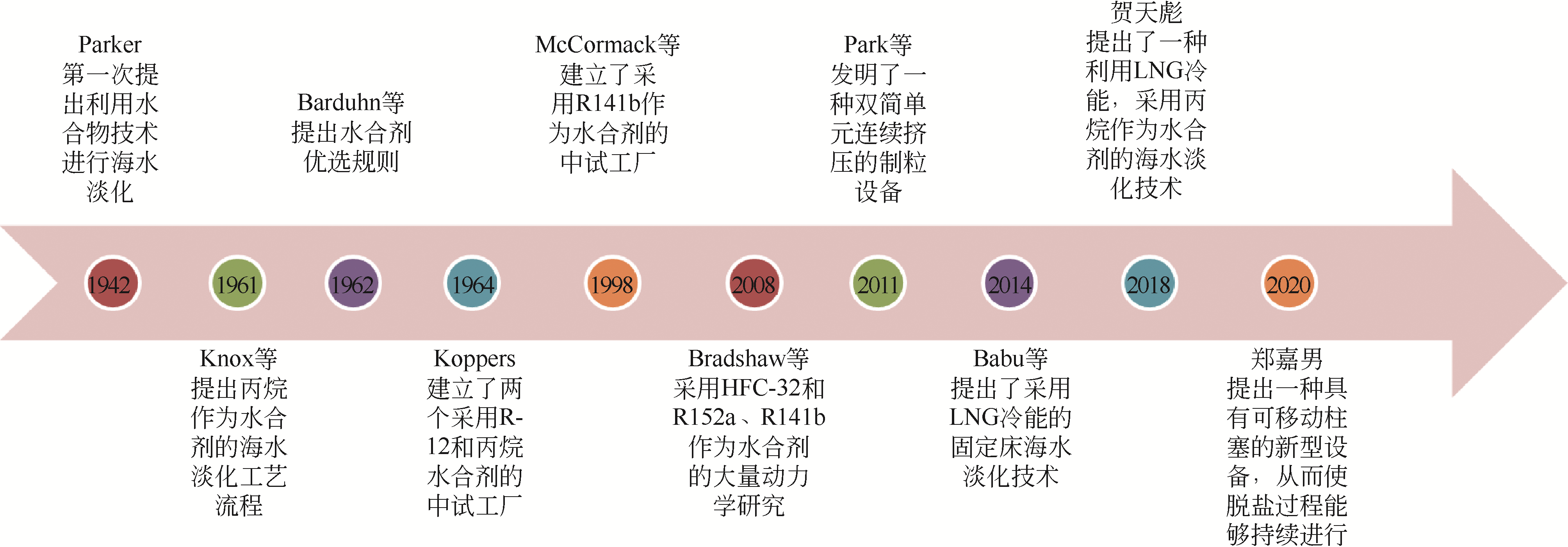
| 7 | PARK K, HONG S Y, LEE J W, et al. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B3+)[J]. Desalination, 2011, 274: 91-96. |
| 8 | LI S F, SHEN Y, LIU D, et al. Concentrating orange juice through CO2 clathrate hydrate technology[J]. Chemical Engineering Research and Design, 2015, 93: 773-778. |
| 9 | PARK Y, KIM D Y, LEE J W, et al. Sequestering carbon dioxide into complex structures of naturally occurring gas hydrates[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(34): 12690-12694. |
| 10 | RIPMEESTER J A, TSE J S, RATCLIFFE C I, et al. A new clathrate hydrate structure[J]. Nature, 1987, 325: 135-136. |
| 11 | KHOKHAR A A, SLOAN E D, GUDMUNDSSON J S. Gas storage in structure H hydrate[J]. Fluid Phase Equilibria, 1998, 151: 383-392. |
| 12 | SLOAN E D. Fundamental principles and applications of natural gas hydrates[J]. Nature, 2003, 426: 353-359. |
| 13 | HAMMERSCHMIDT E G. Formation of gas hydrate in natural gas transmission lines[J]. Industrial and Engineering Chemistry, 1934, 26(8): 851-885. |
| 14 | GLEW D N. Process for liquid recovery and solution concentration: US 3216930[P]. 1966. |
| 15 | HAPPEL J, HNATOW M A, MEYER H. The study of separation nitrogen from methane by hydrate formation using a novel apparatus[J]. Annals of the New York Academy of Sciences, 1994, 715: 412-414. |
| 16 | KANG S P, LEE H. Recovery of CO2 from flue gas using gas hydrate: thermodynamic verification through phase equilibrium measurement [J]. Environmental Science & Technology, 2000, 34(20): 4397-4400. |
| 17 | 陈光进, 程宏远, 樊栓狮. 新型水合物分离技术研究进展[J]. 现代化工, 1999, 19(7): 12-14. |
| CHEN G J, CHENG H Y, FAN S S. New separation technology through forming solid gas hydrates[J]. Modern Chemical Industry, 1999, 19(7): 12-14. | |
| 18 | 陈广印, 孙强, 郭绪强, 等. 水合物法连续分离煤层气实验研究[J]. 高校化学工程学报, 2013(4): 561-566. |
| CHEN G Y, SUN Q, GUO X Q, et al. Experimental study on the continuous separation process of coal bed methane via forming hydrate[J]. Journal of Chemical Engineering of Chinese Universities, 2013(4): 561-566. | |
| 19 | SUN Q, CHEN G Y, GUO X Q, et al. Experiments on the continuous separation of gas mixtures via dissolution and hydrate formation in the presence of THF[J]. Fluid Phase Equilibria, 2014, 361(1): 250-256. |
| 20 | SUN Q, ZHAO Y Y, LIU A X, et al. Continuous separation of CH4/N2 mixture via hydrates formation in the presence of TBAB[J] . Chemical Engineering and Processing: Process Intensification, 2015, 95(9): 284-288. |
| 21 | SUN Q, LIU J, LIU A X, et al. Experiment on the separation of tail gases of ammonia plant via continuous hydrates formation with TBAB[J]. International Journal of Hydrogen Energy, 2015, 40(5): 6358-6364. |
| 22 | WANG Y W, DU M, GUO X Q, et al. Experiments and simulations for continuous recovery of methane from coal seam gas (CSG) utilizing hydrate formation[J]. Energy, 2017, 129: 28-41. |
| 23 | TOMITA S H, AKATSU S, OHMURA R. Experiments and thermodynamic simulations for continuous separation of CO2 from CH4+CO2 gas mixture utilizing hydrate formation[J]. Applied Energy, 2015, 146(1): 104-110. |
| 24 | HORII S, OHMURA R. Continuous separation of CO2 from a H2+CO2 gas mixture using clathrate hydrate[J]. Applied Energy, 2018, 225: 78-84. |
| 25 | KUMAR R, LINGA P, RIPMEESTER J A. Two-stage clathrate hydrate/membrane process for precombustion capture of carbon dioxide and hydrogen[J]. Journal of Environmental Engineering, 2009, 135(6): 411-417. |
| 26 | SUROVTSEVA D, AMIN R, BARIFCANI A. Design and operation of pilot plant for CO2 capture from IGCC flue gases by combined cryogenic and hydrate method[J]. Chemical Engineering Research & Design, 2011, 89(9): 1752-1757. |
| 27 | ZHONG D L, WANG J L, LU Y Y, et al. Precombustion CO2 capture using a hybrid process of adsorption and gas hydrate formation[J]. Energy, 2016, 102: 621-629. |
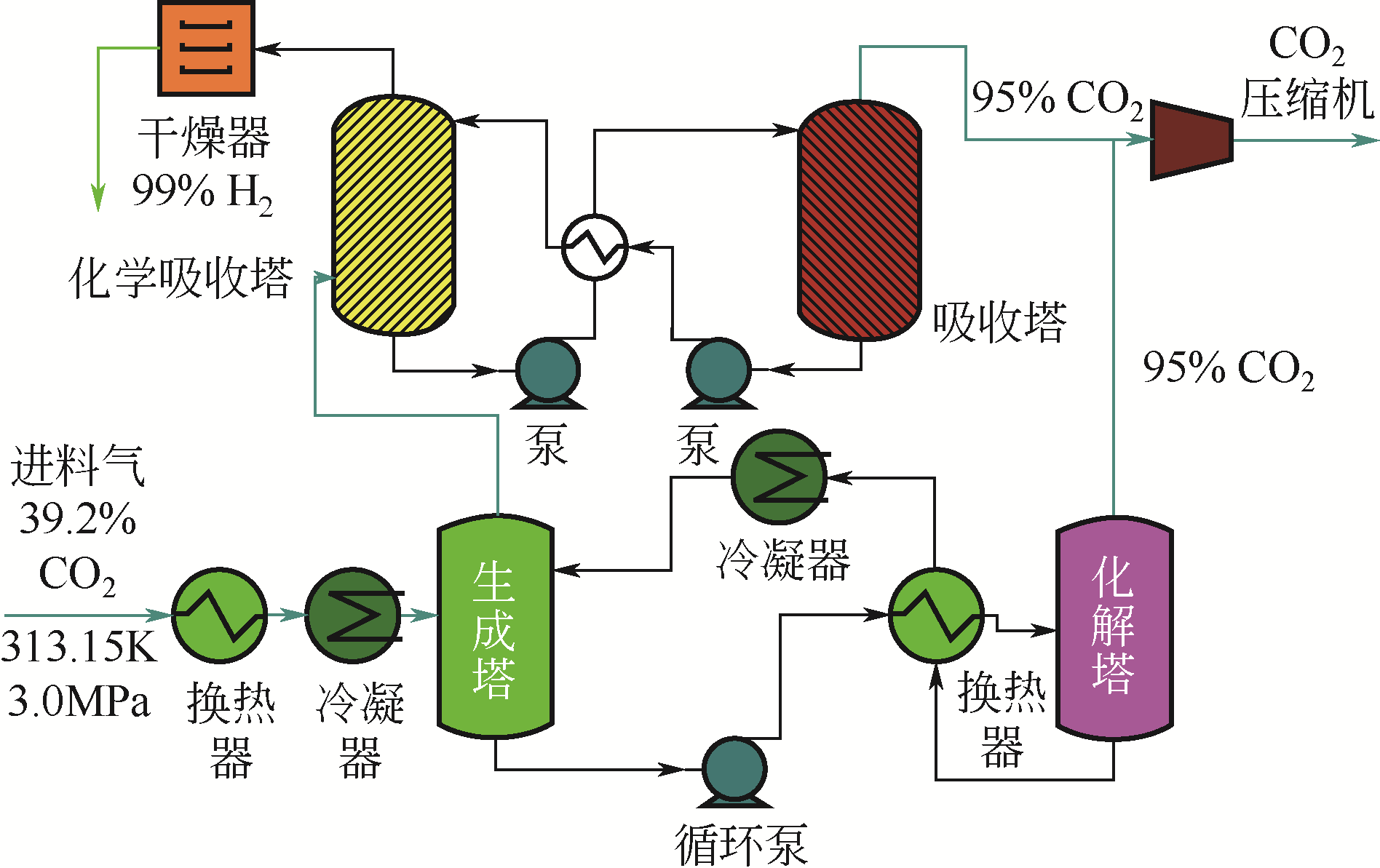
| 28 | XU C G, YU Y S, XIE W J, et al. Study on developing a novel continuous separation device and carbon dioxide separation by process of hydrate combined with chemical absorption[J]. Applied Energy, 2019, 255(8): 1-12. |
| 29 | 李小森, 徐纯刚, 余益松, 等. 一种水合物法联合化学吸收法的CO2气体分离装置及方法: CN201611052184.1[P]. 2017-03-08. |
| LI X S, XU C G, YU Y S, et al. CO2 gas separation device and method of hydrate method combined with chemical absorption method: CN201611052184.1[P]. 2017-03-08. | |
| 30 | RAPHAEL S. Energy issues in desalination processes[J]. Environmental Science & Technology, 2008, 42: 8193-8201. |
| 31 | PARKER A. Potable water from sea-water[J]. Nature, 1942, 149:184-186. |
| 32 | BABU P, NAMBIAR A, HE T B, et al. A review of clathrate hydrate based desalination to strengthen energy-water nexus[J]. ACS Sustainable Chemistry & Engineering, 2018, 6: 8093-8107. |
| 33 | KNOX W G, HESS M, JONES G E. The hydrate process[J]. Chemical Engineering Progress, 1961, 57: 66-71. |
| 34 | 刘昌岭,任宏波,孟庆国,等.添加R141b促进剂的CO2水合物法海水淡化实验研究[J]. 天然气工业, 2013, 33(7): 90-95. |
| LIU C L, REN H B, MENG Q G, et al. An experimental study of CO2 hydrate-based seawater desalination with the R141b as an accelerant[J]. Natural Gas Industry, 2013, 33(7): 90-95. | |
| 35 | BABU P, KUMAR R, LINGA P. Unusual behavior of propane as a co-guest during hydrate formation in silica sand: potential application to seawater desalination and carbon dioxide capture[J]. Chemical Engineering Science, 2014, 117: 342-351. |
| 36 | FAKHARIAN H, GANJI H, NADERIFAR A. Saline produced water treatment using gas hydrates[J]. Journal of Environmental Chemical Engineering, 2017, 5(5): 4269-4273. |
| 37 |
杨亚彬, 谢应明, 耿时江, 等. CO2水合物法海水淡化装置的 及能耗分析[J]. 制冷技术, 2017, 37(1): 23-26. 及能耗分析[J]. 制冷技术, 2017, 37(1): 23-26.
|
| YANG Y B, XIE Y M, GENG S J, et al. Analysis on exergy and energy consumption of seawater desalination device with CO2 hydrate[J]. Refrigeration Technology, 2017, 37(1): 23-26. | |
| 38 | HE T B, NAIR S K, BABU P, et al. A novel conceptual design of hydrate based desalination (HyDesal) process by utilizing LNG cold energy[J]. Applied Energy, 2018, 222: 13-24. |
| 39 | CHONG Z R, HE T B, BABU P, et al. Economic evaluation of energy efficient hydrate based desalination utilizing cold energy from liquefied natural gas (LNG)[J]. Desalination, 2019, 463: 69-80. |
| 40 | SEO S D, HONG S Y, SUM A K, et al. Thermodynamic and kinetic analysis of gas hydrates for desalination of saturated salinity water[J]. Chemical Engineering Journal, 2019, 370: 980-987. |
| 41 | CHOI W J, LEE Y H, MOK J, et al. Thermodynamic and kinetic influences of NaCl on HFC-125a hydrates and their significance in gas hydrate-based desalination[J]. Chemical Engineering Journal, 2019, 358: 598-605. |
| 42 | ZHENG J N, YANG M J. Experimental investigation on novel desalination system via gas hydrate[J]. Desalination, 2020, 478: 114284. |
| 43 | CLAßEN T, SEIDL P, LOEKMAN S, et al. Review on the food technological potentials of gas hydrate technology[J]. Current Opinion in Food Science, 2019, 29: 48-55. |
| 44 | HUANG C P,FENNEMA O,POWRIE W D. Gas hydrates in aqueous-organic systems Ⅱ concentration by gas hydrate formation[J]. Cryobiology, 1966, 2(5): 240-245. |
| 45 | ANDERSEN T B, THOMSEN K. Separation of water through gas hydrate formation[J]. International Sugar Journal, 2009, 111(1330): 632-636. |
| 46 | PURWANTO Y A, OSHITA S, SEO Y, et al. Application study on the concentration of liquid foods by the use of gas hydrate[J]. Journal of Food Engineering, 2011, 47: 133-138. |
| 47 | LI S F, SHEN Y, LIU D, et al. Experimental study of concentration of tomato juice by CO2 hydrate formation[J]. Chemical Industry and Chemical Engineering Quarterly, 2015, 21: 441-446. |
| 48 | LI S F, QI F, SHEN Y M, et al. An energy-efficient juice concentration technology by ethylene hydrate formation[J]. Separation and Purification Technology, 2017, 173: 80-85. |
| 49 | SAFARI S, VARAMINIAN F. Study the kinetics and thermodynamics conditions for CO2 hydrate formation in orange juice concentration[J]. Innovative Food Science and Emerging Technologies, 2019, 57: 102155. |
| 50 | SLOAN E D. Fundamental principles and applications of natural gas hydrates[J]. Nature, 2003, 426(6964): 353-363. |
| 51 | VELUSWAMYA H P, KUMARA A, SEOB Y, et al. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates[J]. Applied Energy, 2018, 216: 262-285. |
| 52 | ZHAO J Z, ZHAO Y S, LIANG W G, et al. Semi-clathrate hydrate process of methane in porous media-mesoporous materials of SBA-15[J]. Fuel, 2018, 220: 446-452. |
| 53 | ZHAO Y Z, ZHAO J Z, LIANG W G, et al. Semi-clathrate hydrate process of methane in porous media-microporous materials of 5A-type zeolites[J]. Fuel, 2018, 220: 185-191. |
| 54 | KUMARA A, VELUSWAMYA H P, KUMARB R, et al. Direct use of seawater for rapid methane storage via clathrate (sⅡ) hydrates[J]. Applied Energy, 2019, 235: 21-30. |
| 55 | KHURANA M, VELUSWAMYA H P, DARABOINAB, et al. Thermodynamic and kinetic modelling of mixed CH4-THF hydrate for methane storage application[J]. Chemical Engineering Journal, 2019, 370: 760-771. |
| 56 | INKONG K, RANGSUNVIGITA P, KULPRATHIPANJA S, et al. Effects of temperature and pressure on the methane hydrate formation with the presence of tetrahydrofuran (THF) as a promoter in an unstirred tank reactor[J]. Fuel, 2019, 255: 115705. |
| 57 | COLLADOS C C, PEREZ J F, ESCANDELL M M, et al. Effect of additives in the nucleation and growth of methane hydrates confined in a high-surface area activated carbon material[J]. Chemical Engineering Journal, 2020, 388: 124224. |
| 58 | VELUSWAMYA H P, KUMAR A, KUMAR R, et al. An innovative approach to enhance methane hydrate formation kinetics with leucine for energy storage application[J]. Applied Energy, 2017, 188: 190-199. |
| 59 | WANG W X, HUANG Z, CHEN H R, et al. Methane hydrates with a high capacity and a high formation rate promoted by bio surfactants[J]. Chemical Communications, 2012, 48(95): 11638-11640. |
| 60 | WANG W X, ZENG P Y, LONG X Y, et al. Methane storage in tea clathrates[J]. Chemical Communications, 2014, 50(10): 1244-1246. |
| 61 | INKONG K, VELUSWAMYA H P, PRAMOCH R, et al. Investigation on the kinetics of methane hydrate formation in the presence of methyl ester sulfonate[J]. Journal of Natural Gas Science and Engineering, 2019, 71: 102999. |
| 62 | FAKHARIAN H, GANJI H, NADERI F A, et al. Potato starch as methane hydrate promoter[J]. Fuel, 2012, 94: 356-360. |
| 63 | LIN Y, VELUSWAMYA H P, LINGA P. Effect of eco-friendly cyclodextrin on the kinetics of mixed methane-tetrahydrofuran hydrate formation[J]. Industrial & Engineering Chemistry Research, 2018, 57(17): 5944-5950. |
| 64 | TIAN L Q, WU G Z. Cyclodextrins as promoter or inhibitor for methane hydrate formation[J]. Fuel, 2020, 264: 116828. |
| 65 | MECH D, GUPTA P, SANGWAI J S. Kinetics of methane hydrate formation in an aqueous solution of thermodynamic promoters (THF and TBAB) with and without kinetic promoter (SDS)[J]. Journal of Natural Gas Science and Engineering B, 2016, 35: 1519-1534. |
| 66 | KUMAR A, KUMAR R, LINGA P. Sodium dodecyl sulfate preferentially promotes enclathration of methane in mixed methanetetrahydrofuran hydrates[J]. iScience, 2019, 14: 136-146. |
| 67 | PAPADIMITRIOU N I, TSIMPANOGIANNIS I N, ECONOMOU I G, et al. Identification of conditions for increased methane storage capacity in sⅡ and sH clathrate hydrates from Monte Carlo simulations[J]. Journal of Chemical Thermodynamics, 2018, 117:128-137. |
| 68 | LEE J D, LEE J W, PARK K N, et al. Double helix type gas hydrate reactor: US20130236368A1[P]. 2015-01-20. |
| 69 | VELUSWAMYA H P, WONG A J H, BABU P, et al. Rapid methane hydrate formation to develop a cost effective large scale energy storage system[J]. Chemical Engineering Journal, 2016, 290: 161-173. |
| 70 | DYADIN Y A, LARIONOV E G, MANAKOV A Y, et al. Clathrate hydrates of hydrogen and neon[J]. Mendeleev Communications, 1999, 5: 209-210. |
| 71 | MAO W L, MAO H K, GONCHAROV A F, et al. Hydrogen clusters in clathrate hydrate[J]. Science, 2002, 297(5590): 2247-2249. |
| 72 | VELUSWAMY H P, KUMAR R, LINGA P. Hydrogen storage in clathrate hydrates: current state of the art and future directions[J]. Applied Energy, 2014, 122: 112-132. |
| 73 | SKIBA S S, LARINONV E G, MANKOV A Y, et al. Double clathrate hydrate of propane and hydrogen[J]. Journal of Inclusion Phenomena & Macrocyclic Chemistry, 2009, 63(3/4): 383-386. |
| 74 | VELUSWAMY H P, YEW J Y, LINGA P. New hydrate phase equilibrium data for two binary gas mixtures of hydrogen and propane coupled with a kinetic study[J]. Journal of Chemical & Engineering Data, 2015, 60(2): 228-237. |
| 75 | MATSUMOTO Y, GRIM R G, KHAN N M, et al. Investigating the thermodynamic stabilities of hydrogen and methane binary gas hydrates[J]. Journal of Physical Chemistry C, 2014, 118(7): 3783-3788. |
| 76 | AHN Y H, SEOKYOON M, KOH D Y, et al. One-step formation of hydrogen clusters in clathrate hydrates stabilized via natural gas blending[J]. Energy Storage Materials, 2020, 24(1): 655-661. |
| 77 | WANG X L, DENNIS M, HOU L X. Clathrate hydrate technology for cold storage in air conditioning systems[J]. Renewable and Sustainable Energy Reviews, 2014, 36: 34-51. |
| 78 | CHEN C X, WANG F, TIAN Y J, et al. Review and prospects of hydrate cold storage technology[J]. Renewable and Sustainable Energy Reviews, 2020, 117: 109492. |
| 79 | 陈伟军, 刘妮, 肖晨, 等. CO2水合物浆在储冷空调中的应用前景[J]. 制冷学报, 2012, 33(3): 1-4. |
| CHEN W J, LIU N, XIAO C, et al. Perspective of CO2 hydrate slurry application in air conditioning system with cool storage[J]. Journal of Refrigeration, 2012, 33(3): 1-4. | |
| 80 | 李刚, 谢应明, 刘道平, 等. 四丁基溴化铵-四氢呋喃系蓄冷水合物[J]. 过程工程学报, 2009, 9(1): 186-189. |
| LI G, XIE Y M, LIU D P, et al. TBAB-THF hydrate mixture as a cold storage medium[J]. The Chinese Journal of Process Engineering, 2009, 9(1): 186-189. | |
| 81 | WANG X L, DENNIS M. Phase equilibrium and formation behaviour of CO2-TBAB semi-clathrate hydrate at low pressures for cold storage air conditioning applications[J]. Chemcal Engineering Science, 2016, 155: 294-305. |
| 82 | MOHAMMADI A, JODAT A. Investigation of the kinetics of TBAB + carbon dioxide semiclathrate hydrate in presence of Tween 80 as a cold storage material[J]. Journal of Molecular Liquids, 2019, 293: 111433. |
| 83 | CHOI S, PARK J, KANG Y T. Experimental investigation on CO2 hydrate formation/dissociation for cold thermal energy harvest and transportation applications[J]. Applied Energy, 2019, 242: 1358-1368. |
| 84 | JAVIDANI M A, FARIZHENDI S A, MOHAMMADI A, et al. Experimental study and kinetic modeling of R410a hydrate formation in presence of SDS, Tween 20, and graphene oxide nanosheets with application in cold storage[J]. Journal of Molecular Liquids, 2020, 304: 112665. |
| 85 | OHGAKI K, TAKANO K, SANGAWA H, et al. Methane exploitation by carbon dioxide from gas hydrates-phase equilibria for CO2-CH4 mixed hydrate system[J]. Journal of Chemical Engineering of Japan, 1996, 29(3): 478-483. |
| 86 | STANWIX P L, RATHNAYAKE N M, DEOBANOS F P, et al. Characterising thermally controlled CH4-CO2 hydrate exchange in unconsolidated sediments[J]. Energy & Environmental Science, 2018, 11: 1828-1840. |
| 87 | LI F G, YUAN Q, LI T D, et al. A review: enhanced recovery of natural gas hydrate reservoirs[J]. Chinese Journal of Chemical Engineering, 2019, 27: 2062-2073. |
| 88 | ZHOU X B, LIANG D Q, LIANG S, et al. Recovering CH4 from natural gas hydrates with the injection of CO2-N2 gas mixtures[J]. Energy & Fuels, 2015, 29(2): 1099-1106. |
| 89 | LIM D, RO H, SEO Y, et al. Thermodynamic stability and guest distribution of CH4/N2/CO2 mixed hydrates for methane hydrate production using N2/CO2 injection[J]. Journal of Chemical Thermodynamics, 2017, 106: 16-21. |
| 90 | MATSUI H, JIA J H, TSUJI T, et al. Microsecond simulation study on the replacement of methane in methane hydrate by carbon dioxide, nitrogen, and carbon dioxide-nitrogen mixtures[J]. Fuel, 2020, 263: 116640. |
| 91 | CHONG Z R, YANG S H B, BABU P, et al. Review of natural gas hydrates as an energy resource: prospects and challenges[J]. Applied Energy, 2016, 162: 1633-1652. |
| 92 | KOH D Y, KANG H, LEE J W, et al. Energy-efficient natural gas hydrate production using gas exchange[J]. Applied Energy, 2016, 162: 114-130. |
| 93 | WANG X H, SUN Y F, WANG Y F, et al. Gas production from hydrates by CH4-CO2/H2 replacement[J]. Applied Energy, 2017, 188: 305-314. |
| 94 | SUN Y F, ZHONG J R, LI R, et al. Natural gas hydrate exploitation by CO2/H2 continuous injection production mode[J]. Applied Energy, 2018, 226: 10-21. |
| 95 | SUN Y F, WANG Y F, ZHONG J R, et al. Gas hydrate exploitation using CO2/H2 mixture gas by semi-continuous injection-production mode[J]. Applied Energy, 2019, 240: 215-225. |
| 96 | ZHANG L X, YANG L, WANG J Q, et al. Enhanced CH4 recovery and CO2 storage via thermal stimulation in the CH4/CO2 replacement of methane hydrate[J]. Chemical Engineering Journal, 2017, 308: 40-49. |
| 97 | TUPSAKHARE S S, CASTALDI M J. Efficiency enhancements in methane recovery from natural gas hydrates using injection of CO2/N2 gas mixture simulating in-situ combustion[J]. Applied Energy, 2019, 236: 825-836. |
| 98 | ZHAO J F, CHEN X Q, SONG Y C, et al. Experimental study on a novel way of methane hydrates recovery: combining CO2 replacement and depressurization[J]. Energy Procedia, 2014, 61: 75-79. |
| 99 | CHEN Y, GAO Y H, CHEN L T, et al. Experimental investigation of the behavior of methane gas hydrates during depressurization-assisted CO2 replacement[J]. Journal of Natural Gas Science and Engineering, 2019, 61: 284-292. |
| [1] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [2] | 李由, 吴越, 钟禹, 林琦璇, 任俊莉. 酸性熔盐水合物预处理麦秆高效制备木糖及其对酶解效率的影响[J]. 化工进展, 2023, 42(9): 4974-4983. |
| [3] | 尹新宇, 皮丕辉, 文秀芳, 钱宇. 特殊浸润性材料在防治油气管道中水合物成核与聚集的应用[J]. 化工进展, 2023, 42(8): 4076-4092. |
| [4] | 汤磊, 曾德森, 凌子夜, 张正国, 方晓明. 相变蓄冷材料及系统应用研究进展[J]. 化工进展, 2023, 42(8): 4322-4339. |
| [5] | 张凯, 吕秋楠, 李刚, 李小森, 莫家媚. 南海海泥中甲烷水合物的形貌及赋存特性[J]. 化工进展, 2023, 42(7): 3865-3874. |
| [6] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [7] | 杨扬, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂OP-13促进HCFC-141b水合物生成[J]. 化工进展, 2023, 42(6): 2854-2859. |
| [8] | 刘佳, 梁德青, 李君慧, 林德才, 吴思婷, 卢富勤. 油水体系水合物浆液流动保障研究进展[J]. 化工进展, 2023, 42(4): 1739-1759. |
| [9] | 王唯, 张东旭, 李遵照, 王晓霖, 黄启玉. 油包水乳状液体系中水合物生长行为研究进展[J]. 化工进展, 2023, 42(3): 1155-1166. |
| [10] | 岳子瀚, 龙臻, 周雪冰, 臧小亚, 梁德青. sⅡ型水合物储氢研究进展[J]. 化工进展, 2023, 42(10): 5121-5134. |
| [11] | 蔡铭威, 王知, 卢小闯, 庄俊伟, 吴嘉豪, 张诗洋, 闵永刚. 聚酰亚胺薄膜在氢气分离中的研究进展[J]. 化工进展, 2023, 42(10): 5232-5248. |
| [12] | 康宇, 苟泽念. 氨基酸和DTAC对CO2水合分离动力学影响[J]. 化工进展, 2023, 42(10): 5067-5075. |
| [13] | 王英梅, 张兆慧, 刘生浩, 焦雯泽, 王立瑾, 滕亚栋, 刘杰. 促进剂体系中二氧化碳水合物常压分解[J]. 化工进展, 2022, 41(S1): 141-149. |
| [14] | 王云飞, 孙长宇, 喻西崇, 李清平, 陈光进. 三维中试装置内水合物降压分解动力学规律[J]. 化工进展, 2022, 41(8): 4111-4119. |
| [15] | 黄婷, 李清平, 李锐, 庞维新, 陈光进. 第一类水合物藏降压开采实验模拟[J]. 化工进展, 2022, 41(8): 4120-4128. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||