化工进展 ›› 2023, Vol. 42 ›› Issue (6): 3281-3291.DOI: 10.16085/j.issn.1000-6613.2022-1519
猪粪厌氧消化进程中重金属与腐殖质的有机结合机制
- 常州大学环境科学与工程学院,江苏 常州 213164
-
收稿日期:2022-08-17修回日期:2022-10-04出版日期:2023-06-25发布日期:2023-06-29 -
通讯作者:张文艺 -
作者简介:庄捷(1998—),男,硕士研究生,研究方向为固体废弃物污染控制。E-mail:375834214@qq.com。 -
基金资助:江苏省科技支撑计划(BE2020761);2021年江苏省研究生科研与实践创新计划(SJCX21-0942)
Organic binding mechanism of heavy metals and humus during anaerobic digestion of pig manure
ZHUANG Jie( ), XUE Jinhui, ZHAO Bincheng, ZHANG Wenyi(
), XUE Jinhui, ZHAO Bincheng, ZHANG Wenyi( )
)
- School of Environmental Science and Engineering, Changzhou University, Changzhou 213164, Jiangsu, China
-
Received:2022-08-17Revised:2022-10-04Online:2023-06-25Published:2023-06-29 -
Contact:ZHANG Wenyi
摘要:
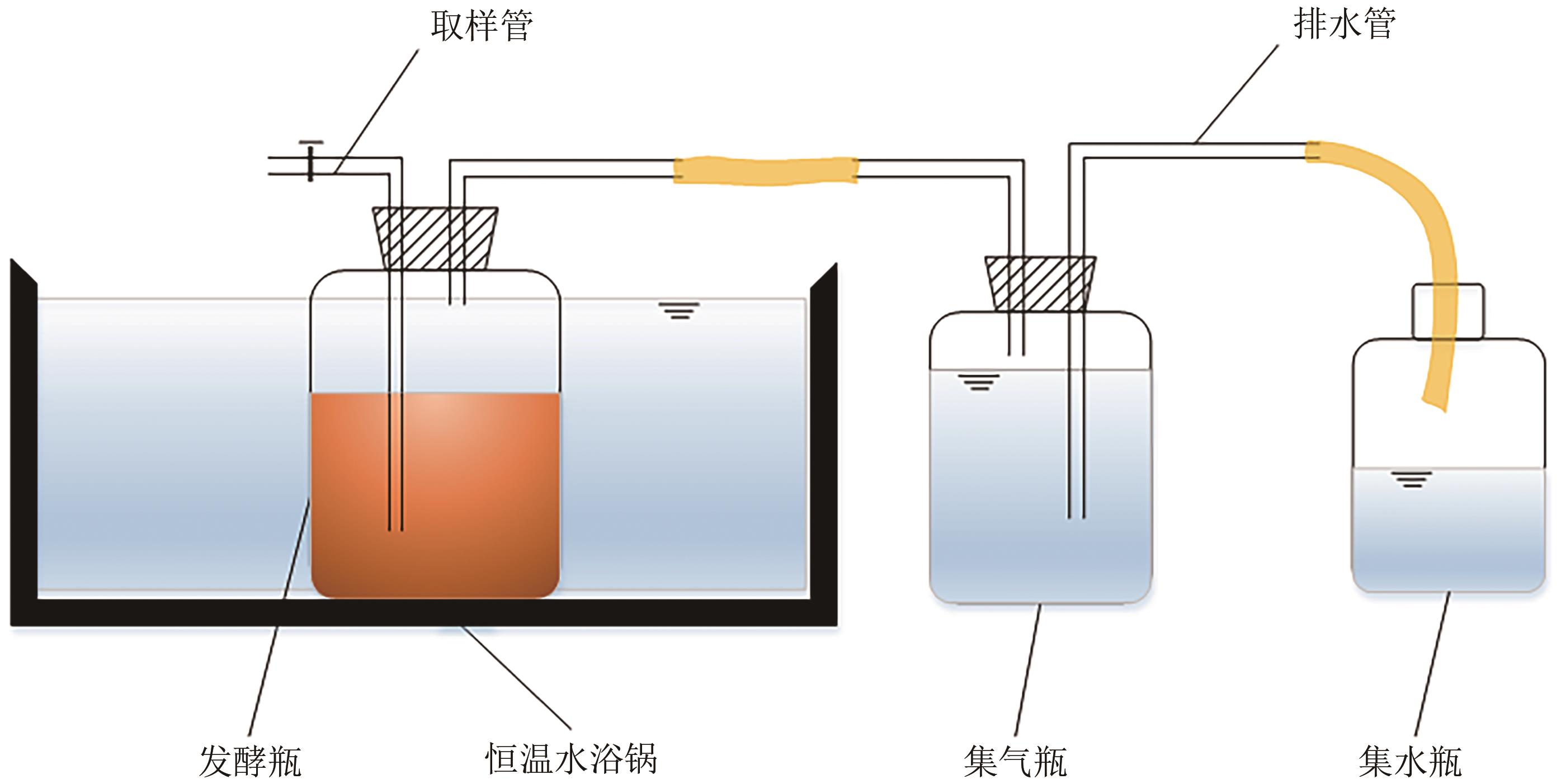
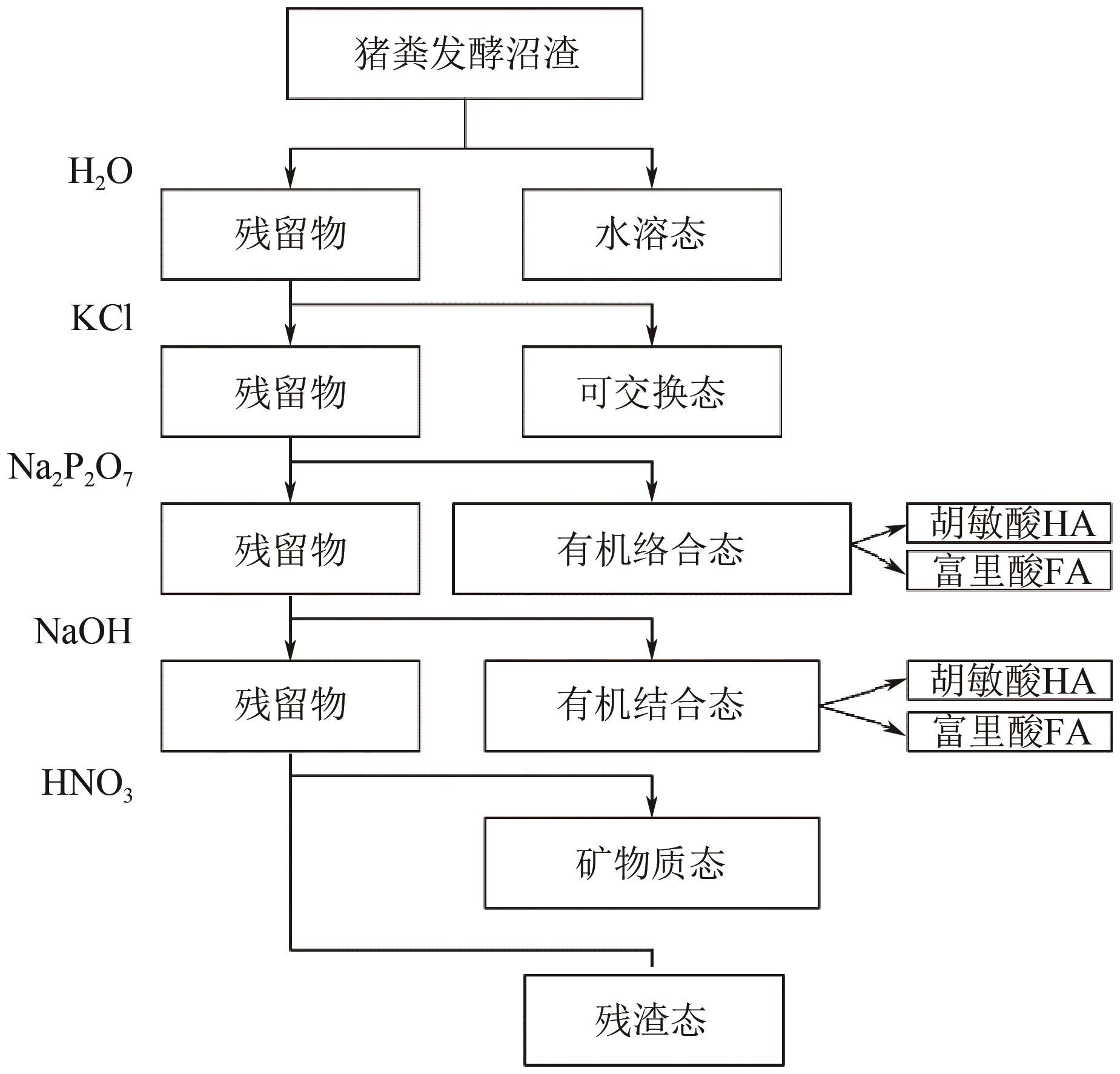
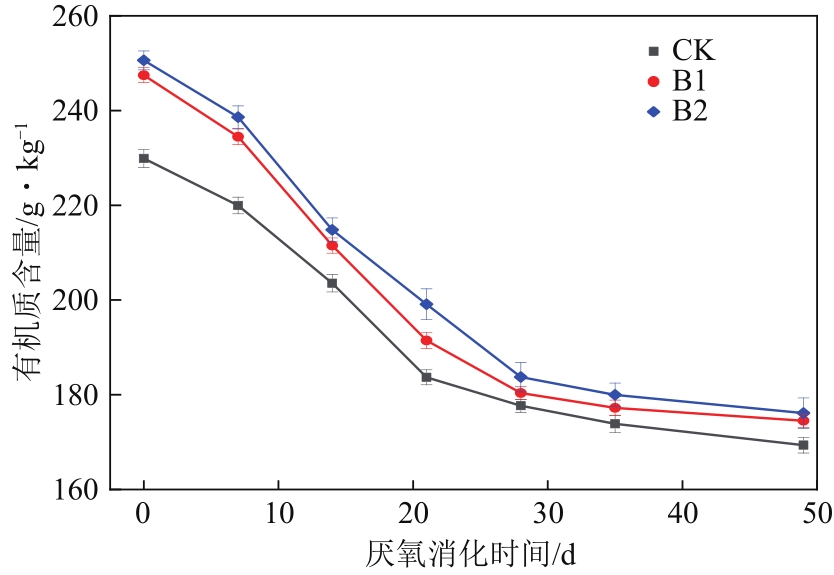
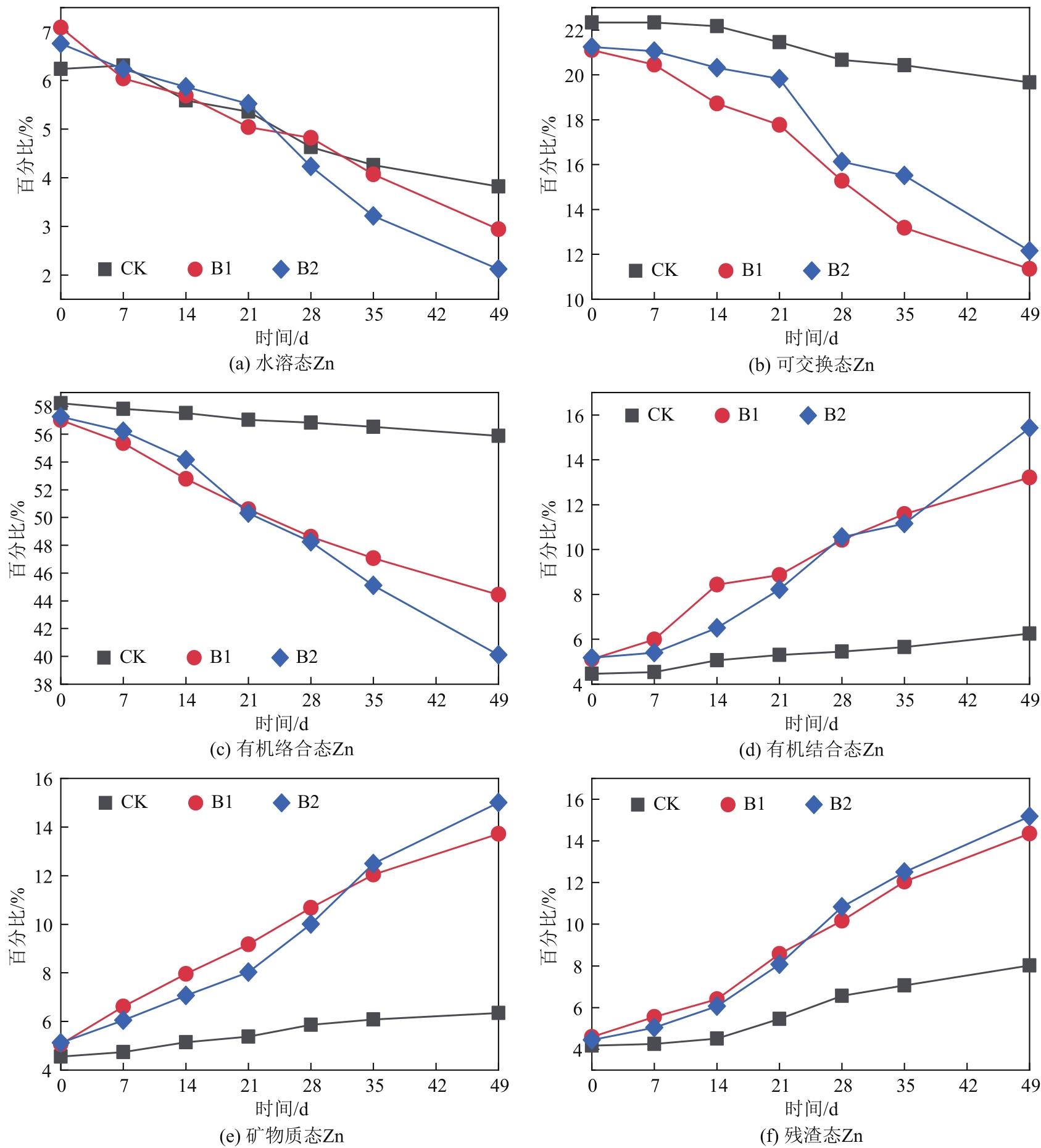
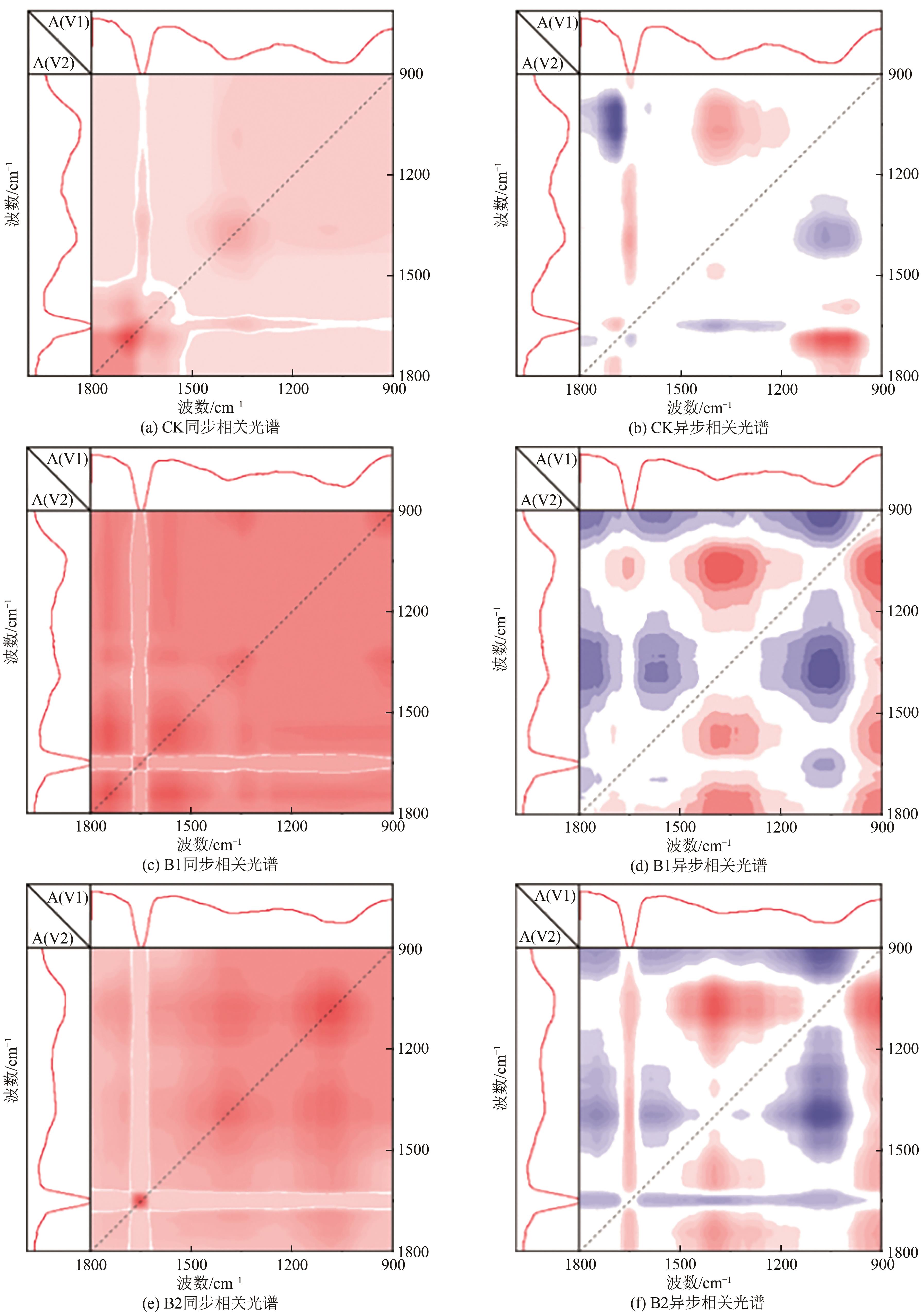
针对猪粪中的重金属污染问题,本文构建以猪粪/玉米秸秆为原料的厌氧消化体系,复配生物炭、腐殖酸、粉煤灰3种钝化剂,使用有机碳分级提取法反应最优钝化组中Cu、Zn形态动态变化过程,探索重金属与腐殖质的结合作用机制。结果表明:对Cu、Zn钝化效果影响最大的分别为腐殖酸、粉煤灰,钝化Cu、Zn最佳复合比分别为B1(腐殖酸∶粉煤灰∶生物炭比例为7.5%∶7.5%∶7.5%)、B2(5.0%∶7.5%∶7.5%)。对腐殖质进行提取发现,体系中的胡敏酸(HA)与富里酸(FA)含量的比值HA/FA呈增加趋势,消化前各处理组沼渣腐殖质中的Cu、Zn主要以FA-Cu、FA-Zn结合形式存在,消化后重金属主要与HA结合,B1、B2中HA-Zn、HA-Cu的占比明显高于对照组(CK),生物可利用性明显低于CK。比较Cu、Zn两种重金属稳定态发现:Zn转化稳定态的幅度偏小,主要是由于Zn是两性重金属,较活泼,易与小分子腐殖质FA结合,而Cu主要与大分子腐殖质HA结合,且结合更为紧密。B1、B2中重金属与腐殖质结合形态更为稳定,因此复合钝化剂可以有效降低厌氧消化进程中Cu、Zn的生物毒性。红外光谱分析发现钝化剂增加底物的芳香性,使得其更容易与重金属结合,芳香族、脂肪族化合物、蛋白质和多糖类化合物是主要的重金属结合点位。
中图分类号:
引用本文
庄捷, 薛锦辉, 赵斌成, 张文艺. 猪粪厌氧消化进程中重金属与腐殖质的有机结合机制[J]. 化工进展, 2023, 42(6): 3281-3291.
ZHUANG Jie, XUE Jinhui, ZHAO Bincheng, ZHANG Wenyi. Organic binding mechanism of heavy metals and humus during anaerobic digestion of pig manure[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3281-3291.
| 材料 | 含水率/% | 总有机碳含量/% | 总氮含量/% | C/N |
|---|---|---|---|---|
| 猪粪 | 79.84 | 9.20 | 0.59 | 15.59 |
| 玉米秸秆 | 4.45 | 15.13 | 0.36 | 42.03 |
表1 猪粪和玉米秸秆主要成分
| 材料 | 含水率/% | 总有机碳含量/% | 总氮含量/% | C/N |
|---|---|---|---|---|
| 猪粪 | 79.84 | 9.20 | 0.59 | 15.59 |
| 玉米秸秆 | 4.45 | 15.13 | 0.36 | 42.03 |
| 材料 | 铜含量/mg·kg-1 | 锌含量/mg·kg-1 |
|---|---|---|
| 猪粪 | 254.17 | 1039.83 |
| 玉米秸秆 | ND | ND |
| 腐殖酸 | 14.20 | 64.33 |
| 粉煤灰 | 71.24 | 118.46 |
| 生物炭 | 6.56 | 59.85 |
表2 消化原料、钝化剂的来源及重金属含量
| 材料 | 铜含量/mg·kg-1 | 锌含量/mg·kg-1 |
|---|---|---|
| 猪粪 | 254.17 | 1039.83 |
| 玉米秸秆 | ND | ND |
| 腐殖酸 | 14.20 | 64.33 |
| 粉煤灰 | 71.24 | 118.46 |
| 生物炭 | 6.56 | 59.85 |
| 水平 | 腐殖酸/% | 粉煤灰/% | 生物炭/% |
|---|---|---|---|
| 1 | 2.5 | 2.5 | 2.5 |
| 2 | 5.0 | 5.0 | 5.0 |
| 3 | 7.5 | 7.5 | 7.5 |
表3 因素水平表
| 水平 | 腐殖酸/% | 粉煤灰/% | 生物炭/% |
|---|---|---|---|
| 1 | 2.5 | 2.5 | 2.5 |
| 2 | 5.0 | 5.0 | 5.0 |
| 3 | 7.5 | 7.5 | 7.5 |
| 编号 | 因素 | 钝化效果 | ||||
|---|---|---|---|---|---|---|
| A | B | C | 空列 | Cu钝化率/% | Zn钝化率/% | |
| S1 | 1 | 1 | 1 | 1 | 43.92 | 23.87 |
| S2 | 2 | 1 | 2 | 2 | 56.96 | 26.85 |
| S3 | 3 | 1 | 3 | 3 | 70.02 | 28.02 |
| S4 | 1 | 2 | 2 | 3 | 49.77 | 26.11 |
| S5 | 2 | 2 | 3 | 1 | 65.17 | 36.24 |
| S6 | 3 | 2 | 1 | 2 | 61.10 | 34.29 |
| S7 | 1 | 3 | 3 | 2 | 54.92 | 35.03 |
| S8 | 2 | 3 | 1 | 3 | 62.11 | 38.30 |
| S9 | 3 | 3 | 2 | 1 | 63.18 | 34.04 |
| Cu钝化极差分析 | ||||||
| K1 | 49.54 | 56.97 | 55.71 | 57.42 | 主次因素 | A>C>B |
| K2 | 61.41 | 58.68 | 56.64 | 57.66 | ||
| K3 | 64.76 | 60.07 | 63.37 | 60.63 | 最优方案 | A3B3C3 |
| R | 15.23 | 3.10 | 7.66 | 3.21 | ||
| Zn钝化极差分析 | ||||||
| K1 | 28.34 | 26.25 | 32.15 | 31.38 | 主次因素 | B>A>C |
| K2 | 33.80 | 32.21 | 29.00 | 32.06 | ||
| K3 | 32.12 | 35.79 | 33.10 | 30.81 | 最优方案 | A2B3C3 |
| R | 5.46 | 9.54 | 4.10 | 1.25 | ||
表4 各处理组消化后Cu、Zn钝化效果及极差分析表
| 编号 | 因素 | 钝化效果 | ||||
|---|---|---|---|---|---|---|
| A | B | C | 空列 | Cu钝化率/% | Zn钝化率/% | |
| S1 | 1 | 1 | 1 | 1 | 43.92 | 23.87 |
| S2 | 2 | 1 | 2 | 2 | 56.96 | 26.85 |
| S3 | 3 | 1 | 3 | 3 | 70.02 | 28.02 |
| S4 | 1 | 2 | 2 | 3 | 49.77 | 26.11 |
| S5 | 2 | 2 | 3 | 1 | 65.17 | 36.24 |
| S6 | 3 | 2 | 1 | 2 | 61.10 | 34.29 |
| S7 | 1 | 3 | 3 | 2 | 54.92 | 35.03 |
| S8 | 2 | 3 | 1 | 3 | 62.11 | 38.30 |
| S9 | 3 | 3 | 2 | 1 | 63.18 | 34.04 |
| Cu钝化极差分析 | ||||||
| K1 | 49.54 | 56.97 | 55.71 | 57.42 | 主次因素 | A>C>B |
| K2 | 61.41 | 58.68 | 56.64 | 57.66 | ||
| K3 | 64.76 | 60.07 | 63.37 | 60.63 | 最优方案 | A3B3C3 |
| R | 15.23 | 3.10 | 7.66 | 3.21 | ||
| Zn钝化极差分析 | ||||||
| K1 | 28.34 | 26.25 | 32.15 | 31.38 | 主次因素 | B>A>C |
| K2 | 33.80 | 32.21 | 29.00 | 32.06 | ||
| K3 | 32.12 | 35.79 | 33.10 | 30.81 | 最优方案 | A2B3C3 |
| R | 5.46 | 9.54 | 4.10 | 1.25 | ||
| 处理 | 时间/d | Na4P2O7 | NaOH | 总HA/FA | ||||
|---|---|---|---|---|---|---|---|---|
| HA | FA | HA/FA | HA | FA | HA/FA | |||
| CK | 0 | 53.57 | 46.43 | 1.15 | 52.63 | 47.37 | 1.11 | 1.13 |
| 7 | 58.82 | 41.18 | 1.43 | 56.25 | 43.75 | 1.29 | 1.36 | |
| 14 | 68.00 | 32.00 | 2.13 | 58.82 | 41.18 | 1.43 | 1.78 | |
| 21 | 70.83 | 29.17 | 2.43 | 60.00 | 40.00 | 1.50 | 1.96 | |
| 28 | 75.00 | 25.00 | 3.00 | 65.22 | 34.78 | 1.88 | 2.44 | |
| 35 | 76.92 | 23.08 | 3.33 | 63.64 | 36.36 | 1.75 | 2.54 | |
| 49 | 78.57 | 21.43 | 3.67 | 68.29 | 31.71 | 2.15 | 2.91 | |
| B1 | 0 | 56.67 | 43.33 | 1.31 | 54.84 | 45.16 | 1.21 | 1.26 |
| 7 | 60.42 | 39.58 | 1.53 | 59.18 | 40.82 | 1.45 | 1.49 | |
| 14 | 60.87 | 39.13 | 1.56 | 62.50 | 37.50 | 1.67 | 1.61 | |
| 21 | 68.18 | 31.82 | 2.14 | 64.71 | 35.29 | 1.83 | 1.99 | |
| 28 | 78.13 | 21.88 | 3.57 | 64.29 | 35.71 | 1.80 | 2.69 | |
| 35 | 80.88 | 19.12 | 4.23 | 67.35 | 32.65 | 2.06 | 3.15 | |
| 49 | 85.42 | 14.58 | 5.86 | 69.23 | 30.77 | 2.25 | 4.05 | |
| B2 | 0 | 57.89 | 42.11 | 1.38 | 54.05 | 45.95 | 1.18 | 1.28 |
| 7 | 61.90 | 38.10 | 1.63 | 60.00 | 40.00 | 1.50 | 1.56 | |
| 14 | 70.00 | 30.00 | 2.33 | 63.27 | 36.73 | 1.72 | 2.03 | |
| 21 | 74.07 | 25.93 | 2.86 | 63.64 | 36.36 | 1.75 | 2.30 | |
| 28 | 77.50 | 22.50 | 3.44 | 65.00 | 35.00 | 1.86 | 2.65 | |
| 35 | 80.36 | 19.64 | 4.09 | 67.92 | 32.08 | 2.12 | 3.10 | |
| 49 | 85.71 | 14.29 | 6.00 | 69.44 | 30.56 | 2.27 | 4.14 | |
表5 厌氧消化进程中有机碳中腐殖质含量表
| 处理 | 时间/d | Na4P2O7 | NaOH | 总HA/FA | ||||
|---|---|---|---|---|---|---|---|---|
| HA | FA | HA/FA | HA | FA | HA/FA | |||
| CK | 0 | 53.57 | 46.43 | 1.15 | 52.63 | 47.37 | 1.11 | 1.13 |
| 7 | 58.82 | 41.18 | 1.43 | 56.25 | 43.75 | 1.29 | 1.36 | |
| 14 | 68.00 | 32.00 | 2.13 | 58.82 | 41.18 | 1.43 | 1.78 | |
| 21 | 70.83 | 29.17 | 2.43 | 60.00 | 40.00 | 1.50 | 1.96 | |
| 28 | 75.00 | 25.00 | 3.00 | 65.22 | 34.78 | 1.88 | 2.44 | |
| 35 | 76.92 | 23.08 | 3.33 | 63.64 | 36.36 | 1.75 | 2.54 | |
| 49 | 78.57 | 21.43 | 3.67 | 68.29 | 31.71 | 2.15 | 2.91 | |
| B1 | 0 | 56.67 | 43.33 | 1.31 | 54.84 | 45.16 | 1.21 | 1.26 |
| 7 | 60.42 | 39.58 | 1.53 | 59.18 | 40.82 | 1.45 | 1.49 | |
| 14 | 60.87 | 39.13 | 1.56 | 62.50 | 37.50 | 1.67 | 1.61 | |
| 21 | 68.18 | 31.82 | 2.14 | 64.71 | 35.29 | 1.83 | 1.99 | |
| 28 | 78.13 | 21.88 | 3.57 | 64.29 | 35.71 | 1.80 | 2.69 | |
| 35 | 80.88 | 19.12 | 4.23 | 67.35 | 32.65 | 2.06 | 3.15 | |
| 49 | 85.42 | 14.58 | 5.86 | 69.23 | 30.77 | 2.25 | 4.05 | |
| B2 | 0 | 57.89 | 42.11 | 1.38 | 54.05 | 45.95 | 1.18 | 1.28 |
| 7 | 61.90 | 38.10 | 1.63 | 60.00 | 40.00 | 1.50 | 1.56 | |
| 14 | 70.00 | 30.00 | 2.33 | 63.27 | 36.73 | 1.72 | 2.03 | |
| 21 | 74.07 | 25.93 | 2.86 | 63.64 | 36.36 | 1.75 | 2.30 | |
| 28 | 77.50 | 22.50 | 3.44 | 65.00 | 35.00 | 1.86 | 2.65 | |
| 35 | 80.36 | 19.64 | 4.09 | 67.92 | 32.08 | 2.12 | 3.10 | |
| 49 | 85.71 | 14.29 | 6.00 | 69.44 | 30.56 | 2.27 | 4.14 | |
| 处理 | 时间/d | Na4P2O7 | NaOH | ||
|---|---|---|---|---|---|
| HA | FA | HA | FA | ||
| CK | 0 | 4.45 | 95.55 | 2.30 | 97.70 |
| 7 | 5.12 | 94.88 | 4.77 | 95.23 | |
| 14 | 8.61 | 91.39 | 6.33 | 93.67 | |
| 21 | 11.85 | 88.15 | 9.62 | 90.38 | |
| 28 | 15.63 | 84.37 | 12.69 | 87.31 | |
| 35 | 16.53 | 83.47 | 13.29 | 86.71 | |
| 49 | 18.26 | 81.74 | 15.82 | 84.18 | |
| B1 | 0 | 5.15 | 94.85 | 4.17 | 95.83 |
| 7 | 8.60 | 91.40 | 7.98 | 92.02 | |
| 14 | 12.94 | 87.06 | 11.23 | 88.77 | |
| 21 | 17.98 | 82.02 | 17.38 | 82.62 | |
| 28 | 23.69 | 76.31 | 23.51 | 76.49 | |
| 35 | 27.90 | 72.10 | 26.65 | 73.35 | |
| 49 | 31.69 | 68.31 | 30.38 | 69.62 | |
| B2 | 0 | 5.79 | 94.21 | 3.80 | 96.20 |
| 7 | 7.88 | 92.12 | 6.82 | 93.18 | |
| 14 | 10.29 | 89.71 | 9.03 | 90.97 | |
| 21 | 15.38 | 84.62 | 12.01 | 87.99 | |
| 28 | 18.41 | 81.59 | 17.71 | 82.29 | |
| 35 | 23.24 | 76.76 | 21.13 | 78.87 | |
| 49 | 27.91 | 72.09 | 25.03 | 74.97 | |
表6 Cu在Na4P2O7和NaOH提取的在腐殖质中的分布
| 处理 | 时间/d | Na4P2O7 | NaOH | ||
|---|---|---|---|---|---|
| HA | FA | HA | FA | ||
| CK | 0 | 4.45 | 95.55 | 2.30 | 97.70 |
| 7 | 5.12 | 94.88 | 4.77 | 95.23 | |
| 14 | 8.61 | 91.39 | 6.33 | 93.67 | |
| 21 | 11.85 | 88.15 | 9.62 | 90.38 | |
| 28 | 15.63 | 84.37 | 12.69 | 87.31 | |
| 35 | 16.53 | 83.47 | 13.29 | 86.71 | |
| 49 | 18.26 | 81.74 | 15.82 | 84.18 | |
| B1 | 0 | 5.15 | 94.85 | 4.17 | 95.83 |
| 7 | 8.60 | 91.40 | 7.98 | 92.02 | |
| 14 | 12.94 | 87.06 | 11.23 | 88.77 | |
| 21 | 17.98 | 82.02 | 17.38 | 82.62 | |
| 28 | 23.69 | 76.31 | 23.51 | 76.49 | |
| 35 | 27.90 | 72.10 | 26.65 | 73.35 | |
| 49 | 31.69 | 68.31 | 30.38 | 69.62 | |
| B2 | 0 | 5.79 | 94.21 | 3.80 | 96.20 |
| 7 | 7.88 | 92.12 | 6.82 | 93.18 | |
| 14 | 10.29 | 89.71 | 9.03 | 90.97 | |
| 21 | 15.38 | 84.62 | 12.01 | 87.99 | |
| 28 | 18.41 | 81.59 | 17.71 | 82.29 | |
| 35 | 23.24 | 76.76 | 21.13 | 78.87 | |
| 49 | 27.91 | 72.09 | 25.03 | 74.97 | |
| 处理 | 时间/d | Na4P2O7 | NaOH | ||
|---|---|---|---|---|---|
| HA | FA | HA | FA | ||
| CK | 0 | 4.42 | 95.58 | 4.47 | 95.53 |
| 7 | 5.82 | 94.18 | 4.99 | 95.01 | |
| 14 | 5.56 | 94.44 | 5.64 | 94.36 | |
| 21 | 6.83 | 93.17 | 6.37 | 93.63 | |
| 28 | 8.59 | 91.41 | 7.44 | 92.56 | |
| 35 | 9.37 | 90.63 | 8.13 | 91.87 | |
| 49 | 10.61 | 89.39 | 10.46 | 89.54 | |
| B1 | 0 | 4.17 | 95.83 | 4.52 | 95.48 |
| 7 | 5.40 | 94.60 | 5.91 | 94.09 | |
| 14 | 6.54 | 93.46 | 6.63 | 93.37 | |
| 21 | 6.91 | 93.09 | 7.79 | 92.21 | |
| 28 | 9.40 | 90.60 | 10.42 | 89.58 | |
| 35 | 11.94 | 88.06 | 11.05 | 88.95 | |
| 49 | 13.03 | 86.97 | 13.40 | 86.60 | |
| B2 | 0 | 4.66 | 95.34 | 4.86 | 95.14 |
| 7 | 5.87 | 94.13 | 6.31 | 93.69 | |
| 14 | 6.05 | 93.95 | 7.90 | 92.10 | |
| 21 | 8.47 | 91.53 | 9.47 | 90.53 | |
| 28 | 11.06 | 88.94 | 12.96 | 87.04 | |
| 35 | 13.98 | 86.02 | 14.87 | 85.13 | |
| 49 | 16.27 | 83.73 | 17.64 | 82.36 | |
表7 在Na4P2O7和NaOH提取的Zn在腐殖质中的分布
| 处理 | 时间/d | Na4P2O7 | NaOH | ||
|---|---|---|---|---|---|
| HA | FA | HA | FA | ||
| CK | 0 | 4.42 | 95.58 | 4.47 | 95.53 |
| 7 | 5.82 | 94.18 | 4.99 | 95.01 | |
| 14 | 5.56 | 94.44 | 5.64 | 94.36 | |
| 21 | 6.83 | 93.17 | 6.37 | 93.63 | |
| 28 | 8.59 | 91.41 | 7.44 | 92.56 | |
| 35 | 9.37 | 90.63 | 8.13 | 91.87 | |
| 49 | 10.61 | 89.39 | 10.46 | 89.54 | |
| B1 | 0 | 4.17 | 95.83 | 4.52 | 95.48 |
| 7 | 5.40 | 94.60 | 5.91 | 94.09 | |
| 14 | 6.54 | 93.46 | 6.63 | 93.37 | |
| 21 | 6.91 | 93.09 | 7.79 | 92.21 | |
| 28 | 9.40 | 90.60 | 10.42 | 89.58 | |
| 35 | 11.94 | 88.06 | 11.05 | 88.95 | |
| 49 | 13.03 | 86.97 | 13.40 | 86.60 | |
| B2 | 0 | 4.66 | 95.34 | 4.86 | 95.14 |
| 7 | 5.87 | 94.13 | 6.31 | 93.69 | |
| 14 | 6.05 | 93.95 | 7.90 | 92.10 | |
| 21 | 8.47 | 91.53 | 9.47 | 90.53 | |
| 28 | 11.06 | 88.94 | 12.96 | 87.04 | |
| 35 | 13.98 | 86.02 | 14.87 | 85.13 | |
| 49 | 16.27 | 83.73 | 17.64 | 82.36 | |
| 1 | JAISWAL A, VERMA A, JAISWAL P. Detrimental effects of heavy metals in soil, plants, and aquatic ecosystems and in humans[J]. Journal of Environmental Pathology, Toxicology and Oncology, 2018, 37(3): 183-197. |
| 2 | LIU Wangrong, ZENG Dong, SHE Lei, et al. Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China[J]. The Science of the Total Environment, 2020, 734: 139023. |
| 3 | 李轶, 刘艳杰, 冯洋洋, 等. 沸石对猪粪沼气发酵特性及沼渣、沼液中重金属Zn的影响[J]. 可再生能源, 2016, 34(6): 943-948. |
| LI Yi, LIU Yanjie, FENG Yangyang, et al. Effects of adding zeolite on pig manure anaerobic fermentation and the change of heavy metal Zinc in digestate[J]. Renewable Energy Resources, 2016, 34(6): 943-948. | |
| 4 | 安登飞. 土壤重金属活化和钝化技术研究进展[J]. 中国资源综合利用, 2021, 39(11): 93-95. |
| AN Dengfei. Research progress on activation and passivation technology of heavy metals in soil[J]. China Resources Comprehensive Utilization, 2021, 39(11): 93-95, 104. | |
| 5 | 蔡旭影. 农田土壤重金属As、Cd污染的钝化剂修复效果及风险评价[D]. 北京: 北京交通大学, 2021. |
| CAI Xuying. Evaluation of remediation effect and risk of heavy metal As and Cd contamination in agricultural soils by passivation agents[D]. Beijing: Beijing Jiaotong University, 2021. | |
| 6 | 曾心雨, 赵欢, 李玉成, 等. Fe2O3对猪粪厌氧发酵及沼渣中Cu、As的钝化效果[J]. 江苏农业科学, 2020, 48(6): 260-264. |
| ZENG Xinyu, ZHAO Huan, LI Yucheng, et al. Effect of Fe2O3 on anaerobic fermentation of pig manure and passivation of Cu and As in biogas dregs[J]. Jiangsu Agricultural Sciences, 2020, 48(6): 260-264. | |
| 7 | 冯晶, 荆勇, 赵立欣, 等. 生物炭强化有机废弃物厌氧发酵技术研究[J]. 农业工程学报, 2019, 35(12): 256-264. |
| FENG Jing, JING Yong, ZHAO Lixin, et al. Research progress on biochar enhanced anaerobic fermentation technology of organic wastes[J]. Transactions of the Chinese Society of Agricultural Engineering, 2019, 35(12): 256-264. | |
| 8 | 周坤渊, 刘仕翔, 罗泽娇. 常见碱性工业废渣稳定化修复重金属污染土壤的研究进展[J]. 安全与环境工程, 2021, 28(6): 174-181. |
| ZHOU Kunyuan, LIU Shixiang, LUO Zejiao. Research progress on stabilization of heavy metals in soil by common alkaline industrial wastes[J].Safety and Environmental Engineering, 2021, 28(6): 174-181. | |
| 9 | HE En, Changwei LYU, HE Jiang, et al. Binding characteristics of Cu2+ to natural humic acid fractions sequentially extracted from the lake sediments[J]. Environmental Science and Pollution Research, 2016, 23(22): 22667-22677. |
| 10 | 何丽霞, 张丰松, 张桂香, 等. 复配钝化剂对稻田重金属有效性及其水稻吸收的影响[J]. 环境工程学报, 2022, 16(2): 565-575. |
| HE Lixia, ZHANG Fengsong, ZHANG Guixiang, et al. Effects of combined passivators on the availability of heavy metals in red paddy soil and their uptake by rice[J]. Chinese Journal of Environmental Engineering, 2022, 16(2): 565-575. | |
| 11 | WANG Xiqing, Tao LYU, DONG Renjie, et al. Dynamic evolution of humic acids during anaerobic digestion: Exploring an effective auxiliary agent for heavy metal remediation[J]. Bioresource Technology, 2021, 320(Pt A): 124331 |
| 12 | HE X T, LOGAN T J, TRAINA S J. Physical and chemical characteristics of selected US municipal solid waste composts[J]. Journal of Environmental Quality, 1995: 543-552. |
| 13 | 巩俊璐, 钝化剂对猪粪/秸秆厌氧消化中铜和锌减害化的影响研究[D]. 沈阳: 沈阳农业大学, 2018. |
| GONG Junlu. Effect of passivating agent on the reduction of Cu and Zn in anaerobic fermentation of pig manure/straw[D]. Shenyang: Shenyang Agricultural University, 2018. | |
| 14 | 王立闯, 郝娇, 李延吉, 等. 玉米秸秆对鸡粪厌氧发酵过程及酶活性的影响规律研究[J]. 可再生能源, 2021, 39(11): 1441-1446. |
| WANG Lichuang, HAO Jiao, LI Yanji, et al. Study on the effect of corn straw on the chicken manure anaerobic digestion process and the change of enzyme activity[J]. Renewable Energy Resources, 2021, 39(11): 1441-1446. | |
| 15 | 李轶, 曲壮壮, 巩俊璐, 等. 海泡石对猪粪秸秆厌氧发酵产物中Cd的钝化效果研究[J]. 农业工程学报, 2018, 34(S1): 1-6. |
| LI Yi, QU Zhuangzhuang, GONG Junlu, et al. Passivation effect of Cd by sepiolite in anaerobic fermentation products with pig manure and straw[J]. Transactions of the Chinese Society of Agricultural Engineering, 2018, 34(S1): 1-6. | |
| 16 | 李轶, 宫兴隆, 郭敬阳, 等. 不同预处理玉米秸秆对猪粪厌氧发酵重金属镉钝化效果[J]. 农业工程学报, 2020, 36(11): 254-260. |
| LI Yi, GONG Xinglong, GUO Jingyang, et al. Effects of various pretreated maize stovers on the passivation of cadmium by anaerobic fermentation of pig manure[J]. Transactions of the Chinese Society of Agricultural Engineering, 2020, 36(11): 254-260. | |
| 17 | 许剑敏, 郭锋, 刘子姣, 等. 不同钝化剂对猪粪菌渣堆肥中Cu、Zn钝化的影响[J]. 山西农业科学, 2019, 47(12): 2155-2158. |
| XU Jianmin, GUO Feng, LIU Zijiao, et al. Effect of different passivation on heavy metals Cu, Zn during composting of pig manure and mushroom[J]. Journal of Shanxi Agricultural Sciences, 2019, 47(12): 2155-2158. | |
| 18 | 黄国锋, 张振钿, 钟流举, 等. 重金属在猪粪堆肥过程中的化学变化[J]. 中国环境科学, 2004, 24(1): 94-99. |
| HUANG Guofeng, ZHANG Zhentian, ZHONG Liuju, et al. Chemical changes of heavy metals in the process of pig manure composting[J]. China Environmental Science, 2004, 24(1): 94-99. | |
| 19 | LU Duian, WANG Lixia, YAN Baixing, et al. Speciation of Cu and Zn during composting of pig manure amended with rock phosphate[J]. Waste Management, 2014, 34(8): 1529-1536. |
| 20 | 张雪英, 周顺桂, 周立祥, 等. 堆肥处理对污泥腐殖物质形态及其重金属分配的影响[J]. 生态学杂志, 2004, 23(1): 30-33. |
| ZHANG Xueying, ZHOU Shungui, ZHOU Lixiang, et al. Component changes of humic substances and heavy metal distribution before and after sewage sludge composting[J]. Chinese Journal of Ecology, 2004, 23(1): 30-33. | |
| 21 | LIU Hongtao, WANG Lixia, ZHONG Rongzhen, et al. Binding characteristics of humic substances with Cu and Zn in response to inorganic mineral additives during swine manure composting[J]. Journal of Environmental Management, 2022, 305: 114387. |
| 22 | 孙向平, 李国学, 肖爱平, 等. 添加不同比例玉米秸秆对猪粪高温堆肥过程中胡敏酸的结构组成及红外光谱特性影响分析[J]. 光谱学与光谱分析, 2014, 34(9): 2413-2418. |
| SUN Xiangping, LI Guoxue, XIAO Aiping, et al. Analysis on the impact of composting with different proportions of corn stalks and pig manure on humic acid fractions and IR spectral feature[J]. Spectroscopy and Spectral Analysis, 2014, 34(9): 2413-2418. | |
| 23 | 栾润宇, 徐应明, 高珊, 等. 不同发酵方式对鸡粪重金属及有机质影响[J]. 中国环境科学, 2020, 40(8): 3486-3494. |
| LUAN Runyu, XU Yingming, GAO Shan, et al. Heavy metal and organic matters in the chicken manure under different types of composting[J]. China Environmental Science, 2020, 40(8): 3486-3494. | |
| 24 | NODA I. Two-dimensional correlation spectroscopy: applications in vibrational and optical spectroscopy[M]. London: John Wiley and Sons Inc., 2004: 36. |
| [1] | 奚永兰, 王成成, 叶小梅, 刘洋, 贾昭炎, 曹春晖, 韩挺, 张应鹏, 田雨. 微纳米气泡在厌氧消化中的应用研究进展[J]. 化工进展, 2023, 42(8): 4414-4423. |
| [2] | 孟晓山, 汤子健, 陈琳, 呼和涛力, 周政忠. 厌氧消化系统酸化预警及调控技术研究进展[J]. 化工进展, 2023, 42(3): 1595-1605. |
| [3] | 祝佳欣, 朱雯喆, 徐俊, 谢靖, 王文标, 谢丽. 基于导电材料强化抗生素胁迫厌氧消化的研究进展[J]. 化工进展, 2023, 42(2): 1008-1019. |
| [4] | 刘亚利, 张宏伟, 康晓荣. 微塑料对污泥厌氧消化的影响和机理[J]. 化工进展, 2022, 41(9): 5037-5046. |
| [5] | 刘洋, 叶小梅, 王成成, 贾昭炎, 杜静, 孔祥平, 奚永兰. 农村有机生活垃圾与不同原料厌氧共发酵工艺优化[J]. 化工进展, 2022, 41(5): 2770-2777. |
| [6] | 邵明帅, 张超, 吴华南, 王宁, 陈钦冬, 徐期勇. 水热耦合厌氧消化技术处理餐厨垃圾沼渣沼液及工艺能耗分析[J]. 化工进展, 2022, 41(5): 2733-2742. |
| [7] | 郑小梅, 林茹晶, 周文静, 徐泠, 张洪宁, 张昕颖, 谢丽. 微生物电解池辅助CO2甲烷化阴极材料的研究进展[J]. 化工进展, 2022, 41(5): 2476-2486. |
| [8] | 阮敏, 孙宇桐, 黄忠良, 李辉, 张轩, 吴希锴, 赵成, 姚世蓉, 张拴保, 张巍, 黄兢. 污泥预处理-厌氧消化体系的能源经济性评价[J]. 化工进展, 2022, 41(3): 1503-1516. |
| [9] | 江汝清, 余广炜, 王玉, 黎长江, 邢贞娇. 酸改性猪粪生物炭的制备及其对直接红23染料的吸附性能[J]. 化工进展, 2022, 41(12): 6489-6499. |
| [10] | 闫雪倩, 裴向军, 杜杰, 米晓慧, 白琳嵚, 马蕊, 张明宽, 钱进. Bi2O2CO3/Fe3O4磁性复合物在环境污染治理领域中的应用[J]. 化工进展, 2021, 40(6): 3515-3525. |
| [11] | 王亭亭, 赵智强, 张耀斌. 碱预处理耦合零价铁强化含油污泥厌氧消化[J]. 化工进展, 2021, 40(1): 534-541. |
| [12] | 邹联沛, 宋琳, 李小伟, 万雨岚, 李曼, 刘建勇, 欧阳创, 奚慧, 钱光人, 戴晓虎. 湿垃圾组分对厌氧消化抑制作用的研究进展[J]. 化工进展, 2020, 39(S2): 362-371. |
| [13] | 傅国志, 郭文阳, 马宗虎, 刘蔚, 李博凯, 孙子滟, 王祯欣, 李叶青. 秸秆与猪粪混合高固厌氧消化产气性能及关键微生物分析[J]. 化工进展, 2020, 39(8): 3386-3394. |
| [14] | 陈思思,杨殿海,庞维海,董滨,戴晓虎. 我国剩余污泥厌氧转化的主要影响因素及影响机制研究进展[J]. 化工进展, 2020, 39(4): 1511-1520. |
| [15] | 张若瑄,王朋,张绪超,段文焱. 土壤中环境持久性自由基的形成与稳定及其影响因素[J]. 化工进展, 2020, 39(4): 1528-1538. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||