化工进展 ›› 2019, Vol. 38 ›› Issue (11): 5015-5023.DOI: 10.16085/j.issn.1000-6613.2019-0049
磷铁渣制备电池级纳米磷酸铁
马毅1,2,3( ),沈文喆1,2,3,袁梅梅1,2,3,王韵珂1,2,3,姚耀春1,2,3(
),沈文喆1,2,3,袁梅梅1,2,3,王韵珂1,2,3,姚耀春1,2,3( )
)
- 1. 昆明理工大学冶金与能源工程学院,云南 昆明 650093
2. 节能与新能源汽车动力电池及关键材料研究中心,云南 昆明 650093
3. 昆明理工大学真空冶金国家工程实验室,云南 昆明 650093
Preparation of battery grade nano iron phosphate by using ferro-phosphorus as raw material
Yi MA1,2,3( ),Wenzhe SHEN1,2,3,Meimei YUAN1,2,3,Yunke WANG1,2,3,Yaochun YAO1,2,3(
),Wenzhe SHEN1,2,3,Meimei YUAN1,2,3,Yunke WANG1,2,3,Yaochun YAO1,2,3( )
)
- 1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
2. Energy Saving and New Energy Automobile Power Battery and Key Materials Research Center, Kunming 650093, Yunnan, China
3. National Engineering Laboratory for Vacuum Metallurgy, Kunming 650093, Yunnan, China
摘要:
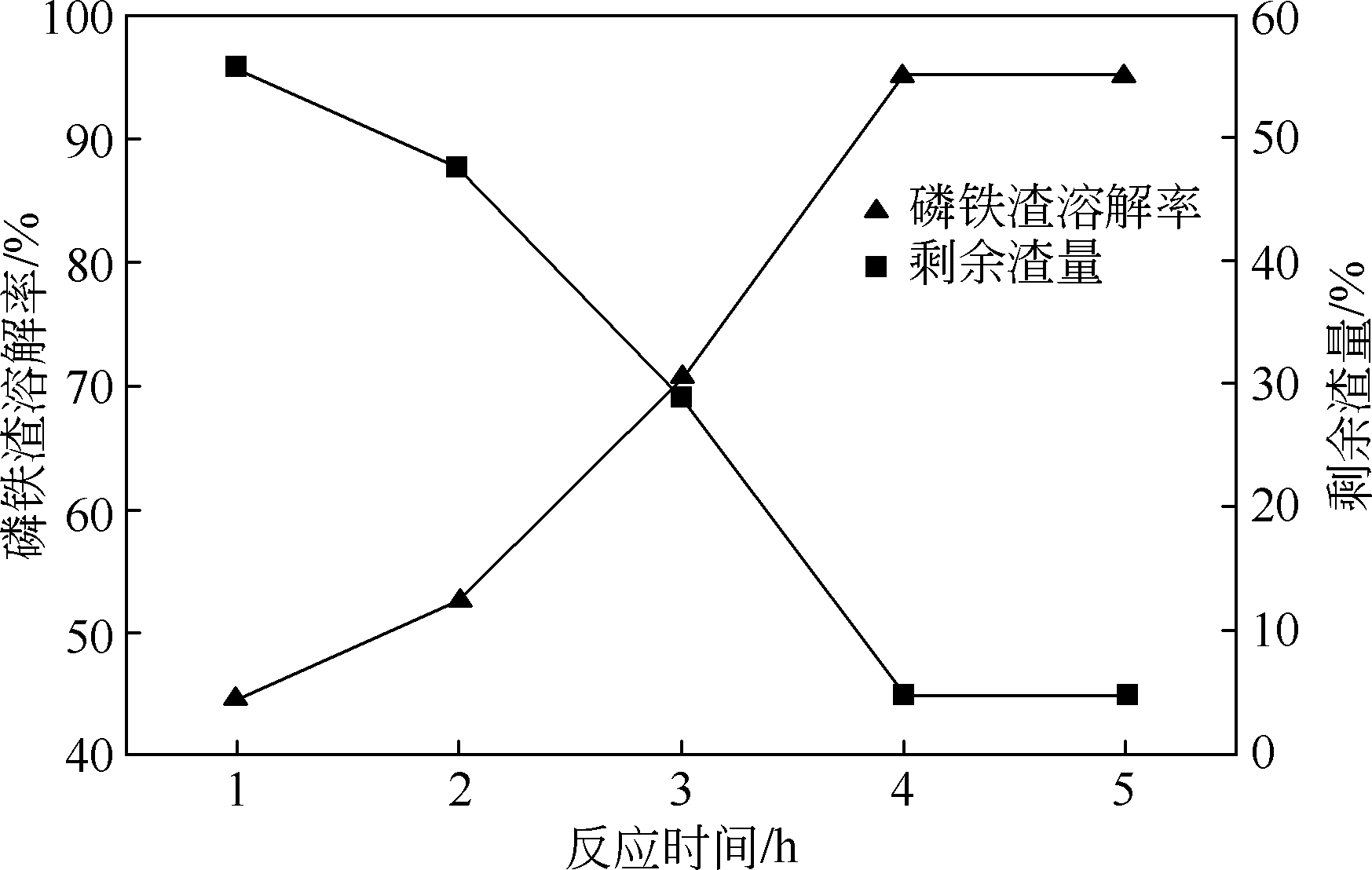
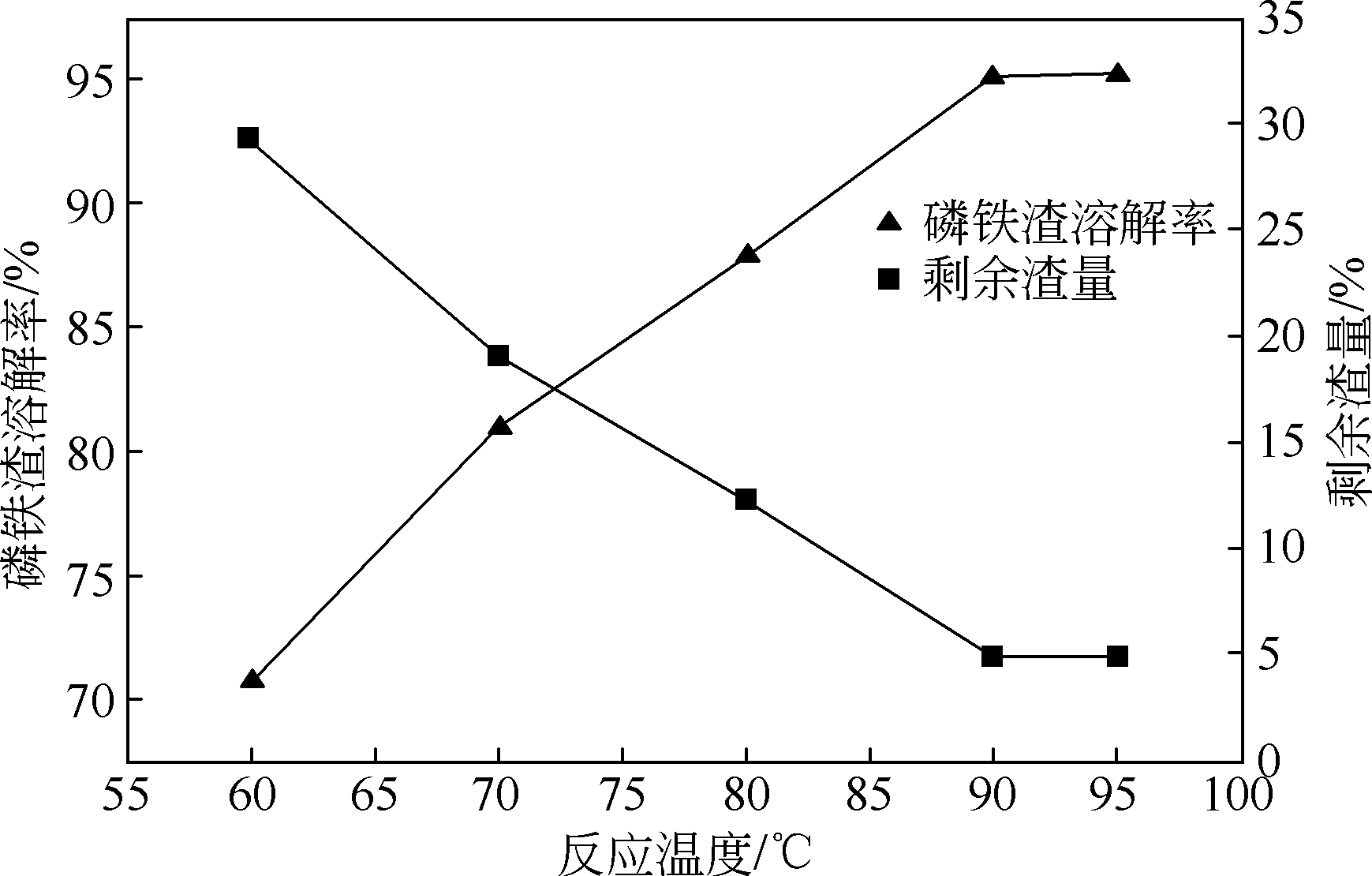
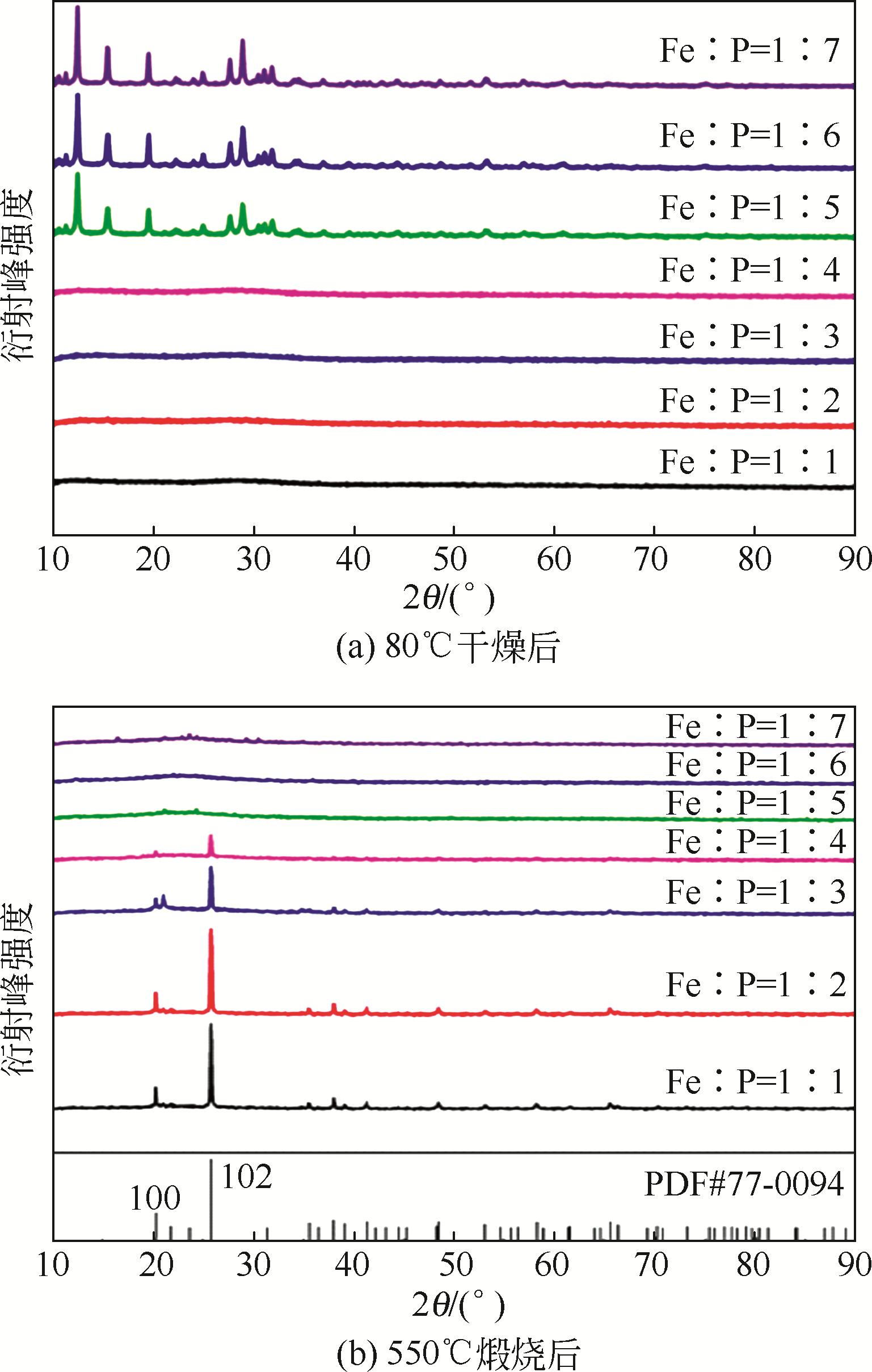
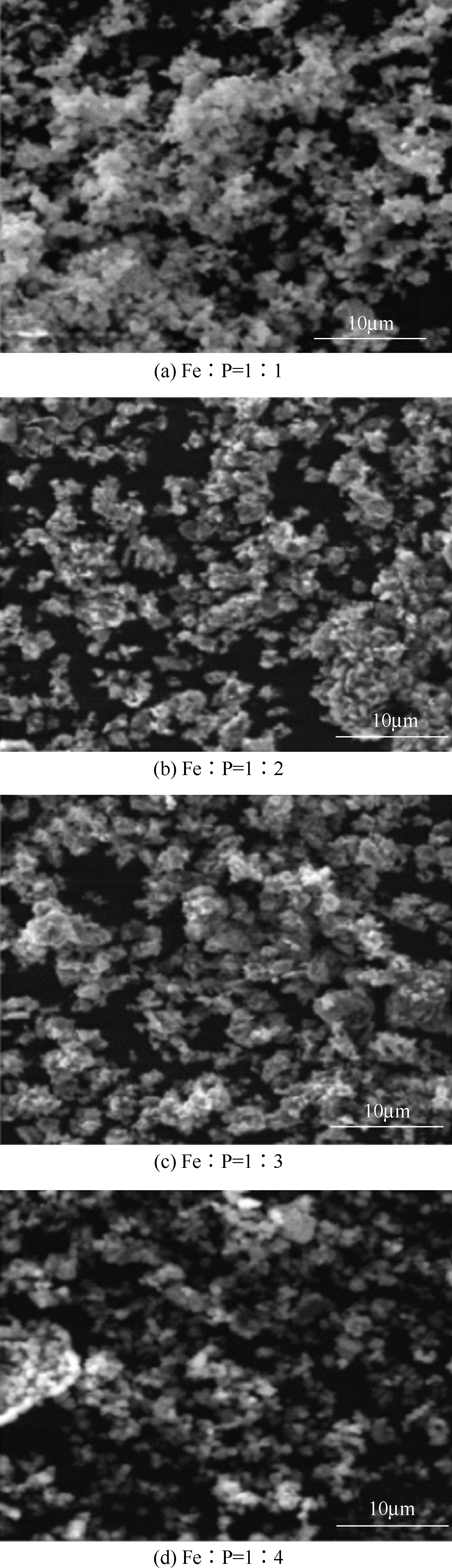
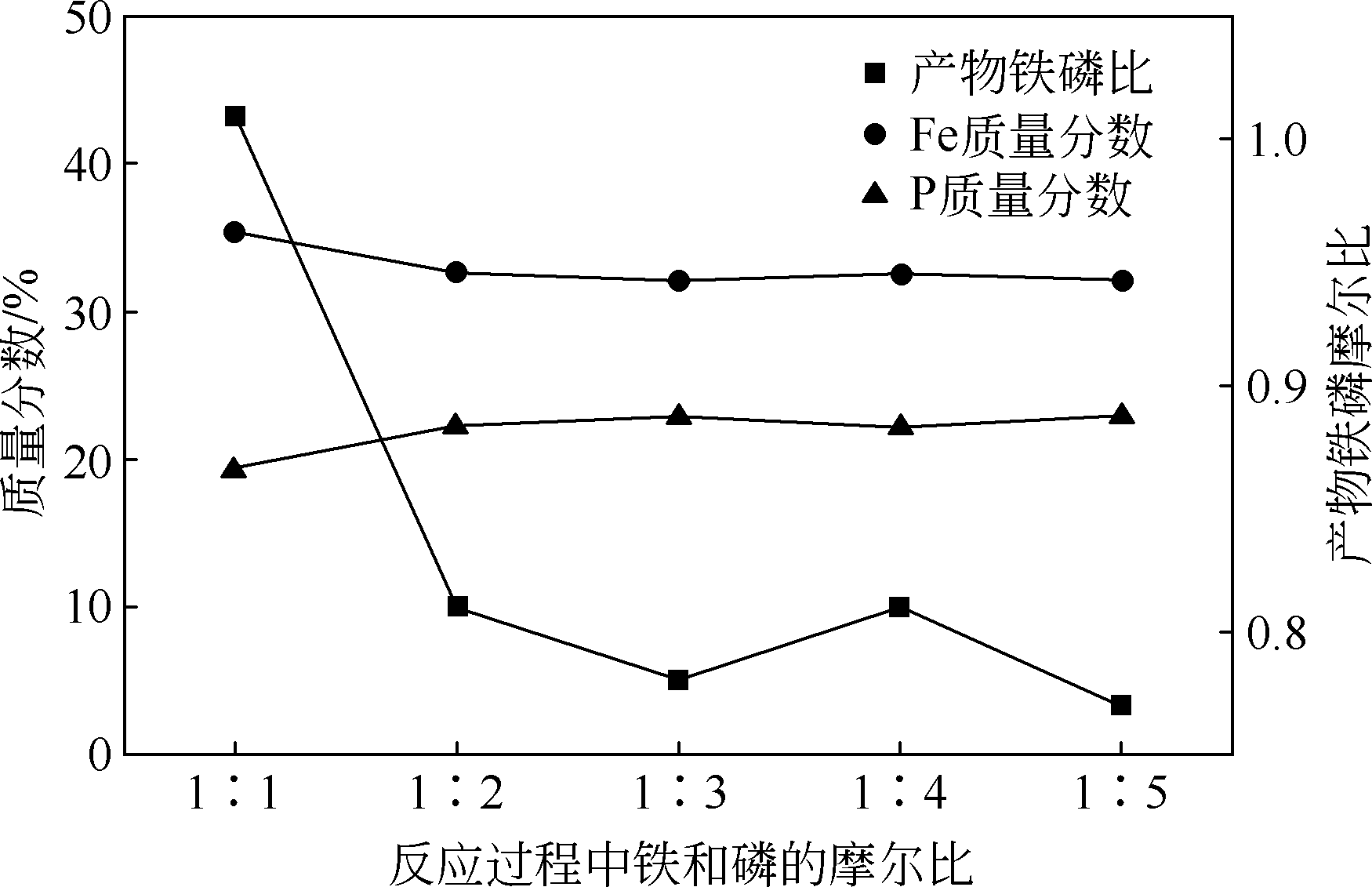
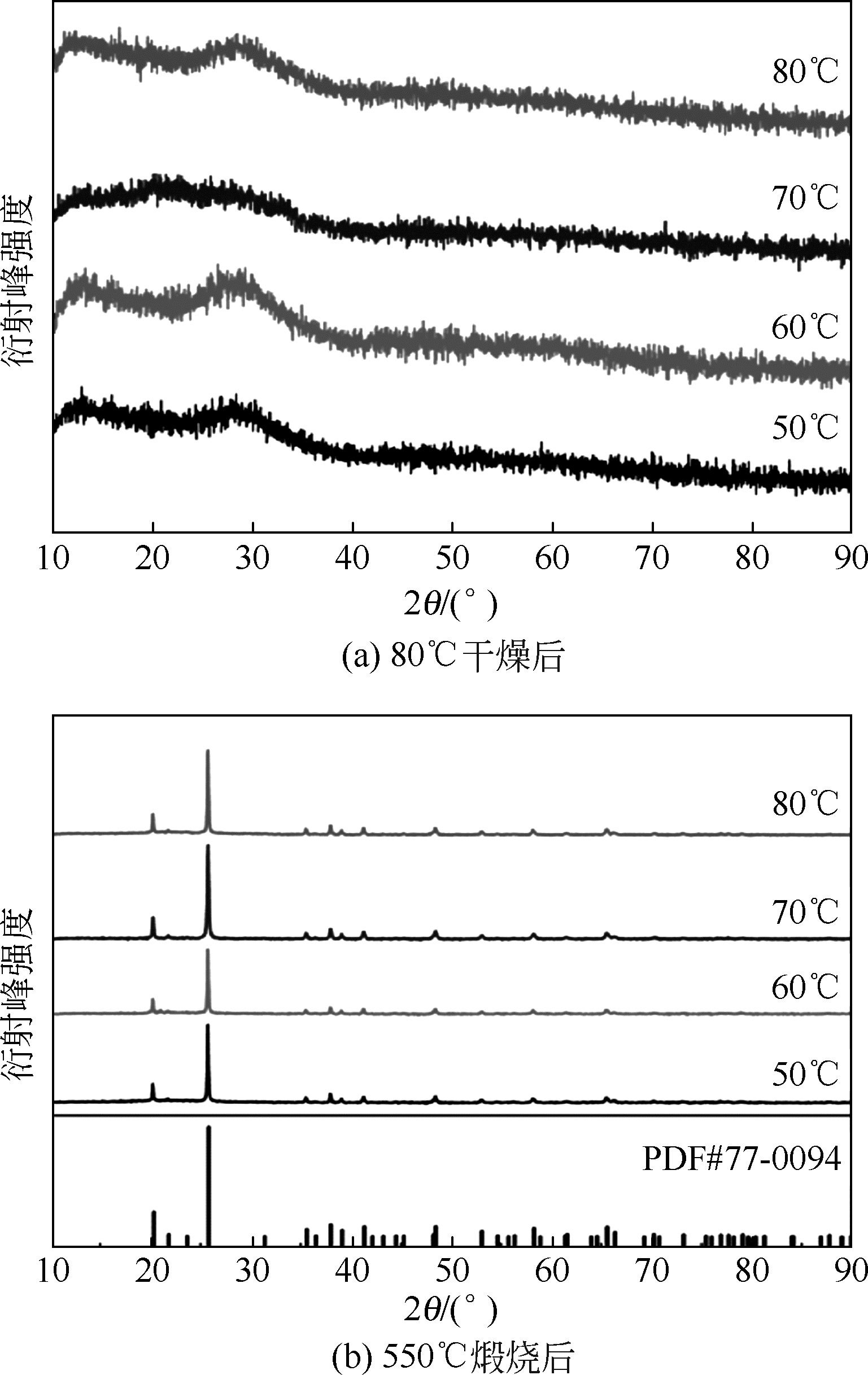
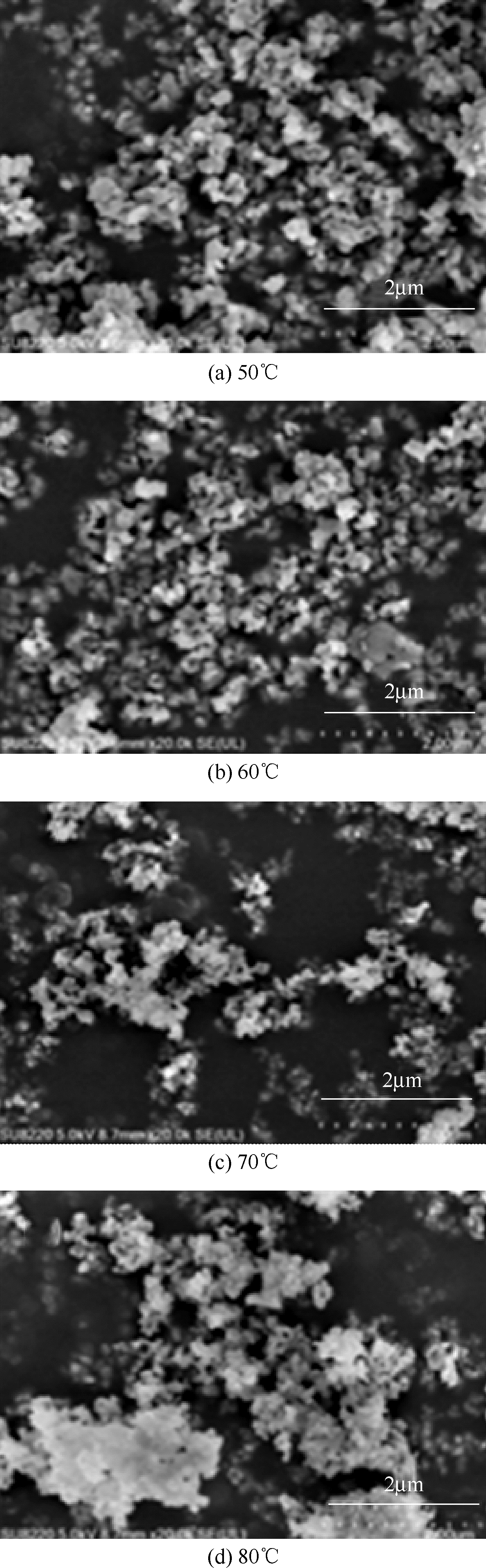
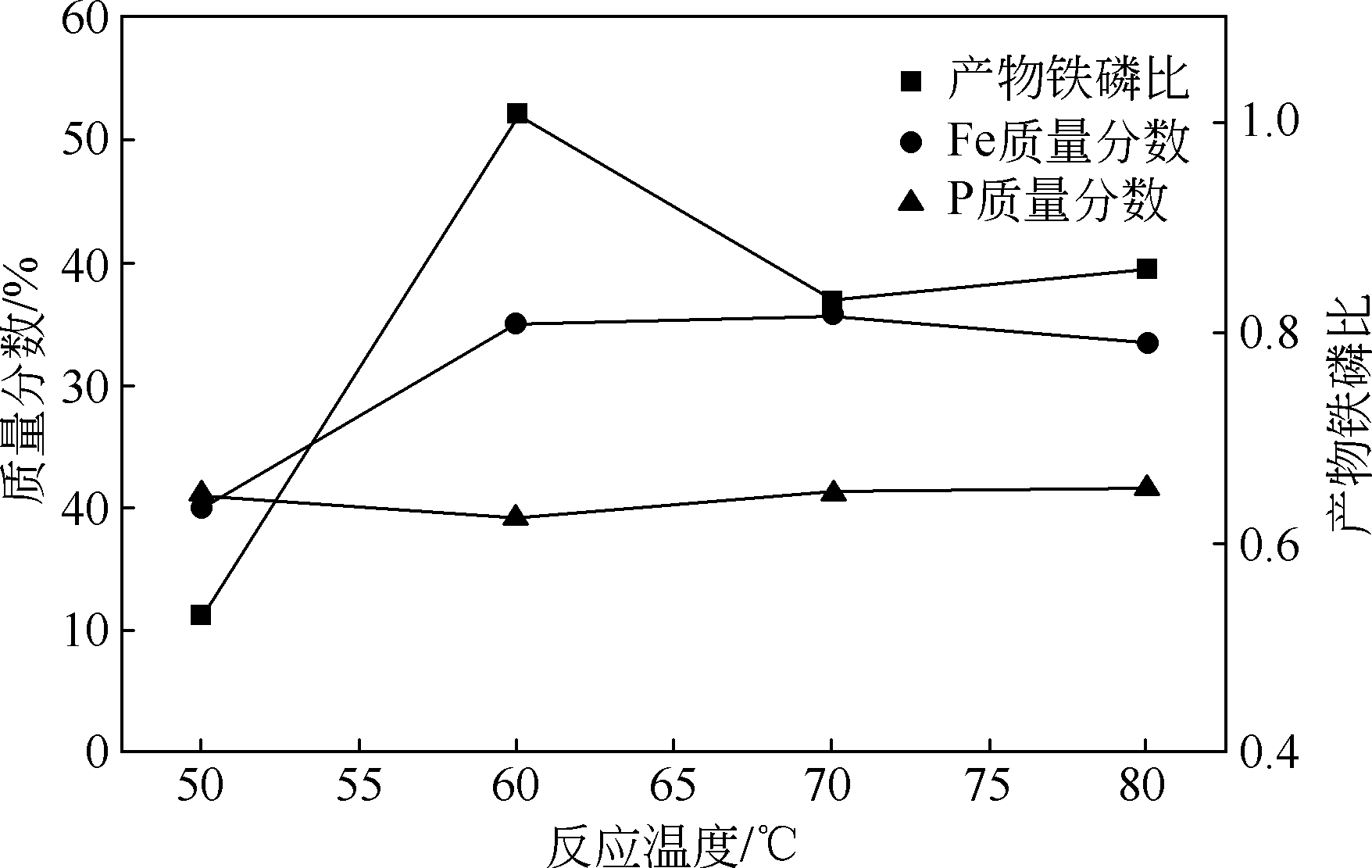
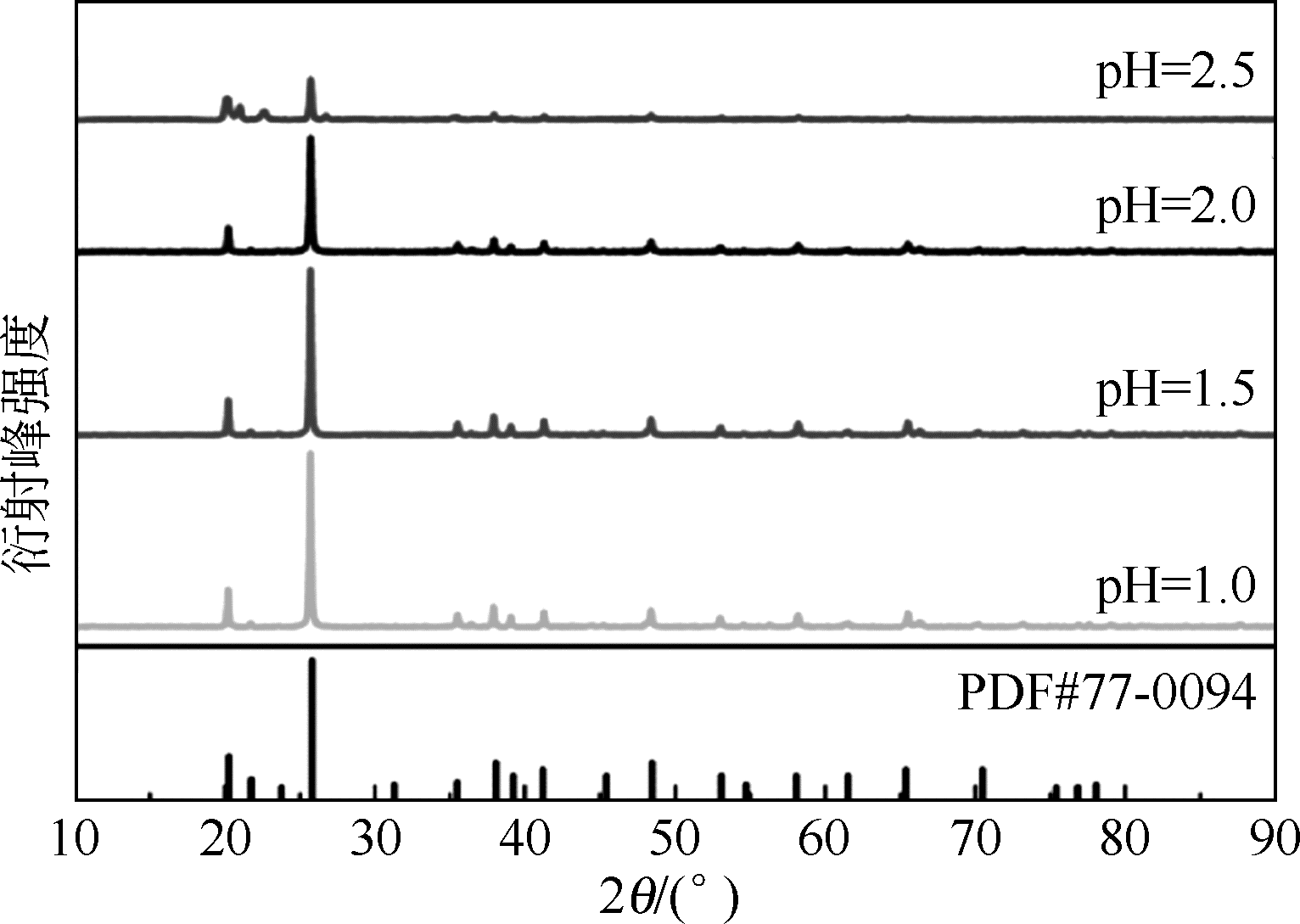
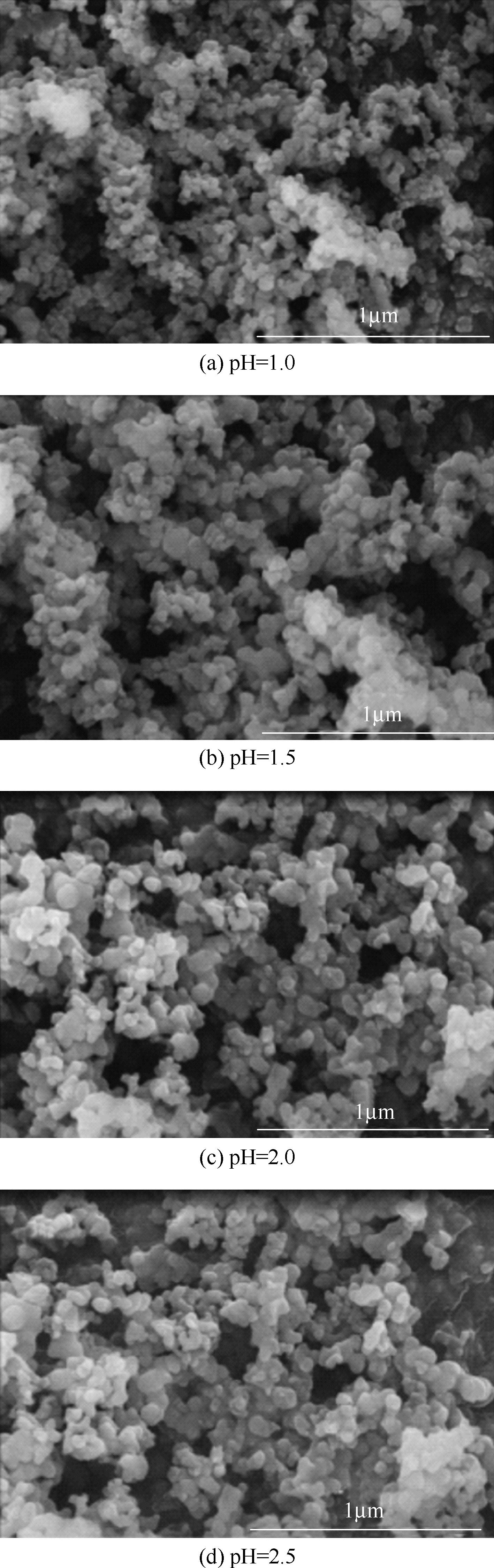
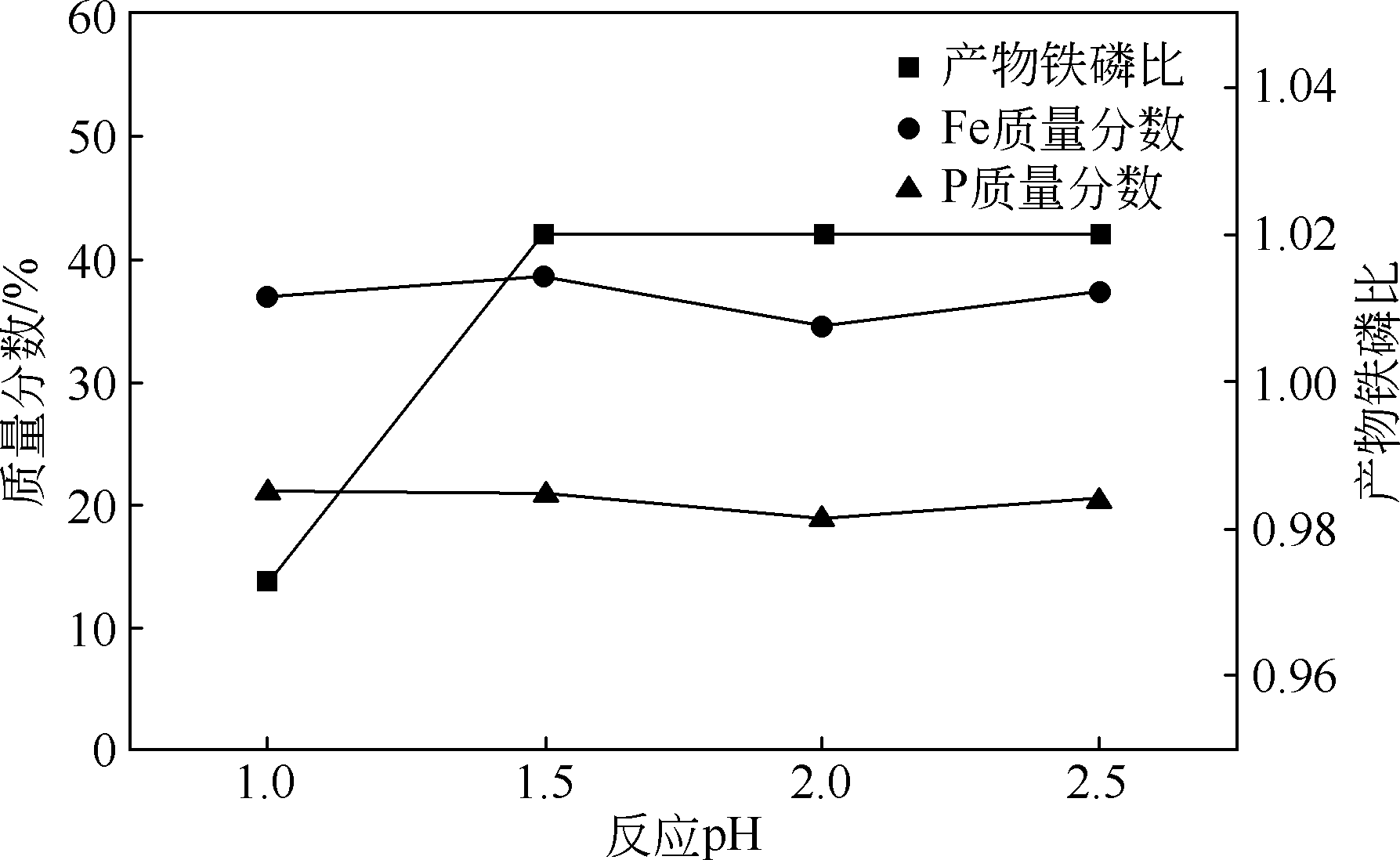
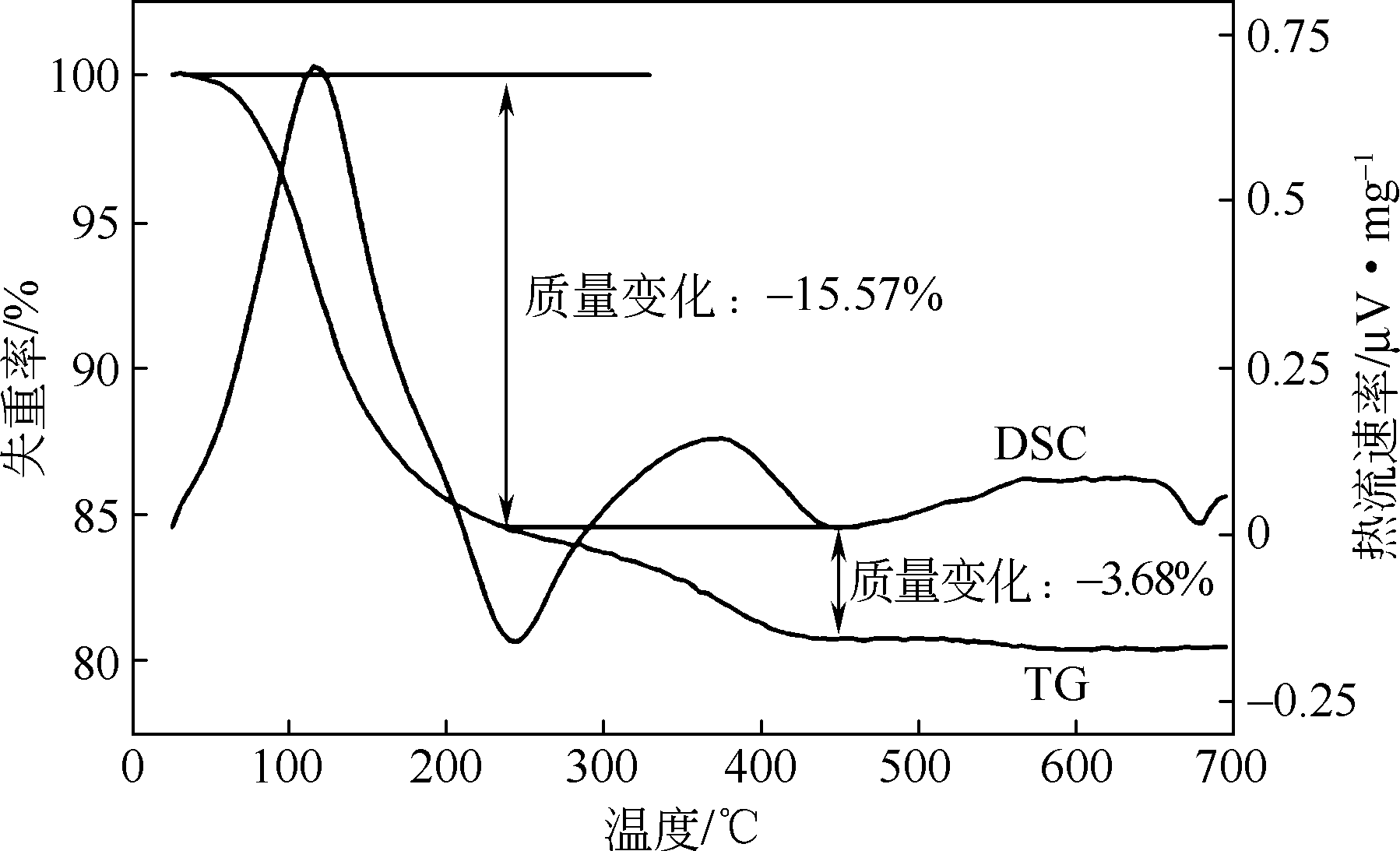
以磷铁废渣为原料提供磷源和铁源,用硝酸和硫酸混合溶液浸出磷铁渣中的铁和磷元素,并通过沉淀法制备电池级纳米磷酸铁。探究了硝酸浓度、反应时间、反应温度对磷铁渣溶解率的影响,并研究了反应过程中铁磷比、温度和pH对制备的磷酸铁性能影响。利用X衍射分析仪、扫描电子显微镜、热重分析仪、红外光谱仪和电感耦合等离子体发射光谱仪等分析手段对磷酸铁的形貌、晶体结构与化学成分进行了表征。实验结果表明,磷铁渣浸出最佳的实验条件为:硝酸浓度1.5mol/L,反应时间4h,反应温度90℃,此条件下磷铁的溶解率为95.11%;磷酸铁制备过程中的最佳实验条件为:铁磷比1∶1,反应温度60℃,反应pH=1.0,所制备的FePO4结晶度高,颗粒形貌规整,分散均匀,一次颗粒粒径为100~200nm,铁磷摩尔比为0.97,杂质元素含量符合电池级磷酸铁的要求。
中图分类号: