化工进展 ›› 2023, Vol. 42 ›› Issue (12): 6507-6517.DOI: 10.16085/j.issn.1000-6613.2023-0180
• 资源与环境化工 • 上一篇
天然气藏中酸气H2S与CO2协同转化研究进展
于姗1,2( ), 张洪华1,2, 付梦瑶1,2, 段元刚1,2, 段超1,2, 黄靖元1,2, 唐春1,2, 黄泽皑1,2, 周莹1,2(
), 张洪华1,2, 付梦瑶1,2, 段元刚1,2, 段超1,2, 黄靖元1,2, 唐春1,2, 黄泽皑1,2, 周莹1,2( )
)
- 1.油气藏地质及开发工程国家重点实验室,四川 成都 610500
2.西南石油大学新能源与材料学院,四川 成都 610500
-
收稿日期:2023-02-13修回日期:2023-04-15出版日期:2023-12-25发布日期:2024-01-08 -
通讯作者:于姗,周莹 -
作者简介:于姗(1986—),女,博士,副教授,硕士生导师,研究方向为天然气资源的清洁利用。E-mail:yushan@swpu.edu.cn。 -
基金资助:国家自然科学基金(22002123);四川省科技厅项目(2022YFSY0052)
Research progress on synergistic conversion of acid gas H2S and CO2 in natural gas
YU Shan1,2( ), ZHANG Honghua1,2, FU Mengyao1,2, DUAN Yuangang1,2, DUAN Chao1,2, HUANG Jingyuan1,2, TANG Chun1,2, HUANG Ze’ai1,2, ZHOU Ying1,2(
), ZHANG Honghua1,2, FU Mengyao1,2, DUAN Yuangang1,2, DUAN Chao1,2, HUANG Jingyuan1,2, TANG Chun1,2, HUANG Ze’ai1,2, ZHOU Ying1,2( )
)
- 1.State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Chengdu 610500, Sichuan, China
2.School of New Energy and Materials, Southwest Petroleum University, Chengdu 610500, Sichuan, China
-
Received:2023-02-13Revised:2023-04-15Online:2023-12-25Published:2024-01-08 -
Contact:YU Shan, ZHOU Ying
摘要:
为保障国家能源安全,减少我国对国外油气资源的依存度,必须加大对国内天然气等资源的开发。在天然气的开采净化过程中,往往会产生酸气H2S与CO2等。现有酸气处理技术主要通过克劳斯工艺回收得到H2S中的硫黄,并未对CO2进行处理,造成了氢资源的浪费和严重的碳排放。如果能将H2S与CO2协同转化,则有望在减少碳排放的同时得到氢气、合成气和硫黄高值化学品。本文基于国内外30多年相关领域的实验研究和理论模拟,总结阐述了H2S与CO2协同转化的发展历史,并分别从热反应(直接热反应、工艺流程和经济性评估、催化热分解)、光催化、电催化和等离子体催化角度详细综述了H2S与CO2协同转化的研究进展。从催化剂、反应条件和反应产物分布等方面展开了细致的分析,对比了各种技术的优缺点。展望了H2S与CO2协同转化的发展趋势,近期可考虑使用绿电进行电催化技术的小规模应用示范;同时可以考虑通过太阳光储热的方式减小热催化技术的碳排放,或通过太阳能聚光等方式提升光催化技术的催化效率等。此外,在后续研究中,必须结合酸性气藏的特点,考虑不同的H2S/CO2比例或是H2O等杂质分子对反应过程的影响。
中图分类号:
引用本文
于姗, 张洪华, 付梦瑶, 段元刚, 段超, 黄靖元, 唐春, 黄泽皑, 周莹. 天然气藏中酸气H2S与CO2协同转化研究进展[J]. 化工进展, 2023, 42(12): 6507-6517.
YU Shan, ZHANG Honghua, FU Mengyao, DUAN Yuangang, DUAN Chao, HUANG Jingyuan, TANG Chun, HUANG Ze’ai, ZHOU Ying. Research progress on synergistic conversion of acid gas H2S and CO2 in natural gas[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6507-6517.
| 反应体系 | CO2转化率 /% | H2S转化率 /% | CO选择性 /% | S2选择性 /% | H2选择性 /% |
|---|---|---|---|---|---|
| 热力学平衡值 | 26.5 | 33.9 | 69.7 | 76.1 | 21.5 |
| 空白实验 | 1.7 | 2.2 | 15.1 | 18.2 | 1.4 |
| SiO2 | 4.6 | 4.8 | 42.7 | 42.8 | 4.0 |
| CaO | 26.0 | 26.4 | 53.9 | 57.4 | 14.6 |
| γ-Al2O3 | 24.3 | 24.8 | 51.1 | 56.6 | 14.5 |
| NiO/γ-Al2O3 | 24.8 | 27.6 | 52.7 | 56.7 | 22.1 |
| NiO/MgO | 25.6 | 27.9 | 53.3 | 58.1 | 16.6 |
| MgO | 25.2 | 25.4 | 50.2 | 51.5 | 8.2 |
表1 1073K、100000h-1空速条件下,V(H2S)∶V(CO2)∶V(N2)=3∶3∶14时,使用不同催化剂时H2S与CO2的转换率以及各种产物的选择性(均为摩尔分数)[18]
| 反应体系 | CO2转化率 /% | H2S转化率 /% | CO选择性 /% | S2选择性 /% | H2选择性 /% |
|---|---|---|---|---|---|
| 热力学平衡值 | 26.5 | 33.9 | 69.7 | 76.1 | 21.5 |
| 空白实验 | 1.7 | 2.2 | 15.1 | 18.2 | 1.4 |
| SiO2 | 4.6 | 4.8 | 42.7 | 42.8 | 4.0 |
| CaO | 26.0 | 26.4 | 53.9 | 57.4 | 14.6 |
| γ-Al2O3 | 24.3 | 24.8 | 51.1 | 56.6 | 14.5 |
| NiO/γ-Al2O3 | 24.8 | 27.6 | 52.7 | 56.7 | 22.1 |
| NiO/MgO | 25.6 | 27.9 | 53.3 | 58.1 | 16.6 |
| MgO | 25.2 | 25.4 | 50.2 | 51.5 | 8.2 |
| 催化剂 | 反应产物生成速率(以催化剂面积计)/mmol·m-2 | |||
|---|---|---|---|---|
| H2 | CO | SO2 | S2 | |
| FeS | 0.889 | 0.027 | 0.025 | 0.252 |
| RuO2 | 0.009 | 0.001 | 0.007 | 0.003 |
| MoS2 | 0.014 | 0.070 | 0.004 | 0.014 |
| Nb2O5 | 0.011 | 0.093 | 0.028 | 0.074 |
| IrO2 | 0.027 | 0.003 | 0.001 | 0.012 |
| V2O5 | 0.141 | 0.210 | 0.013 | 0.730 |
| CdS | 0.005 | 0.017 | 0.001 | 0.017 |
| PbS | 0.037 | 0.054 | 0.023 | 0.001 |
| Mn3O4 | 0.038 | 0.065 | 0.011 | 0.199 |
| CoS | 0.217 | 0.143 | 0.069 | 0.001 |
| NiS | 1.502 | 0.285 | 0.051 | 0.001 |
| WS2 | 0.032 | 0.019 | 0.005 | 0.001 |
表2 873K下V(H2S)∶V(CO2)∶V(H2O)=60∶60∶1时不同催化剂5h内催化H2S与CO2协同转化的效率[25]
| 催化剂 | 反应产物生成速率(以催化剂面积计)/mmol·m-2 | |||
|---|---|---|---|---|
| H2 | CO | SO2 | S2 | |
| FeS | 0.889 | 0.027 | 0.025 | 0.252 |
| RuO2 | 0.009 | 0.001 | 0.007 | 0.003 |
| MoS2 | 0.014 | 0.070 | 0.004 | 0.014 |
| Nb2O5 | 0.011 | 0.093 | 0.028 | 0.074 |
| IrO2 | 0.027 | 0.003 | 0.001 | 0.012 |
| V2O5 | 0.141 | 0.210 | 0.013 | 0.730 |
| CdS | 0.005 | 0.017 | 0.001 | 0.017 |
| PbS | 0.037 | 0.054 | 0.023 | 0.001 |
| Mn3O4 | 0.038 | 0.065 | 0.011 | 0.199 |
| CoS | 0.217 | 0.143 | 0.069 | 0.001 |
| NiS | 1.502 | 0.285 | 0.051 | 0.001 |
| WS2 | 0.032 | 0.019 | 0.005 | 0.001 |
| 反应体系 | 产率/µmol·g-1·h-1 | |||
|---|---|---|---|---|
| 气相产物 | 液相产物 | |||
| CH4 | CO | H2 | HCOOH | |
| 纯水溶液无CO2 | — | — | — | — |
| 纯水溶液 | 0.3 | 0.3 | 0.6 | — |
| NaOH(100mmol/L) | — | 0.3 | — | — |
| NaCl(500mmol/L) | — | 0.8 | 1.8 | — |
| NaBr(500mmol/L) | — | 0.5 | 0.7 | — |
| Na2S(100mmol/L) | — | 2.8 | 209.4 | 25.7 |
表3 Cu-TiO2在不同反应溶质中的光催化CO2还原产率[39]
| 反应体系 | 产率/µmol·g-1·h-1 | |||
|---|---|---|---|---|
| 气相产物 | 液相产物 | |||
| CH4 | CO | H2 | HCOOH | |
| 纯水溶液无CO2 | — | — | — | — |
| 纯水溶液 | 0.3 | 0.3 | 0.6 | — |
| NaOH(100mmol/L) | — | 0.3 | — | — |
| NaCl(500mmol/L) | — | 0.8 | 1.8 | — |
| NaBr(500mmol/L) | — | 0.5 | 0.7 | — |
| Na2S(100mmol/L) | — | 2.8 | 209.4 | 25.7 |
| 处理工艺 | 碳税/每吨CO2 | ||||
|---|---|---|---|---|---|
| 0 | 10 | 25 | 50 | 100 | |
| MDEA + LO-CAT | 0.00948 | 0.0124 | 0.0168 | 0.0242 | 0.0389 |
| 双塔络合法脱硫 | 0.01103 | 0.0140 | 0.0184 | 0.0258 | 0.0405 |
| 干法脱硫 | 0.06636 | 0.0693 | 0.0737 | 0.0811 | 0.0958 |
| 电催化系统(50mA/cm2,商用电) | 0.01123 | 0.01123 | 0.01123 | 0.01123 | 0.01123 |
| 电催化系统(50mA/cm2,绿电) | -0.02285 | -0.02285 | -0.02285 | -0.02285 | -0.02285 |
表4 常用处理工艺和H2S与CO2电催化系统估算的每立方米气体运行成本[54]
| 处理工艺 | 碳税/每吨CO2 | ||||
|---|---|---|---|---|---|
| 0 | 10 | 25 | 50 | 100 | |
| MDEA + LO-CAT | 0.00948 | 0.0124 | 0.0168 | 0.0242 | 0.0389 |
| 双塔络合法脱硫 | 0.01103 | 0.0140 | 0.0184 | 0.0258 | 0.0405 |
| 干法脱硫 | 0.06636 | 0.0693 | 0.0737 | 0.0811 | 0.0958 |
| 电催化系统(50mA/cm2,商用电) | 0.01123 | 0.01123 | 0.01123 | 0.01123 | 0.01123 |
| 电催化系统(50mA/cm2,绿电) | -0.02285 | -0.02285 | -0.02285 | -0.02285 | -0.02285 |
| 处理工艺 | 优势 | 劣势 |
|---|---|---|
| 直接热转化技术 | 经济性评估体系完善,工艺改造成本低 | 能耗高 |
| 热催化技术 | 处理量大,工艺改造成本低 | 能耗较高,反应过程认识不明确 |
| 光催化技术 | 能量来源广,绿色无污染 | 转化效率低,产物复杂,机理研究不充分 |
| 电催化技术 | 反应条件温和,活性高 | 电极材料成本高,工艺开发不够完善,机理研究不充分 |
| 等离子体催化技术 | 操作简单,装置体积小,能量效率高 | 湿度、反应气体纯度影响大、能耗较高 |
表5 H2S与CO2协同转化技术对比
| 处理工艺 | 优势 | 劣势 |
|---|---|---|
| 直接热转化技术 | 经济性评估体系完善,工艺改造成本低 | 能耗高 |
| 热催化技术 | 处理量大,工艺改造成本低 | 能耗较高,反应过程认识不明确 |
| 光催化技术 | 能量来源广,绿色无污染 | 转化效率低,产物复杂,机理研究不充分 |
| 电催化技术 | 反应条件温和,活性高 | 电极材料成本高,工艺开发不够完善,机理研究不充分 |
| 等离子体催化技术 | 操作简单,装置体积小,能量效率高 | 湿度、反应气体纯度影响大、能耗较高 |
| 1 | 黄黎明. 高含硫气藏安全清洁高效开发技术新进展[J]. 天然气工业, 2015, 35(4): 1-6. |
| HUANG Liming. New progresses in safe, clean and efficient development technologies for high-sulfur gas reservoirs[J]. Natural Gas Industry, 2015, 35(4): 1-6. | |
| 2 | 张婧, 张铁, 孙峰, 等. 硫化氢直接分解制取氢气和硫黄研究进展[J]. 化工进展, 2017, 36(4): 1448-1459. |
| ZHANG Jing, ZHANG Tie, SUN Feng, et al. Research progress on hydrogen and sulfur production from direct decomposition of hydrogen sulfide[J]. Chemical Industry and Engineering Progress, 2017, 36(4): 1448-1459. | |
| 3 | FELLMUTH P, LUTZ W, BÜLOW M. Influence of weakly coordinated cations and basic sites upon the reaction of H2S and CO2 on zeolites[J]. Zeolites, 1987, 7(4): 367-371. |
| 4 | LUTZ W, SEIDEL A, BODDENBERG B. On the formation of COS from H2S and CO2 in the presence of zeolite/salt compounds[J]. Adsorption Science & Technology, 1998, 16(7): 577-581. |
| 5 | BOWMAN M G. Thermochemical cycle for splitting hydrogen sulfide: US4999178[P]. 1991-03-12. |
| 6 | TOWLER G P, LYNN S. Development of a zero-emissions sulfur-recovery process. 1. Thermochemistry and reaction kinetics of mixtures of hydrogen sulfide and carbon dioxide at high temperature[J]. Industrial & Engineering Chemistry Research, 1993, 32(11): 2800-2811. |
| 7 | TOWLER G P, LYNN S. Development of a zero-emissions sulfur-recovery process. 2. Sulfur-recovery process based on the reactions of hydrogen sulfide and carbon dioxide at high temperature[J]. Industrial & Engineering Chemistry Research, 1993, 32(11): 2812-2819. |
| 8 | TOWLER G P, LYNN S. Sulfur recovery with reduced emissions, low capital investment and hydrogen co-production[J]. Chemical Engineering Communications, 1996, 155(1): 113-143. |
| 9 | BASSANI A, MANENTI F, RANZI E, et al. Novel coal gasification process: Improvement of syngas yield and cut of emissions[J]. Chemical Engineering Transactions, 2015, 43: 1483-1488. |
| 10 | MANENTI. CO2 as feedstock: a new pathway to syngas[M]. Computer Aided Chemical Engineering, 2015, 37: 1049-1054. |
| 11 | BASSANI A, PIROLA C, MAGGIO E, et al. Acid Gas to Syngas (AG2S™) technology applied to solid fuel gasification: Cutting H2S and CO2 emissions by improving syngas production[J]. Applied Energy, 2016, 184: 1284-1291. |
| 12 | EL-MELIH A M, IBRAHIM S, GUPTA A K, et al. Experimental examination of syngas recovery from acid gases[J]. Applied Energy, 2016, 164: 64-68. |
| 13 | SELIM H, IBRAHIM S, SHOAIBI A AL, et al. Investigation of sulfur chemistry with acid gas addition in hydrogen/air flames[J]. Applied Energy, 2014, 113: 1134-1140. |
| 14 | IBRAHIM S, RAJ A. Kinetic simulation of acid gas (H2S and CO2) destruction for simultaneous syngas and sulfur recovery[J]. Industrial & Engineering Chemistry Research, 2016, 55(24): 6743-6752. |
| 15 | 闫天兰, 闫玥儿, 张亚红, 等. 二硫化钼加氢脱硫催化剂研究进展[J]. 复旦学报(自然科学版), 2022, 61(6): 683-698. |
| YAN Tianlan, YAN Yue’er, ZHANG Yahong, et al. Research progress of MoS2 hydrodesulfurization catalysts[J]. Journal of Fudan University (Natural Science), 2022, 61(6): 683-698. | |
| 16 | SORIANO D, KEENER T C, KHANG Soon-Jai. Catalytic production of elemental sulfur from the thermal decomposition of H2S in the presence of CO2 [J]. Chemical Engineering Communications, 1996, 143(1): 73-89. |
| 17 | MALOKA I, ALIWI S, NAMAN S. Thermal reduction of CO2 in the presence of H2S[J]. Petroleum Science and Technology, 2006, 24(1): 117-127. |
| 18 | SU Hui, LI Yuyang, LI Ping, et al. Simultaneous recovery of carbon and sulfur resources from reduction of CO2 with H2S using catalysts[J]. Journal of Energy Chemistry, 2016, 25(1): 110-116. |
| 19 | MA Jun, SUN Nannan, ZHANG Xuelan, et al. A short review of catalysis for CO2 conversion[J]. Catalysis Today, 2009, 148(3/4): 221-231. |
| 20 | SHI Chunkai, ZHANG Peng. Role of MgO over γ-Al2O3-supported Pd catalysts for carbon dioxide reforming of methane[J]. Applied Catalysis B: Environmental, 2015, 170/171: 43-52. |
| 21 | OMAE I. Aspects of carbon dioxide utilization[J]. Catalysis Today, 2006, 115(1/2/3/4): 33-52. |
| 22 | TAIRA K. Dry reforming reactions of CH4 over CeO2/MgO catalysts at high concentrations of H2S, and behavior of CO2 at the CeO2-MgO interface[J]. Journal of Catalysis, 2022, 407: 29-43. |
| 23 | LIU Xiaozhan, ZHAO Lu, LI Ying, et al. Ni-Mo sulfide semiconductor catalyst with high catalytic activity for one-step conversion of CO2 and H2S to syngas in non-thermal plasma[J]. Catalysts, 2019, 9(6): 525. |
| 24 | KARAN K, MEHROTRA A K, BEHIE L A. A high-temperature experimental and modeling study of homogeneous gas-phase COS reactions applied to Claus plants[J]. Chemical Engineering Science, 1999, 54(15/16): 2999-3006. |
| 25 | ALDERMAN N P, PENEAU V, VIASUS C J, et al. Syn-gas from waste: The reduction of CO2 with H2S[J]. Reaction Chemistry & Engineering, 2019, 4(4): 763-771. |
| 26 | ZHANG Fenglian, WEI Zheng, JIANG Guoxia, et al. Synergistic conversion of acid gases (H2S and CO2) to valuable chemicals: Carbonyl sulfide synthesis over vacancy-defective CoMo sulfide catalysts[J]. Applied Catalysis B: Environmental, 2022, 319: 121912. |
| 27 | FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38. |
| 28 | BORGARELLO E, KALYANASUNDARAM K, GRÄTZEL M, et al. Visible light induced generation of hydrogen from H2S in CdS-dispersions, hole transfer catalysis by RuO2 [J]. Helvetica Chimica Acta, 1982, 65(1): 243-248. |
| 29 | DAN Meng, YU Shan, LI Yi, et al. Hydrogen sulfide conversion: How to capture hydrogen and sulfur by photocatalysis[J]. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 2020, 42: 100339. |
| 30 | DAN Meng, ZHANG Qian, YU Shan, et al. Noble-metal-free MnS/In2S3 composite as highly efficient visible light driven photocatalyst for H2 production from H2S[J]. Applied Catalysis B: Environmental, 2017, 217: 530-539. |
| 31 | DAN Meng, WEI Shiqian, DORONKIN D E, et al. Novel MnS/(In x Cu1- x )2S3 composite for robust solar hydrogen sulphide splitting via the synergy of solid solution and heterojunction[J]. Applied Catalysis B: Environmental, 2019, 243: 790-800. |
| 32 | YU Shan, FAN Xiangbing, WANG Xian, et al. Efficient photocatalytic hydrogen evolution with ligand engineered all-inorganic InP and InP/ZnS colloidal quantum dots[J]. Nature Communications, 2018, 9(1): 4009. |
| 33 | YU Shan, WU Fan, ZOU Pengkun, et al. Highly value-added utilization of H2S in Na2SO3 solution over Ca-CdS nanocrystal photocatalysts[J]. Chemical Communications, 2020, 56(91): 14227-14230. |
| 34 | DAN Meng, XIANG Jianglai, YANG Jian, et al. Beyond hydrogen production: Solar-driven H2S-donating value-added chemical production over Mn x Cd1- x S/Cd y Mn1- y S catalyst[J]. Applied Catalysis B: Environmental, 2021, 284: 119706. |
| 35 | 淡猛, 蔡晴, 向将来, 等. 用于光催化分解硫化氢制氢的金属硫化物[J]. 化学进展, 2020, 32(7): 917-926. |
| DAN Meng, CAI Qing, XIANG Jianglai, et al. Metal sulfide semiconductors for photocatalytic hydrogen production from waste hydrogen sulfide[J]. Progress in Chemistry, 2020, 32(7): 917-926. | |
| 36 | LI Xin, YU Jiaguo, JARONIEC M, et al. Cocatalysts for selective photoreduction of CO2 into solar fuels[J]. Chemical Reviews, 2019, 119(6): 3962-4179. |
| 37 | WANG Yi’ou, CHEN Enqi, TANG Junwang. Insight on reaction pathways of photocatalytic CO2 conversion[J]. ACS Catalysis, 2022, 12(12): 7300-7316. |
| 38 | LIN Huiwen, LUO Shunqin, ZHANG Huabin, et al. Toward solar-driven carbon recycling[J]. Joule, 2022, 6(2): 294-314. |
| 39 | NAVARRO-JAÉN S, VIRGINIE M, BONIN J, et al. Highlights and challenges in the selective reduction of carbon dioxide to methanol[J]. Nature Reviews Chemistry, 2021, 5(8): 564-579. |
| 40 | TRAN Duyen P H, PHAM Minh-Thuan, Xuan-Thanh BUI, et al. CeO2 as a photocatalytic material for CO2 conversion: A review[J]. Solar Energy, 2022, 240: 443-466. |
| 41 | 郭立行, 庞蔚莹, 马克遥, 等. 层序空间多孔结构TiO2实现高效光催化CO2还原[J]. 化工进展, 2023, 42(7): 3643-3651. |
| GUO Lixing, PANG Weiying, MA Keyao, et al. Hierarchically multilayered TiO2 with spatial pore-structure for efficient photocatalytic CO2 reduction[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3643-3651. | |
| 42 | CAO Yuehan, GUO Lan, DAN Meng, et al. Modulating electron density of vacancy site by single Au atom for effective CO2 photoreduction[J]. Nature Communications, 2021, 12(1): 1-10. |
| 43 | BERTON M, MELLO R, GONZÁLEZ-NÚÑEZ M E. Iodide-photocatalyzed reduction of carbon dioxide to formic acid with thiols and hydrogen sulfide[J]. ChemSusChem, 2016, 9(24): 3397-3400. |
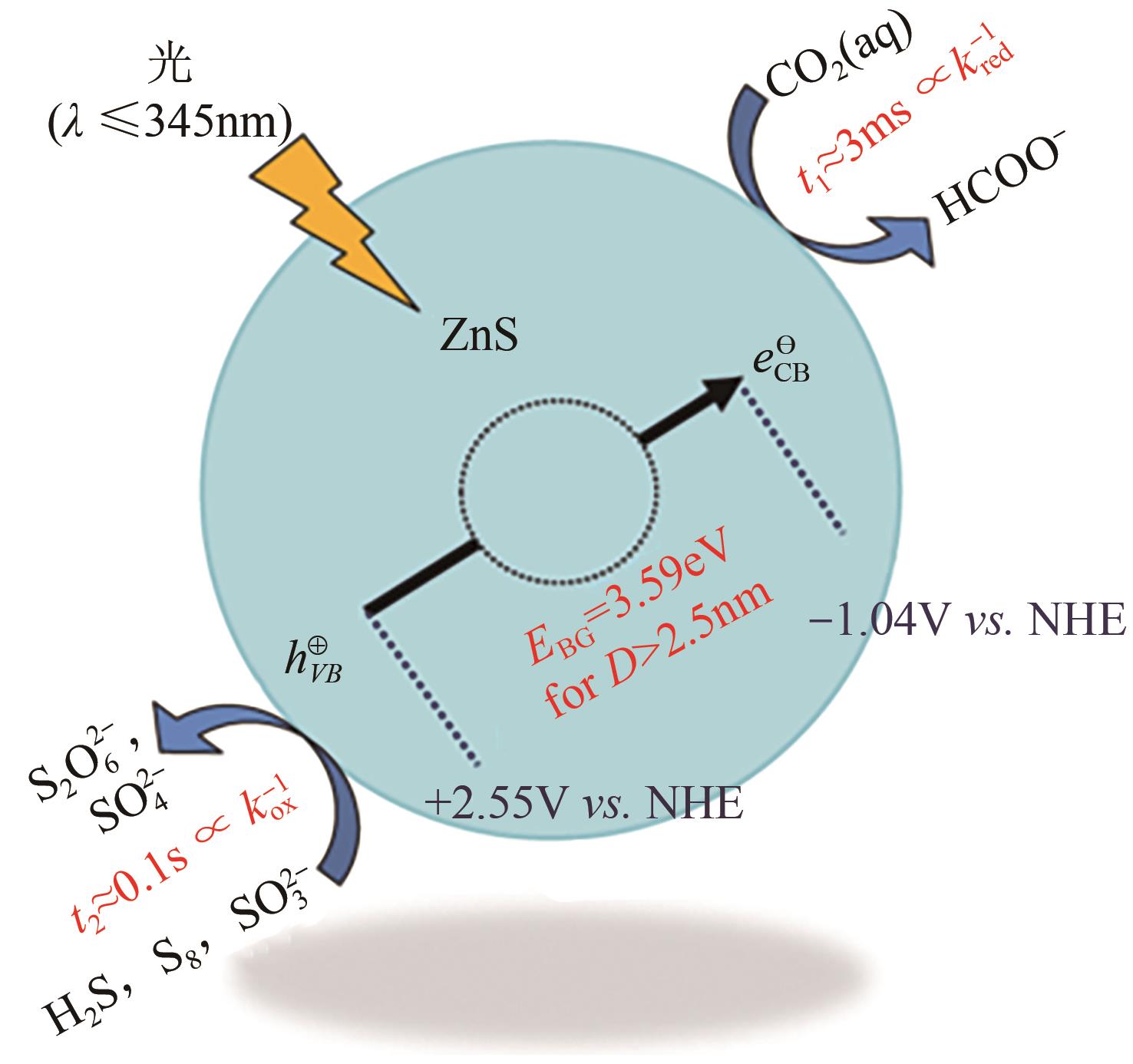
| 44 | ZHOU Ruixin, GUZMAN M I. CO2 reduction under periodic illumination of ZnS[J]. The Journal of Physical Chemistry C, 2014, 118(22): 11649-11656. |
| 45 | GONELL F, PUGA A V, JULIÁN-LÓPEZ B, et al. Copper-doped titania photocatalysts for simultaneous reduction of CO2 and production of H2 from aqueous sulfide[J]. Applied Catalysis B: Environmental, 2016, 180: 263-270. |
| 46 | LI Kai, CAI Yanming, YANG Xiaohan, et al. H2S involved photocatalytic system: A novel syngas production strategy by boosting the photoreduction of CO2 while recovering hydrogen from the environmental toxicant[J]. Advanced Functional Materials, 2022, 32(20): 2113002. |
| 47 | 段超, 唐春, 吴梦南, 等. 电催化分解硫化氢制氢脱硫研究进展[J]. 天然气化工(C1化学与化工), 2021, 46(S1): 24-30. |
| DUAN Chao, TANG Chun, WU Mengnan, et al. Progress in electrocatalytic decomposition of hydrogen sulfide to hydrogen and sulfur[J]. Natural Gas Chemical Industry, 2021, 46(S1): 24-30. | |
| 48 | DUAN Chao, TANG Chun, YU Shan, et al. Efficient electrocatalytic desulfuration and synchronous hydrogen evolution from H2S via anti-sulfuretted NiSe nanowire array catalyst[J]. Applied Catalysis B: Environmental, 2023, 324: 122255. |
| 49 | ZHANG Mo, GUAN Jing, TU Yunchuan, et al. Highly efficient H2 production from H2S via a robust graphene-encapsulated metal catalyst[J]. Energy & Environmental Science, 2020, 13(1): 119-126. |
| 50 | WANG Genxiang, CHEN Junxiang, DING Yichun, et al. Electrocatalysis for CO2 conversion: From fundamentals to value-added products[J]. Chemical Society Reviews, 2021, 50(8): 4993-5061. |
| 51 | 彭仁杰, 周继承, 罗羽裳, 等. 硫化氢分解制取氢和单质硫研究进展[J]. 天然气化工(C1化学与化工), 2015, 40(1): 89-94. |
| PENG Renjie, ZHOU Jicheng, LUO Yushang, et al. Research progress in hydrogen and sulfur production from hydrogen sulfide[J]. Natural Gas Chemical Industry, 2015, 40(1): 89-94. | |
| 52 | 华亚妮, 冯少广, 党欣悦, 等. CO2电催化还原产合成气研究进展[J]. 化工进展, 2022, 41(3): 1224-1240. |
| HUA Yani, FENG Shaoguang, DANG Xinyue, et al. Research progress of CO2 electrocatalytic reduction to syngas[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1224-1240. | |
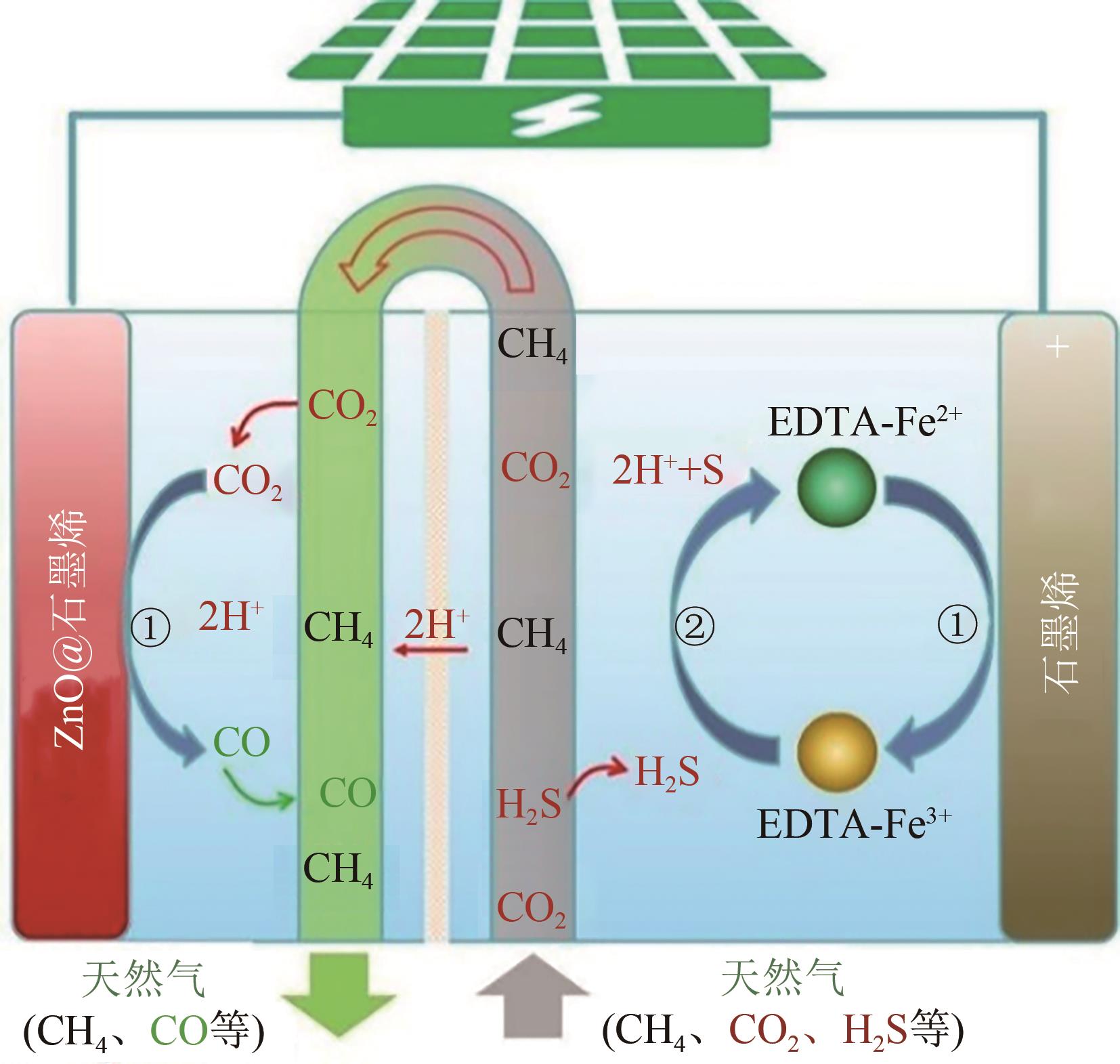
| 53 | MA Weiguang, WANG Hong, YU Wei, et al. Achieving simultaneous CO2 and H2S conversion via a coupled solar-driven electrochemical approach on non-precious-metal catalysts[J]. Angewandte Chemie International Edition, 2018, 57(13): 3473-3477. |
| 54 | ZHANG Bo, BAI Jing, ZHANG Yan, et al. High yield of CO and synchronous S recovery from the conversion of CO2 and H2S in natural gas based on a novel electrochemical reactor[J]. Environmental Science & Technology, 2021, 55(21): 14854-14862. |
| 55 | FU Xianzhong, LI Jie, PAN Xinrong, et al. A single microbial electrochemical system for CO2 reduction and simultaneous biogas purification, upgrading and sulfur recovery[J]. Bioresource Technology, 2020, 297: 122448. |
| 56 | 王乾浩, 赵璐, 孙付琳, 等. ZSM-5催化剂与低温等离子体协同转化H2S-CO2制合成气[J]. 化工学报, 2022, 73(1): 255-265. |
| WANG Qianhao, ZHAO Lu, SUN Fulin, et al. Production of syngas derived from H2S-CO2 via synergy of ZSM-5 catalyst and non-thermal plasma[J]. CIESC Journal, 2022, 73(1): 255-265. | |
| 57 | ZHAO Lu, LIU Xiaozhan, MU Xiaoliang, et al. Highly selective conversion of H2S-CO2 to syngas by combination of non-thermal plasma and MoS2/Al2O3 [J]. Journal of CO2 Utilization, 2020, 37: 45-54. |
| 58 | GUTSOL K, NUNNALLY T, RABINOVICH A, et al. Plasma assisted dissociation of hydrogen sulfide[J]. International Journal of Hydrogen Energy, 2012, 37(2): 1335-1347. |
| 59 | ZHAO Guibing, JOHN Sanil, ZHANG Jijun, et al. Production of hydrogen and sulfur from hydrogen sulfide in a nonthermal-plasma pulsed corona discharge reactor[J]. Chemical Engineering Science, 2007, 62(8): 2216-2227. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 徐晨阳, 都健, 张磊. 基于图神经网络的化学反应优劣评价[J]. 化工进展, 2023, 42(S1): 205-212. |
| [3] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [4] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [5] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [6] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [7] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [8] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [9] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [10] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [11] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [12] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [13] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [14] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [15] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||