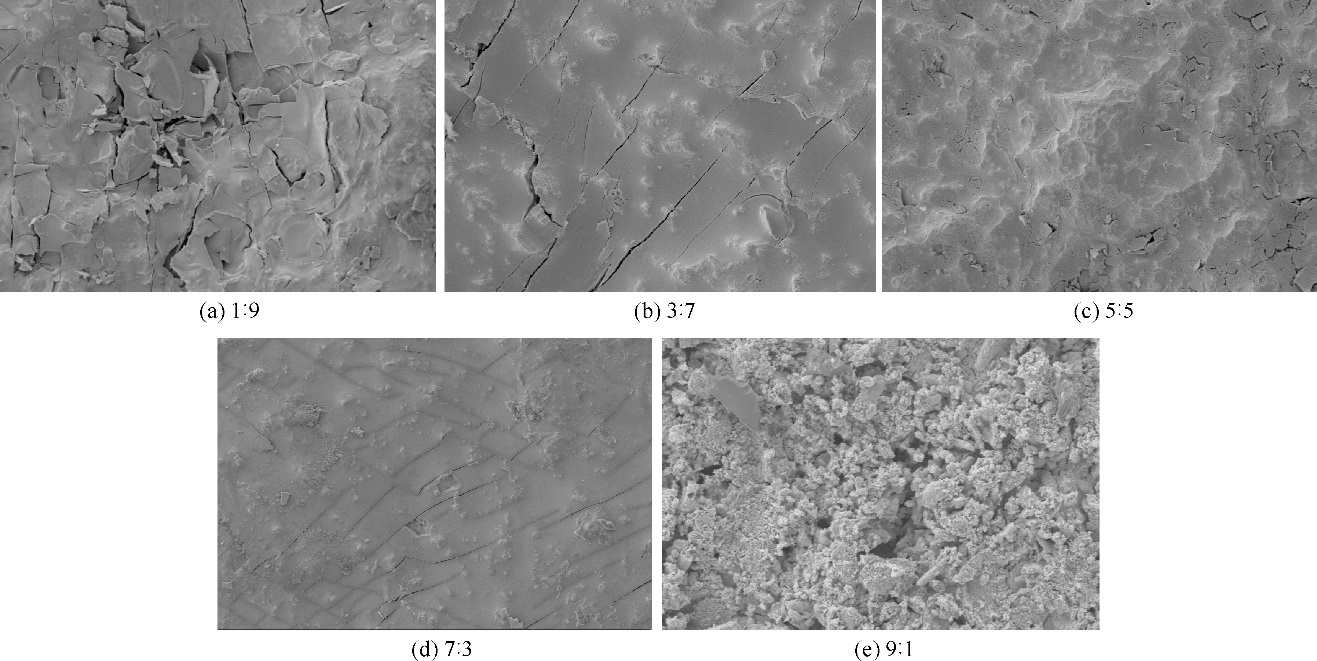
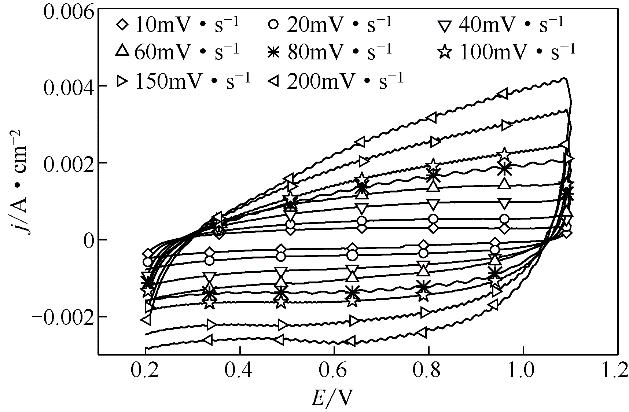
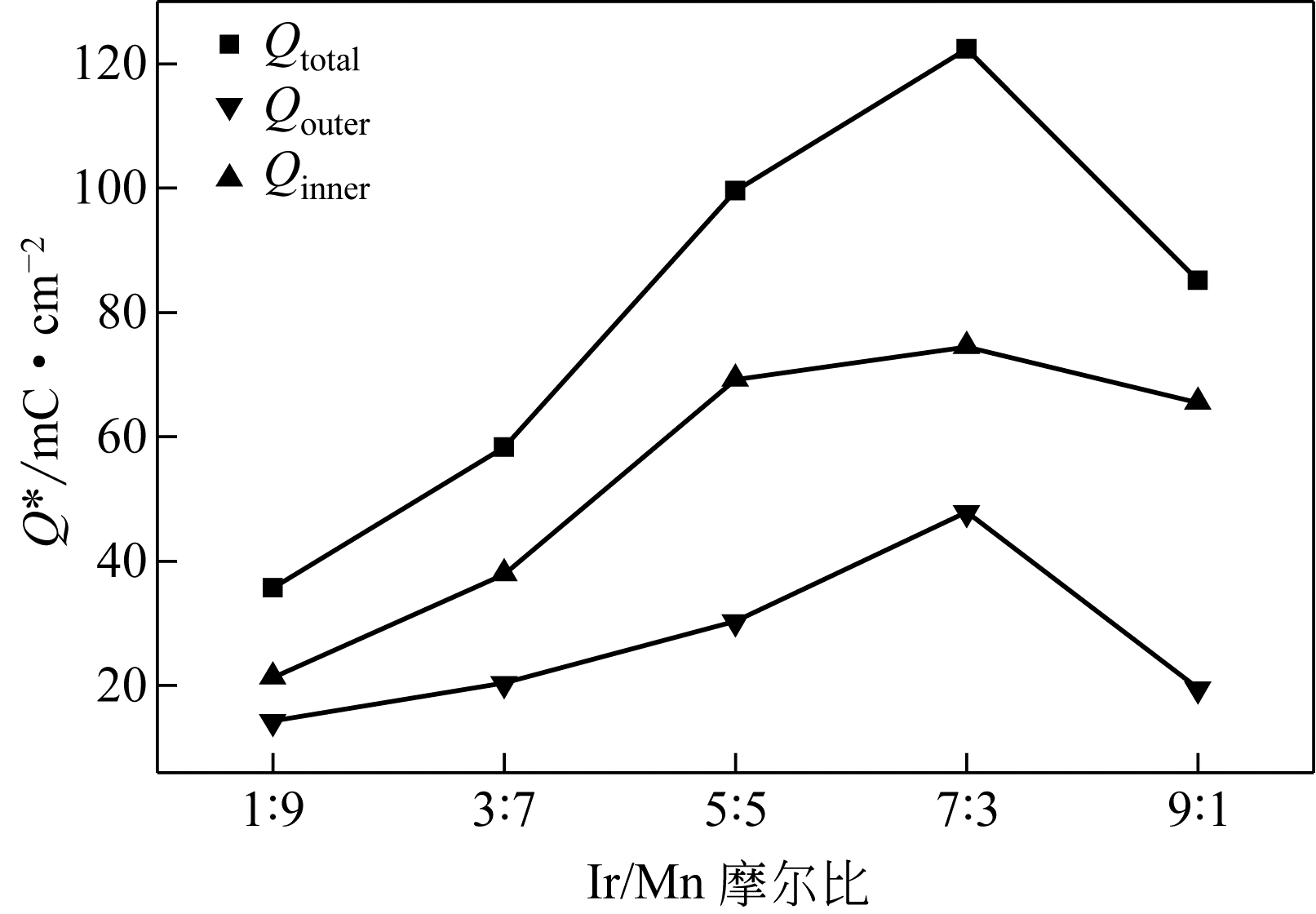
| 1 |
YE Z G , HUANG G B , LIU G W , et al . Influence of preparation process on electrocatalytic activity of Ti/IrO2+MnO2 anodes for oxygen evolution in 0.5M Na2SO4 solution[J]. Materials Research Innovations, 2014, 18(s2): 440-446.
|
| 2 |
高洁, 朱玉婵, 任占冬, 等 . Ir0.5Pt0.5O2阳极的电催化活性及氧化电解水制备[J]. 化工学报, 2015, 66(3): 992-1000.
|
|
GAO Jie , ZHU Yuchan , REN Zhandong , et al . Electrocatalytic performance of Ir0.5Pt0.5O2 anode and preparation of electrolyzed oxidizing water[J]. Journal of Chemical Industry and Engineering, 2014, 24(10): 2553-2558.
|
| 3 |
ZHANG J J , HU J M , ZHANG J Q , CAO C N . IrO2-SiO2 binary oxide films: geometric or kinetic interpretation of the improved electrocatalytic activity for the oxygen evolution reaction[J]. International Journal of Hydrogen Energy, 2011, 36(9): 5218-5226.
|
| 4 |
MARSHALL A , BORRESEN B , HAGEN G , et al . Electrochemical characterisation of Ir x Sn1- x O2 powders as oxygen evolution electrocatalysts[J]. Electrochimica Acta, 2006, 51(15): 3161-3167.
|
| 5 |
KATO Z , KASHIMA R , TATSUMI K , et al . The influence of coating solution and calcination condition on the durability of Ir1- x Sn x O2/Ti anodes for oxygen evolution[J]. Applied Surface Science, 2016, 388:640-644.
|
| 6 |
孔德生, 吕文华, 冯媛媛, 等 . DSA电极电催化性能研究及尚待深入探究的几个问题[J]. 化学进展, 2009, 21(6): 1107-1117.
|
|
KONG Desheng , Wenhua LǛ , FENG Yuanyuan , et al . Advances and some problems in electrocatalysis of DSA electrodes[J]. Progress in Chemistry, 2009, 21(6): 1107-1117.
|
| 7 |
HU W , CHEN S , XIA Q . IrO2/Nb-TiO2 electrocatalyst for oxygen evolution reaction in acidic medium[J]. International Journal of Hydrogen Energy, 2014, 39(13): 6967-6976.
|
| 8 |
朱学军, 邓俊, 张毅 . Ti/MnO2电极制备及对甲醇催化氧化[J]. 化工进展, 2016, 35(s2): 244-247.
|
|
ZHU Xuejun , DENG Jun , ZHANG Yi . Preparation of Ti/MnO2 electrode for the catalytic oxidation of methanol[J]. Chemical Industry and Engineering Progress, 2016, 35(s2): 244-247.
|
| 9 |
TRASATTI S . Physical electrochemistry of ceramic oxides[J]. Electrochimica Acta, 1991, 36(2): 225-241.
|
| 10 |
KIM K W , LEE E H, KIM J S , et al . Study on the electro-activity and non-stochiometry of a Ru-based mixed oxide electrode[J]. Electrochimica Acta, 2002, 46(6): 915-921.
|
| 11 |
WANG X M , HU J M , ZHANG J Q . IrO2-SiO2 binary oxide films: preparation, physiochemical characterization and their electrochemical properties[J]. Electrochimica Acta, 2010, 55(15): 4587-4593
|
| 12 |
WANG Y Q , TONG H Y , XU W L . Electrocatalytic activity of Ti/TiO2 electrodes in H2SO4 solution[J]. Journal of Process Engineering, 2003, 3(4): 356-360.
|
| 13 |
COMNINELLIS C H , VERCESI G P . Characterization of DSA®-type oxygen evolving electrodes: choice of a coating[J]. Journal of Applied Electrochemistry, 1991, 21(4): 335-345.
|
| 14 |
XU J , LIU G , LI J . The electrocatalytic properties of an IrO2/SnO2 catalyst using SnO2 as a support and an assisting reagent for the oxygen evolution reaction[J]. Electrochimica Acta, 2012, 59:105-112.
|
| 15 |
GRUPIONI A A F , ARASHIRO E , LASSAIL T A F . Voltammetric characterization of an iridium oxide-based system: the pseudocapacitive nature of the Ir0.3Mn0.7O2 electrode[J]. Electrochimica Acta, 2003, 48(4): 407-418.
|
| 16 |
SPIOLO G , ARDIZZONE S , TRASATTI S . Surface characterization of Co3O4 electrodes prepared by the sol-gel method[J]. Journal of Electroanalytical Chemistry, 1997, 423(1): 49-57.
|
| 17 |
VOGT H . Note on a method to interrelate inner and outer electrode areas[J]. Electrochimica Acta, 1994, 39(13): 1981-1983.
|
| 18 |
ARDIZZONE S , FREGONARA G , TRASATTI S . Inner and outer active surface of RuO2 electrodes[J]. Electrochimica Acta, 1990, 35(1): 263-267.
|
| 19 |
PAULI C P DE , TRASATTI S . Electrochemical surface characterization of IrO2+SnO2 mixed oxide electrocatalysts[J]. Journal of Electroanalytical Chemistry, 1995, 396(1): 161-168.
|
| 20 |
LOCATELLI C , MINGUZZI A , VERTOVA A . IrO2-SnO2 mixtures as electrocatalysts for the oxygen reduction reaction in alkaline media[J]. Journal of Applied Electrochemistry, 2013, 43(2): 171-179.
|
| 21 |
SILVA L M DA , FARIA L A DE , BOODTS J F C . Electrochemical impedance spectroscopic (EIS) investigation of the deactivation mechanism, surface and electrocatalytic properties of Ti/RuO2(x)+Co3O4(1-x) electrodes[J]. Journal of Electroanalytical Chemistry, 2002, 532(1): 141-150.
|
 ),Wenxue GUAN1,2,Sanfan WANG1,2,Xueming ZHANG1,2
),Wenxue GUAN1,2,Sanfan WANG1,2,Xueming ZHANG1,2