化工进展 ›› 2019, Vol. 38 ›› Issue (11): 5142-5150.DOI: 10.16085/j.issn.1000-6613.2019-0302
生物炭对重金属(Zn)的吸附特性及动力学
- 南京师范大学能源与机械工程学院,江苏 南京 210023
-
收稿日期:2019-02-28出版日期:2019-11-05发布日期:2019-11-05 -
通讯作者:卢平 -
作者简介:王昱璇(1995—),女,硕士研究生,主要从事生物质和固体废弃物能源化与资源化利用技术的研究。E-mail:xgaza301@163.com 。 -
基金资助:国家自然科学基金(51476079)
Adsorption and kinetics of heavy metal (Zn) over biochars in solution
Yuxuan WANG( ),Hong WANG,Ping LU(
),Hong WANG,Ping LU( )
)
- School of Energy and Mechanical Engineering, Nanjing Normal University, Nanjing 210023, Jiangsu, China
-
Received:2019-02-28Online:2019-11-05Published:2019-11-05 -
Contact:Ping LU
摘要:
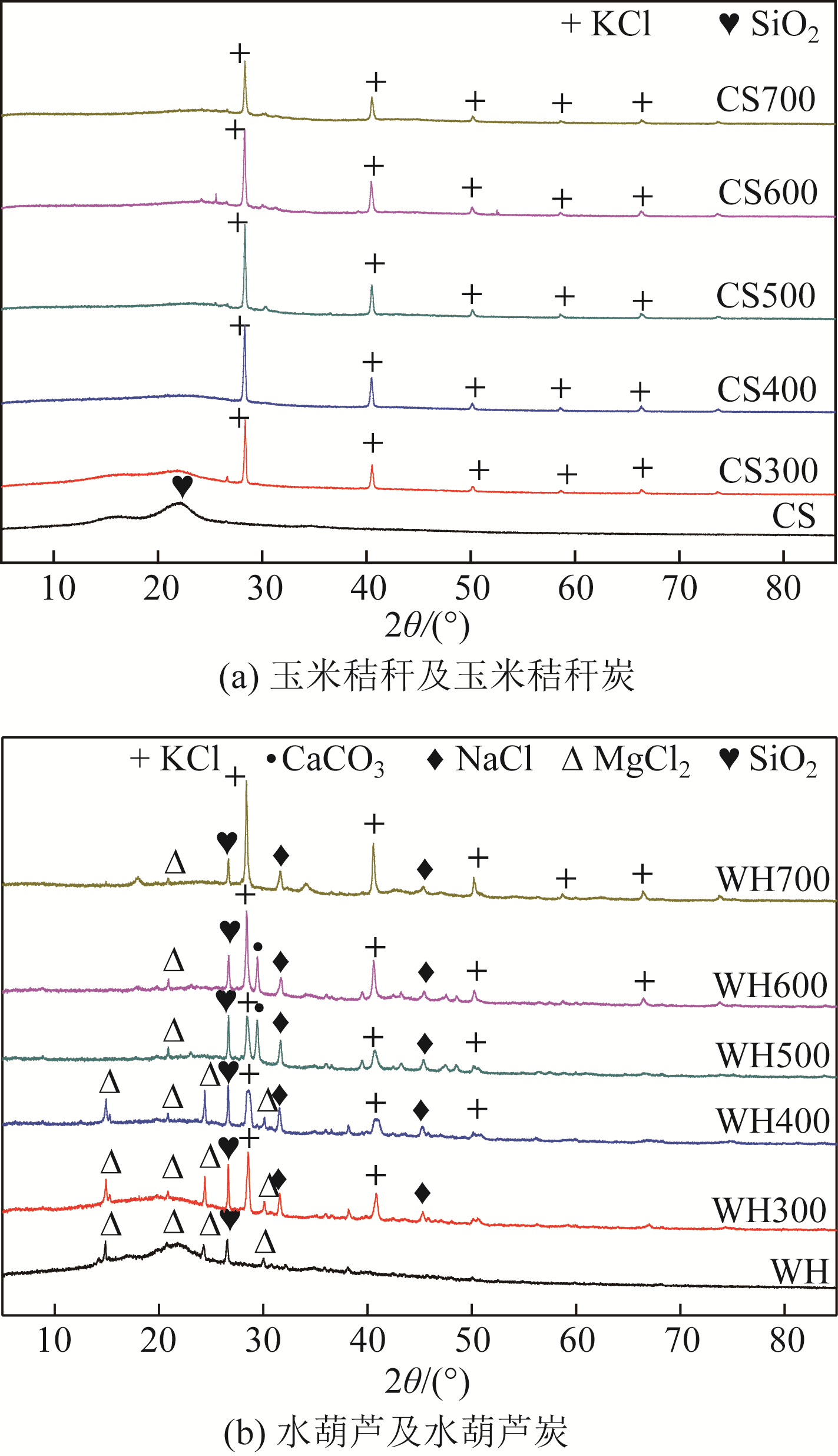
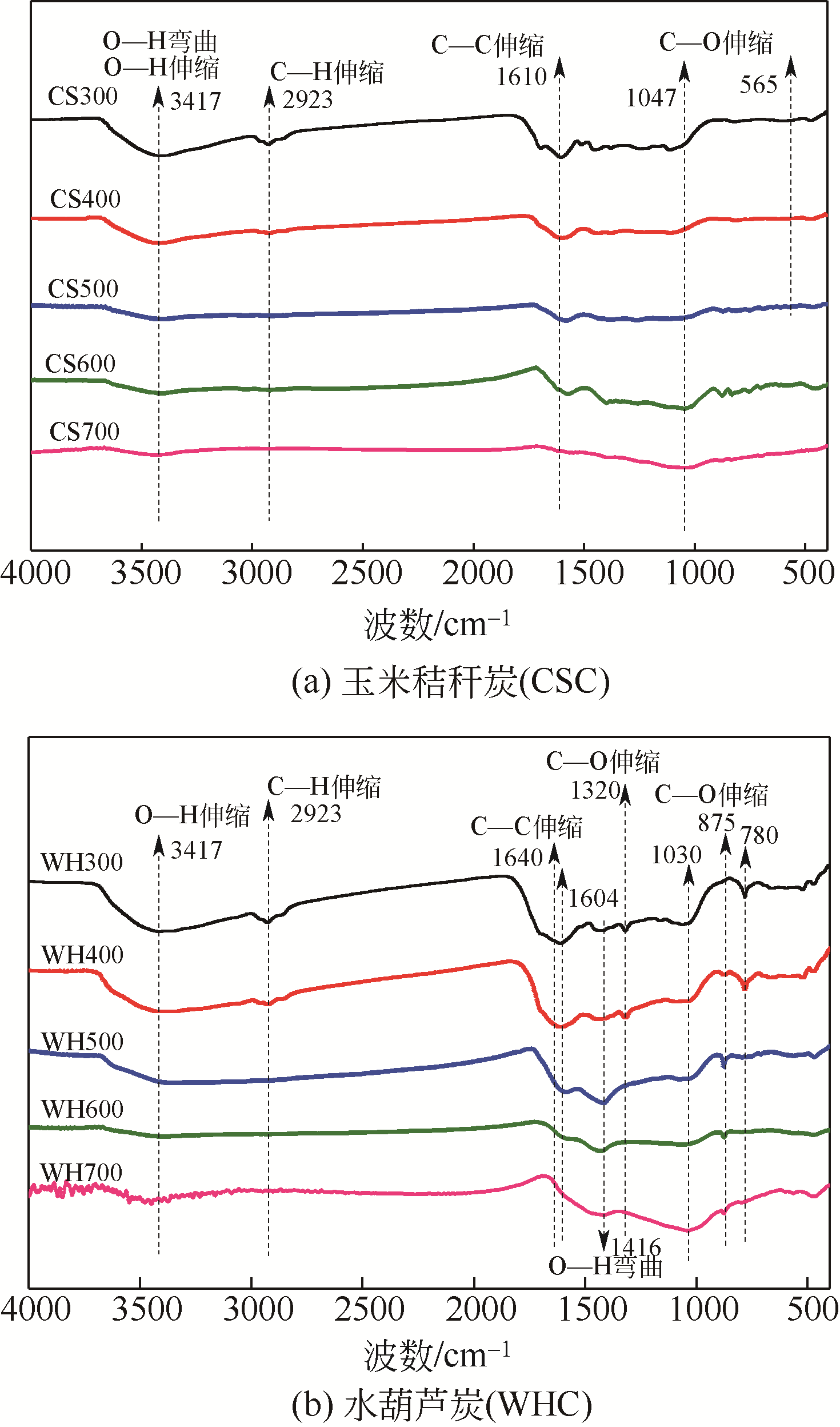
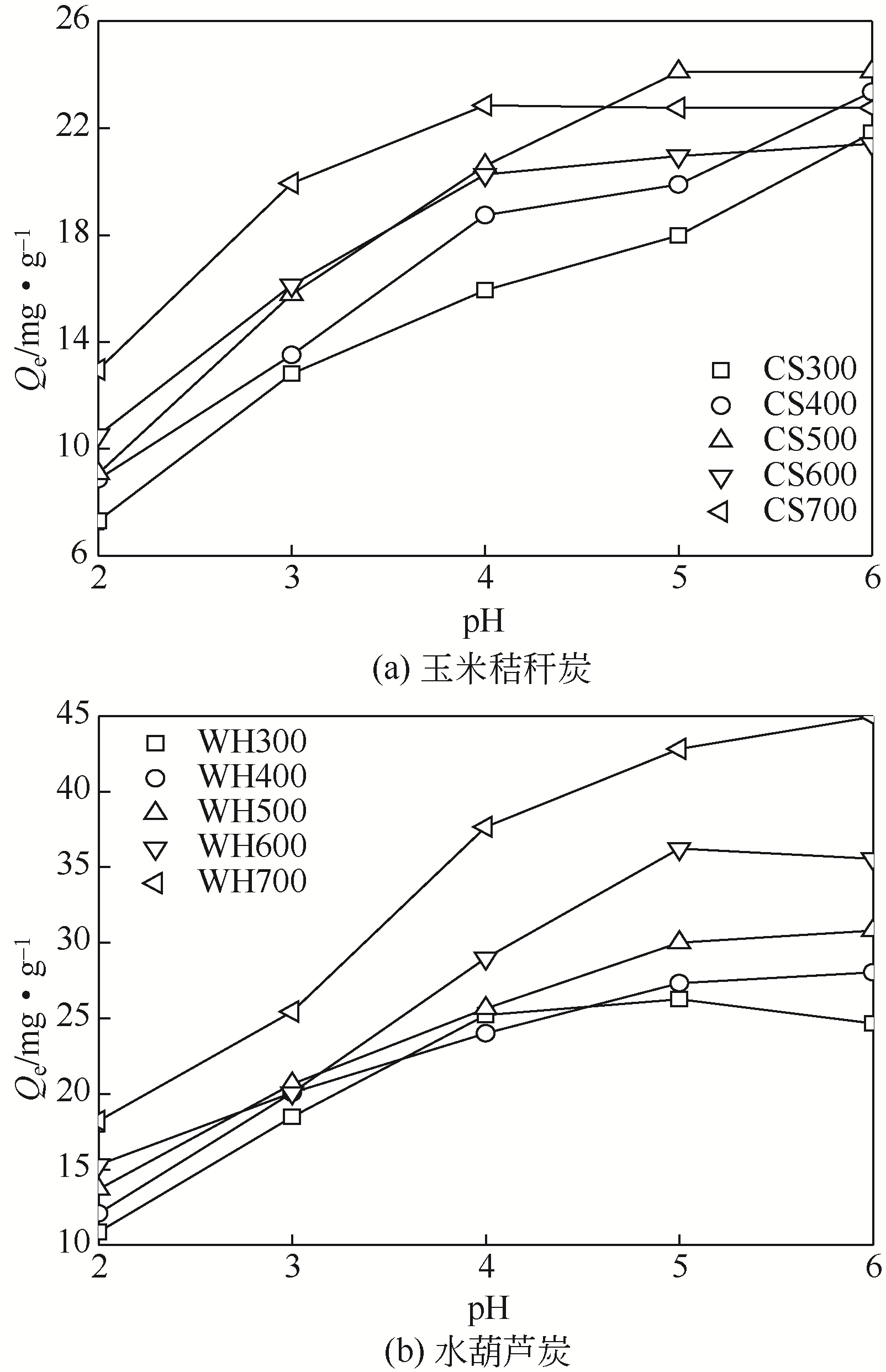
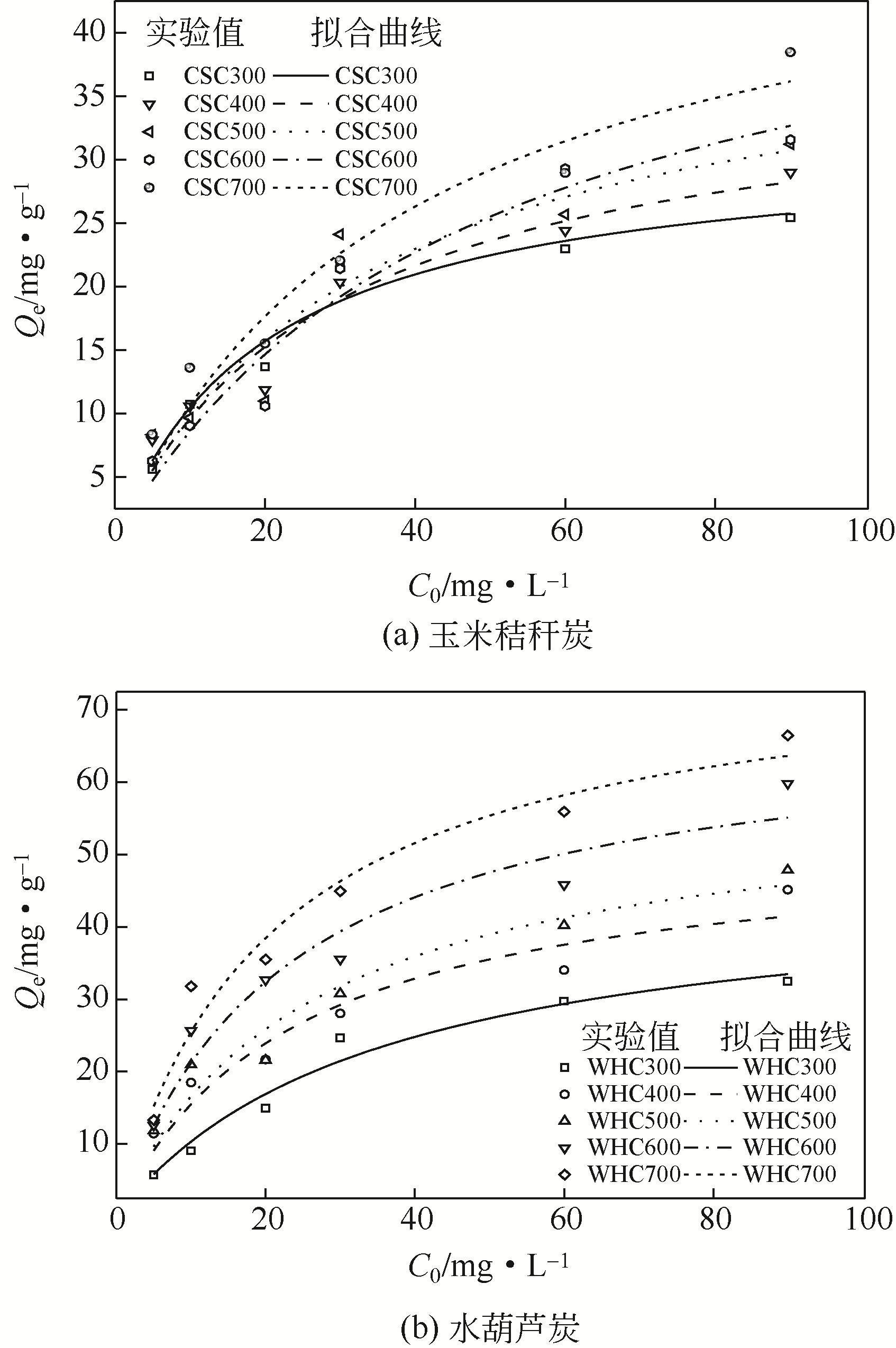
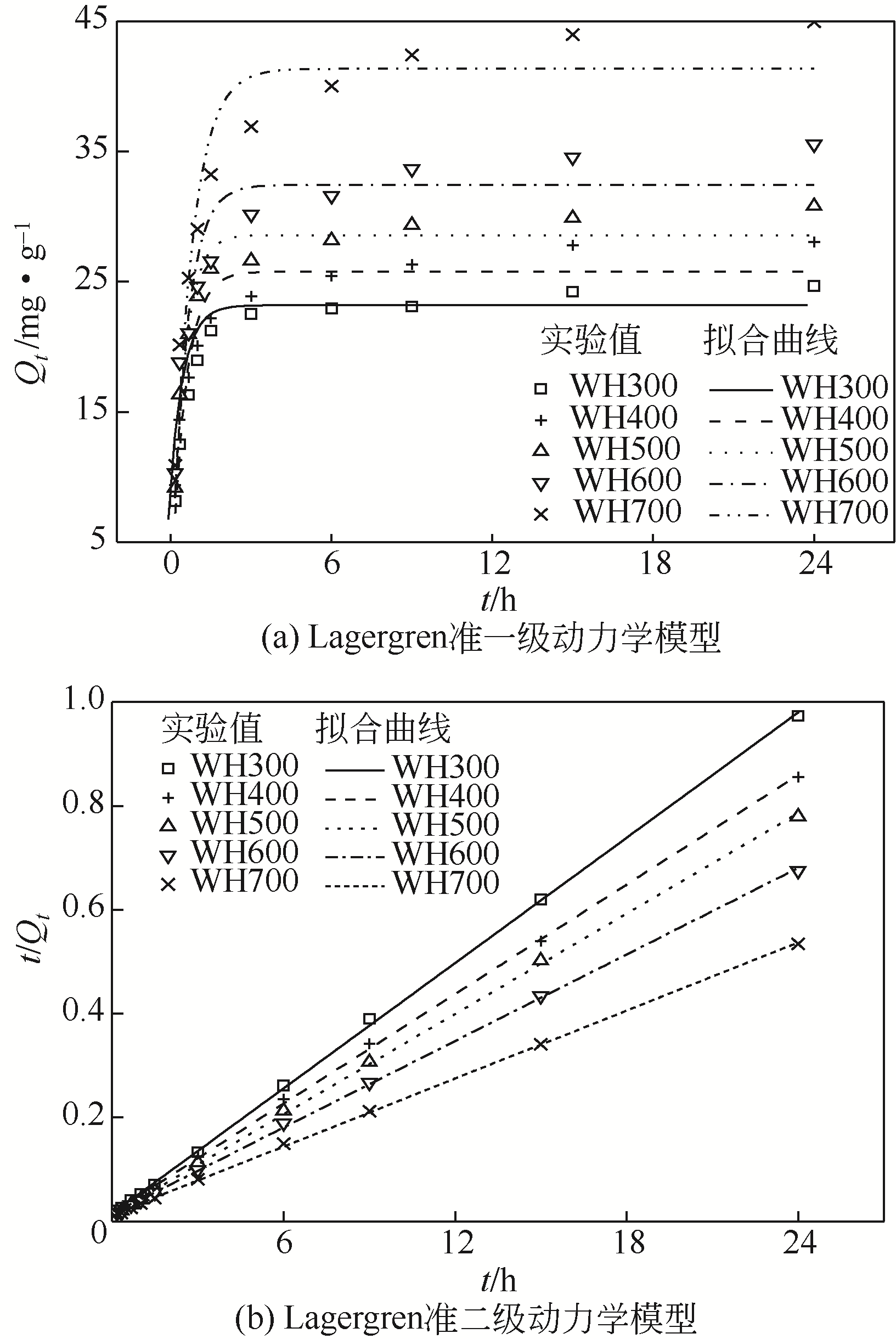
在300~700℃下制备了水葫芦炭和玉米秸秆炭,研究了生物质种类、热解温度、溶液初始pH和Zn(Ⅱ)初始浓度对两种生物炭吸附溶液中Zn(Ⅱ)的影响,并结合吸附过程曲线拟合获得了吸附动力学模型。结果表明:随着热解温度的升高,生物炭理化特性发生显著变化,生物炭的挥发分、氧含量、氢含量以及O/C和H/C显著降低,而固定碳、灰分和热值显著升高,生物炭的比表面积、总孔容、微孔容、pH以及KCl等盐类物质均得到了显著增加。随着溶液初始pH增加,生物炭对Zn(Ⅱ)的吸附能力呈现先快速增加然后逐步趋于稳定或稍有下降的趋势,不同生物炭的最大平衡吸附量出现在pH=4~6之间。Zn(Ⅱ)初始浓度<30mg/L时,生物炭对Zn(Ⅱ)平衡吸附量随溶液Zn(Ⅱ)初始浓度的增加呈线性快速增长,而当Zn(Ⅱ)初始浓度>30mg/L,其平衡吸附量增长趋势变缓。在相同Zn(Ⅱ)初始浓度下,随着热解温度的提高,生物炭对溶液中Zn(Ⅱ)平衡吸附量逐渐提高,且在同一热解温度下制备的水葫芦炭对Zn(Ⅱ)的平衡吸附量显著高于玉米秸秆炭。两种生物炭对溶液Zn(Ⅱ)的吸附符合Lagergren准二级动力学模型,其吸附过程均受化学吸附控制,水葫芦炭和玉米秸秆炭对Zn(Ⅱ)吸附机制主要包括含氧官能团的络合作用和无机盐离子的沉淀作用。
中图分类号:
引用本文
王昱璇,王红,卢平. 生物炭对重金属(Zn)的吸附特性及动力学[J]. 化工进展, 2019, 38(11): 5142-5150.
Yuxuan WANG,Hong WANG,Ping LU. Adsorption and kinetics of heavy metal (Zn) over biochars in solution[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5142-5150.
| 样品 | 工业分析/% | 元素分析/% | Q net,ad | H/C | O/C | (O+N)/C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Vad | FCad | Aad | Cad | Had | Oad ① | Nad | Sad | /MJ·kg-1 | ||||||
| CS | 13.14 | 64.68 | 15.82 | 6.36 | 40.71 | 5.64 | 33.59 | 0.50 | 0.06 | 14.11 | 0.14 | 0.83 | 0.84 | ||
| CSC300 | — | 42.67 | 43.57 | 13.76 | 58.24 | 4.70 | 22.75 | 0.49 | 0.06 | 22.76 | 0.08 | 0.39 | 0.40 | ||
| CSC400 | — | 23.08 | 59.78 | 17.13 | 64.89 | 4.01 | 13.48 | 0.48 | 0.01 | 20.58 | 0.06 | 0.21 | 0.22 | ||
| CSC500 | — | 13.09 | 66.23 | 20.68 | 67.77 | 3.06 | 8.01 | 0.47 | 0.01 | 22.84 | 0.05 | 0.12 | 0.13 | ||
| CSC600 | — | 8.41 | 70.36 | 21.23 | 68.96 | 2.56 | 6.78 | 0.46 | 0.01 | 25.37 | 0.04 | 0.10 | 0.11 | ||
| CSC700 | — | 6.99 | 70.34 | 22.68 | 69.20 | 1.84 | 5.79 | 0.43 | 0.06 | 24.73 | 0.03 | 0.08 | 0.09 | ||
| WH | 13.93 | 60.79 | 12.84 | 12.45 | 36.94 | 5.63 | 29.56 | 1.33 | 0.16 | 10.52 | 0.15 | 0.80 | 0.84 | ||
| WHC300 | — | 45.55 | 33.12 | 21.32 | 50.15 | 4.34 | 21.86 | 2.22 | 0.11 | 19.20 | 0.09 | 0.44 | 0.48 | ||
| WHC400 | — | 29.67 | 42.59 | 27.75 | 52.31 | 3.72 | 13.96 | 2.19 | 0.07 | 19.71 | 0.07 | 0.27 | 0.31 | ||
| WHC500 | — | 18.93 | 48.50 | 32.57 | 51.89 | 2.79 | 10.59 | 2.05 | 0.11 | 18.95 | 0.05 | 0.20 | 0.24 | ||
| WHC600 | — | 13.66 | 51.13 | 35.20 | 53.24 | 1.98 | 7.65 | 1.92 | 0.01 | 19.21 | 0.04 | 0.14 | 0.18 | ||
| WHC700 | — | 9.22 | 54.82 | 35.96 | 55.57 | 1.66 | 5.20 | 1.51 | 0.10 | 19.35 | 0.03 | 0.09 | 0.12 | ||
表1 生物质和生物炭的工业分析、元素分析和热值
| 样品 | 工业分析/% | 元素分析/% | Q net,ad | H/C | O/C | (O+N)/C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Vad | FCad | Aad | Cad | Had | Oad ① | Nad | Sad | /MJ·kg-1 | ||||||
| CS | 13.14 | 64.68 | 15.82 | 6.36 | 40.71 | 5.64 | 33.59 | 0.50 | 0.06 | 14.11 | 0.14 | 0.83 | 0.84 | ||
| CSC300 | — | 42.67 | 43.57 | 13.76 | 58.24 | 4.70 | 22.75 | 0.49 | 0.06 | 22.76 | 0.08 | 0.39 | 0.40 | ||
| CSC400 | — | 23.08 | 59.78 | 17.13 | 64.89 | 4.01 | 13.48 | 0.48 | 0.01 | 20.58 | 0.06 | 0.21 | 0.22 | ||
| CSC500 | — | 13.09 | 66.23 | 20.68 | 67.77 | 3.06 | 8.01 | 0.47 | 0.01 | 22.84 | 0.05 | 0.12 | 0.13 | ||
| CSC600 | — | 8.41 | 70.36 | 21.23 | 68.96 | 2.56 | 6.78 | 0.46 | 0.01 | 25.37 | 0.04 | 0.10 | 0.11 | ||
| CSC700 | — | 6.99 | 70.34 | 22.68 | 69.20 | 1.84 | 5.79 | 0.43 | 0.06 | 24.73 | 0.03 | 0.08 | 0.09 | ||
| WH | 13.93 | 60.79 | 12.84 | 12.45 | 36.94 | 5.63 | 29.56 | 1.33 | 0.16 | 10.52 | 0.15 | 0.80 | 0.84 | ||
| WHC300 | — | 45.55 | 33.12 | 21.32 | 50.15 | 4.34 | 21.86 | 2.22 | 0.11 | 19.20 | 0.09 | 0.44 | 0.48 | ||
| WHC400 | — | 29.67 | 42.59 | 27.75 | 52.31 | 3.72 | 13.96 | 2.19 | 0.07 | 19.71 | 0.07 | 0.27 | 0.31 | ||
| WHC500 | — | 18.93 | 48.50 | 32.57 | 51.89 | 2.79 | 10.59 | 2.05 | 0.11 | 18.95 | 0.05 | 0.20 | 0.24 | ||
| WHC600 | — | 13.66 | 51.13 | 35.20 | 53.24 | 1.98 | 7.65 | 1.92 | 0.01 | 19.21 | 0.04 | 0.14 | 0.18 | ||
| WHC700 | — | 9.22 | 54.82 | 35.96 | 55.57 | 1.66 | 5.20 | 1.51 | 0.10 | 19.35 | 0.03 | 0.09 | 0.12 | ||
| 样品 | S BET /m2·g-1 | S D-R /m2·g-1 | V T /mm3·g-1 | V M /mm3·g-1 | X /% | D a /nm |
|---|---|---|---|---|---|---|
| CS | 1.54 | 2.39 | 2.9 | 0.9 | 31.30 | 7.53 |
| CS300 | 6.80 | 11.34 | 9.4 | 4.3 | 45.99 | 5.53 |
| CS400 | 10.51 | 16.57 | 11.7 | 5.9 | 50.43 | 4.45 |
| CS500 | 13.88 | 20.82 | 13.1 | 7.4 | 56.49 | 3.78 |
| CS600 | 60.50 | 81.25 | 61.2 | 25.1 | 41.01 | 4.05 |
| CS700 | 20.92 | 30.35 | 21.1 | 10.8 | 51.18 | 4.03 |
| WH | 2.26 | 3.36 | 5.8 | 1.2 | 20.83 | 10.27 |
| WH300 | 2.79 | 5.33 | 8.2 | 1.9 | 23.17 | 11.76 |
| WH400 | 3.80 | 5.69 | 8.3 | 2.0 | 24.10 | 8.74 |
| WH500 | 8.19 | 12.13 | 16.9 | 4.2 | 24.85 | 8.24 |
| WH600 | 37.46 | 52.85 | 39.2 | 18.8 | 47.96 | 4.19 |
| WH700 | 53.74 | 78.14 | 43.6 | 27.8 | 63.76 | 3.25 |
表2 生物质和生物炭的孔隙结构参数
| 样品 | S BET /m2·g-1 | S D-R /m2·g-1 | V T /mm3·g-1 | V M /mm3·g-1 | X /% | D a /nm |
|---|---|---|---|---|---|---|
| CS | 1.54 | 2.39 | 2.9 | 0.9 | 31.30 | 7.53 |
| CS300 | 6.80 | 11.34 | 9.4 | 4.3 | 45.99 | 5.53 |
| CS400 | 10.51 | 16.57 | 11.7 | 5.9 | 50.43 | 4.45 |
| CS500 | 13.88 | 20.82 | 13.1 | 7.4 | 56.49 | 3.78 |
| CS600 | 60.50 | 81.25 | 61.2 | 25.1 | 41.01 | 4.05 |
| CS700 | 20.92 | 30.35 | 21.1 | 10.8 | 51.18 | 4.03 |
| WH | 2.26 | 3.36 | 5.8 | 1.2 | 20.83 | 10.27 |
| WH300 | 2.79 | 5.33 | 8.2 | 1.9 | 23.17 | 11.76 |
| WH400 | 3.80 | 5.69 | 8.3 | 2.0 | 24.10 | 8.74 |
| WH500 | 8.19 | 12.13 | 16.9 | 4.2 | 24.85 | 8.24 |
| WH600 | 37.46 | 52.85 | 39.2 | 18.8 | 47.96 | 4.19 |
| WH700 | 53.74 | 78.14 | 43.6 | 27.8 | 63.76 | 3.25 |
| 生物炭 | C 0 /mg·g-1 | 实验平衡吸附量Q e1/mg·g-1 | 一级动力学吸附模型 | 二级动力学吸附模型 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Q e2 /mg·g-1 | K 1 /h-1 | R 2 | Q e2 /mg·g-1 | K 2 /g·mg-1·h-1 | R 2 | ||||
| CS300 | 30 | 21.82 | 19.44 | 1.458 | 0.9026 | 22.12 | 0.064 | 0.9983 | |
| CS400 | 30 | 20.35 | 17.99 | 1.877 | 0.8773 | 20.57 | 0.077 | 0.9978 | |
| CS500 | 30 | 24.12 | 22.34 | 2.104 | 0.9060 | 23.69 | 0.073 | 0.9979 | |
| CS600 | 30 | 21.41 | 20.47 | 2.979 | 0.9748 | 21.47 | 0.214 | 0.9998 | |
| CS700 | 30 | 22.06 | 20.88 | 3.639 | 0.9738 | 21.97 | 0.215 | 0.9994 | |
| WH300 | 30 | 24.66 | 23.19 | 2.018 | 0.9588 | 24.88 | 0.107 | 0.9997 | |
| WH400 | 30 | 28.05 | 25.77 | 1.924 | 0.8934 | 28.48 | 0.075 | 0.9996 | |
| WH500 | 30 | 30.80 | 28.58 | 2.124 | 0.9488 | 31.05 | 0.080 | 0.9996 | |
| WH600 | 30 | 35.55 | 32.42 | 1.764 | 0.8782 | 36.03 | 0.053 | 0.9995 | |
| WH700 | 30 | 44.95 | 41.35 | 1.409 | 0.9220 | 45.84 | 0.036 | 0.9997 | |
表3 基于Lagergren准一级和准二级动力学模型对生物炭Zn(Ⅱ)吸附的拟合参数
| 生物炭 | C 0 /mg·g-1 | 实验平衡吸附量Q e1/mg·g-1 | 一级动力学吸附模型 | 二级动力学吸附模型 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Q e2 /mg·g-1 | K 1 /h-1 | R 2 | Q e2 /mg·g-1 | K 2 /g·mg-1·h-1 | R 2 | ||||
| CS300 | 30 | 21.82 | 19.44 | 1.458 | 0.9026 | 22.12 | 0.064 | 0.9983 | |
| CS400 | 30 | 20.35 | 17.99 | 1.877 | 0.8773 | 20.57 | 0.077 | 0.9978 | |
| CS500 | 30 | 24.12 | 22.34 | 2.104 | 0.9060 | 23.69 | 0.073 | 0.9979 | |
| CS600 | 30 | 21.41 | 20.47 | 2.979 | 0.9748 | 21.47 | 0.214 | 0.9998 | |
| CS700 | 30 | 22.06 | 20.88 | 3.639 | 0.9738 | 21.97 | 0.215 | 0.9994 | |
| WH300 | 30 | 24.66 | 23.19 | 2.018 | 0.9588 | 24.88 | 0.107 | 0.9997 | |
| WH400 | 30 | 28.05 | 25.77 | 1.924 | 0.8934 | 28.48 | 0.075 | 0.9996 | |
| WH500 | 30 | 30.80 | 28.58 | 2.124 | 0.9488 | 31.05 | 0.080 | 0.9996 | |
| WH600 | 30 | 35.55 | 32.42 | 1.764 | 0.8782 | 36.03 | 0.053 | 0.9995 | |
| WH700 | 30 | 44.95 | 41.35 | 1.409 | 0.9220 | 45.84 | 0.036 | 0.9997 | |
| 1 | LIANG J Y , LIU Y Y , ZOU J , et al . Inhibitory effect of zinc on human prostatic carcinoma cell growth[J]. The Prostate, 1999, 40(3): 200-207. |
| 2 | ALQADAMI A A , NAUSHAD M , ABDALLA M A , et al . Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: a study of adsorption parameters and interaction mechanism[J]. Journal of Cleaner Production, 2017, 156: 426-436. |
| 3 | COLLA T S , ANDREAZZA R , BUCKER F , et al . Bioremediation assessment of diesel-biodiesel-contaminated soil using an alternative bioaugmentation strategy[J]. Environmental Science and Pollution Research, 2014, 21(4): 2592-2602. |
| 4 | ZHANG Y , LUO W . Adsorptive removal of heavy metal from acidic wastewater with biochar produced from anaerobically digested residues: kinetics and surface complexation modeling[J]. Bioresources, 2014, 9(2): 2484-2499. |
| 5 | INYANG M , GAO B , YAO Y , et al . A review of biochar as a low-cost adsorbent for aqueous heavy metal removal[J]. Critical Reviews in Environmental Science and Technology, 2016, 46(4): 406-433. |
| 6 | 王怀臣, 冯雷雨, 陈银广 . 废物资源化制备生物质炭及其应用的研究进展[J]. 化工进展, 2012, 31(4): 907-914. |
| WANG H C , FENG L Y , CHEN Y G . Advances in biochar production from wastes and its applications[J]. Chemical Industry and Engineering Progress, 2012, 31(4): 907-914. | |
| 7 | GUL S, WHALEN J K , THOMAS B W , et al . Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions[J]. Agriculture, Ecosystems & Environment, 2015, 206: 46-59. |
| 8 | ZGANG G , GUO X , ZHAO Z , et al . Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil[J]. Environmental Pollution, 2016, 218: 513-522. |
| 9 | TAN X , LIU Y , ZENG G , et al . Application of biochar for the removal of pollutants from aqueous solutions[J]. Chemosphere, 2015,125: 70-85. |
| 10 | 王重庆, 王晖, 江小燕, 等 . 生物炭吸附重金属离子的研究进展[J]. 化工进展, 2019, 38(1): 692-706. |
| WANG C Q , WANG H , JIANG X Y , et al . Research advances on adsorption of heavy metals by biochar[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 692-706. | |
| 11 | CHEN X , CHEN G , CHEN L , et al . Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution[J]. Bioresource Technology, 2011, 102(19): 8877-8884. |
| 12 | 谢伟雪, 刘孝敏, 李小东, 等 . 废毛发生物炭的特性及其对Ni(Ⅱ)和Zn(Ⅱ)的吸附研究[J]. 环境工程技术学报, 2018, 8(6): 656-662. |
| XIE W X , LIU X M , LI X D , et al . Characteristics of waste hair biochar and its adsorption to Ni(Ⅱ) and Zn(Ⅱ)[J]. Journal of Environmental Engineering Technology, 2018, 8(6): 656-662. | |
| 13 | 王震宇, 刘国成, XING Monica , 等 .不同热解温度生物炭对Cd(Ⅱ)的吸附特性 [J]. 环境科学, 2014, 35(12): 4735-4744. |
| WANG Z Y , LIU G C , XING M , et al . Adsorption of Cd(Ⅱ) varies with biochars derived at different pyrolysis temperatures[J]. Environmental Science, 2014, 35(12): 4735-4744. | |
| 14 | LI Q , TANG L , HU J , et al . Removal of toxic metals from aqueous solution by biochars derived from long-root Eichhornia crassipes[J]. Royal Society Open Science, 2018, 5(10): 180966. |
| 15 | QIAN T , WU P , QIN Q , et al . Screening of wheat straw biochars for the remediation of soils polluted with Zn (Ⅱ) and Cd (Ⅱ)[J]. Journal of Hazardous Materials, 2019, 365: 311-317. |
| 16 | JIANG S S , HUANG L B , NGUYEN T A , et al . Copper and zinc adsorption by softwood and hardwood biochars under elevated sulphate-induced salinity and acidic pH conditions[J]. Chemosphere, 2016, 142: 64-71. |
| 17 | XU X , CAO X , ZHAO L , et al . Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar[J]. Environmental Science and Pollution Research, 2013, 20(1): 358-368. |
| 18 | KOLODYŃSKA D , KRUKOWSKA J , THOMAS P . Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon [J]. Chemical Engineering Journal, 2017, 307: 353-363. |
| 19 | 国家质量技术监督局 . 木质活性炭试验方法pH值的测定:GB/T 12496.7―1999 [S]. 北京:中国标准出版社, 2000. |
| State Bureau of Quality Technical . Test methods of wooden activated carbon-Determination of pH: GB/T 12496.7—1999 [S]. Beijing: Standards Press of China, 2000. | |
| 20 | MASULILIA, UTOMO W H , SYECHFANI M S , et al . Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and its influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia[J]. The Journal of Agricultural Science, 2010, 2(1): 39-47. |
| 21 | HO Y S, MCKAY G . Pseudo-second order model for sorption processes[J]. Process Biochemistry, 1999, 34(5): 451-465. |
| 22 | 安增莉, 侯艳伟, 蔡超, 等 . 水稻秸秆生物炭对Pb(Ⅱ)的吸附特性[J]. 环境化学, 2011, 30(11): 1851-1857. |
| AN Z L , HOU Y W , CAI C , et al . Lead (Ⅱ) adsorption characteristics on different biochars derived from rice straw[J]. Environmental Chemistry, 2011, 30(11): 1851-1857. | |
| 23 | HO Y S . Review of second-order models for adsorption systems[J]. Journal of Hazardous Materials, 2006, 136(3): 681-689. |
| 24 | NOVAK J M , LIMA I , XING B , et al . Characterization of designer biochar produced at different temperatures and their effects on a Loamy sand[J]. Annals of Environmental Science, 2009, 3: 195-206. |
| 25 | GASKIN J W , STEINER C , HARRIS K , et al . Effect of low-temperature pyrolysis conditions on biochar for agricultural use[J]. Transactions of the ASABE, 2008, 51(6): 2061-2069. |
| 26 | AHMAD M , RAJAPAKSHA A U , LIM J E, et al . Biochar as a sorbent for contaminant management in soil and water: a review[J]. Chemosphere, 2014, 99: 19-33. |
| 27 | 谢超然, 王兆炜, 朱俊民, 等 . 核桃青皮生物炭对重金属铅、铜的吸附特性研究[J]. 环境科学学报, 2016, 36(4): 1190-1198. |
| XIE C R , WANG Z W , ZHU J M , et al . Adsorption of lead and copper from aqueous solutions on biochar produced from walnut[J]. Acta Scientiae Circumstantiae, 2016, 36(4): 1190-1198. | |
| 28 | 郭素华, 许中坚, 李方文,等 . 生物炭对水中Pb(Ⅱ)和Zn(Ⅱ)的吸附特征[J]. 环境工程学报, 2015, 9(7): 3215-3222. |
| GUO S H , XU Z J , LI F W , et al . Adsorption of Pb (Ⅱ) , Zn (Ⅱ) from aqueous solution by biochars[J]. Chinese Journal of Environmental Engineering, 2015, 9(7): 3215-3222. | |
| 29 | ZHU Q , WU J , WANG L , et al . Adsorption characteristics of Pb2+ onto wine lees-derived biochar[J]. Bulletin of Environmental Contamination and Toxicology, 2016, 97(2): 294-299. |
| 30 | EL-ASHTOUKHY E S Z , AMIN N K , ABDELWAHAB O . Removal of lead (Ⅱ) and copper (Ⅱ) from aqueous solution using pomegranate peel as a new adsorbent [J]. Desalination, 2008, 223(1/2/3): 162-173. |
| 31 | 邓金环,郜礼阳,周皖婉,等 . 不同温度制备香根草生物炭对 Cd2+的吸附特性与机制[J]. 农业环境科学学报, 2018, 37(2): 340-349. |
| DENG J H , GAO L Y , ZHOU W W , et al . Adsorption characteristics and mechanisms of Cd2+ in biochar derived from vetiver grass under different pyrolysis temperatures[J]. Journal of Agro-Environment Science, 2018, 37(2): 340-349. | |
| 32 | CAO X , MA L , GAO B , et al . Dairy-manure derived biochar effectively sorbs lead and atrazine[J]. Environmental Science & Technology, 2009, 43(9): 3285-3291. |
| 33 | LI H , DONG X , SILVA E B , et al . Mechanisms of metal sorption by biochars: biochar characteristics and modifications[J]. Chemosphere, 2017,178: 466-478. |
| [1] | 黄益平, 李婷, 郑龙云, 戚傲, 陈政霖, 史天昊, 张新宇, 郭凯, 胡猛, 倪泽雨, 刘辉, 夏苗, 主凯, 刘春江. 三级环流反应器中气液流动与传质规律[J]. 化工进展, 2023, 42(S1): 175-188. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [4] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [5] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [6] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [7] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [8] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [9] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [10] | 汪鹏, 张洋, 范兵强, 何登波, 申长帅, 张贺东, 郑诗礼, 邹兴. 高碳铬铁盐酸浸出过程工艺及动力学[J]. 化工进展, 2023, 42(S1): 510-517. |
| [11] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [12] | 刘阳, 王云刚, 修浩然, 邹立, 白彦渊. 基于动力学分析的核桃壳最佳炭化工艺[J]. 化工进展, 2023, 42(S1): 94-103. |
| [13] | 董佳宇, 王斯民. 超声强化对二甲苯结晶特性及调控机理实验[J]. 化工进展, 2023, 42(9): 4504-4513. |
| [14] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [15] | 杨斌, 王晓冬, 王燕, 仪桂云, 王铁狼, 时闯, 张战营. 纳米Pt/ZnO异质结构的制备及其气敏性能[J]. 化工进展, 2023, 42(9): 4817-4827. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||