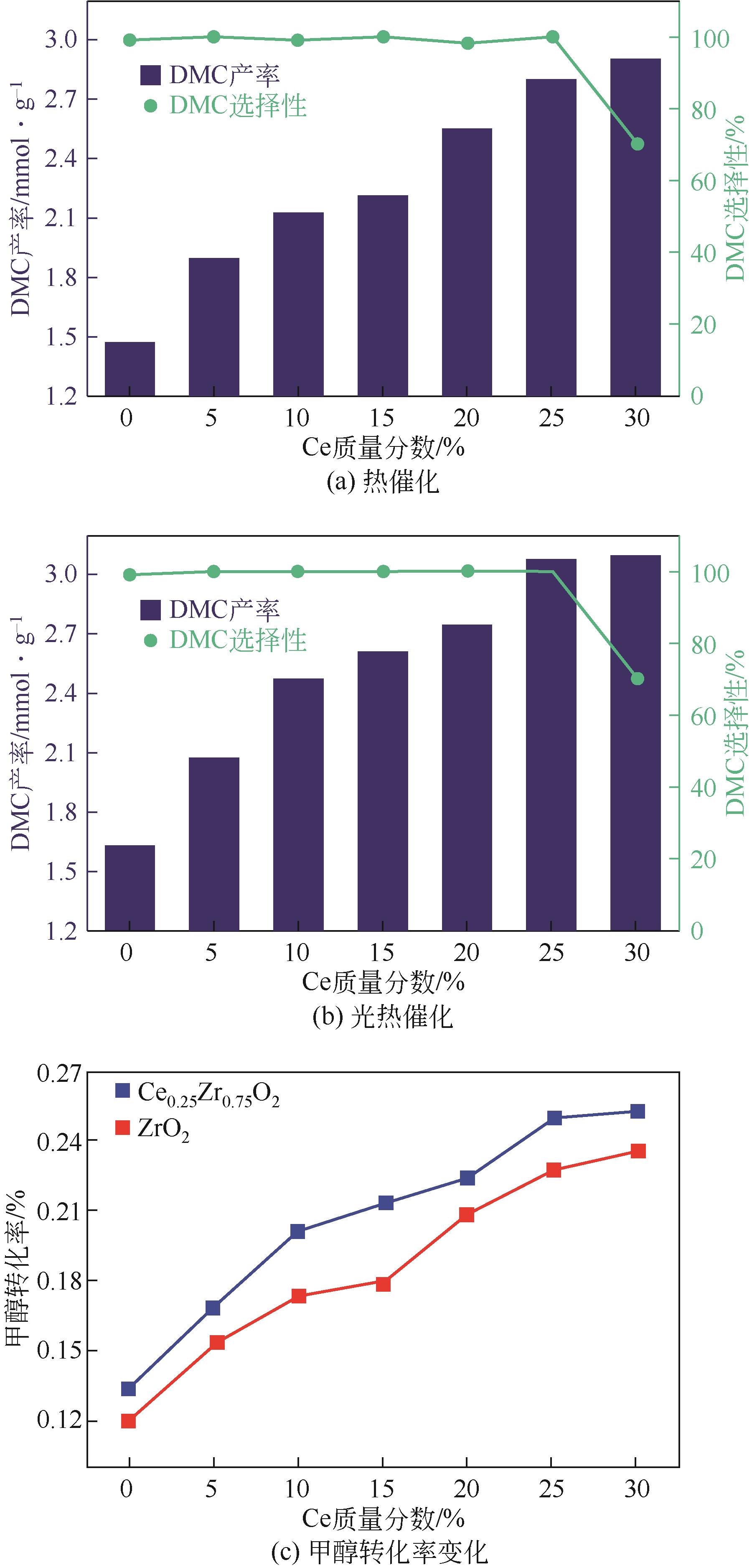
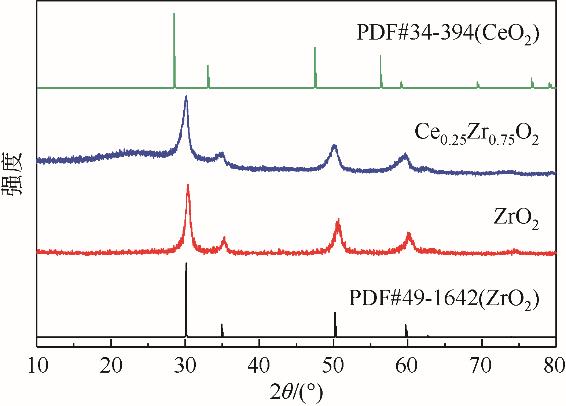
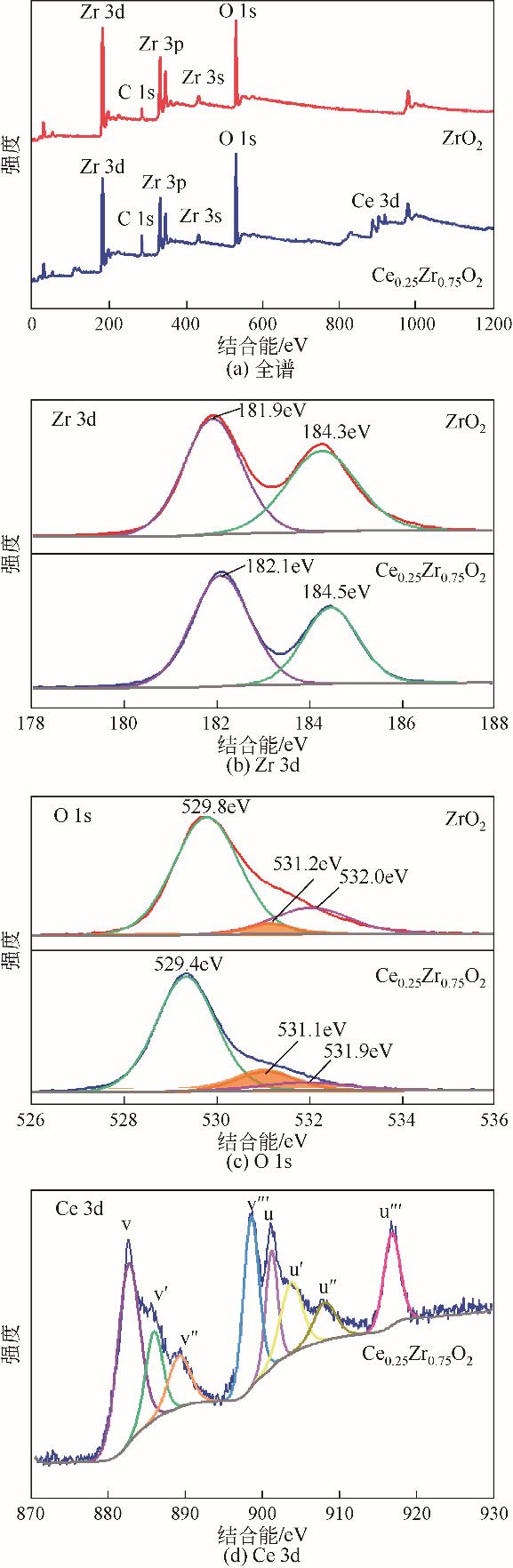
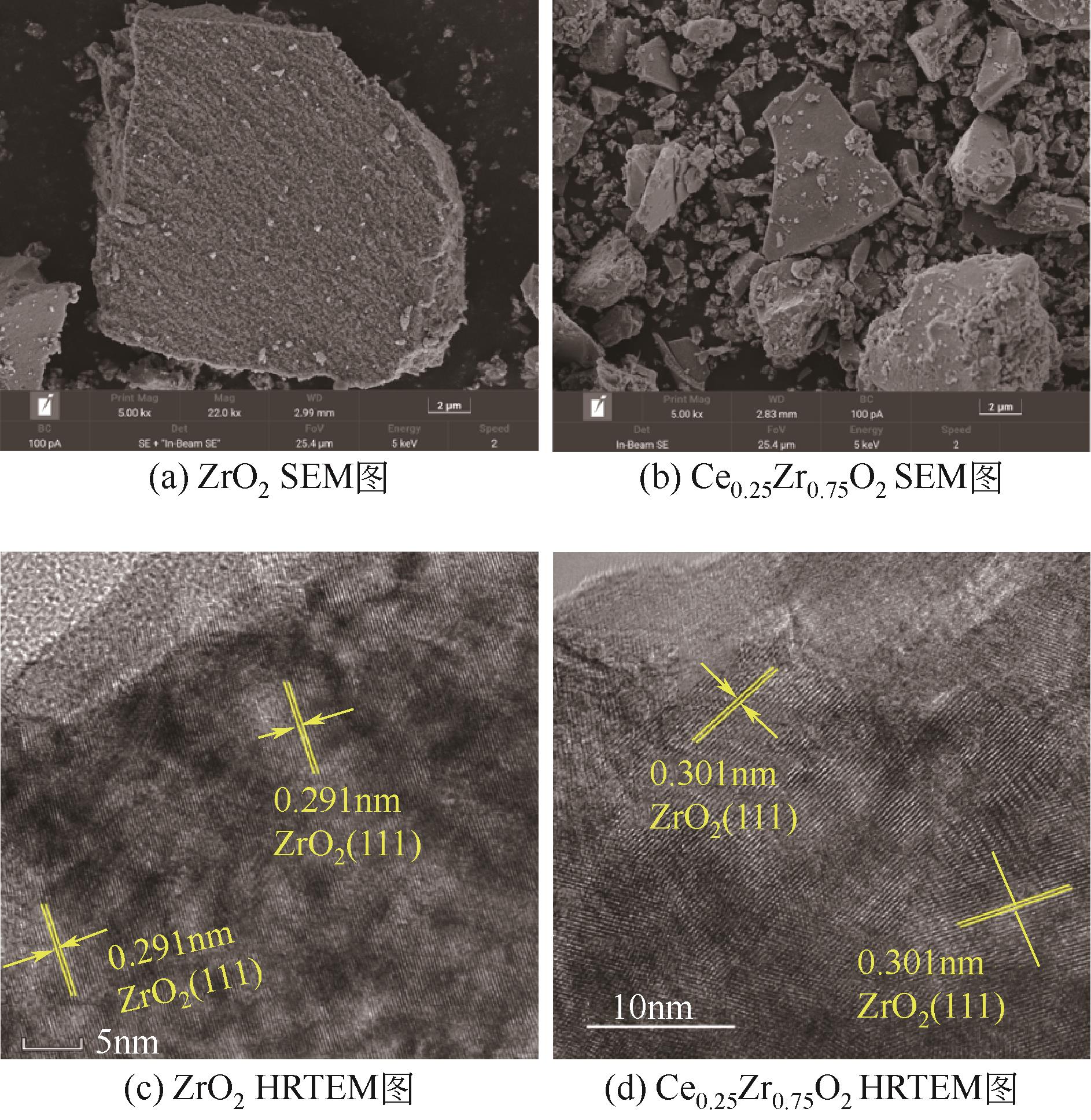
| 1 |
LIU Kangli, ZHANG Xiaochao, ZHANG Changming, et al. Enhanced photocatalytic reduction of CO2 to CO over BiOBr assisted by phenolic resin-based activated carbon spheres[J]. RSC Advances, 2019, 9(25): 14391-14399.
|
| 2 |
SANTOS B A, SILVA V M, LOUREIRO J M, et al. Review for the direct synthesis of dimethyl carbonate[J]. ChemBioEng. Reviews, 2014, 1(5): 214-229.
|
| 3 |
LIU Kai, LIU Chun. Synthesis of dimethyl carbonate from methanol and CO2 under low pressure[J]. RSC Advances, 2021, 11(57): 35711-35717.
|
| 4 |
ZHAO Tiansheng, HAN Yizhuo, SUN Yuhan. Novel reaction route for dimethyl carbonate synthesis from CO2 and methanol[J]. Fuel Processing Technology, 2000, 62(2/3): 187-194.
|
| 5 |
SONG Yu, HE Xing, YU Bing, et al. Protic ionic liquid-promoted synthesis of dimethyl carbonate from ethylene carbonate and methanol[J]. Chinese Chemical Letters, 2020, 31(3): 667-672.
|
| 6 |
SHI Dichao, SVETLANA Heyte, Capron MICKAËL, et al. Catalytic processes for the direct synthesis of dimethyl carbonate from CO2 and methanol: A review[J]. Green Chemistry, 2022, 24(3): 1067-1089.
|
| 7 |
KUENEN H J, MENGERS H J, NIJMEIJER D C, et al. Techno-economic evaluation of the direct conversion of CO2 to dimethyl carbonate using catalytic membrane reactors[J]. Computers & Chemical Engineering, 2016, 86: 136-147.
|
| 8 |
天津大学物理化学教研室. 物理化学(上册)[M]. 5版. 北京: 高等教育出版社, 2009.
|
|
Physical Chemistry Teaching and Research Office of Tianjin University. Physical chemistry[M]. 5th ed. Beijing: Higher Education Press, 2009.
|
| 9 |
WANG X J, XIAO M, WANG S J, et al. Direct synthesis of dimethyl carbonate from carbon dioxide and methanol using supported copper (Ni, V, O) catalyst with photo-assistance[J]. Journal of Molecular Catalysis A: Chemical, 2007, 278(1/2): 92-96.
|
| 10 |
孔令丽, 钟顺和, 柳荫. Cu/NiO-MoO3/SiO2光催化CO2与CH3OH合成碳酸二甲酯的反应性能[J]. 催化学报, 2005, 26(10): 917-922.
|
|
KONG Lingli, ZHONG Shunhe, LIU Yin. Photocatalytic reaction for synthesis of dimethyl carbonate from CO2 and CH3OH over Cu/NiO-MoO3/SiO2 catalyst[J]. Chinese Journal of Catalysis, 2005, 26(10): 917-922.
|
| 11 |
CHEN Shichuan, WANG Hui, KANG Zhixiong, et al. Oxygen vacancy associated single-electron transfer for photofixation of CO2 to long-chain chemicals[J]. Nature Communications, 2019, 10: 788.
|
| 12 |
SANTOS B A, PEREIRA C S, SILVA V M, et al. Kinetic study for the direct synthesis of dimethyl carbonate from methanol and CO2 over CeO2 at high pressure conditions[J]. Applied Catalysis A: General, 2013, 455: 219-226.
|
| 13 |
TOMISHIGE K, SAKAIHORI T, IKEDA Y, et al. A novel method of direct synthesis of dimethyl carbonate from methanol and carbon dioxide catalyzed by zirconia[J]. Catalysis Letters, 1999, 58(4): 225-229.
|
| 14 |
BAI Jiaqi, Lingling LYU, LIU Jiuyi, et al. Control of CeO2 defect sites for photo- and thermal- synergistic catalysis of CO2 and methanol to DMC[J]. Catalysis Letters, 2022: 1-10.
|
| 15 |
XU Chong, ZHOU Qin, HUANG Weiya, et al. Constructing Z-scheme β-Bi2O3/ZrO2 heterojunctions with 3D mesoporous SiO2 nanospheres for efficient antibiotic remediation via synergistic adsorption and photocatalysis[J]. Rare Metals, 2022, 41(6): 2094-2107.
|
| 16 |
WANG Qing, KAVEH Edalati, YUTA Koganemaru, et al. Photocatalytic hydrogen generation on low-bandgap black zirconia (ZrO2) produced by high-pressure torsion[J]. Journal of Materials Chemistry A, 2020, 8(7): 3643-3650.
|
| 17 |
CHEN Shiping, LI Xin, LIN Liqin, et al. Carbon template synthesis of CeO2 catalyst for direct conversion of methanol and carbon dioxide to dimethyl carbonate[J]. Journal of the Energy Institute, 2023, 107: 101190.
|
| 18 |
FU Zhongwei, YU Yuehong, LI Zhen, et al. Surface reduced CeO2 nanowires for direct conversion of CO2 and methanol to dimethyl carbonate: Catalytic performance and role of oxygen vacancy[J]. Catalysts, 2018, 8(4): 164.
|
| 19 |
TOMASZEWSKI Henryk, GODWOD Krzysztof. Influence of oxygen partial pressure on the metastability of undoped zirconia dispersed in alumina matrix[J]. Journal of the European Ceramic Society, 1995, 15(1): 17-23.
|
| 20 |
LIU Bin, LI Congming, ZHANG Guoqiang, et al. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-doped CeO2 nanorods[J]. ACS Catalysis, 2018, 8(11): 10446-10456.
|
| 21 |
LING Yang, TAN Shaoqing, WANG Daolei, et al. An experimental and DFT study on enhanced elemental mercury removal performance via cerium chloride modified carbon aerogel: A synergistic effect between chemical adsorption and thermal catalysis[J]. Chemical Engineering Journal, 2021, 425: 127344.
|
| 22 |
TAO Xueyu, MA Jie, HOU Ruilin, et al. Template-free synthesis of star-like ZrO2 nanostructures and their application in photocatalysis[J]. Advances in Materials Science and Engineering, 2018, 2018: 1-10.
|
| 23 |
LIU Bin, LI Congming, ZHANG Guoqiang, et al. Direct synthesis of dimethyl carbonate from CO2 and methanol over CaO-CeO2 catalysts: The role of acid-base properties and surface oxygen vacancies[J]. New Journal of Chemistry, 2017, 41(20): 12231-12240.
|
| 24 |
ZHU Chengzhang, WEI Xiaoqian, LI Wanqin, et al. Crystal-plane effects of CeO2{110} and CeO2{100} on photocatalytic CO2 reduction: Synergistic interactions of oxygen defects and hydroxyl groups[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(38): 14397-14406.
|
| 25 |
YANG Min, SHEN Genli, WANG Qi, et al. Roles of oxygen vacancies of CeO2 and Mn-doped CeO2 with the same morphology in benzene catalytic oxidation[J]. Molecules, 2021, 26(21): 6363.
|
| 26 |
MI Shu, XIE Yong, LI Yuanyuan, et al. The effect of thickness-tunable ZrO2 shell on enhancing the tunneling magnetoresistance of Fe3O4 supraparticles[J]. Advanced Materials Interfaces, 2018, 5(12): 1800236.
|
| 27 |
SHA Feng, TANG Shan, TANG Chizhou, et al. The role of surface hydroxyls on ZnZrO x solid solution catalyst in CO2 hydrogenation to methanol[J]. Chinese Journal of Catalysis, 2023, 45: 162-173.
|
| 28 |
PIUMETTI Marco, BENSAID Samir, RUSSO Nunzio, et al. Investigations into nanostructured ceria-zirconia catalysts for soot combustion[J]. Applied Catalysis B: Environmental, 2016, 180: 271-282.
|
| 29 |
林忻, 何小波, 银凤翔, 等. Ce0.25Zr0.75O2固溶体的制备及其电化学合成氨催化性能[J]. 北京化工大学学报(自然科学版), 2020, 47(4): 9-15.
|
|
LIN Xin, HE Xiaobo, YIN Fengxiang, et al. Catalytic performance of Ce0.25Zr0.75O2 solid solution in the electrochemical synthesis of ammonia[J]. Journal of Beijing University of Chemical Technology (Natural Science Edition), 2020, 47(4): 9-15.
|
| 30 |
REDDY C V, NEELAKANTA R I, RAVINDRANADH K, et al. Ni-dopant concentration effect of ZrO2 photocatalyst on photoelectrochemical water splitting and efficient removal of toxic organic pollutants[J]. Separation and Purification Technology, 2020, 252: 117352.
|
| 31 |
ZHANG Xiaochao, XUE Tingting, ZHANG Changming, et al. In situ synthesis of hydrangea finch coral-like Bi12SiO20 film with highly effective photocatalytic CO2 reduction performance[J]. ACS Applied Energy Materials, 2021, 4(1): 15-19.
|
| 32 |
FAKHRI Ali, BEHROUZ Sajjad, TYAGI Inderjeet, et al. Synthesis and characterization of ZrO2 and carbon-doped ZrO2 nanoparticles for photocatalytic application[J]. Journal of Molecular Liquids, 2016, 216: 342-346.
|
| 33 |
GUAN Xiushuai, ZHANG Xiaochao, ZHANG Changming, et al. In situ hydrothermal synthesis of metallic Bi self-deposited Bi2SiO5 with enhanced photocatalytic CO2 reduction performance[J]. Solar RRL, 2022, 6(9): 2200346.
|
| 34 |
RADHA G, SAMARESH L, DEVENDRA D P. Synthesis and characterization of guanine-functionalized mesoporous silica[SBA-16-G]: A metal-free and recyclable heterogeneous solid base catalyst for synthesis of pyran-annulated heterocyclic compounds[J]. Research on Chemical Intermediates, 2019, 45(3): 1619-1637.
|
| 35 |
LI Yingxuan, WEN Miaomiao, WANG Ying, et al. Plasmonic hot electrons from oxygen vacancies for infrared light-driven catalytic CO2 reduction on Bi2O3- x [J]. Angewandte Chemie (International Ed in English), 2021, 60(2): 910-916.
|
| 36 |
WANG Min, SHEN Meng, JIN Xixiong, et al. Mild generation of surface oxygen vacancies on CeO2 for improved CO2 photoreduction activity[J]. Nanoscale, 2020, 12(23): 12374-12382.
|
| 37 |
WANG Yaqi, ZHANG Xiaochao, ZHANG Changming, et al. Novel Bi4Ti3O12 hollow-spheres with highly-efficient CO2 photoreduction activity[J]. Inorganic Chemistry Communications, 2020, 116: 107931.
|
| 38 |
IKEDA Yoshiki, ASADULLAH Mohammad, FUJIMOTO Kaoru, et al. Structure of the active sites on H3PO4/ZrO2 catalysts for dimethyl carbonate synthesis from methanol and carbon dioxide[J]. The Journal of Physical Chemistry B, 2001, 105(43): 10653-10658.
|
 ), 官修帅1, 靳山彪1, 张长明2, 张小超1(
), 官修帅1, 靳山彪1, 张长明2, 张小超1( )
)
 ), GUAN Xiushuai1, JIN Shanbiao1, ZHANG Changming2, ZHANG Xiaochao1(
), GUAN Xiushuai1, JIN Shanbiao1, ZHANG Changming2, ZHANG Xiaochao1( )
)