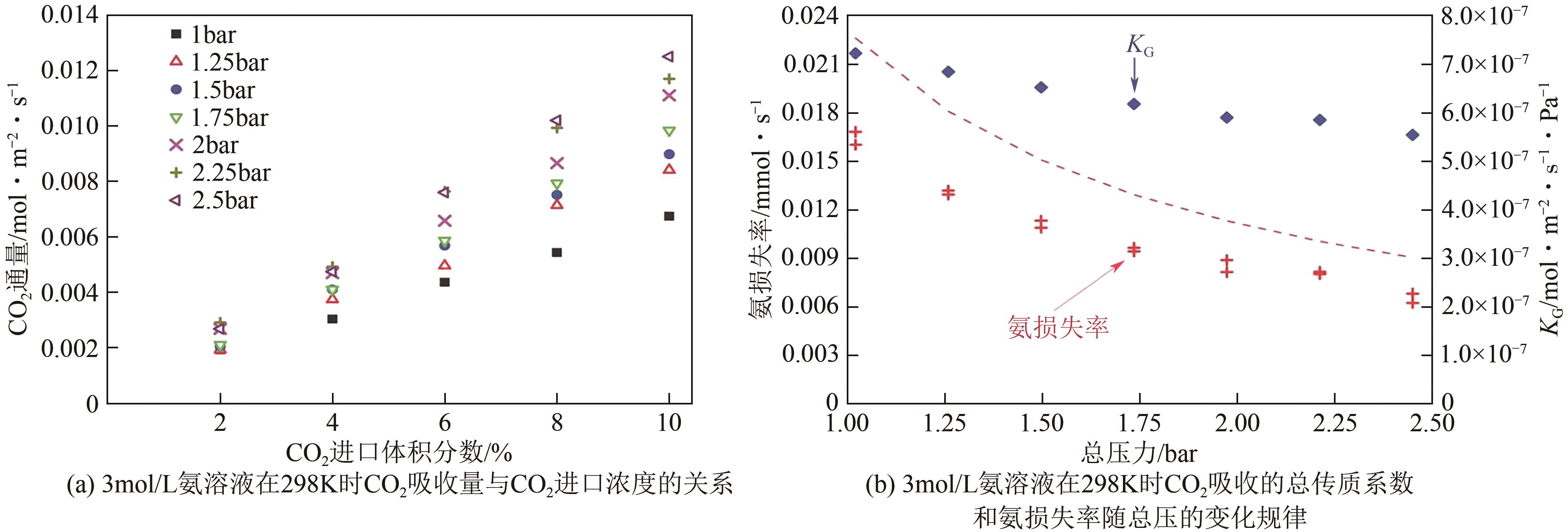
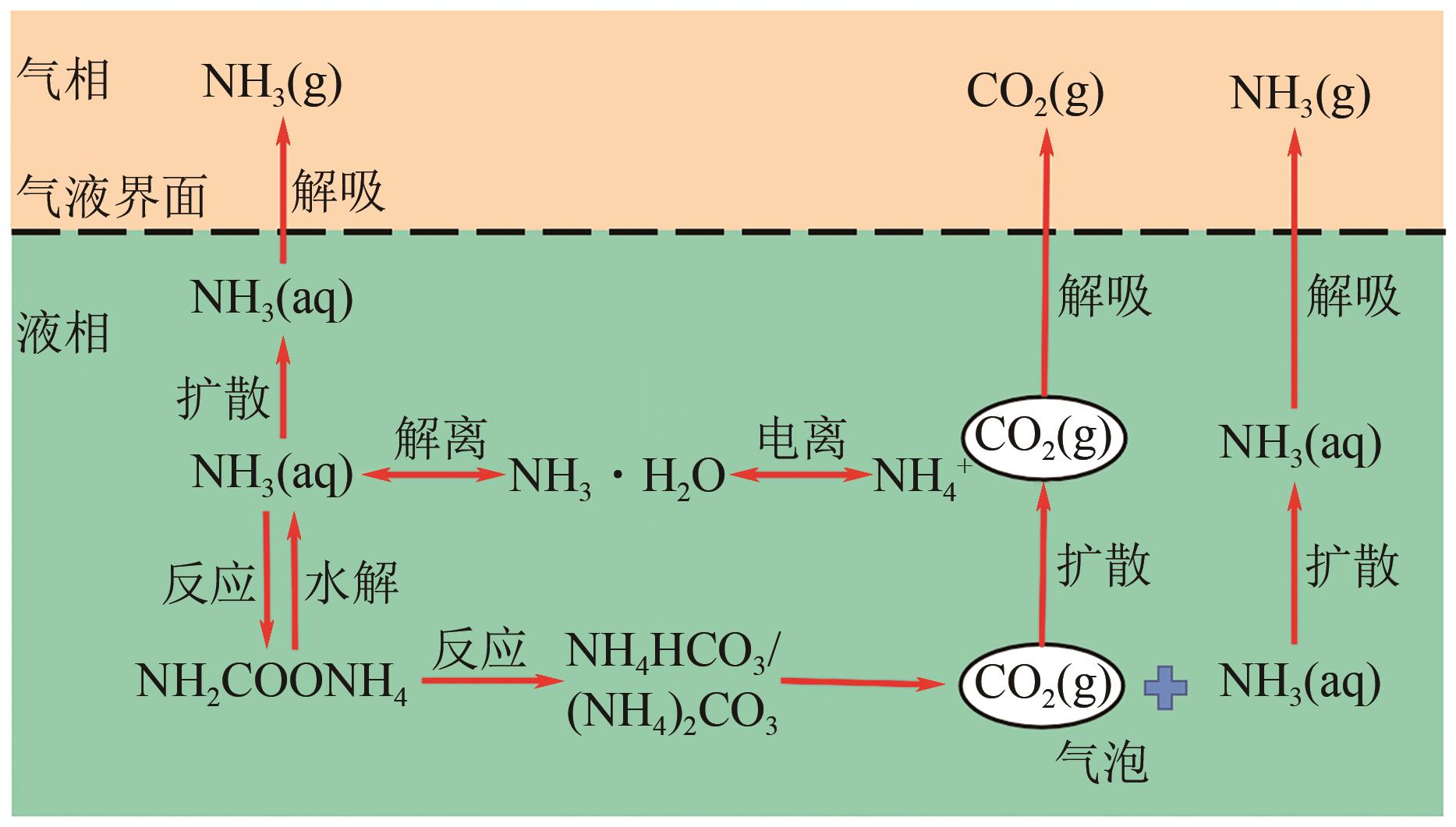
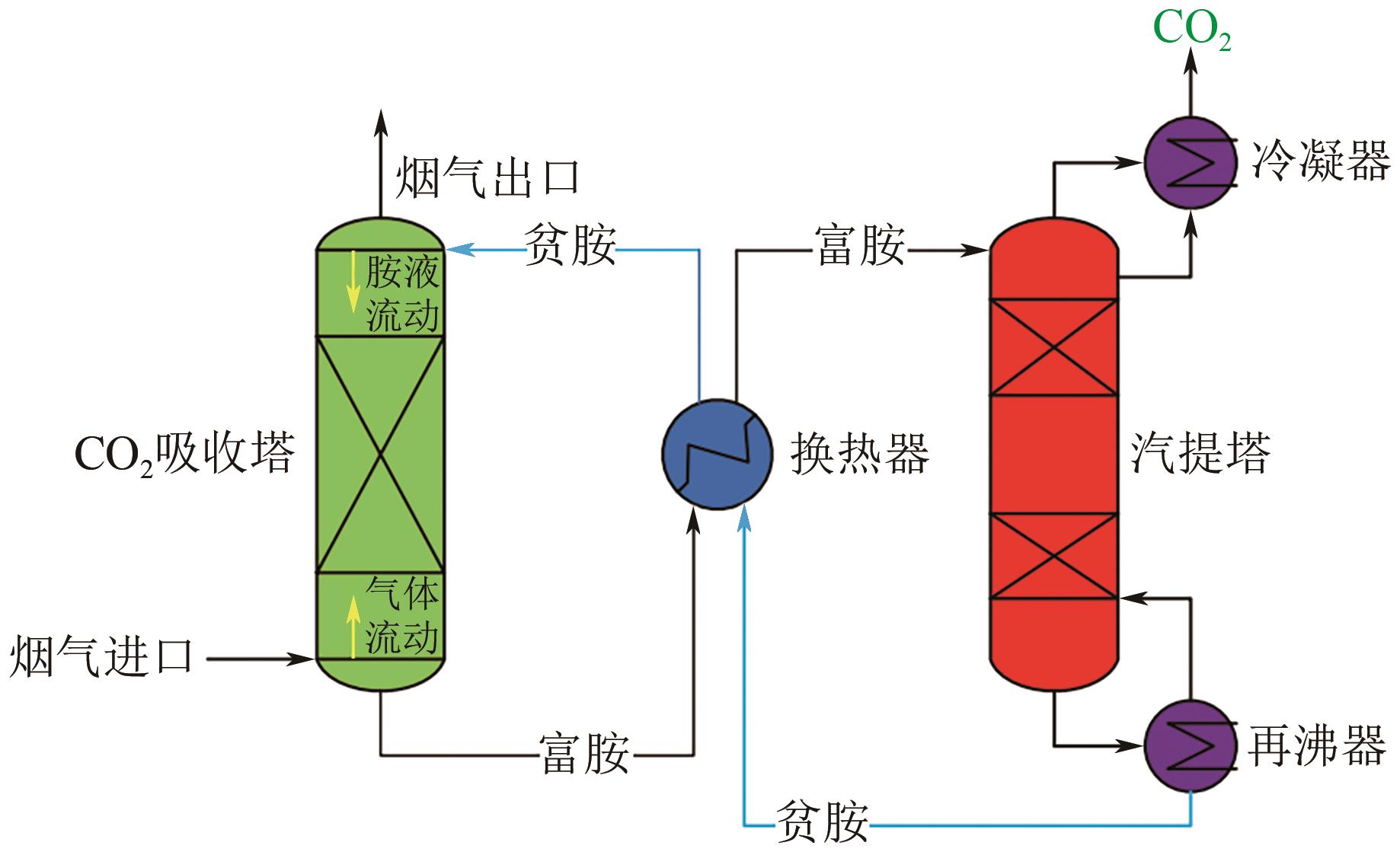
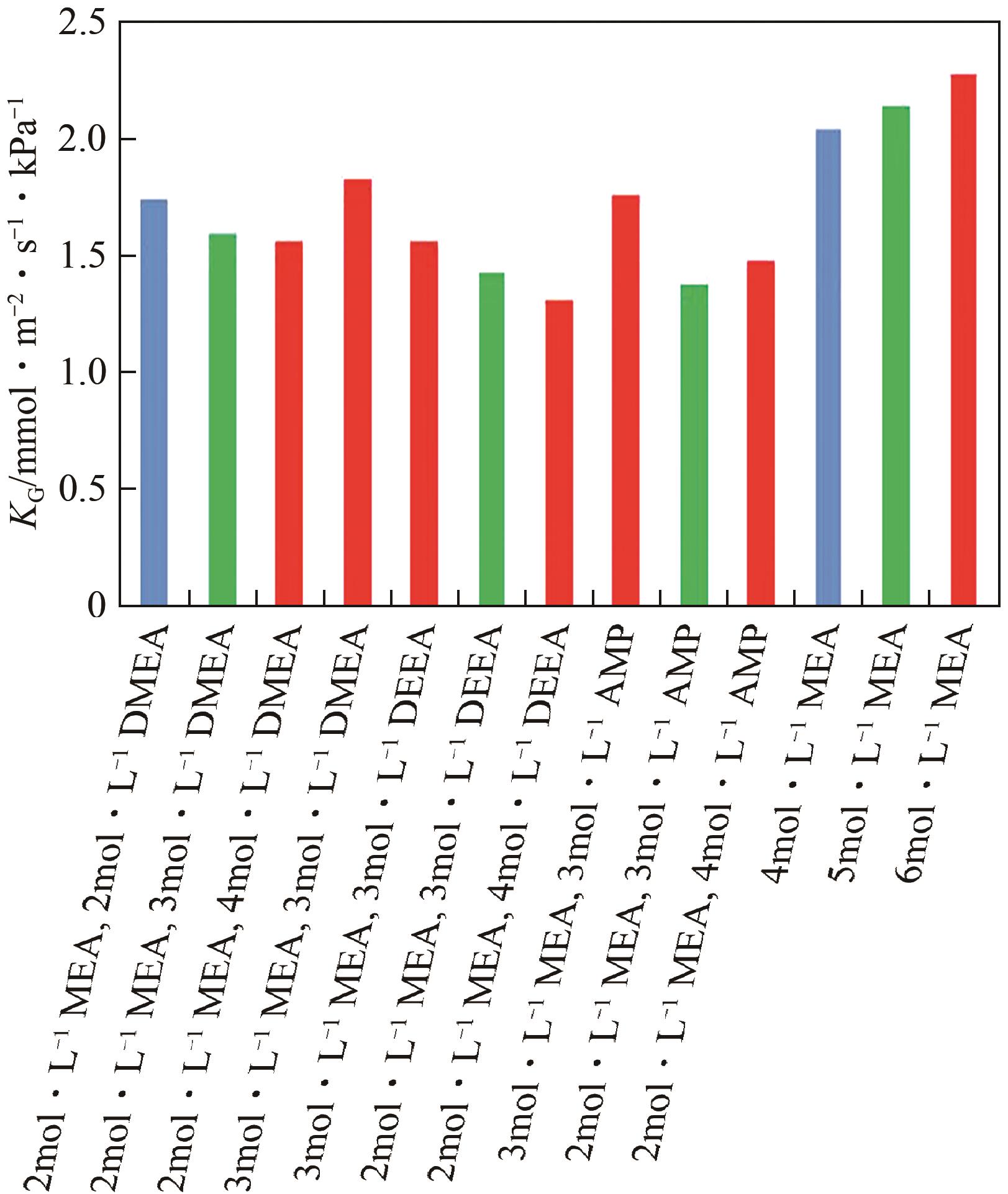
化工进展 ›› 2024, Vol. 43 ›› Issue (10): 5734-5747.DOI: 10.16085/j.issn.1000-6613.2023-1523
• 资源与环境化工 • 上一篇
液体吸收剂捕集燃烧后CO2的研究进展
- 1.上海交通大学中英国际低碳学院,上海 200240
2.上海交通大学机械与动力工程学院,上海 200240
-
收稿日期:2023-09-01修回日期:2023-11-05出版日期:2024-10-15发布日期:2024-10-29 -
通讯作者:王恩禄 -
作者简介:苏辉辉(1997—),男,硕士研究生,研究方向为CO2捕集技术。E-mail:suhui1997@sjtu.edu.cn。 -
基金资助:上海交通大学校企合作项目(22H010104472)
Advances in research on capture of post-combustion carbon dioxide by liquid adsorbents
SU Huihui1( ), WANG Enlu1,2(
), WANG Enlu1,2( ), XU Yifei2
), XU Yifei2
- 1.China UK Low Carbon college, Shanghai Jiao Tong University, Shanghai 200240, China
2.School of Mechanical and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China
-
Received:2023-09-01Revised:2023-11-05Online:2024-10-15Published:2024-10-29 -
Contact:WANG Enlu
摘要:
近年来,以胺溶液、氨溶液、碳酸盐溶液等为代表的液体吸收剂在捕集CO2方面表现出了处理能力大、价格廉价、技术成熟和应用广泛等特点,未来有望成为更经济、高效、绿色和可持续的碳捕集材料。本文首先综述了目前使用液体吸收剂捕集燃烧后CO2的现状,介绍了液体吸收剂捕集CO2的机理、影响因素以及增强捕集性能的策略和挑战。然后对比了各种液体吸收剂在捕集CO2方面的优缺点,并展望了液体吸收剂未来发展的前景。分析表明,单一液体吸收剂在捕集CO2方面性能相对较差,可以通过添加剂、抑制剂和共混物等手段进行改良。对现有工艺改进并结合新技术可以降低捕集过程中的能耗,但经济评估仍需加强。此外,当前的研究主要集中于单组分气体或双组分混合气体,未来的研究应多开展使用液体吸收剂捕集多组分混合气体中的CO2。
中图分类号:
引用本文
苏辉辉, 王恩禄, 徐逸飞. 液体吸收剂捕集燃烧后CO2的研究进展[J]. 化工进展, 2024, 43(10): 5734-5747.
SU Huihui, WANG Enlu, XU Yifei. Advances in research on capture of post-combustion carbon dioxide by liquid adsorbents[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5734-5747.
| 名称 | 化学式 | 摩尔质量/g·mol-1 | 密度/g·cm-3 | 黏度(293K)/mPa·s | 沸点/K | 数字识别号(CAS编号) |
|---|---|---|---|---|---|---|
| 单乙醇胺(MEA) | C2H7NO | 61.08 | 1.012 | 24.10 | 443 | 141-43-5 |
| 二乙醇胺(DEA) | C4H11NO2 | 105.14 | 1.097 | 380 | 544.2 | 111-42-2 |
| 甲基二乙醇胺(MDEA) | C12H17NO2 | 119.16 | 1.038 | 101 | 520.2 | 105-59-9 |
| 二异丙胺(DIPA) | C6H15N | 101.10 | 0.722 | 0.40(298K) | 357 | 108-18-9 |
| 2-氨基-2-甲基-1-丙醇(AMP) | C4H11NO | 89.14 | 0.934 | 102(303K) | 438.6 | 124-68-5 |
| 二甲基乙醇胺(DMEA) | C4H11NO | 89.14 | 0.888 | 3.80 | 407.4 | 108-01-0 |
| 二乙胺乙醇(DEEA) | C6H15NO | 117.19 | 0.884 | 5.10 | 434.2 | 100-37-8 |
| 2-甲基-1,5-二氨基戊烷(DA2MP) | C6H16N2 | 116.21 | 0.86 | 2.63 | 465 | 15520-10-2 |
| 3-甲氨基丙胺(MAPA) | C4H12N2 | 88.15 | 0.840 | 1.60 | 412-414 | 6291-84-5 |
| 二乙烯三胺(DETA) | C4H13N3 | 103.17 | 0.955 | 7.14 | 477.2 | 111-40-0 |
| 羟乙基乙二胺(AEEA) | C4H12N2O | 104.15 | 1.030 | 155 | 516 | 111-41-1 |
表1 常见胺的特性
| 名称 | 化学式 | 摩尔质量/g·mol-1 | 密度/g·cm-3 | 黏度(293K)/mPa·s | 沸点/K | 数字识别号(CAS编号) |
|---|---|---|---|---|---|---|
| 单乙醇胺(MEA) | C2H7NO | 61.08 | 1.012 | 24.10 | 443 | 141-43-5 |
| 二乙醇胺(DEA) | C4H11NO2 | 105.14 | 1.097 | 380 | 544.2 | 111-42-2 |
| 甲基二乙醇胺(MDEA) | C12H17NO2 | 119.16 | 1.038 | 101 | 520.2 | 105-59-9 |
| 二异丙胺(DIPA) | C6H15N | 101.10 | 0.722 | 0.40(298K) | 357 | 108-18-9 |
| 2-氨基-2-甲基-1-丙醇(AMP) | C4H11NO | 89.14 | 0.934 | 102(303K) | 438.6 | 124-68-5 |
| 二甲基乙醇胺(DMEA) | C4H11NO | 89.14 | 0.888 | 3.80 | 407.4 | 108-01-0 |
| 二乙胺乙醇(DEEA) | C6H15NO | 117.19 | 0.884 | 5.10 | 434.2 | 100-37-8 |
| 2-甲基-1,5-二氨基戊烷(DA2MP) | C6H16N2 | 116.21 | 0.86 | 2.63 | 465 | 15520-10-2 |
| 3-甲氨基丙胺(MAPA) | C4H12N2 | 88.15 | 0.840 | 1.60 | 412-414 | 6291-84-5 |
| 二乙烯三胺(DETA) | C4H13N3 | 103.17 | 0.955 | 7.14 | 477.2 | 111-40-0 |
| 羟乙基乙二胺(AEEA) | C4H12N2O | 104.15 | 1.030 | 155 | 516 | 111-41-1 |
| 组成成分(质量分数) | 温度/K | 压力 | CO2吸收能力 | 参考文献 |
|---|---|---|---|---|
| 30%单乙醇胺 + 10%~60% 1-丙醇 | 293~318 | 1.09bar | 0~2.24mol/L | [ |
| 5%~15%单乙醇胺 + 30%~50%二甲氨基异丙醇 | 303~323 | 1bar | 0.32~0.71mol/mol | [ |
| 25%~40%单乙醇胺 + 5%哌嗪 | 303 | 1bar | 0.13~0.25mol/mol | [ |
| 9∶21, 15∶15, 21∶9羟乙基乙二胺∶二乙醇胺 | 393.15 | 477.4kPa | 0.15~0.33mol/mol | [ |
| 4.5%甲基二乙醇胺 + 0.5%哌嗪 | 313 | 1bar | 0.7mol/mol | [ |
| 0.1~0.3mol/L哌嗪 + 1.5~4mol/L 2-氨基-2-甲基-1-丙醇 | 293~323 | 90.66kPa | 0.13~0.94mol/mol | [ |
| 0.5~2mol/L二乙胺乙醇 + 0.05~0.2mol/L 3-甲氨基丙胺 | 313.15 | 0~35MPa | 0.05~0.15mol/mol | [ |
| 5%~25%二乙烯三胺 + 5%~25%哌嗪 | 313 | 1bar | 0.3~0.483mol/kg | [ |
| 5%~10%单乙醇胺 + 1% 2-氨基-2-甲基-1-丙醇 + 1%~5%甲基二乙醇胺 | 313 | — | 0.1~0.44mol/mol | [ |
| 0.5mol/L二乙烯三胺 + 1.5mol/L 2-氨基-2-甲基-1-丙醇 + 3mol/L五甲基二乙烯三胺 | 313 | 1bar | 3.17mol/L | [ |
表2 胺溶液吸收CO2性能
| 组成成分(质量分数) | 温度/K | 压力 | CO2吸收能力 | 参考文献 |
|---|---|---|---|---|
| 30%单乙醇胺 + 10%~60% 1-丙醇 | 293~318 | 1.09bar | 0~2.24mol/L | [ |
| 5%~15%单乙醇胺 + 30%~50%二甲氨基异丙醇 | 303~323 | 1bar | 0.32~0.71mol/mol | [ |
| 25%~40%单乙醇胺 + 5%哌嗪 | 303 | 1bar | 0.13~0.25mol/mol | [ |
| 9∶21, 15∶15, 21∶9羟乙基乙二胺∶二乙醇胺 | 393.15 | 477.4kPa | 0.15~0.33mol/mol | [ |
| 4.5%甲基二乙醇胺 + 0.5%哌嗪 | 313 | 1bar | 0.7mol/mol | [ |
| 0.1~0.3mol/L哌嗪 + 1.5~4mol/L 2-氨基-2-甲基-1-丙醇 | 293~323 | 90.66kPa | 0.13~0.94mol/mol | [ |
| 0.5~2mol/L二乙胺乙醇 + 0.05~0.2mol/L 3-甲氨基丙胺 | 313.15 | 0~35MPa | 0.05~0.15mol/mol | [ |
| 5%~25%二乙烯三胺 + 5%~25%哌嗪 | 313 | 1bar | 0.3~0.483mol/kg | [ |
| 5%~10%单乙醇胺 + 1% 2-氨基-2-甲基-1-丙醇 + 1%~5%甲基二乙醇胺 | 313 | — | 0.1~0.44mol/mol | [ |
| 0.5mol/L二乙烯三胺 + 1.5mol/L 2-氨基-2-甲基-1-丙醇 + 3mol/L五甲基二乙烯三胺 | 313 | 1bar | 3.17mol/L | [ |
| 捕集材料 | 缺点 | 优点 | 提高吸收性能的方法 |
|---|---|---|---|
| 胺吸收液 | |||
| 单乙醇胺(MEA) | ● 高腐蚀性 ● 再生能量需求高 | ● 高吸收率 ● 可规模化应用 ● 成本低 | ● 使用两胺或者多胺混合物 ● 使用非水溶剂 ● 添加促进剂 ● 改进工艺 |
| 二乙醇胺(DEA) | ● 氧气存在时产生腐蚀性酸 ● 不能携带低压气体 | ● 低腐蚀性 ● 低发泡性 | |
| 三乙醇胺(TEA) | ● 相对单乙醇胺,吸收率低 | ● 成本低 ● 再生能量需求低 | |
| 二异丙胺(DIPA) | ● CO2吸收效果差 | ● 无腐蚀性 ● 再生能量需求低 | |
| 哌嗪(PZ) | ● 浓度使用有限 | ● 高的吸收能力,是MEA的两倍 | |
| 羟乙基乙二胺(AEEA) | ● 溶剂降解 | ● 高的CO2吸收能力 ● 高的吸收循环能力 | |
| 2-氨基-2-甲基-1-丙醇(AMP) | ● 与单乙醇胺相比,传质效果差 | ● 高的CO2吸收能力 ● 低腐蚀性 | |
| 氨溶液 | ● NH3的高挥发性 ● 反应速率慢 ● 与胺反应相比更复杂 ● 不能将产品气体中的二氧化碳含量降低到非常低的水平 | ● 与单乙醇胺相比,反应过程的热量低 ● 不受COS、CS2、HCN和H2S的影响 ● 除CO2外,还可以去除SO2、NO x 和汞 ● 生产增值化学品,如硫酸铵和硝酸铵 | ● 酸洗和水洗 ● 膜法 ● 添加抑制剂 ● 参数优化 ● 工艺改进 |
| 碳酸盐溶液 | ● 孔隙易堵塞 ● 再生温度高 ● 反应速度慢,传质小 ● 不适合从低CO2分压源捕获CO2,因为碳酸盐和碳酸氢盐的溶解度有限 | ● 成本低 ● 降解和腐蚀性低 ● 高温吸收,易解吸 | ● 添加促进剂 ● 腐蚀抑制剂(重铬酸钾、乙二胺四乙酸、碳酸铜) ● 改进反应动力学 ● 改善工艺 |
| 离子液体 | ● 高黏度,限制了传质速率 ● 新的离子液合成成本相对较高 | ● 非挥发性 ● 高CO2溶解度 ● 高CO2选择性 | ● 增加阳离子上的烷基链 ● 添加或接枝官能团(胺、氨基酸、酚、唑类) |
| 相变吸收剂溶液 | ● 溶剂的动力学和热力学的研究不够完善 ● CO2负载量的增加导致富相溶剂黏度变高 | ● 显著降低了用于CO2捕集的能量使用和设备成本 | ● 添加促进剂 ● 双相溶剂 |
| 碱性氢氧化物溶液 | ● 容易在工艺再沸器及管线内沉淀 ● 与碳酸盐相比,氢氧化物不容易用温和的热或压力摆动产生 | ● 低溶剂成本 ● 高可及性 ● 低毒性和非挥发性 | ● 参数优化 ● 添加促进剂 |
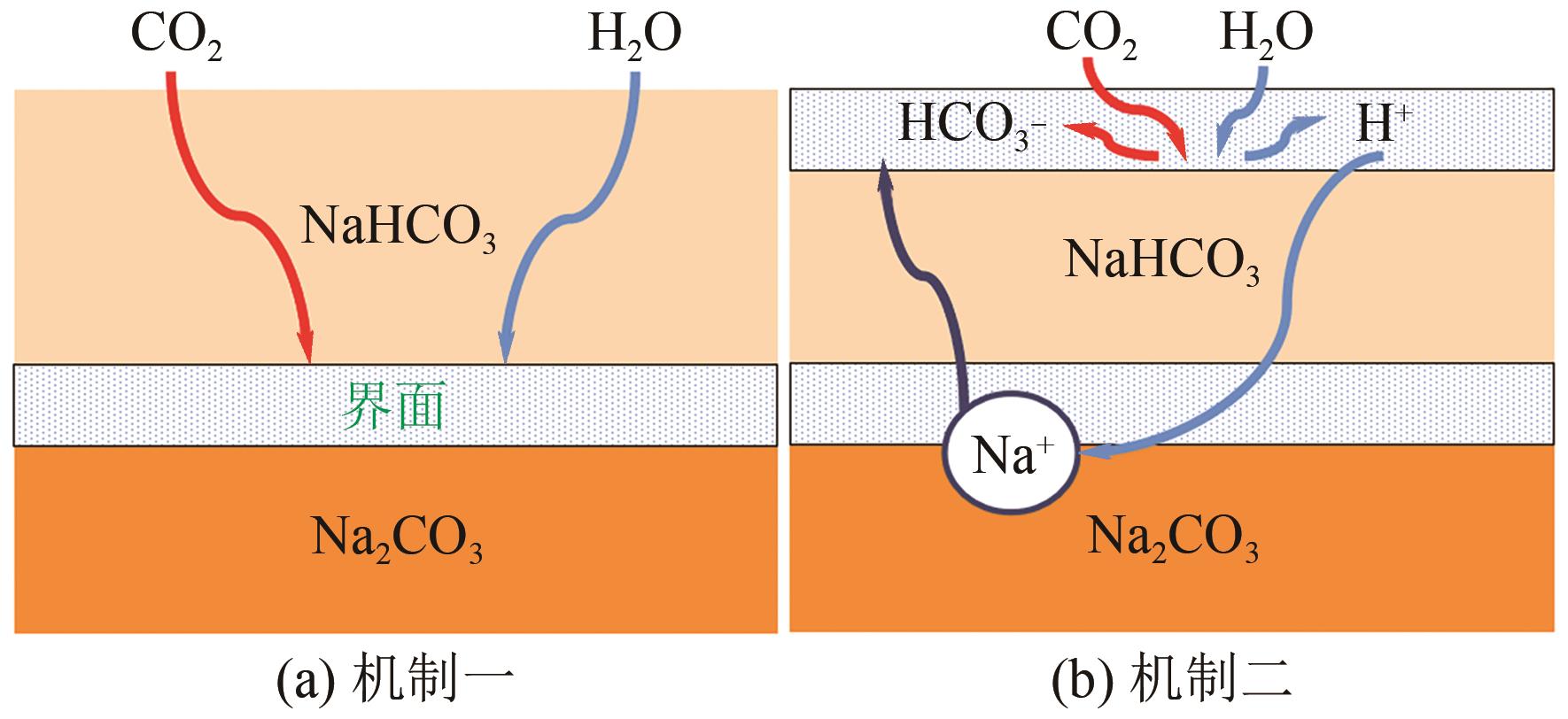
表3 不同吸收剂捕集CO2的对比[122-123]
| 捕集材料 | 缺点 | 优点 | 提高吸收性能的方法 |
|---|---|---|---|
| 胺吸收液 | |||
| 单乙醇胺(MEA) | ● 高腐蚀性 ● 再生能量需求高 | ● 高吸收率 ● 可规模化应用 ● 成本低 | ● 使用两胺或者多胺混合物 ● 使用非水溶剂 ● 添加促进剂 ● 改进工艺 |
| 二乙醇胺(DEA) | ● 氧气存在时产生腐蚀性酸 ● 不能携带低压气体 | ● 低腐蚀性 ● 低发泡性 | |
| 三乙醇胺(TEA) | ● 相对单乙醇胺,吸收率低 | ● 成本低 ● 再生能量需求低 | |
| 二异丙胺(DIPA) | ● CO2吸收效果差 | ● 无腐蚀性 ● 再生能量需求低 | |
| 哌嗪(PZ) | ● 浓度使用有限 | ● 高的吸收能力,是MEA的两倍 | |
| 羟乙基乙二胺(AEEA) | ● 溶剂降解 | ● 高的CO2吸收能力 ● 高的吸收循环能力 | |
| 2-氨基-2-甲基-1-丙醇(AMP) | ● 与单乙醇胺相比,传质效果差 | ● 高的CO2吸收能力 ● 低腐蚀性 | |
| 氨溶液 | ● NH3的高挥发性 ● 反应速率慢 ● 与胺反应相比更复杂 ● 不能将产品气体中的二氧化碳含量降低到非常低的水平 | ● 与单乙醇胺相比,反应过程的热量低 ● 不受COS、CS2、HCN和H2S的影响 ● 除CO2外,还可以去除SO2、NO x 和汞 ● 生产增值化学品,如硫酸铵和硝酸铵 | ● 酸洗和水洗 ● 膜法 ● 添加抑制剂 ● 参数优化 ● 工艺改进 |
| 碳酸盐溶液 | ● 孔隙易堵塞 ● 再生温度高 ● 反应速度慢,传质小 ● 不适合从低CO2分压源捕获CO2,因为碳酸盐和碳酸氢盐的溶解度有限 | ● 成本低 ● 降解和腐蚀性低 ● 高温吸收,易解吸 | ● 添加促进剂 ● 腐蚀抑制剂(重铬酸钾、乙二胺四乙酸、碳酸铜) ● 改进反应动力学 ● 改善工艺 |
| 离子液体 | ● 高黏度,限制了传质速率 ● 新的离子液合成成本相对较高 | ● 非挥发性 ● 高CO2溶解度 ● 高CO2选择性 | ● 增加阳离子上的烷基链 ● 添加或接枝官能团(胺、氨基酸、酚、唑类) |
| 相变吸收剂溶液 | ● 溶剂的动力学和热力学的研究不够完善 ● CO2负载量的增加导致富相溶剂黏度变高 | ● 显著降低了用于CO2捕集的能量使用和设备成本 | ● 添加促进剂 ● 双相溶剂 |
| 碱性氢氧化物溶液 | ● 容易在工艺再沸器及管线内沉淀 ● 与碳酸盐相比,氢氧化物不容易用温和的热或压力摆动产生 | ● 低溶剂成本 ● 高可及性 ● 低毒性和非挥发性 | ● 参数优化 ● 添加促进剂 |
| 1 | LIU Zhu, DENG Zhu, DAVIS Steven J, et al. Monitoring global carbon emissions in 2021[J]. Nature Reviews Earth & Environment, 2022, 3(4): 217-219. |
| 2 | VAN VUUREN D P, MEINSHAUSEN M, PLATTNER G K, et al. Temperature increase of 21st century mitigation scenarios[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(40): 15258-15262. |
| 3 | JACOBSON Tyler A, KLER Jasdeep S, HERNKE Michael T, et al. Direct human health risks of increased atmospheric carbon dioxide[J]. Nature Sustainability, 2019, 2(8): 691-701. |
| 4 | SINGH Gurwinder, LEE Jangmee, KARAKOTI Ajay, et al. Emerging trends in porous materials for CO2 capture and conversion[J]. Chemical Society Reviews, 2020, 49(13): 4360-4404. |
| 5 | RAJABLOO Talieh, VALEE Joris, MARENNE Yves, et al. Carbon capture and utilization for industrial applications[J]. Energy Reports, 2023, 9: 111-116. |
| 6 | MA Jinfeng, LI Lin, WANG Haofan, et al. Carbon capture and storage: History and the road ahead[J]. Engineering, 2022, 14: 33-43. |
| 7 | BONAVENTURA D, CHACARTEGUI R, Valverde JM, et al. Carbon capture and utilization for sodium bicarbonate production assisted by solar thermal power[J]. Energy Conversion and Management, 2017, 149: 860-874. |
| 8 | SVENDSEN Hallvard F, HESSEN Erik T, MEJDELL Thor. Carbon dioxide capture by absorption, challenges and possibilities[J]. Chemical Engineering Journal, 2011, 171(3): 718-724. |
| 9 | Ahmed AL-MAMOORI, KRISHNAMURTHY Anirudh, ROWNAGHI Ali A, et al. Carbon capture and utilization update[J]. Energy Technology, 2017, 5(6): 834-849. |
| 10 | Mohammad Chehrazi, Moghadas Bahareh Kamyab. A review on CO2 capture with chilled ammonia and CO2 utilization in urea plant[J]. Journal of CO2 Utilization, 2022, 61: 102030. |
| 11 | TAN Xinyi, Nielsen Jens. The integration of bio-catalysis and electrocatalysis to produce fuels and chemicals from carbon dioxide[J]. Chemical Society Reviews, 2022, 51(11): 4763-4785. |
| 12 | Mehmet ÇOPUR, ŞENOL Ayşe Merve, PEKDEMIR Turgay, et al. Industrial symbiosis: CO2 utilization by producing disodium octaborate tetrahydrate and baking soda from borax[J]. Industrial & Engineering Chemistry Research, 2023, 62(34): 13340-13354. |
| 13 | WANG Xianfeng, BAO Zhenghong, AKHMEDOV Novruz G, et al. Unique biological amino acids turn CO2 emission into novel nanomaterials with three switchable product pathways[J]. Environmental Technology & Innovation, 2023, 32: 103279. |
| 14 | Martin NAĎ, BRUMMER Vladimír, Pavel LOŠÁK, et al. Waste-to-energy plants flue gas CO2 mitigation using a novel tubular photobioreactor while producing Chlorella algae[J]. Journal of Cleaner Production, 2023, 385: 135721. |
| 15 | IGLINA Tatyana, IGLIN Pavel, PASHCHENKO Dmitry. Industrial CO2 capture by algae: A review and recent advances[J]. Sustainability, 2022, 14(7): 3801. |
| 16 | PERES Christiano B, RESENDE Pedro M R, NUNES Leonel J R, et al. Advances in carbon capture and use (CCU) technologies: A comprehensive review and CO2 mitigation potential analysis[J]. Clean Technologies, 2022, 4(4): 1193-1207. |
| 17 | Federica Raganati, Francesco Miccio, Paola Ammendola. Adsorption of carbon dioxide for post-combustion capture: A review[J]. Energy & Fuels, 2021, 35(16): 12845-12868. |
| 18 | SANZ-PÉREZ Eloy S, MURDOCK Christopher R, DIDAS Stephanie A, et al. Direct capture of CO2 from ambient air[J]. Chemical Reviews, 2016, 116(19): 11840-11876. |
| 19 | TZIMAS Evangelos, MERCIER Arnaud, CORMOS Calin-Cristian, et al. Trade-off in emissions of acid gas pollutants and of carbon dioxide in fossil fuel power plants with carbon capture[J]. Energy Policy, 2007, 35(8): 3991-3998. |
| 20 | WANG Xiaoxing, SONG Chunshan. Carbon capture from flue gas and the atmosphere: A perspective[J]. Frontiers in Energy Research, 2020, 8: 560849. |
| 21 | Zhe Lun OOI, TAN Pui Yee, TAN Lian See, et al. Amine-based solvent for CO2 absorption and its impact on carbon steel corrosion: A perspective review[J]. Chinese Journal of Chemical Engineering, 2020, 28(5): 1357-1367. |
| 22 | TIWARI S C, BHARDWAJ A, NIGAM K D P, et al. A strategy of development and selection of absorbent for efficient CO2 capture: An overview of properties and performance[J]. Process Safety and Environmental Protection, 2022, 163: 244-273. |
| 23 | ZHAO Bingtao, SU Yaxin, TAO Wenwen, et al. Post-combustion CO2 capture by aqueous ammonia: A state-of-the-art review[J]. International Journal of Greenhouse Gas Control, 2012, 9: 355-371. |
| 24 | FU Lipei, REN Zhangkun, SI Wenzhe, et al. Research progress on CO2 capture and utilization technology[J]. Journal of CO2 Utilization, 2022, 66: 102260. |
| 25 | ATZORI Federico, BARZAGLI Francesco, VARONE Alberto, et al. CO2 absorption in aqueous NH3 solutions: Novel dynamic modeling of experimental outcomes[J]. Chemical Engineering Journal, 2023, 451: 138999. |
| 26 | KIM You Jeong, YOU Jong Kyun, HONG Won Hi, et al. Characteristics of CO2 Absorption into aqueous ammonia[J]. Separation Science and Technology, 2008, 43(4): 766-777. |
| 27 | ZHANG Minkai, GUO Yincheng. Rate based modeling of absorption and regeneration for CO2 capture by aqueous ammonia solution[J]. Applied Energy, 2013, 111: 142-152. |
| 28 | FANG Mengxiang, XIANG Qunyang, YU Chunjiang, et al. Experimental study on CO2 absorption by aqueous ammonia solution at elevated pressure to enhance CO2 absorption and suppress ammonia vaporization[J]. Greenhouse Gases: Science and Technology, 2015, 5(2): 210-221. |
| 29 | 马双忱, 王梦璇, 孟亚男, 等. 氨法吸收烟气中二氧化碳与脱碳后溶液解吸研究进展[J]. 化工进展, 2012, 31(5): 1143-1148, 1159. |
| MA Shuangchen, WANG Mengxuan, MENG Yanan, et al. Research on the absorption of CO2 from flue gas and the desorption of decarbonization solution using ammonia method[J]. Chemical Industry and Engineering Progress, 2012, 31(5): 1143-1148, 1159. | |
| 30 | AS B, NN D. Upflow anaerobic sludge blanket reactor a review[J]. Indian journal of environmental health, 2001, 43(2): 1-82. |
| 31 | WANG Fu, ZHAO Jun, MIAO He, et al. Current status and challenges of the ammonia escape inhibition technologies in ammonia-based CO2 capture process[J]. Applied Energy, 2018, 230: 734-749. |
| 32 | MA Shuangchen, SONG Huihui, WANG Mengxuan, et al. Research on mechanism of ammonia escaping and control in the process of CO2 capture using ammonia solution[J]. Chemical Engineering Research and Design, 2013, 91(7): 1327-1334. |
| 33 | HAO Xiaochen, DI Yinlu, XU Qingquan, et al. Multi-objective prediction for denitration systems in cement: An approach combining process analysis and bi-directional long short-term memory network[J]. Environmental Science and Pollution Research, 2023, 30(11): 30408-30429. |
| 34 | KHAKHARIA Purvil, HUIZINGA Arjen, JURADO LOPEZ Cristina, et al. Acid wash scrubbing as a countermeasure for ammonia emissions from a postcombustion CO2 capture plant[J]. Industrial & Engineering Chemistry Research, 2014, 53(33): 13195-13204. |
| 35 | FANG Mengxiang, MA Qinhui, WANG Zhen, et al. A novel method to recover ammonia loss in ammonia-based CO2 capture system: Ammonia regeneration by vacuum membrane distillation[J]. Greenhouse Gases: Science and Technology, 2015, 5(4): 487-498. |
| 36 | MAKHLOUFI Camel, LASSEUGUETTE Elsa, REMIGY Jean Christophe, et al. Ammonia based CO2 capture process using hollow fiber membrane contactors[J]. Journal of Membrane Science, 2014, 455: 236-246. |
| 37 | CUI Zheng, DE MONTIGNY David. Experimental study of carbon dioxide absorption into aqueous ammonia with a hollow fiber membrane contactor[J]. Journal of Membrane Science, 2017, 540: 297-306. |
| 38 | MA Shuangchen, SONG Huihui, ZANG Bin, et al. Experimental study of Co(Ⅱ) additive on ammonia escape in carbon capture using renewable ammonia[J]. Chemical Engineering Journal, 2013, 234: 430-436. |
| 39 | KANG Min-Kyoung, JEON Soo-Bin, LEE Min-Ho, et al. Improvement in CO2 absorption and reduction of absorbent loss in aqueous NH3/triethanolamine/2-amino-2-methyl-1-propanol blends[J]. Korean Journal of Chemical Engineering, 2013, 30(6): 1171-1180. |
| 40 | CHU Fengming, GAO Qianhong, LI Shang, et al. Mass transfer characteristic of ammonia escape and energy penalty analysis in the regeneration process[J]. Applied Energy, 2020, 258: 113975. |
| 41 | DARDE Victor, THOMSEN Kaj, VAN WELL Willy J M, et al. Chilled ammonia process for CO2 capture[J]. Energy Procedia, 2009, 1(1): 1035-1042. |
| 42 | Rodrigo RIVERA-TINOCO, BOUALLOU Chakib. Comparison of absorption rates and absorption capacity of ammonia solvents with MEA and MDEA aqueous blends for CO2 capture[J]. Journal of Cleaner Production, 2010, 18(9): 875-880. |
| 43 | MOLINA Carol Toro, BOUALLOU Chakib. Assessment of different methods of CO2 capture in post-combustion using ammonia as solvent[J]. Journal of Cleaner Production, 2015, 103: 463-468. |
| 44 | JIANG Kaiqi, LI Kangkang, YU Hai, et al. Advancement of ammonia based post-combustion CO2 capture using the advanced flash stripper process[J]. Applied Energy, 2017, 202: 496-506. |
| 45 | OBEK Christine Ann, AYITTEY Foster Kofi, SAPTORO Agus. Improved process modifications of aqueous ammonia-based CO2 capture system[J]. MATEC Web of Conferences, 2019, 268: 02004. |
| 46 | ISHAQ Hafsa, Usman ALI, SHER Farooq, et al. Process analysis of improved process modifications for ammonia-based post-combustion CO2 capture[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104928. |
| 47 | MENG Fanzhi, MENG Yuan, JU Tongyao, et al. Research progress of aqueous amine solution for CO2 capture: A review[J]. Renewable and Sustainable Energy Reviews, 2022, 168: 112902. |
| 48 | OCHEDI Friday O, YU Jianglong, YU Hai, et al. Carbon dioxide capture using liquid absorption methods: A review[J]. Environmental Chemistry Letters, 2021, 19(1): 77-109. |
| 49 | HAMDY Louise B, Goel Chitrakshi, RUDD Jennifer A, et al. The application of amine-based materials for carbon capture and utilisation: An overarching view[J]. Materials Advances, 2021, 2(18): 5843-5880. |
| 50 | SAID Ridha BEN, KOLLE Joel Motaka, ESSALAH Khaled, et al. A unified approach to CO2–amine reaction mechanisms[J]. ACS Omega, 2020, 5(40): 26125-26133. |
| 51 | AGHEL Babak, JANATI Sara, WONGWISES Somchai, et al. Review on CO2 capture by blended amine solutions[J]. International Journal of Greenhouse Gas Control, 2022, 119: 103715. |
| 52 | MENG Fanzhi, JU Tongyao, HAN Siyu, et al. Novel low energy mixed amine biphasic solvent for carbon dioxide capture in biogas upgrading[J]. Separation and Purification Technology, 2023, 326: 124724. |
| 53 | WANG Rujie, LIU Shanshan, WANG Lidong, et al. Superior energy-saving splitter in monoethanolamine-based biphasic solvents for CO2 capture from coal-fired flue gas[J]. Applied Energy, 2019, 242: 302-310. |
| 54 | WANG Lemeng, TIAN Xiangfeng, FU D, et al. Experimental investigation on CO2 absorption capacity and viscosity for high concentrated 1-dimethylamino-2-propanol-monoethanolamine aqueous blends[J]. The Journal of Chemical Thermodynamics, 2019, 139: 105865. |
| 55 | ORANGI S, AROMADA S, NEDA Razi, et al. Simulation and economic analysis of MEA+PZ and MDEA+MEA blends in post-combustion CO2 capture plant[C]// Proceedings of SIMS EUROSIM 2021, Finland, 2021. |
| 56 | MAZARI Shaukat ALI, KANG Tae-Ha, DEVKOTA Sijan, et al. Investigating the effect of blending of diamine and alkanolamine for CO2 capture: Experiment and thermodynamic modeling of CO2-AEEA-DEA-H2O system[J]. Chemical Engineering Journal, 2023, 470(8): 144141. |
| 57 | LEE Woo Yun, PARK Sung Yeol, LEE Ki Bong, et al. Simultaneous removal of CO2 and H2S from biogas by blending amine absorbents: A performance comparison study[J]. Energy & Fuels, 2020, 34(2): 1992-2000. |
| 58 | HASSANKIADEH Mojtaba Nabipoor, JAHANGIRI Alireza. Application of aqueous blends of AMP and piperazine to the low CO2 partial pressure capturing: New experimental and theoretical analysis[J]. Energy, 2018, 165: 164-178. |
| 59 | SOBALA Katarzyna, Hanna KIERZKOWSKA-PAWLAK. Heat of absorption of CO2 in aqueous N,N-diethylethanolamine + N-methyl-1,3-propanediamine solutions at 313K[J]. Chinese Journal of Chemical Engineering, 2019, 27(3): 628-633. |
| 60 | LIU Ji, LI Xiaoshan, ZHANG Zewu, et al. Promotion of CO2 capture performance using piperazine (PZ) and diethylenetriamine (DETA) bi-solvent blends[J]. Greenhouse Gases: Science and Technology, 2019, 9(2): 349-359. |
| 61 | LIU Y, FAN W, WANG K, et al. Studies of CO2 absorption/regeneration performances of novel aqueous monothanlamine (MEA)-based solutions[J]. Journal of Cleaner Production, 2016, 112: 4012-4021. |
| 62 | ZHOU Xiaobin, JING Guohua, Bihong LYU, et al. Low-viscosity and efficient regeneration of carbon dioxide capture using a biphasic solvent regulated by 2-amino-2-methyl-1-propanol[J]. Applied Energy, 2019, 235: 379-390. |
| 63 | CONWAY William, BRUGGINK Stefan, BEYAD Yaser, et al. CO2 absorption into aqueous amine blended solutions containing monoethanolamine (MEA), N,N-dimethylethanolamine (DMEA), N,N-diethylethanolamine (DEEA) and 2-amino-2-methyl-1-propanol (AMP) for post-combustion capture processes[J]. Chemical Engineering Science, 2015, 126: 446-454. |
| 64 | HADRI Nabil EL, QUANG Dang Viet, GOETHEER Earl L V, et al. Aqueous amine solution characterization for post-combustion CO2 capture process[J]. Applied Energy, 2017, 185: 1433-1449. |
| 65 | WANG Nan, PENG Zhengqi, GAO Hongxia, et al. New insight and evaluation of secondary Amine/N-butanol biphasic solutions for CO2 Capture: Equilibrium Solubility, phase separation Behavior, absorption Rate, desorption Rate, energy consumption and ion species[J]. Chemical Engineering Journal, 2022, 431: 133912. |
| 66 | ISOGAI Hirotaka, NAKAGAKI Takao. Mechanistic analysis of post-combustion CO2 capture performance during amine degradation[J]. International Journal of Greenhouse Gas Control, 2022, 114: 103597. |
| 67 | LEPAUMIER Helene, PICQ Dominique, CARRETTE Pierre-Louis. New amines for CO2 capture. Ⅰ. Mechanisms of amine degradation in the presence of CO2 [J]. Industrial & Engineering Chemistry Research, 2009, 48(20): 9061-9067. |
| 68 | WANG Shuo, LONG Qinghai, SHEN Shufeng. Regulating phase change behaviors of water-lean absorbents containing potassium prolinate and 2-butoxyethanol for CO2 capture: Effect of water content[J]. Separation and Purification Technology, 2022, 301: 122059. |
| 69 | LIU Xiangwei, AO Qian, SHI Shengyou, et al. CO2 capture by alcohol ammonia based deep eutectic solvents with different water content[J]. Materials Research Express, 2022, 9(1): 015504. |
| 70 | GUO Hui, LI Chenxu, SHI Xiaoqin, et al. Nonaqueous amine-based absorbents for energy efficient CO2 capture[J]. Applied Energy, 2019, 239: 725-734. |
| 71 | HWANG Junhyeok, KIM Jeongnam, LEE Hee Won, et al. An experimental based optimization of a novel water lean amine solvent for post combustion CO2 capture process[J]. Applied Energy, 2019, 248: 174-184. |
| 72 | YE Jiexu, JIANG Chenkai, CHEN Han, et al. Novel biphasic solvent with tunable phase separation for CO2 capture: Role of water content in mechanism, kinetics, and energy penalty[J]. Environmental Science & Technology, 2019, 53(8): 4470-4479. |
| 73 | MENG Fanzhi, HAN Siyu, MENG Yuan, et al. A comparative study of the effects of aqueous mixed amines on biogas upgrading based on 13C nuclear magnetic resonance (NMR) analysis[J]. Journal of Cleaner Production, 2022, 369: 133288. |
| 74 | MOHAN Shaurya, UPRETI Namrata, VAIDYA Prakash D. Improved CO2 separation using aqueous solutions of 2-amino-2-hydroxymethyl-1,3-propanediol promoted with piperazine[J]. Energy & Fuels, 2023, 37(9): 6651-6660. |
| 75 | HE Xinwei, HE Hang, BARZAGLI Francesco, et al. Analysis of the energy consumption in solvent regeneration processes using binary amine blends for CO2 capture[J]. Energy, 2023, 270: 126903. |
| 76 | GAUTAM Ashish, KUMAR MONDAL Monoj. Post-combustion capture of CO2 using novel aqueous Triethylenetetramine and 2-Dimethylaminoethanol amine blend: Equilibrium CO2 loading-empirical model and optimization, CO2 desorption, absorption heat, and 13C NMR analysis[J]. Fuel, 2023, 331: 125864. |
| 77 | ZHANG Rui, LI Yufan, HE Xinwei, et al. Investigation of the improvement of the CO2 capture performance of aqueous amine sorbents by switching from dual-amine to trio-amine systems[J]. Separation and Purification Technology, 2023, 316: 123810. |
| 78 | JERNG Sung Eun, GALLANT Betar M. Electrochemical reduction of CO2 in the captured state using aqueous or nonaqueous amines[J]. iScience, 2022, 25(7): 104558. |
| 79 | Elena PÉREZ-GALLENT, VANKANI Chirag, Carlos SÁNCHEZ-MARTÍNEZ, et al. Integrating CO2 capture with electrochemical conversion using amine-based capture solvents as electrolytes[J]. Industrial & Engineering Chemistry Research, 2021, 60(11): 4269-4278. |
| 80 | CHEN Lu, LI Fengwang, ZHANG Ying, et al. Electrochemical reduction of carbon dioxide in a monoethanolamine capture medium[J]. ChemSusChem, 2017, 10(20): 4109-4118. |
| 81 | LEE Geonhui, LI Yuguang C, KIM Ji-Yong, et al. Electrochemical upgrade of CO2 from amine capture solution[J]. Nature Energy, 2020, 6(1): 46-53. |
| 82 | LANGIE Kezia Megagita Gerby, Kyungjae TAK, KIM Changsoo, et al. Toward economical application of carbon capture and utilization technology with near-zero carbon emission[J]. Nature Communications, 2022, 13: 7482. |
| 83 | SULLIVAN Ian, GORYACHEV Andrey, DIGDAYA Ibadillah A, et al. Coupling electrochemical CO2 conversion with CO2 capture[J]. Nature Catalysis, 2021, 4(11): 952-958. |
| 84 | HASAN Saman, ABBAS Abubakar Jibrin, NASR Ghasem Ghavami. Improving the carbon capture efficiency for gas power plants through amine-based absorbents[J]. Sustainability, 2020, 13(1): 72. |
| 85 | DANACI David, Mai BUI, PETIT Camille, et al. En route to zero emissions for power and industry with amine-based post-combustion capture[J]. Environmental Science & Technology, 2021, 55(15): 10619-10632. |
| 86 | FERON Paul H M, COUSINS Ashleigh, JIANG Kaiqi, et al. An update of the benchmark post-combustion CO2-capture technology[J]. Fuel, 2020, 273: 117776. |
| 87 | ROMEO Luis M, MINGUELL Diego, SHIRMOHAMMADI Reza, et al. Comparative analysis of the efficiency penalty in power plants of different amine-based solvents for CO2 capture[J]. Industrial & Engineering Chemistry Research, 2020, 59(21): 10082-10092. |
| 88 | MUMFORD Kathryn A, WU Yue, SMITH Kathryn H, et al. Review of solvent based carbon-dioxide capture technologies[J]. Frontiers of Chemical Science and Engineering, 2015, 9(2): 125-141. |
| 89 | CHAI Slyvester Yew Wang, Lock Hei NGU, Bing Shen HOW. Review of carbon capture absorbents for CO2 utilization[J]. Greenhouse Gases: Science and Technology, 2022, 12(3): 394-427. |
| 90 | CAI Yuanhao, WANG Weilin, LI Liang, et al. Effective capture of carbon dioxide using hydrated sodium carbonate powders[J]. Materials, 2018, 11(2): 183. |
| 91 | PARK Sang-Wook, SUNG Deok-Ho, CHOI Byoung-Sik, et al. Sorption of carbon dioxide onto sodium carbonate[J]. Separation Science and Technology, 2006, 41(12): 2665-2684. |
| 92 | KNUUTILA Hanna, JULIUSSEN Olav, SVENDSEN Hallvard F. Kinetics of the reaction of carbon dioxide with aqueous sodium and potassium carbonate solutions[J]. Chemical Engineering Science, 2010, 65(23): 6077-6088. |
| 93 | CAI Tianyi, CHEN Xiaoping, Karl JOHNSON J, et al. Understanding and improving the kinetics of bulk carbonation on sodium carbonate[J]. The Journal of Physical Chemistry C, 2020, 124(42): 23106-23115. |
| 94 | CAI Tianyi, Karl JOHNSON J, WU Ye, et al. Toward understanding the kinetics of CO2 capture on sodium carbonate[J]. ACS Applied Materials & Interfaces, 2019, 11(9): 9033-9041. |
| 95 | GUO Yincheng, NIU Zhenqi, LIN Wenyi. Comparison of removal efficiencies of carbon dioxide between aqueous ammonia and NaOH solution in a fine spray column[J]. Energy Procedia, 2011, 4: 512-518. |
| 96 | DINUL Fadhilah Ikhsan, NURDIN Hendri, RAHMADIAWAN Dieter, et al. Comparison of NaOH and Na2CO3 as absorbents for CO2 absorption in carbon capture and storage technology[J]. Journal of Engineering Researcher and Lecturer, 2023, 2(1): 28-34. |
| 97 | VALLURI Sriram, KAWATRA S K. Use of frothers to improve the absorption efficiency of dilute sodium carbonate slurry for post combustion CO2 capture[J]. Fuel Processing Technology, 2021, 212: 106620. |
| 98 | HORNBOSTEL K, NGUYEN D, BOURCIER W, et al. Packed and fluidized bed absorber modeling for carbon capture with micro-encapsulated sodium carbonate solution[J]. Applied Energy, 2019, 235: 1192-1204. |
| 99 | NASIMAN Tuerxun, KANOH Hirofumi. Preparation of the Na2CO3-carbon nanocomposite and its CO2 capture[J]. Energy & Fuels, 2018, 32(12): 12689-12694. |
| 100 | BARZAGLI F, GIORGI C, MANI F, et al. CO2 capture by aqueous Na2CO3 integrated with high-quality CaCO3 formation and pure CO2 release at room conditions[J]. Journal of CO2 Utilization, 2017, 22: 346-354. |
| 101 | WU Ying, CHEN Xiaoping, MA Jiliang, et al. System integration optimization for coal-fired power plant with CO2 capture by Na2CO3 dry sorbents[J]. Energy, 2020, 211: 118554. |
| 102 | BONAVENTURA D, CHACARTEGUI R, VALVERDE J M, et al. Dry carbonate process for CO2 capture and storage: Integration with solar thermal power[J]. Renewable and Sustainable Energy Reviews, 2018, 82: 1796-1812. |
| 103 | ROGERS Robin D, VOTH Gregory A. Ionic liquids[J]. Accounts of Chemical Research, 2007, 40(11): 1077-1078. |
| 104 | LIAN Shaohan, SONG Chunfeng, LIU Qingling, et al. Recent advances in ionic liquids-based hybrid processes for CO2 capture and utilization[J]. Journal of Environmental Sciences, 2021, 99: 281-295. |
| 105 | PAN M, CAO N, LIN W, et al. Reversible CO2 capture by conjugated ionic liquids through dynamic covalent carbon-oxygen bonds[J]. ChemSusChem, 2016, 9(17): 2351-2357. |
| 106 | YOON Bohak, VOTH Gregory A. Elucidating the molecular mechanism of CO2 capture by amino acid ionic liquids[J]. Journal of the American Chemical Society, 2023, 145(29): 15663-15667. |
| 107 | Bihong LYU, JING Guohua, QIAN Yuhao, et al. An efficient absorbent of amine-based amino acid-functionalized ionic liquids for CO2 capture: High capacity and regeneration ability[J]. Chemical Engineering Journal, 2016, 289: 212-218. |
| 108 | FAISAL ELMOBARAK Wamda, ALMOMANI Fares, TAWALBEH Muhammad, et al. Current status of CO2 capture with ionic liquids: Development and progress[J]. Fuel, 2023, 344: 128102. |
| 109 | AVILA Jocasta, Fernando LEPRE L, SANTINI Catherine C, et al. High-performance porous ionic liquids for low-pressure CO2 capture[J]. Angewandte Chemie, 2021, 133(23): 12986-12992. |
| 110 | LAN Youshi, YAN Tongan, TONG Minman, et al. Large-scale computational assembly of ionic liquid/MOF composites: Synergistic effect in the wire-tube conformation for efficient CO2/CH4 separation[J]. Journal of Materials Chemistry A, 2019, 7(20): 12556-12564. |
| 111 | LI Xueqin, CHEN Kai, GUO Ruili, et al. Ionic liquids functionalized MOFs for adsorption[J]. Chemical Reviews, 2023, 123(16): 10432-10467. |
| 112 | BUDZIANOWSKI, WOJCIECH M. Energy efficient solvents for CO2 capture by gas-liquid absorption:compounds,blends and advanced solvent systems[M]. Poland: Springer, 2016: 16-17. |
| 113 | ZHANG Shihan, SHEN Yao, WANG Lidong, et al. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges[J]. Applied Energy, 2019, 239: 876-897. |
| 114 | PAPADOPOULOS Athanasios I, TZIRAKIS Fragkiskos, TSIVINTZELIS Ioannis, et al. Phase-change solvents and processes for postcombustion CO2 capture: A detailed review[J]. Industrial & Engineering Chemistry Research, 2019, 58(13): 5088-5111. |
| 115 | HU Liang. CO2 capture from flue gas by phase transitional absorption[R]. Hampton Univ., Hampton, VA (United States), 2009. |
| 116 | 符乐, 杨阳, 徐文青, 等. 新型相变有机胺吸收捕集CO2技术研究进展[J]. 化工进展, 2023, 42(4): 2068-2080. |
| FU Le, YANG Yang, XU Wenqing, et al. Research progress in CO2 capture technology using novel biphasic organic amine absorbent[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2068-2080. | |
| 117 | BIAN Yangyang, SHEN Shufeng. CO2 absorption into a phase change absorbent: Water-lean potassium prolinate/ethanol solution[J]. Chinese Journal of Chemical Engineering, 2018, 26(11): 2318-2326. |
| 118 | YOO Miran, HAN Sangjun, Jung-Ho WEE. Carbon dioxide capture capacity of sodium hydroxide aqueous solution[J]. Journal of Environmental Management, 2013, 114: 512-519. |
| 119 | HAN Sangjun, YOO Miran, KIM Dong-Woo, et al. Carbon dioxide capture using calcium hydroxide aqueous solution as the absorbent[J]. Energy & Fuels, 2011, 25(8): 3825-3834. |
| 120 | RASTEGAR Zahra, GHAEMI Ahad. CO2 absorption into potassium hydroxide aqueous solution: Experimental and modeling[J]. Heat and Mass Transfer, 2022, 58(3): 365-381. |
| 121 | Raktim SEN, GOEPPERT Alain, KAR Sayan, et al. Hydroxide based integrated CO2 capture from air and conversion to methanol[J]. Journal of the American Chemical Society, 2020, 142(10): 4544-4549. |
| 122 | SPIGARELLI Brett P, Komar KAWATRA S. Opportunities and challenges in carbon dioxide capture[J]. Journal of CO2 Utilization, 2013, 1: 69-87. |
| 123 | BORHANI Tohid N, WANG Meihong. Role of solvents in CO2 capture processes: The review of selection and design methods[J]. Renewable and Sustainable Energy Reviews, 2019, 114: 109299. |
| [1] | 陆诗建, 张娟娟, 杨菲, 刘玲, 陈思铭, 康国俊, 房芹芹. 化学吸收法胺液逃逸控制技术研究进展[J]. 化工进展, 2024, 43(8): 4562-4570. |
| [2] | 狄子琛, 雷飞霞, 常成功, 陈文慧, 程芳琴. 焦化行业碳氢资源利用潜力与低碳路径评价[J]. 化工进展, 2024, 43(5): 2862-2871. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [5] | 舒斌, 陈建宏, 熊健, 吴其荣, 喻江涛, 杨平. 碳中和目标下推动绿色甲醇发展的必要性分析[J]. 化工进展, 2023, 42(9): 4471-4478. |
| [6] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [7] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [8] | 陆诗建, 张媛媛, 吴文华, 杨菲, 刘玲, 康国俊, 李清方, 陈宏福, 王宁, 王风, 张娟娟. 百万吨级CO2捕集项目亚硝胺污染物扩散健康风险评估[J]. 化工进展, 2023, 42(6): 3209-3216. |
| [9] | 桑伟, 唐建峰, 花亦怀, 陈洁, 孙培源, 许义飞. 物理溶剂及有机胺的性质对相变吸收性能的影响[J]. 化工进展, 2023, 42(4): 2151-2159. |
| [10] | 符乐, 杨阳, 徐文青, 耿錾卜, 朱廷钰, 郝润龙. 新型相变有机胺吸收捕集CO2技术研究进展[J]. 化工进展, 2023, 42(4): 2068-2080. |
| [11] | 李文秀, 杨宇航, 黄艳, 王涛, 王镭, 方梦祥. 二氧化碳矿化高钙基固废制备微细碳酸钙研究进展[J]. 化工进展, 2023, 42(4): 2047-2057. |
| [12] | 孔祥如, 张肖阳, 孙鹏翔, 崔琳, 董勇. 直接空气捕碳固体多孔材料的研究进展[J]. 化工进展, 2023, 42(3): 1471-1483. |
| [13] | 孙晖, 孟祥海, 魏景海, 周红军, 徐春明. 绿电制氢生产氨的新场景与实践[J]. 化工进展, 2023, 42(2): 1098-1102. |
| [14] | 胡鹏, 赵丹, 纪红兵. 温控构筑仿生契合辨识机制强化合成气提纯[J]. 化工进展, 2023, 42(12): 6133-6135. |
| [15] | 曹明敏, 韩铖乐, 杨芳, 陈玉焕. 离子液体/金属有机框架复合材料捕集分离CO2[J]. 化工进展, 2023, 42(11): 5831-5841. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||