化工进展 ›› 2025, Vol. 44 ›› Issue (5): 2655-2666.DOI: 10.16085/j.issn.1000-6613.2024-1955
利用可再生原料生物合成脂肪族短链二元胺与醇的研究进展
- 南京工业大学生物与制药工程学院,江苏 南京 211816
-
收稿日期:2024-11-28修回日期:2025-01-16出版日期:2025-05-25发布日期:2025-05-20 -
通讯作者:陈可泉 -
作者简介:冯娇(1988—),女,硕士生导师,研究方向为生物催化。E-mail:fengjiao88@njtech.edu.cn。 -
基金资助:国家自然科学基金(U21B2097);江苏省合成生物基础研究中心(BK20233003)
Research progress in the biosynthesis of aliphatic short-chain diamines and diols from renewable feedstocks
FENG Jiao( ), LIU Mingming, LIU Yao, WANG Xin, CHEN Kequan(
), LIU Mingming, LIU Yao, WANG Xin, CHEN Kequan( )
)
- College of Biotechnology and Pharmaceutical Engineering,Nanjing Tech University, Nanjing 211816, Jiangsu, China
-
Received:2024-11-28Revised:2025-01-16Online:2025-05-25Published:2025-05-20 -
Contact:CHEN Kequan
摘要:
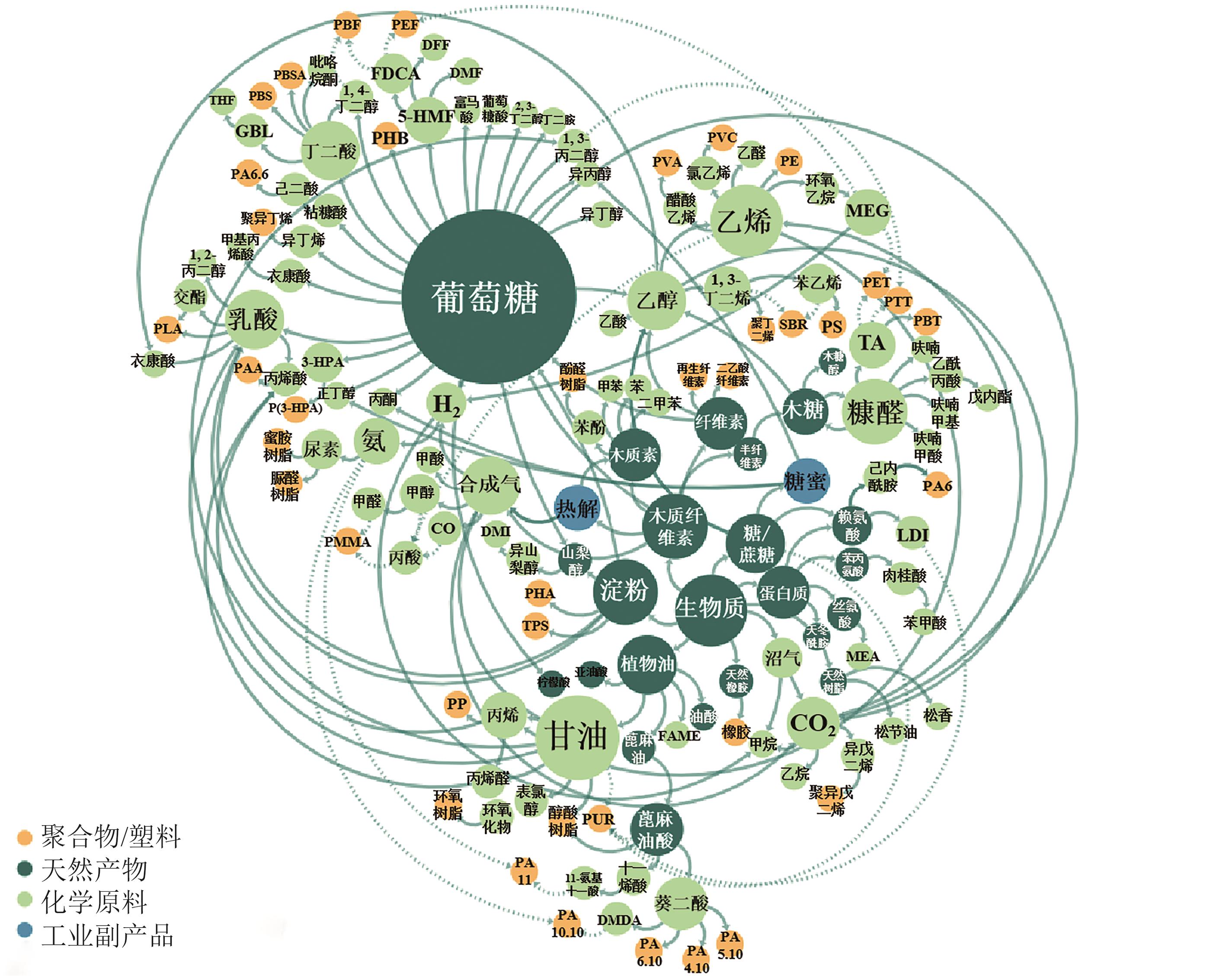
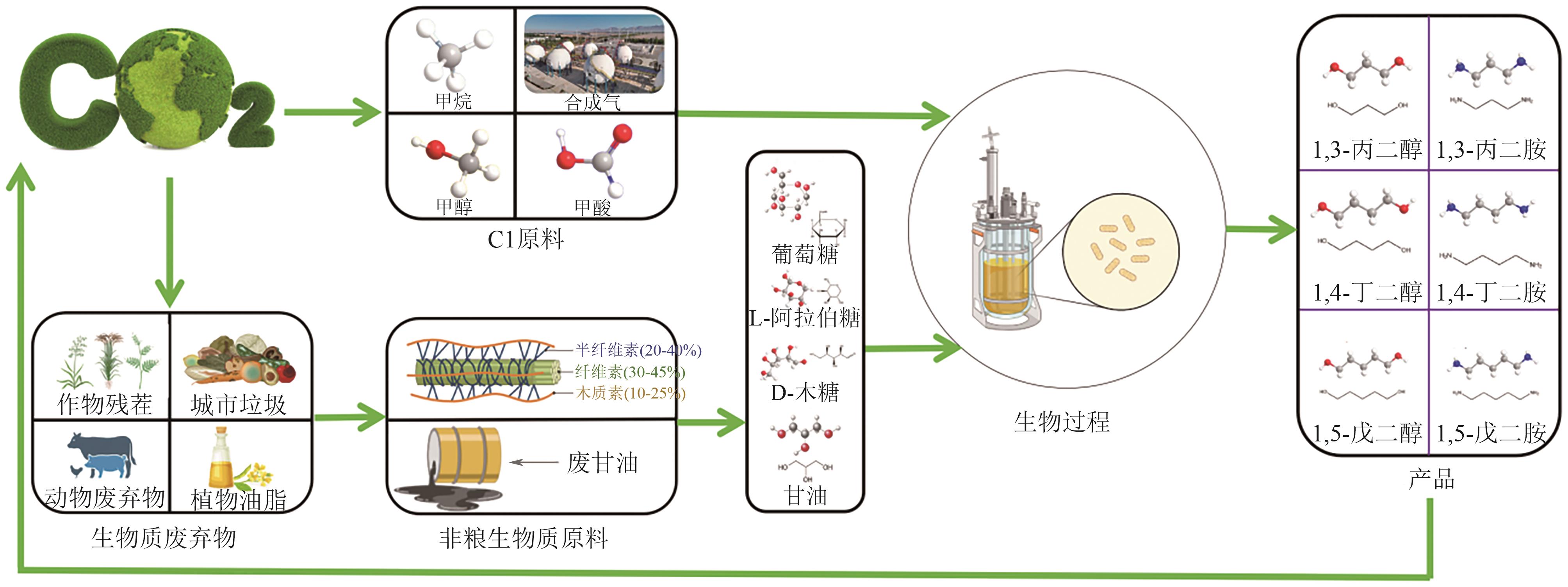
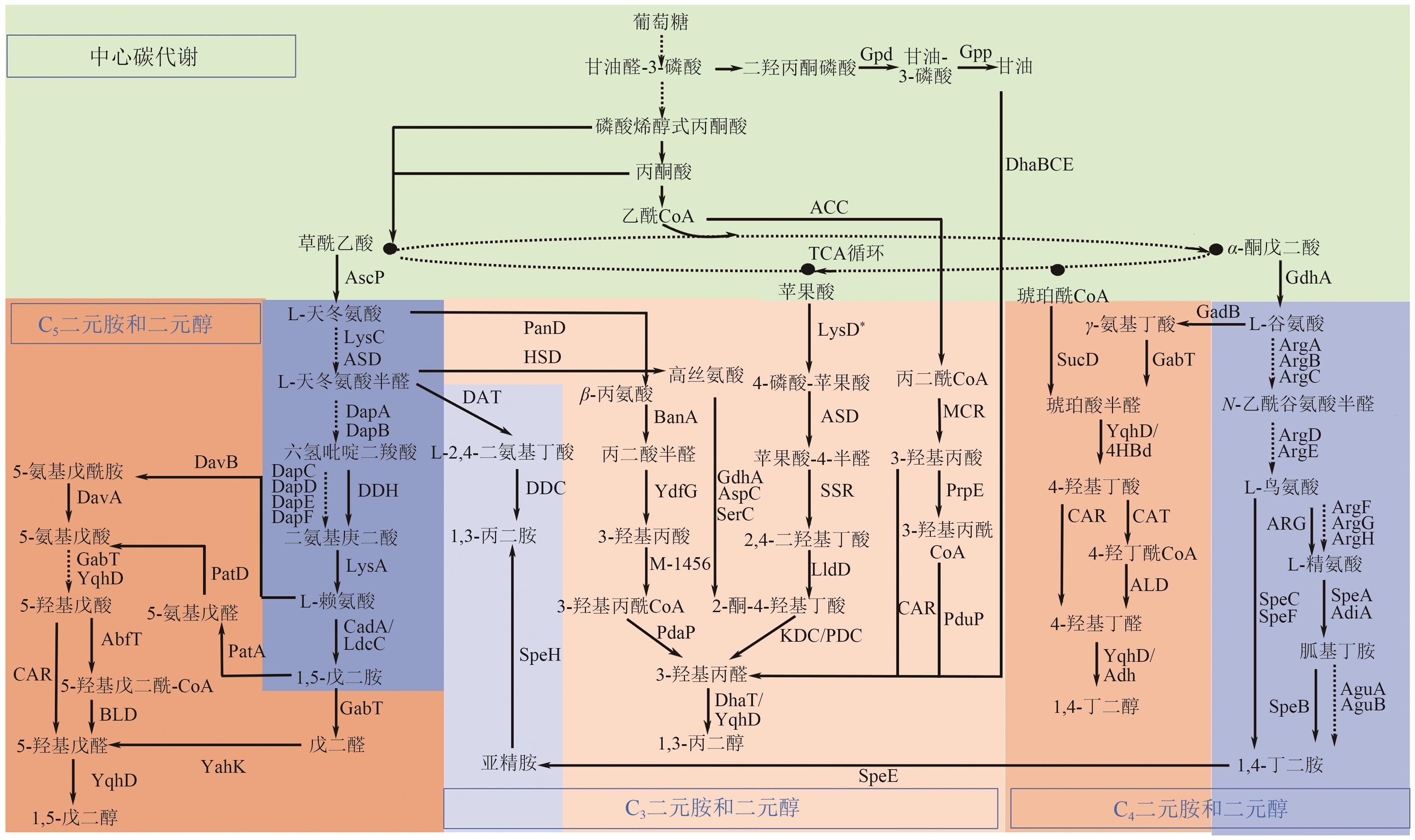
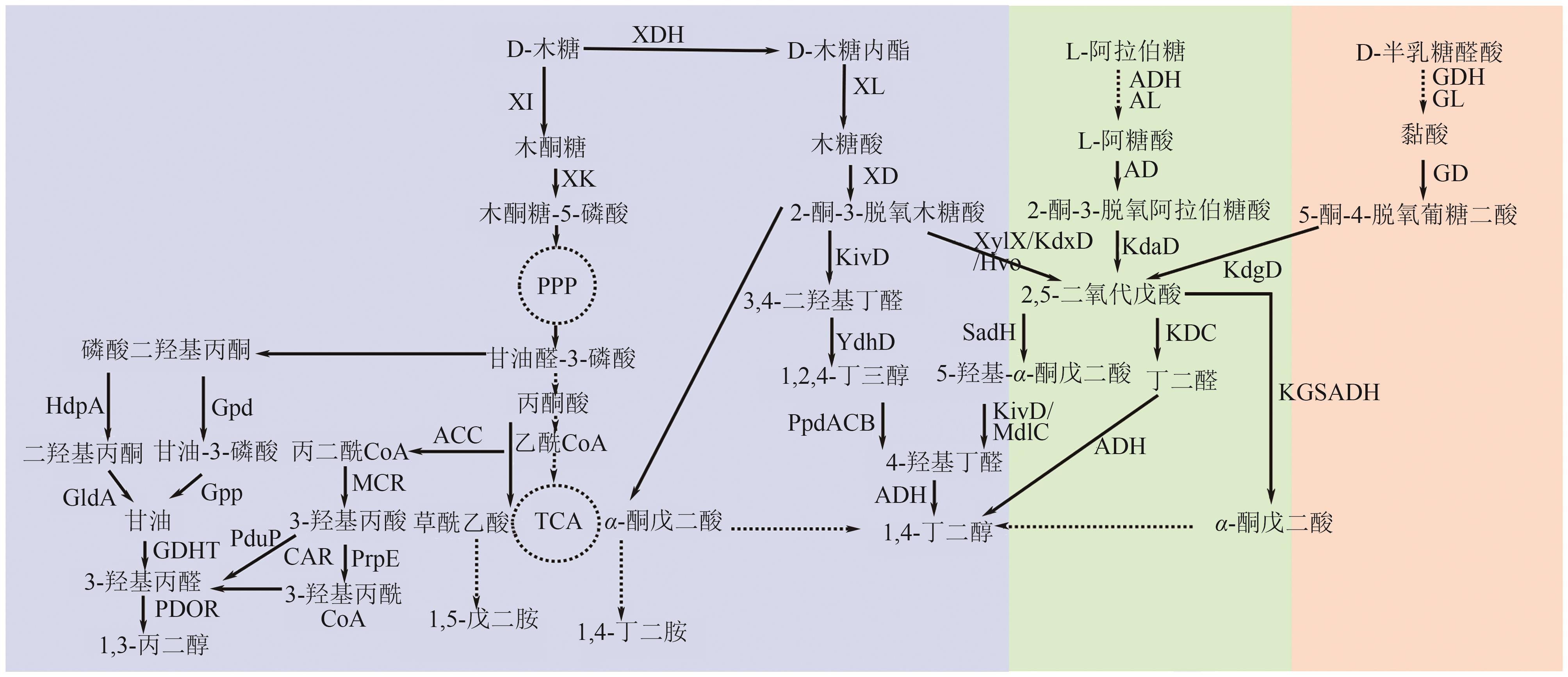
绿色生物制造是以生物质等可再生原料通过生物过程和生物系统生产生物基材料、化学品、能源、医药和食品等,其绿色清洁的生产工艺有助于改善传统化工行业对化石资源高依赖以及工艺高能耗、高排放等问题。脂肪族短链二元胺和醇是重要的大宗化学品,可作为聚合单体应用于合成聚酯、聚氨酯、聚酰胺等高分子材料,同时在化妆品、制药等领域具有广泛应用。本文全面探讨了利用可再生原料生物合成1,3-丙二胺、1,4-丁二胺、1,5-戊二胺、1,3-丙二醇、1,4-丁二醇、1,5-戊二醇的研究进展,概况了可再生原料生物制造的碳循环和代谢途径,总结了脂肪族短链二元胺和二元醇的从头生物合成路线,阐述了利用木质纤维素水解糖、甘油、一碳化合物原料合成短链二元胺和醇的研究现状。并对脂肪族短链二元胺和醇生物制造主要面临的挑战进行了相关讨论和展望。
中图分类号:
引用本文
冯娇, 刘明明, 刘耀, 王昕, 陈可泉. 利用可再生原料生物合成脂肪族短链二元胺与醇的研究进展[J]. 化工进展, 2025, 44(5): 2655-2666.
FENG Jiao, LIU Mingming, LIU Yao, WANG Xin, CHEN Kequan. Research progress in the biosynthesis of aliphatic short-chain diamines and diols from renewable feedstocks[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2655-2666.
| 产品 | 化学合成法 | 生物合成法 | ||||||
|---|---|---|---|---|---|---|---|---|
| 原料 | 工艺 | 优点 | 缺点 | 原料 | 工艺 | 优点 | 缺点 | |
| 1,3-丙二胺 | 丙烯睛、丙二醇等 | 丙烯睛氨化还原法、丙二醇氨解法等 | 工艺简单、原料来源广泛 | 催化剂价格昂贵、副反应多、产物分离困难 | 葡萄糖 | 发酵法 | 原料可再生、绿色可持续 | 产率较低、无法满足工业化需求 |
| 1,4-丁二胺 | 丙烯腈 | 丙烯腈法 | 反应规模大、产量高 | 原料不可再生、催化剂价格、氰化物毒性高、反应条件苛刻且易燃易爆安全性低 | 葡萄糖、精氨酸等 | 发酵法、全细胞催化法 | 成本低廉,副产物少 | 工艺复杂、产率较低、产品不易分离 |
| 1,5-戊二胺 | 戊二腈 | 戊二腈法 | 产量较高 | 生产成本高,有副产物生成,反应过程剧烈,污染较大 | 葡萄糖、赖氨酸等 | 发酵法、全细胞催化法 | 酶催化收率高、反应时间短、过程简单 | 发酵法的葡萄糖转化率低,发酵体系成分复杂 |
| 1,3-丙二醇 | 丙烯醛、 环氧乙烷 | 丙烯醛水合氢化法、环氧乙烷氢甲酰化法 | 工艺路线较成熟、反应条件温和 | 丙烯醛剧毒且易燃易爆、HPA选择性和收率低、易发生副反应 | 葡萄糖、甘油等 | 发酵法 | 反应条件温和、绿色环保、成本低 | 仍需降低生产成本 |
| 1,4-丁二醇 | 乙炔和甲醛、顺酐等 | 炔醛法、顺酐法等 | 工艺简单、技术成熟 | 受乙炔、顺酐等原料的限制 | 葡萄糖 | 发酵法 | 可持续、原料清洁、已工业化 | 副产物积累、路径酶的特异性仍需提升 |
| 1,5-戊二醇 | 戊二酸、四氢糠醇等 | 戊二酸加氢法、糠醇法等 | 工艺流程简单 | 加氢反应压力大、酸加氢副产物复杂 | 葡萄糖 | 发酵法 | 绿色可持续、原料清洁可再生 | 产量相对较低、副产物积累 |
表1 脂肪族短链二元胺和醇常用化学合成法和生物合成法的对比
| 产品 | 化学合成法 | 生物合成法 | ||||||
|---|---|---|---|---|---|---|---|---|
| 原料 | 工艺 | 优点 | 缺点 | 原料 | 工艺 | 优点 | 缺点 | |
| 1,3-丙二胺 | 丙烯睛、丙二醇等 | 丙烯睛氨化还原法、丙二醇氨解法等 | 工艺简单、原料来源广泛 | 催化剂价格昂贵、副反应多、产物分离困难 | 葡萄糖 | 发酵法 | 原料可再生、绿色可持续 | 产率较低、无法满足工业化需求 |
| 1,4-丁二胺 | 丙烯腈 | 丙烯腈法 | 反应规模大、产量高 | 原料不可再生、催化剂价格、氰化物毒性高、反应条件苛刻且易燃易爆安全性低 | 葡萄糖、精氨酸等 | 发酵法、全细胞催化法 | 成本低廉,副产物少 | 工艺复杂、产率较低、产品不易分离 |
| 1,5-戊二胺 | 戊二腈 | 戊二腈法 | 产量较高 | 生产成本高,有副产物生成,反应过程剧烈,污染较大 | 葡萄糖、赖氨酸等 | 发酵法、全细胞催化法 | 酶催化收率高、反应时间短、过程简单 | 发酵法的葡萄糖转化率低,发酵体系成分复杂 |
| 1,3-丙二醇 | 丙烯醛、 环氧乙烷 | 丙烯醛水合氢化法、环氧乙烷氢甲酰化法 | 工艺路线较成熟、反应条件温和 | 丙烯醛剧毒且易燃易爆、HPA选择性和收率低、易发生副反应 | 葡萄糖、甘油等 | 发酵法 | 反应条件温和、绿色环保、成本低 | 仍需降低生产成本 |
| 1,4-丁二醇 | 乙炔和甲醛、顺酐等 | 炔醛法、顺酐法等 | 工艺简单、技术成熟 | 受乙炔、顺酐等原料的限制 | 葡萄糖 | 发酵法 | 可持续、原料清洁、已工业化 | 副产物积累、路径酶的特异性仍需提升 |
| 1,5-戊二醇 | 戊二酸、四氢糠醇等 | 戊二酸加氢法、糠醇法等 | 工艺流程简单 | 加氢反应压力大、酸加氢副产物复杂 | 葡萄糖 | 发酵法 | 绿色可持续、原料清洁可再生 | 产量相对较低、副产物积累 |
| 1 | 谭天伟, 苏海佳, 陈必强, 等. 绿色生物制造[J]. 北京化工大学学报(自然科学版), 2018, 45(5): 107-118. |
| TAN Tianwei, SU Haijia, CHEN Biqiang, et al. Green bio-manufacturing[J]. Journal of Beijing University of Chemical Technology (Natural Science Edition), 2018, 45(5): 107-118. | |
| 2 | 谭天伟, 陈必强, 张会丽, 等. 加快推进绿色生物制造助力实现“碳中和”[J]. 化工进展, 2021, 40(3): 1137-1141. |
| TAN Tianwei, CHEN Biqiang, ZHANG Huili, et al. Accelerate promotion of green bio-manufacturing to help achieve “carbon neutrality”[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1137-1141. | |
| 3 | CHAE Tong Un, Jung Ho AHN, Yoo Sung KO, et al. Metabolic engineering for the production of dicarboxylic acids and diamines[J]. Metabolic Engineering, 2020, 58: 2-16. |
| 4 | WANG Jian, LI Chenyi, ZOU Yusong, et al. Bacterial synthesis of C3-C5 diols via extending amino acid catabolism[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(32): 19159-19167. |
| 5 | BURGARD Anthony, BURK Mark J, OSTERHOUT Robin, et al. Development of a commercial scale process for production of 1,4-butanediol from sugar[J]. Current Opinion in Biotechnology, 2016, 42: 118-125. |
| 6 | CEN Xuecong, DONG Yang, LIU Dehua, et al. New pathways and metabolic engineering strategies for microbial synthesis of diols[J]. Current Opinion in Biotechnology, 2022, 78: 102845. |
| 7 | ZHU Fanghuan, LIU Dehua, CHEN Zhen. Recent advances in biological production of 1,3-propanediol: New routes and engineering strategies[J]. Green Chemistry, 2022, 24(4): 1390-1403. |
| 8 | 薛敏敏. 凯赛生物公司扩产生物基新材料产能[J]. 合成纤维, 2018, 47 (12): 51. |
| XUE Minmin. Expansion of bio-based new material production capacity of Cathay Biotech Inc.[J]. Synthetic Fiber in China, 2018, 47 (12): 51. | |
| 9 | LEE Ji Young, LEE Sung Eun, LEE Dong Woo. Current status and future prospects of biological routes to bio-based products using raw materials, wastes, and residues as renewable resources[J]. Critical Reviews in Environmental Science and Technology, 2022, 52(14): 2453-2509. |
| 10 | LIU Zihe, WANG Kai, CHEN Yun, et al. Third-generation biorefineries as the means to produce fuels and chemicals from CO2 [J]. Nature Catalysis, 2020, 3(3): 274-288. |
| 11 | Seng Hon KEE, CHIONGSON Justin Brian V, SALUDES Jonel P, et al. Bioconversion of agro-industry sourced biowaste into biomaterials via microbial factories—A viable domain of circular economy[J]. Environmental Pollution, 2021, 271: 116311. |
| 12 | SITARA Angeliki, HOCQ Rémi, HORVATH Josef, et al. Industrial biotechnology goes thermophilic: Thermoanaerobes as promising hosts in the circular carbon economy[J]. Bioresource Technology, 2024, 408: 131164. |
| 13 | DENG Weiping, FENG Yunchao, FU Jie, et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels[J]. Green Energy & Environment, 2023, 8(1): 10-114. |
| 14 | HE Niling, CHEN Mingxing, QIU Zhongyang, et al. Simultaneous and rate-coordinated conversion of lignocellulose derived glucose, xylose, arabinose, mannose, and galactose into D-lactic acid production facilitates D-lactide synthesis[J]. Bioresource Technology, 2023, 377: 128950. |
| 15 | FRANCOIS Jean Marie, ALKIM Ceren, MORIN Nicolas. Engineering microbial pathways for production of bio-based chemicals from lignocellulosic sugars: Current status and perspectives[J]. Biotechnology for Biofuels, 2020, 13: 118. |
| 16 | KUMAR Vinod, AGRAWAL Deepti, BOMMAREDDY Rajesh Reddy, et al. Arabinose as an overlooked sugar for microbial bioproduction of chemical building blocks[J]. Critical Reviews in Biotechnology, 2024, 44(6): 1103-1120. |
| 17 | SAXENA Ayush, HUSSAIN Akhtar, PARVEEN Fouziya, et al. Current status of metabolic engineering of microorganisms for bioethanol production by effective utilization of pentose sugars of lignocellulosic biomass[J]. Microbiological Research, 2023, 276: 127478. |
| 18 | Sun Ae JUN, MOON Chuloo, KANG Cheol Hee, et al. Microbial fed-batch production of 1,3-propanediol using raw glycerol with suspended and immobilized Klebsiella pneumoniae [J]. Applied Biochemistry and Biotechnology, 2010, 161(1/2/3/4/5/6/7/8): 491-501. |
| 19 | WILKENS Erik, RINGEL Anne Katrin, HORTIG Diana, et al. High-level production of 1,3-propanediol from crude glycerol by Clostridium butyricum AKR102a[J]. Applied Microbiology and Biotechnology, 2012, 93(3): 1057-1063. |
| 20 | Stefan PFLÜGL, MARX Hans, MATTANOVICH Diethard, et al. 1,3-Propanediol production from glycerol with Lactobacillus diolivorans [J]. Bioresource Technology, 2012, 119: 133-140. |
| 21 | Yuki DOI. Glycerol metabolism and its regulation in lactic acid bacteria[J]. Applied Microbiology and Biotechnology, 2019, 103(13): 5079-5093. |
| 22 | JIANG Wei, WANG Shizhen, WANG Yuanpeng, et al. Key enzymes catalyzing glycerol to 1,3-propanediol[J]. Biotechnology for Biofuels, 2016, 9: 57. |
| 23 | International Energy Agency. CO2 emissions in 2023[R]. Paris: IEA, 2024. https://www.iea.org/reports/co2-emissions-in-2023. |
| 24 | 姚伦, 周雍进. 一碳化合物生物利用和转化研究进展[J]. 化工进展, 2023, 42(1): 16-29. |
| YAO Lun, ZHOU Yongjin. Progress in microbial utilization of one-carbon feedstocks for biomanufacturing[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 16-29. | |
| 25 | 郭姝媛, 吴良焕, 刘香健, 等. 微生物中一碳代谢网络构建的进展与挑战[J]. 合成生物学, 2022, 3(1): 116-137. |
| GUO Shuyuan, WU Lianghuan, LIU Xiangjian, et al. Developing C1-based metabolic network in methylotrophy for biotransformation[J]. Synthetic Biology Journal, 2022, 3(1): 116-137. | |
| 26 | CHAE Tong Un, KIM Won Jun, CHOI Sol, et al. Metabolic engineering of Escherichia coli for the production of 1,3-diaminopropane, a three carbon diamine[J]. Scientific Reports, 2015, 5: 13040. |
| 27 | YE Pan, WANG Tiantian, XU Xue, et al. Mining of aminotransferase genes for efficient bio-production of 1,3-diaminopropane[J]. Biochemical Engineering Journal, 2024, 209: 109377. |
| 28 | KURIAN Joseph V. A new polymer platform for the future—Sorona® from corn derived 1,3-propanediol[J]. Journal of Polymers and the Environment, 2005, 13(2): 159-167. |
| 29 | ENDAH Yohana Kurnia, HAN Sang Hoon, KIM Jae Hoon, et al. Solid-state polymerization and characterization of a copolyamide based on adipic acid, 1,4-butanediamine, and 2,5-furandicarboxylic acid[J]. Journal of Applied Polymer Science, 2016, 133(18): 43391. |
| 30 | WANG Jing, DU Min, WANG Xin, et al. Highly efficient bio-production of putrescine from L-arginine with arginase and L-ornithine decarboxylase in engineered Escherichia coli [J]. Bioresource Technology, 2024, 413: 131471. |
| 31 | QIAN Zhigang, XIA Xiaoxia, LEE Sang Yup. Metabolic engineering of Escherichia coli for the production of putrescine: A four carbon diamine[J]. Biotechnology and Bioengineering, 2009, 104(4): 651-662. |
| 32 | Minho NOH, YOO Seung Min, KIM Won Jun, et al. Gene expression knockdown by modulating synthetic small RNA expression in Escherichia coli [J]. Cell Systems, 2017, 5(4): 418-426. |
| 33 | GUO Hui, LIU Huan, JIN Yuhan, et al. Advances in research on the bio-production of 1,4-butanediol by the engineered microbes[J]. Biochemical Engineering Journal, 2022, 185: 108478. |
| 34 | ZHANG Ye, LIU Dehua, CHEN Zhen. Production of C2-C4 diols from renewable bioresources: New metabolic pathways and metabolic engineering strategies[J]. Biotechnology for Biofuels, 2017, 10: 299. |
| 35 | Harry YIM, HASELBECK Robert, NIU Wei, et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol[J]. Nature Chemical Biology, 2011, 7(7): 445-452. |
| 36 | BARTON Nelson R, BURGARD Anthony P, BURK Mark J, et al. An integrated biotechnology platform for developing sustainable chemical processes[J]. Journal of Industrial Microbiology and Biotechnology, 2015, 42(3): 349-360. |
| 37 | LI Zhen, LIU Jianzhong. Transcriptomic changes in response to putrescine production in metabolically engineered Corynebacterium glutamicum [J]. Frontiers in Microbiology, 2017, 8: 1987. |
| 38 | KIM Hee Taek, BARITUGO Kei-Anne, Young Hoon OH, et al. Metabolic engineering of Corynebacterium glutamicum for the high-level production of cadaverine that can be used for the synthesis of biopolyamide 510[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(4): 5296-5305. |
| 39 | KIM Hee Taek, BARITUGO Kei-Anne, HYUN Sung Min, et al. Development of metabolically engineered Corynebacterium glutamicum for enhanced production of cadaverine and its use for the synthesis of bio-polyamide 510[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(1): 129-138. |
| 40 | CEN Xuecong, LIU Yu, CHEN Bo, et al. Metabolic engineering of Escherichia coli for de novo production of 1,5-Pentanediol from glucose[J]. ACS Synthetic Biology, 2021, 10(1): 192-203. |
| 41 | CEN Xuecong, LIU Yanjuan, ZHU Fanghuan, et al. Metabolic engineering of Escherichia coli for high production of 1,5-pentanediol via a cadaverine-derived pathway[J]. Metabolic Engineering, 2022, 74: 168-177. |
| 42 | HUA Wenfeng, LIANG Bo, ZHOU Suhui, et al. An integrated cofactor and co-substrate recycling pathway for the biosynthesis of 1,5-pentanediol[J]. Microbial Cell Factories, 2024, 23(1): 132. |
| 43 | TAI Yishu, XIONG Mingyong, JAMBUNATHAN Pooja, et al. Engineering nonphosphorylative metabolism to generate lignocellulose-derived products[J]. Nature Chemical Biology, 2016, 12(4): 247-253. |
| 44 | LIU Huaiwei, LU Ting. Autonomous production of 1,4-butanediol via a de novo biosynthesis pathway in engineered Escherichia coli [J]. Metabolic Engineering, 2015, 29: 135-141. |
| 45 | WANG Jia, JAIN Rachit, SHEN Xiaolin, et al. Rational engineering of diol dehydratase enables 1,4-butanediol biosynthesis from xylose[J]. Metabolic Engineering, 2017, 40: 148-156. |
| 46 | LI Zihua, WU Ziyi, CEN Xuecong, et al. Efficient production of 1,3-propanediol from diverse carbohydrates via a non-natural pathway using 3-hydroxypropionic acid as an intermediate[J]. ACS Synthetic Biology, 2021, 10(3): 478-486. |
| 47 | LI Zihua, DONG Yufei, LIU Yu, et al. Systems metabolic engineering of Corynebacterium glutamicum for high-level production of 1,3-propanediol from glucose and xylose[J]. Metabolic Engineering, 2022, 70: 79-88. |
| 48 | MEISWINKEL Tobias M, GOPINATH Vipin, LINDNER Steffen N, et al. Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine[J]. Microbial Biotechnology, 2013, 6(2): 131-140. |
| 49 | YANG Fengyu, QIU Kangling, ZHU Yichun, et al. Production of putrescine in metabolic engineering Corynebacterium crenatum by mixed sugar fermentation[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(44): 14407-14416. |
| 50 | BUSCHKE Nele, Hartwig SCHRÖDER, WITTMANN Christoph. Metabolic engineering of Corynebacterium glutamicum for production of 1,5-diaminopentane from hemicellulose[J]. Biotechnology Journal, 2011, 6(3): 306-317. |
| 51 | BUSCHKE Nele, BECKER Judith, Recker SCHÄFER, et al. Systems metabolic engineering of xylose-utilizing Corynebacterium glutamicum for production of 1,5-diaminopentane[J]. Biotechnology Journal, 2013, 8(5): 557-570. |
| 52 | IMAO Kenta, KONISHI Rie, KISHIDA Mayumi, et al. 1,5-Diaminopentane production from xylooligosaccharides using metabolically engineered Corynebacterium glutamicum displaying beta-xylosidase on the cell surface[J]. Bioresource Technology, 2017, 245: 1684-1691. |
| 53 | LI Lu, ZOU Dian, JI Anying, et al. Multilevel metabolic engineering of Bacillus amyloliquefaciens for production of the platform chemical putrescine from sustainable biomass hydrolysates[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(5): 2147-2157. |
| 54 | XU Yunzhen, GUO Nini, ZHENG Zongming, et al. Metabolism in 1,3‐propanediol fed‐batch fermentation by a D‐lactate deficient mutant of Klebsiella pneumoniae [J]. Biotechnology and Bioengineering, 2009, 104(5): 965-972. |
| 55 | RAJAGOPALAN Gobinath, JAIN Akanksha, POOSARLA Venkata Giridhar, et al. High level of 1,3-propanediol production from crude glycerol by Clostridium strain BOH3: Critical roles of inoculum and substrate concentration[J]. Journal of Chemical Technology & Biotechnology, 2024, 99(6): 1445-1458. |
| 56 | TANG Xueming, TAN Yongsong, ZHU Hong, et al. Microbial conversion of glycerol to 1,3-propanediol by an engineered strain of Escherichia coli [J]. Applied and Environmental Microbiology, 2009, 75(6): 1628-1634. |
| 57 | ZHANG Ye, LI Zihua, LIU Yu, et al. Systems metabolic engineering of Vibrio natriegens for the production of 1,3-propanediol[J]. Metabolic Engineering, 2021, 65: 52-65. |
| 58 | ZHANG Ye, SUN Qing, LIU Yu, et al. Development of a plasmid stabilization system in Vibrio natriegens for the high production of 1,3-propanediol and 3-hydroxypropionate[J]. Bioresources and Bioprocessing, 2021, 8(1): 125. |
| 59 | LIU Huan, LIU Shuang, NING Yuchen, et al. Metabolic engineering of Escherichia coli for efficient production of 1,4-butanediol from crude glycerol[J]. Journal of Environmental Chemical Engineering, 2024, 12(1): 111660. |
| 60 | MEISWINKEL Tobias M, RITTMANN Doris, LINDNER Steffen N, et al. Crude glycerol-based production of amino acids and putrescine by Corynebacterium glutamicum [J]. Bioresource Technology, 2013, 145: 254-258. |
| 61 | FREUDENBERG Robert A, WITTEMEIER Luisa, EINHAUS Alexander, et al. Advanced pathway engineering for phototrophic putrescine production[J]. Plant Biotechnology Journal, 2022, 20(10): 1968-1982. |
| 62 | NGUYEN Linh Thanh, LEE Eun Yeol. Biological conversion of methane to putrescine using genome-scale model-guided metabolic engineering of a methanotrophic bacterium Methylomicrobium alcaliphilum 20Z[J]. Biotechnology for Biofuels, 2019, 12: 147. |
| 63 | NAERDAL Ingemar, PFEIFENSCHNEIDER Johannes, BRAUTASET Trygve, et al. Methanol-based cadaverine production by genetically engineered Bacillus methanolicus strains[J]. Microbial Biotechnology, 2015, 8(2): 342-350. |
| 64 | NAERDAL Ingemar, PFEIFENSCHNEIDER Johannes, BRAUTASET Trygve, et al. Methanol-based cadaverine production by genetically engineered Bacillus methanolicus strains (vol 8, pg 342, 2015)[J]. Microbial Biotechnology, 2019, 12(1): 182-183. |
| 65 | NGUYEN Thu Thi, LEE Ok Kyung, NAIZABEKOV Sanzhar, et al. Bioconversion of methane to cadaverine and lysine using an engineered type Ⅱ methanotroph, Methylosinus trichosporium OB3b[J]. Green Chemistry, 2020, 22(22): 7803-7811. |
| 66 | HIROKAWA Yasutaka, MAKI Yuki, HANAI Taizo. Improvement of 1,3-propanediol production using an engineered cyanobacterium, Synechococcus elongatus by optimization of the gene expression level of a synthetic metabolic pathway and production conditions[J]. Metabolic Engineering, 2017, 39: 192-199. |
| 67 | LIU Hongyu, NI Jun, XU Ping, et al. Enhancing light-driven 1,3-propanediol production by using natural compartmentalization of differentiated cells[J]. ACS Synthetic Biology, 2018, 7(10): 2436-2446. |
| 68 | MENG Hao, WANG Chuang, YUAN Qipeng, et al. An aldolase-based new pathway for bioconversion of formaldehyde and ethanol into 1,3-propanediol in Escherichia coli [J]. ACS Synthetic Biology, 2021, 10(4): 799-809. |
| 69 | FENG Jia, HAN Ye, XU Shuang, et al. Engineering RuBisCO-based shunt for improved cadaverine production in Escherichia coli [J]. Bioresource Technology, 2024, 398: 130529. |
| 70 | GAO Siyuan, LU Jiachen, WANG Tongtao, et al. A novel co-production of cadaverine and succinic acid based on a thermal switch system in recombinant Escherichia coli [J]. Microbial Cell Factories, 2022, 21(1): 248. |
| 71 | WANG Jing, MAO Jingwen, TIAN Weilong, et al. Coproduction of succinic acid and cadaverine using lysine as a neutralizer and CO2 donor with L-lysine decarboxylase overexpressed Escherichia coli AFP111[J]. Green Chemistry, 2018, 20(12): 2880-2887. |
| [1] | 曹湘洪, 周峰, 姜睿, 刘诗哲, 方向晨, 亢万忠, 乔金樑, 聂红. 加快我国生物基材料产业发展的对策[J]. 化工进展, 2025, 44(5): 2385-2393. |
| [2] | 王媛媛, 张翀, 韩双艳, 邢新会. 毕赤酵母利用甲醇生产重组蛋白技术的研究进展[J]. 化工进展, 2025, 44(5): 2441-2450. |
| [3] | 盛华康, 张博, 申晓林, 孙新晓, 王佳, 袁其朋. 微生物合成白藜芦醇及其衍生物[J]. 化工进展, 2025, 44(5): 2463-2474. |
| [4] | 钟家伟, 谭涛, 谢君, 陈勇. 生物质高值能源转换技术[J]. 化工进展, 2025, 44(5): 2524-2528. |
| [5] | 聂红, 习远兵, 葛泮珠, 丁石, 张登前. 可持续航空燃料生产路线与展望——以中石化石科院为例[J]. 化工进展, 2025, 44(5): 2529-2534. |
| [6] | 王水众, 宋国勇. 木质素选择性氢解制备高功能化单酚及其高值利用[J]. 化工进展, 2025, 44(5): 2535-2540. |
| [7] | 陈彦君, 戴杰, 单军强, 张思欣, 计磊, 朱晨杰, 应汉杰. 我国纤维素乙醇的研究进展和发展趋势[J]. 化工进展, 2025, 44(5): 2541-2562. |
| [8] | 乔凯, 张震宇, 马会霞, 傅杰, 周峰. 生物基呋喃二甲酸关键技术路线和产业发展现状[J]. 化工进展, 2025, 44(5): 2577-2586. |
| [9] | 许镇浩, 易子骁, 曾晨, 王宇辰, 严凯. 生物质基平台分子转化升级的研究进展[J]. 化工进展, 2025, 44(5): 2642-2654. |
| [10] | 孙仲顺, 刘根, 程春昱, 李美昕, 杨宪坛, 吴志强, 杨伯伦. 生物质热化学转化制备绿氢研究进展[J]. 化工进展, 2025, 44(5): 2667-2682. |
| [11] | 艾佳臻, 张振磊, 詹国雄, 马龙巍, 史国靖, 尹海川, 张香平. “木质素优先”还原催化分馏工艺与模拟研究进展[J]. 化工进展, 2025, 44(5): 2683-2693. |
| [12] | 丁阿静, 周巧巧, 顾学红. 膜反应器中杨木催化气化制清洁合成气[J]. 化工进展, 2025, 44(5): 2716-2723. |
| [13] | 乔金樑. 原料替代助力我国石化产业高质量发展[J]. 化工进展, 2025, 44(5): 2803-2805. |
| [14] | 夏猛, 赵雪冰, 蒋国强, 卢滇楠, 刘铮. 电-酶催化CO2转化生产化学品的研究展望[J]. 化工进展, 2025, 44(5): 2825-2833. |
| [15] | 孙涛, 汪鑫, 孙美莉, 王凯峰, 纪晓俊. 合成生物学优化酵母代谢过程中的碳保存和碳固定[J]. 化工进展, 2025, 44(5): 2834-2845. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||