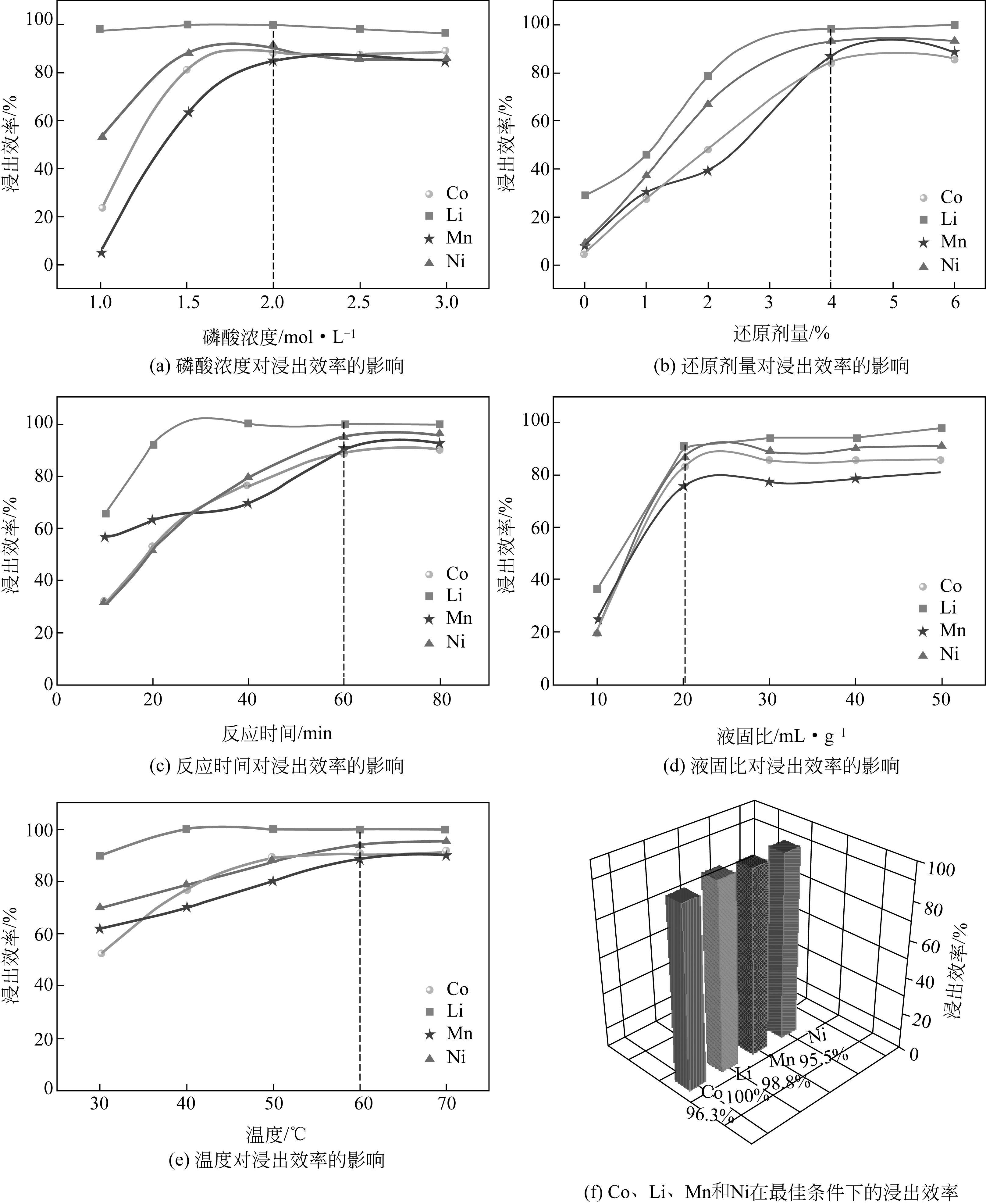
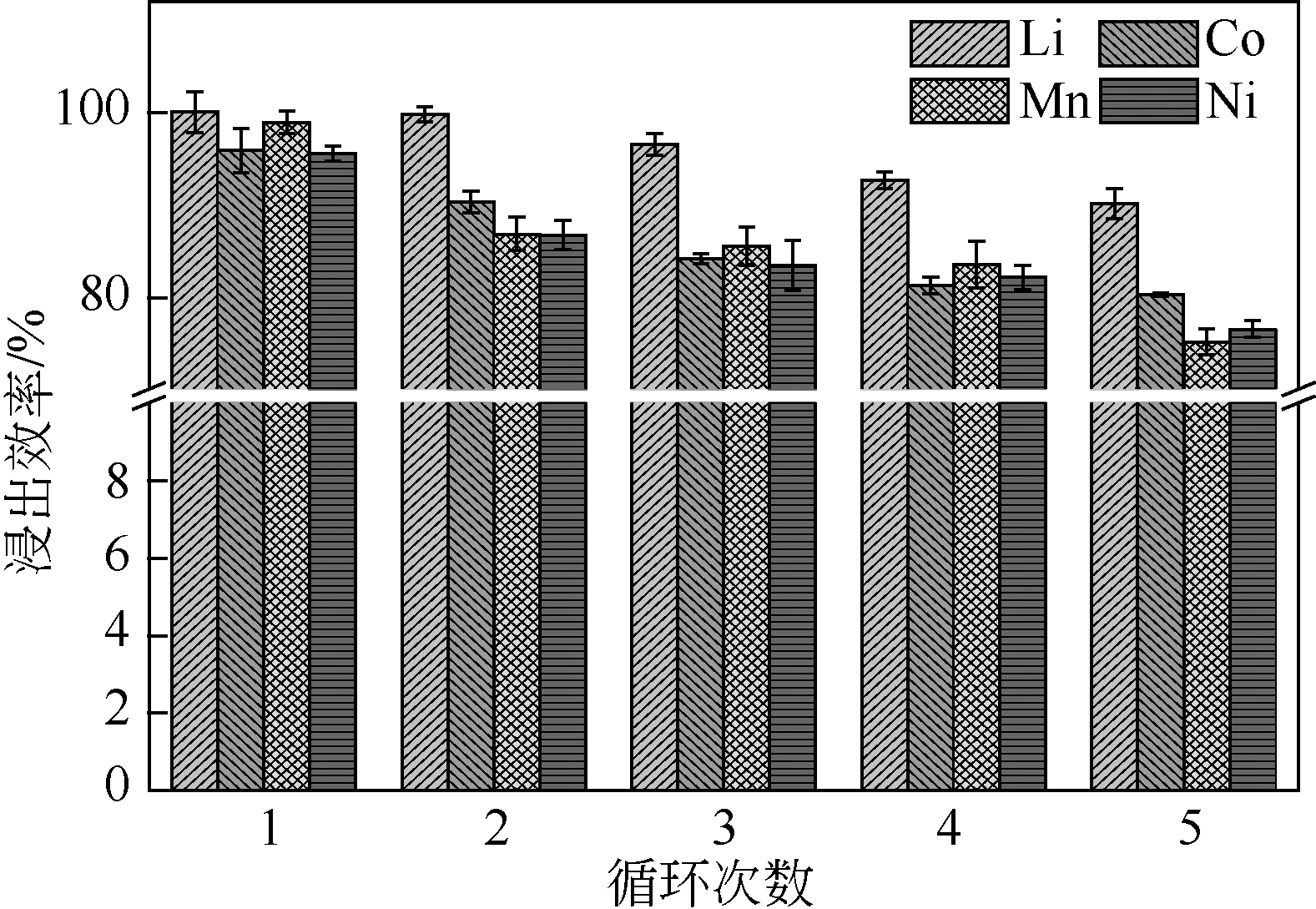
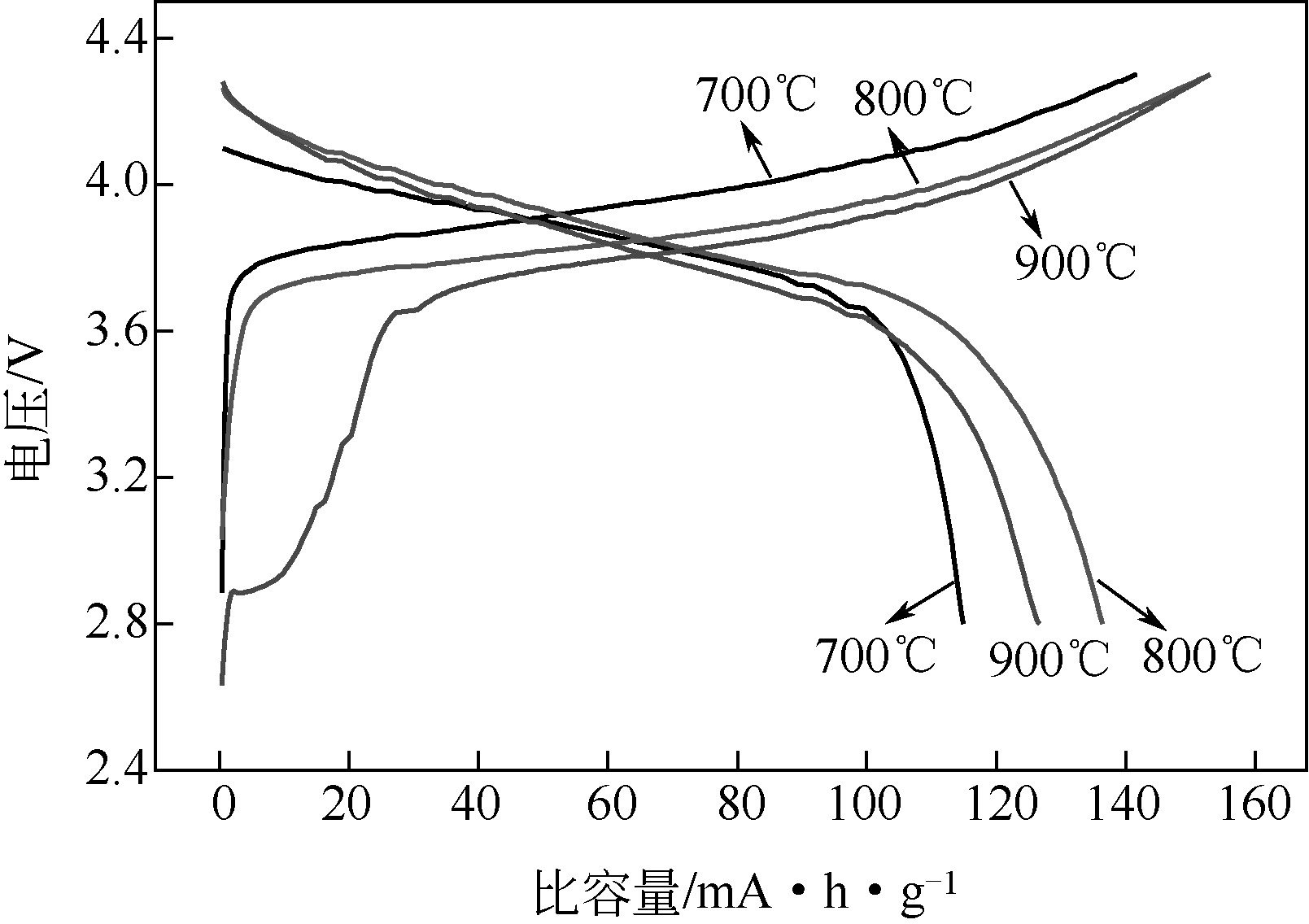
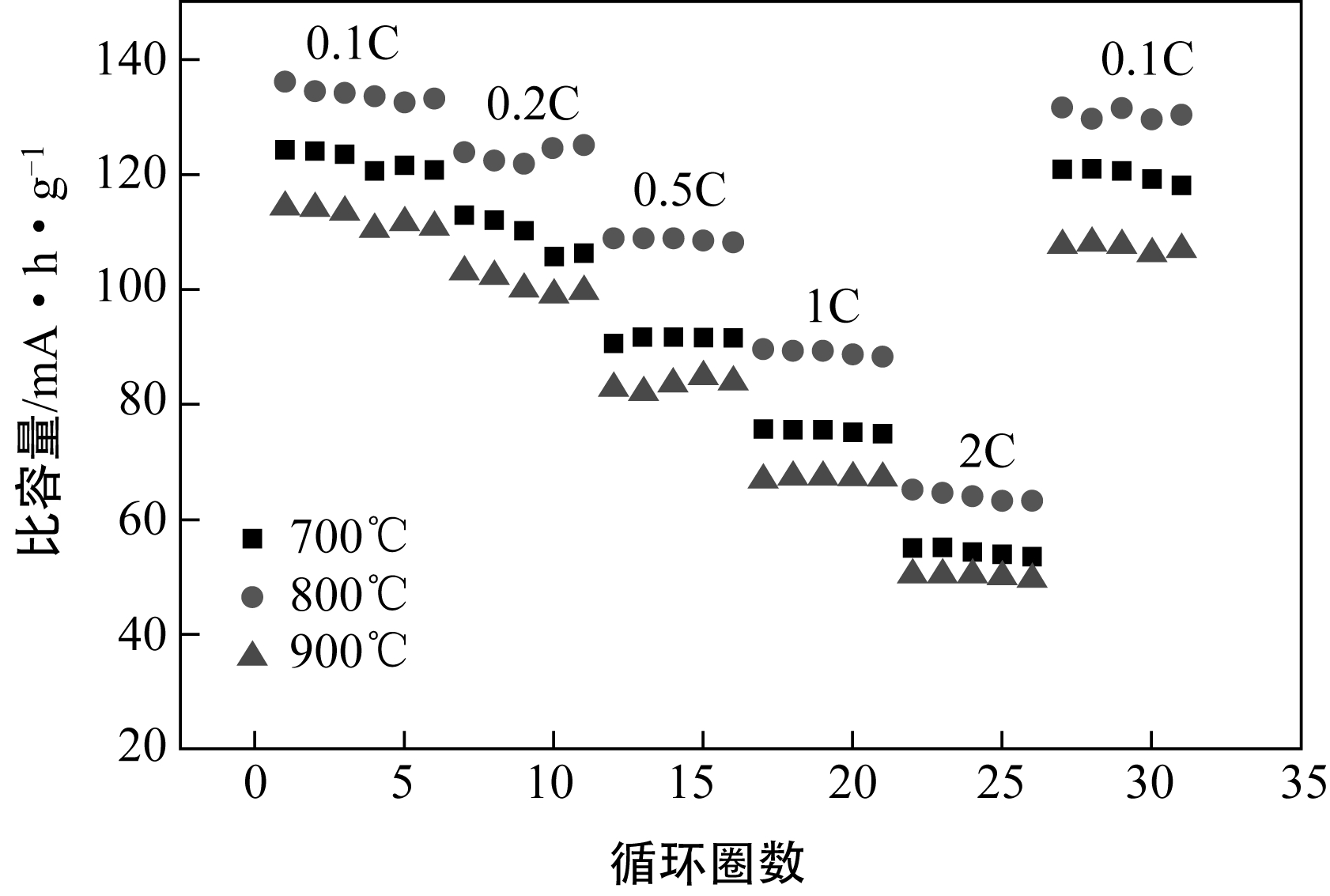
| 1 |
BERTUOLD A, TONIASSOC, JIMENEZ, et al.Application of spouted bed elutriation in the recycling of lithium ion batteries[J].Journal of Power Sources, 2015, 275: 627-632.
|
| 2 |
ZHANGT, HEY, WANGF, et al.Chemical and process mineralogical characterizations of spent lithium-ion batteries: an approach by multi-analytical techniques[J].Waste Management, 2014, 34(6): 1051-1058.
|
| 3 |
JHA M K, KUMARIA, JHA A K, et al.Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone[J]. Waste Management, 2013, 33(9): 1890-1897.
|
| 4 |
CHENX P, LUOC B, ZHANGJ X, et al. Sustainable recovery of metals from spent lithium-ion batteries: a green process[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(12): 3104-3113.
|
| 5 |
CHENL, TABGX, ZHANGY, et al. Process for the recovery of cobalt oxalate from spent lithium-ion batteries[J]. Hydrometallurgy, 2011, 108(1/2): 80-86.
|
| 6 |
XUJ, THOMASH R, FRANCISR W, et al. A review of processes and technologies for the recycling of lithium-ion secondary batteries[J]. Journal of Power Sources, 2008, 177(2): 512-527.
|
| 7 |
CHENX P, FANB L, XULP, et al. An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries[J]. Journal of Cleaner Production, 2016, 112: 3562-3570.
|
| 8 |
ZENGX, LIJ, SHENB. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid[J]. Journal of Hazardous Materials, 2015, 295: 112-118.
|
| 9 |
CHENX, ZHOUT. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media[J]. Waste Management Research, 2014, 32(11): 1083-1093.
|
| 10 |
LIL, GEJ, CHENR J, et al. Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries[J]. Waste Management, 2010, 30(12): 2615-2621.
|
| 11 |
PAULINOJ F, BUSNARDON G, AFONSOJ C. Recovery of valuable elements from spent Li-batteries[J]. Journal of Hazardous Materials, 2008, 150(3): 843-849.
|
| 12 |
ZENGG, DENGX, LUOS, et al. A copper-catalyzed bioleaching process for enhancement of cobalt dissolution from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2012, 199/200: 164-169.
|
| 13 |
ERUSTC, AKCILA, GAHANC S, et al. Biohydrometallurgy of secondary metal resources: a potential alternative approach for metal recovery[J]. Journal of Chemical Technology & Biotechnology, 2013, 88(12): 2115-2132.
|
| 14 |
JOULIEM, LAUCOURNETR, BILLYE. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries[J]. Journal of Power Sources, 2014, 247: 551-555.
|
| 15 |
OU Z, LIJ, WANGZ. Application of mechanochemistry to metal recovery from second-hand resources: a technical overview[J]. Environmental Science: Processes & Impacts, 2015, 17(9): 1522-1530.
|
| 16 |
SUNZ, CAOH, XIAOY, et al. Toward sustainability for recovery of critical metals from electronic waste: the hydrochemistry processes[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(1): 21-24.
|
| 17 |
ZHANGX, XIEY, CAOH, et al. A novel process for recycling and resynthesizing LiNi1/3Co1/3Mn1/3O2 from the cathode scraps intended for lithium-ion batteries[J]. Waste Management, 2014, 34(9): 1715-1724.
|
| 18 |
LEE C K, RHEEK I. Preparation of LiCoO2 from spent lithium-ion batteries[J]. Journal of Power Sources, 2002, 109(1): 17-21.
|
| 19 |
LIL, FANE, GUANY, et al. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5224-5233.
|
| 20 |
KIM D S, SOHNJ S, LEE C K, et al. Simultaneous separation and renovation of lithium cobalt oxide from the cathode of spent lithium ion rechargeable batteries[J]. Journal of Power Sources, 2004, 132(1-2): 145-149.
|
| 21 |
WENGY, XUS, HUANGG, et al. Synthesis and performance of Li[(Ni1/3Co1/3Mn1/3)1−xMgx]O2 prepared from spent lithium ion batteries[J]. Journal of Hazardous Materials, 2013, 246-247: 163-172.
|
| 22 |
ZHANGX, CAOH, XIEY, et al. A closed-loop process for recycling LiNi1/3Co1/3Mn1/3O2 from the cathode scraps of lithium-ion batteries: process optimization and kinetics analysis[J]. Separation and Purification Technology, 2015, 150: 186-195.
|
| 23 |
SAKULTUNGS, PRUKSATHORNK, HUNSOMM. Simultaneous recovery of valuable metals from spent mobile phone battery by an acid leaching process[J]. Korean Journal of Chemical Engineering, 2007, 24(2): 272-277.
|
| 24 |
谌谷春, 唐新村, 王志敏, 等. 废旧锂电池中镍钴锰的回收及正极材料LiCo1/3Ni1/3Mn1/3O2的制备[J]. 无机化学学报, 2011, 27(10): 1987-1992.
|
|
CHENGuchun, TANGXincun, WANGZhimin, et al. Recovery of nickel, cobalt and manganese in waste lithium batteries and preparation of cathode material LiCo1/3Ni1/3Mn1/3O2 [J]. Journal of Inorganic Chemistry, 2011, 27(10): 1987-1992.
|
| 25 |
史红彩. 废旧锂离子动力电池中镍钴锰酸锂正极材料的回收及再利用[D]. 郑州: 郑州大学, 2017.
|
|
SHIHongcai. Recovery and reuse of nickel-cobalt-manganate cathode materials in waste lithium ion power batteries [D]. Zhengzhou: Zhengzhou University, 2017.
|
 ),刘雅丽2,康铎之1,李佳竺1,陈湘萍1(
),刘雅丽2,康铎之1,李佳竺1,陈湘萍1( ),马宏瑞1
),马宏瑞1
 ),Yali LIU2,Duozhi KANG1,Jiazhu LI1,Xiangping CHEN1(
),Yali LIU2,Duozhi KANG1,Jiazhu LI1,Xiangping CHEN1( ),Hongrui MA1
),Hongrui MA1