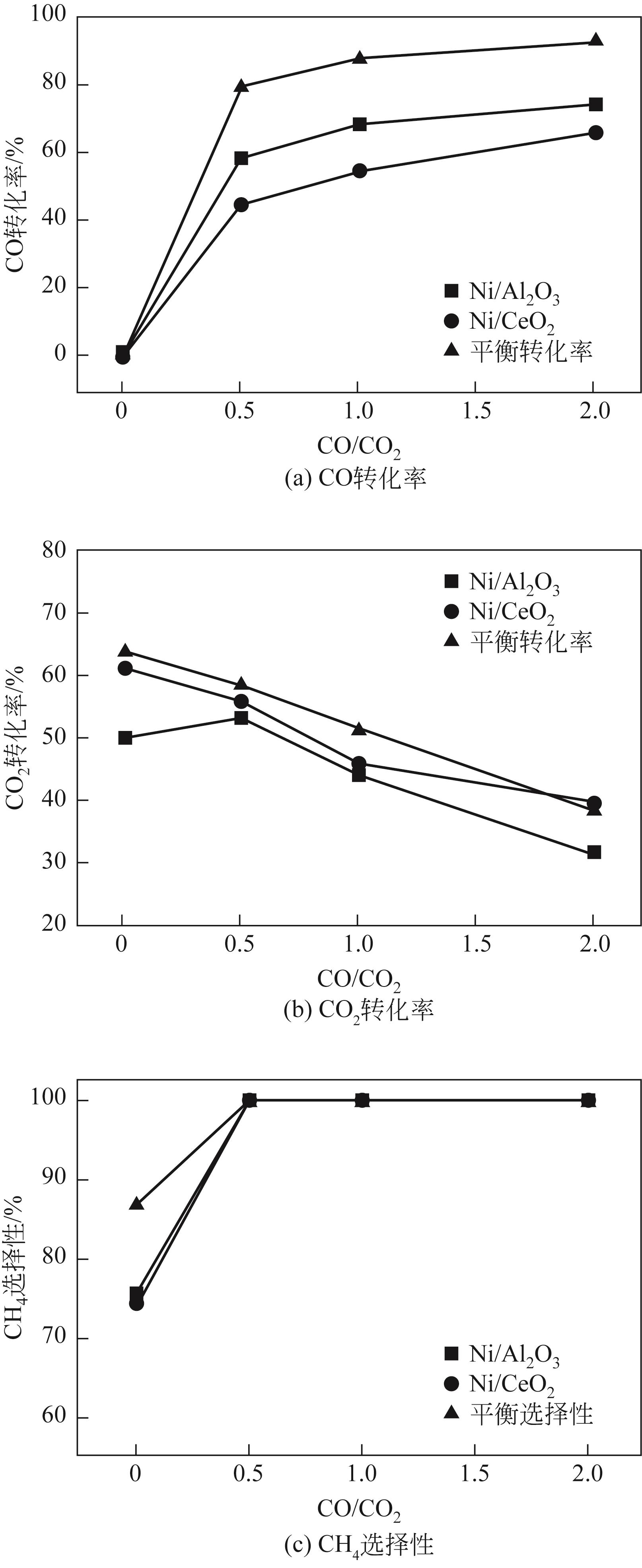
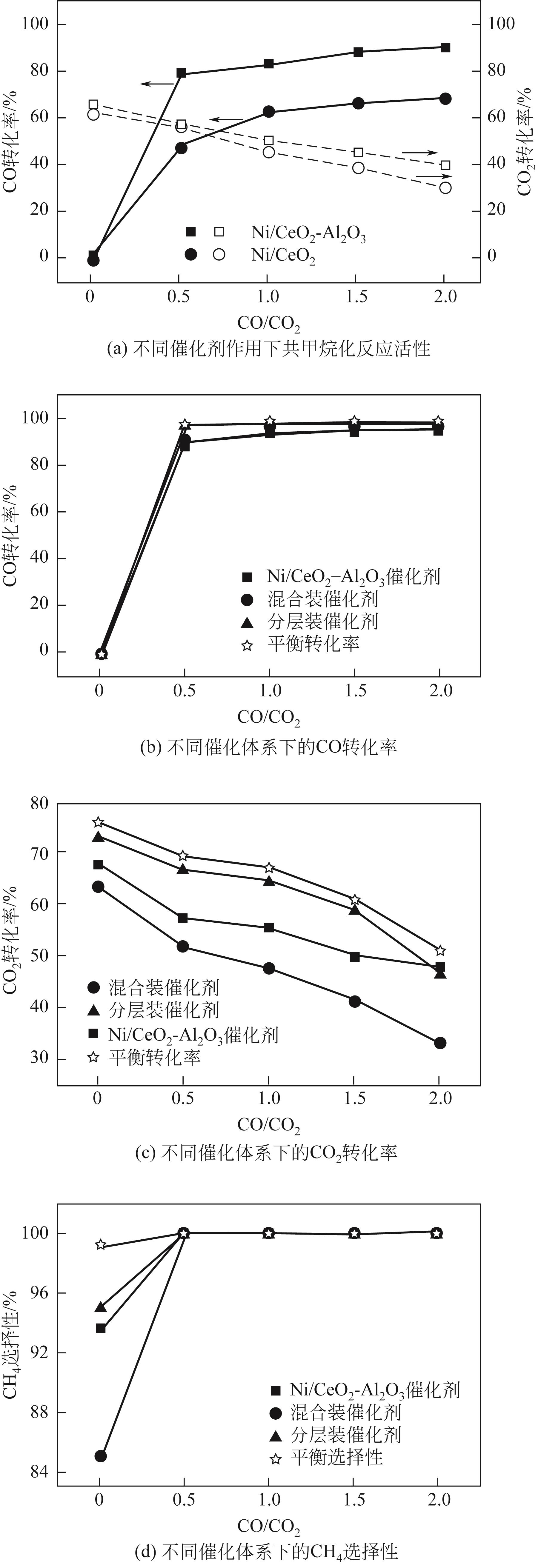
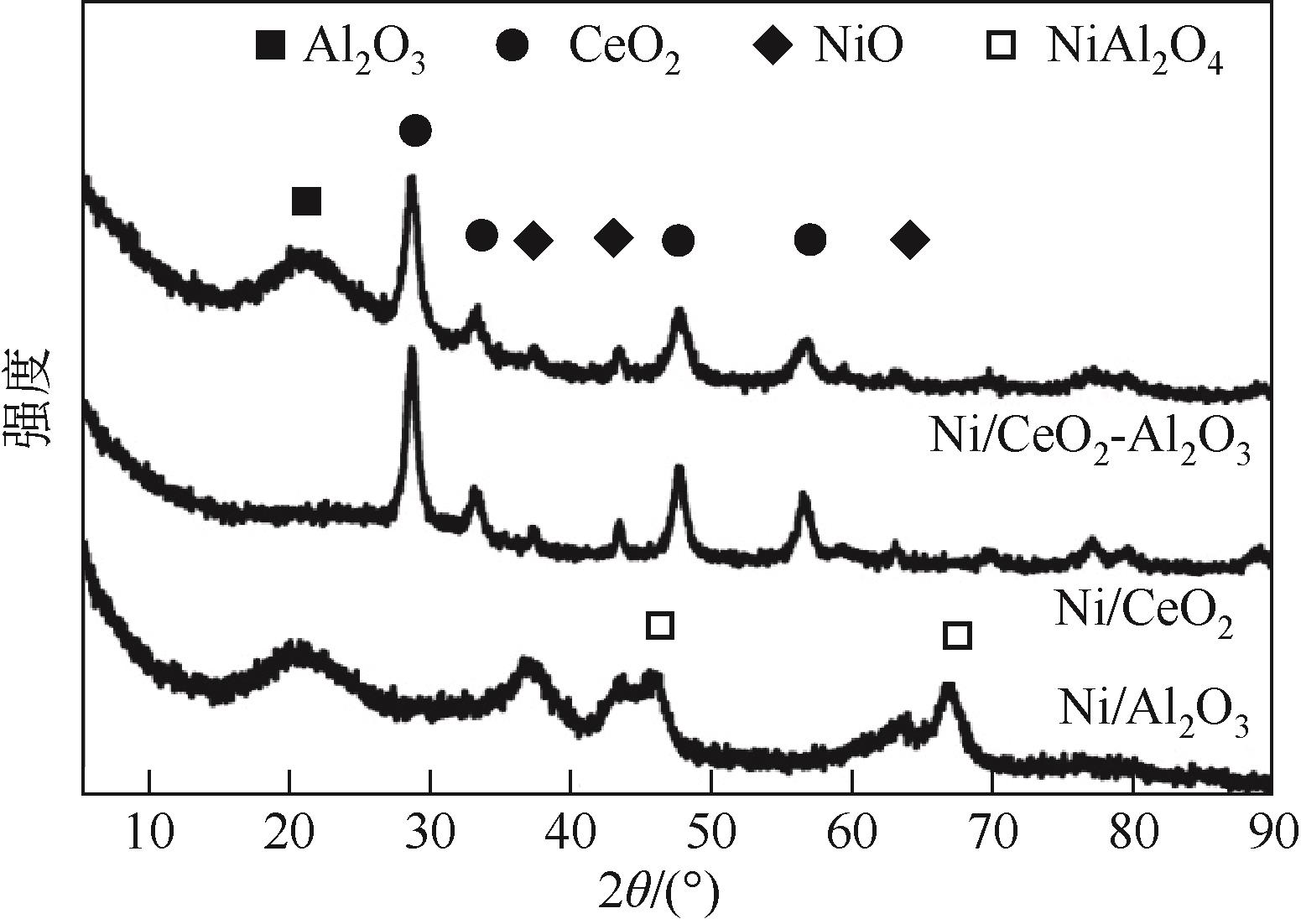
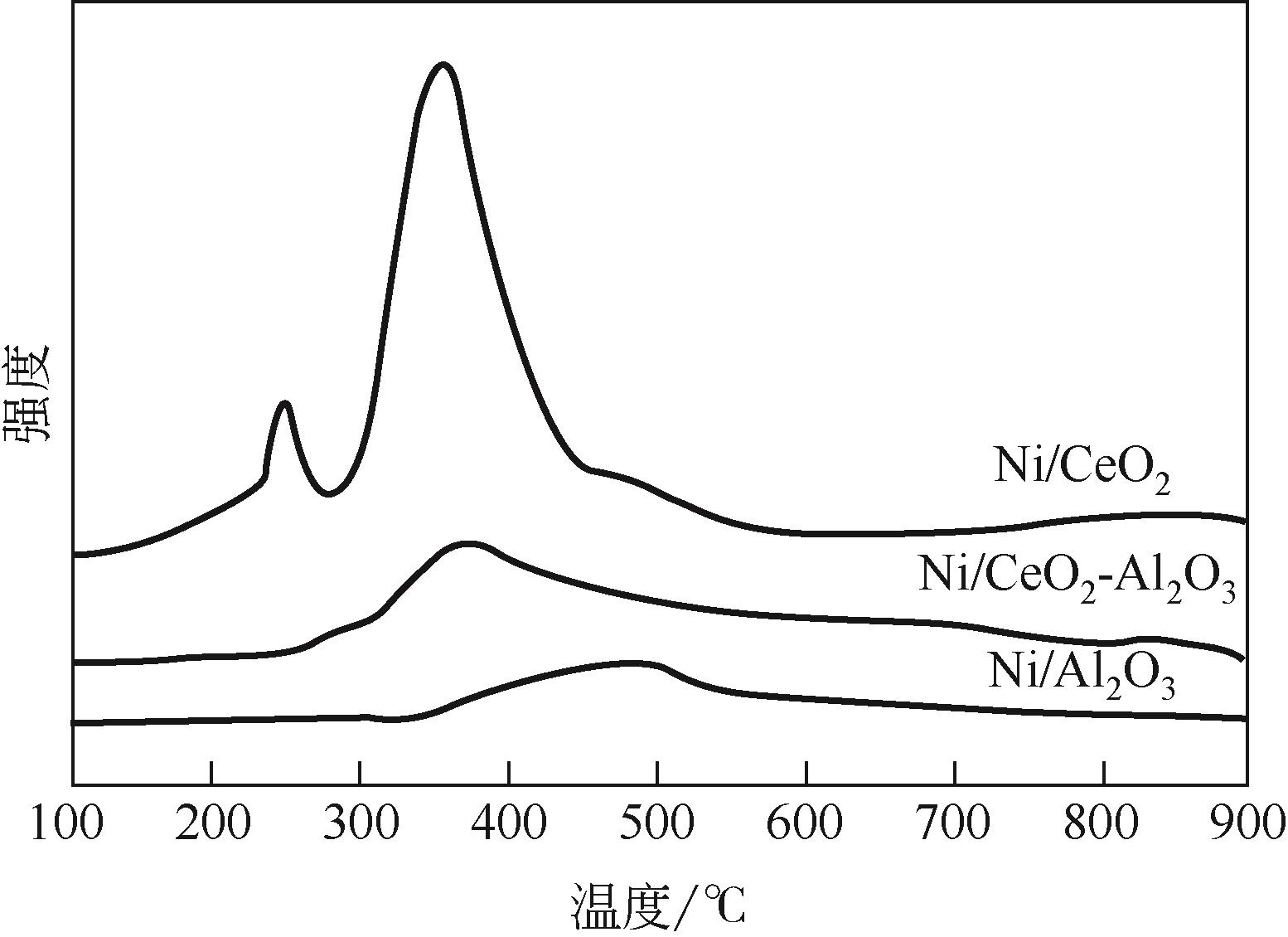
| 1 |
CHOUDHURY M B I, AHMED S, SHALABI M A, et al. Preferential methanation of CO in a syngas involving CO2 at lower temperature range[J]. Applied Catalysis A: General, 2006, 314(1): 47-53.
|
| 2 |
ECKLE S, ANFANG H, BEHM R J. What drives the selectivity for CO methanation in the methanation of CO2-rich reformate gases on supported Ru catalysts[J]. Applied Catalysis A: Genenal, 2011, 391(1/2): 325-333.
|
| 3 |
ABDEL-MAGEED A M, ECKLE S, ANFANG H G, et al. Selective CO methanation in CO2-rich H2 atmospheres over a Ru/zeolite catalyst: the influence of catalyst calcination[J]. Journal of Catalysis, 2013, 298:148-160.
|
| 4 |
TADA Shaohei, SHIMIZU Teruyuki, KAMEYAMA Hiromichi, et al. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures[J]. International Journal of Hydrogen Energy, 2012, 37: 5527-5531.
|
| 5 |
TADA Shohei, OCHIENG Ochieng James, KIKUCHI Ryuji, et al. Promotion of CO2 methanation activity and CH4 selectivity at low temperatures over Ru/CeO2/Al2O3 catalysts[J]. International Journal of Hydrogen Energy, 2014, 39: 10090-10100.
|
| 6 |
SHARMA Sudhanshu, HU Zhenpeng, ZHANG Peng, et al. CO2 methanation on Ru-doped ceria[J]. Journal of Catalysis, 2011, 278: 297-309.
|
| 7 |
WANG Fei, HE Shan, CHEN Hao, et al. Active site dependent reaction mechanism over Ru/CeO2 catalyst toward CO2 methanation[J]. Journal of the American Chemical Society, 2016, 138(19): 6298-6305.
|
| 8 |
MA Yuan, LIU Jiao, CHU Mo, et al. Cooperation between active metal and basic support in Ni-based catalyst for low-temperature CO2 methanation[J]. Catalysis Letters, 2020, 150: 1418-1426.
|
| 9 |
GÓMEZ L, MARTÍNEZ I, NAVARRO M V, et al. Sorption-enhanced CO and CO2 methanation (SEM) for the production of high purity methane[J]. Chemical Engineering Journal, 2022, 440: 135842- 135854.
|
| 10 |
CAO X Y, PU T C, LIS B M, et al. Controlling the reconstruction of Ni/CeO2 catalyst during reduction for enhanced CO methanation[J]. Engineering, 2022, 14: 94-99.
|
| 11 |
ZHANG Tengfei, WANG Weiwei, GU Fangna, et al. Enhancing the low-temperature CO2 methanation over Ni/La-CeO2 catalyst: The effects of surface oxygen vacancy and basic site on the catalytic performance[J]. Applied Catalysis B: Environmental, 2022, 312: 121385-121401.
|
| 12 |
SAWAHARA Keito, YATAGAI Kohei, BOLL Torben, et al. Role of atomic hydrogen supply on the onset of CO2 methanation over La-Ni based hydrogen storage alloys studied by in-situ approach[J]. International Journal of Hydrogen Energy, 2022, 47: 19051-19061.
|
| 13 |
CHEN Jianhui, SHEN Xuqiang, WANG Qianjuan, et al. CO2 methanation over γ-Al2O3 nanosheets-stabilized Ni catalysts: Effects of MnO x and MoO x additives on catalytic performance and reaction pathway[J]. Journal of CO2 Utilization, 2022, 63: 102113-102125.
|
| 14 |
Sergio LÓPEZ-RODRÍGUEZ, Arantxa DAVÓ-QUÍNONERO, Esther BAILÓN-GARCÍA, et al. Monitoring by in situ NAP-XPS of active sites for CO2 methanation on a Ni/CeO2 catalyst[J]. Journal of CO2 Utilization, 2022, 60: 101980-101990.
|
| 15 |
YI Huilin, XUE Qiangqiang, LU Shuliang, et al. Effect of pore structure on Ni/Al2O3 microsphere catalysts for enhanced CO2 methanation[J]. Fuel, 2022, 315: 123262-123273.
|
| 16 |
GAO Wenli, YIN Qiangfeng, MENG Xin, et al. Excellent behaviors of highly dispersed Ni-based catalyst in CO methanation synthesized by in-situ hydrothermal method with carbon quantum dots assisted[J]. Fuel, 2022: DOI: 10.106/j.fuel.2021.121813.
|
| 17 |
VRIJBURG W L, GARBARINO G, CHEN W, et al. Ni-Mn catalysts on silica-modified alumina for CO2 methanation[J]. Journal of Catalysis, 2020, 382: 358-371.
|
| 18 |
KARELOVIC Alejandro, RUIZ Patricio. Mechanistic study of low temperature CO2 methanation over Rh/TiO2 catalysts[J]. Journal of Catalysis, 2013, 301(5): 141-153.
|
| 19 |
DAMYANOVA S, BUENO J M C. Effect of CeO2 loading on the surface and catalytic behaviors of CeO2-Al2O3-supported Pt catalysts[J]. Applied Catalysis A: General, 2003, 253(1): 135-150.
|
| 20 |
PINO Lidia, VITA Antonio, CIPITI Francesco, et al. Hydrogen production by methane tri-reforming process over Ni-ceria catalysts: effect of La-doping[J]. Applied Catalysis B: Environmental, 2011, 104: 64-73.
|
| 21 |
王国栋, 郭亚飞, 李佳媛, 等. 碱/碱土金属修饰Ni基催化剂的CO2吸附与甲烷化性能[J]. 化工进展, 2021, 40(12): 6925-6933.
|
|
WANG Guodong, GUO Yafei, LI Jiayuan, et al. CO2 adsorption and methanation performance of nickel-based catalysts modified with alkali/alkaline-earth metals[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6925-6933.
|
| 22 |
彭超, 陈建富, 王海丰, 等. Ni/Ce0.75Zr0.25O2界面催化CO2甲烷化密度泛函理论研究[J]. 中国科学: 化学, 2015, 45: 1291-1298.
|
|
PENG Chao, CHEN Jianfu, WANG Haifeng, et al. Density functional theory study of catalytic methanation of CO2 at the Ni/Ce0.75Zr0.25O2 interface[J]. Scientia Sinica (Chimica), 2015, 45: 1291-1298.
|
| 23 |
张荣斌, 徐校燕, 王亮, 等. 酸化及碱化膨润土负载镍催化CO2甲烷化反应[J]. 应用化学, 2011, 28: 203-208.
|
|
ZHANG Rongbin, XU Xiaoyan, WANG Liang, et al. Catalytic methanation of CO2 over Ni supported on acidified and alkalified bentontie[J]. Chinese Journal of Applied Chemistry, 2011, 28: 203-208.
|
| 24 |
RAZZAQ Rauf, ZHU Hongwei, JIANG Li, et al. Catalytic methanation of CO and CO2 in coke oven gas over Ni-Co/ZrO2-CeO2 [J]. Industrial Engineering Chemistry Research, 2013, 52: 2247-2256.
|
| 25 |
PAN Qiushi, PENG Jiaxi, SUN Tianjun, et al. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites[J]. Catalysis Communications, 2014, 45(1): 74-78.
|
| 26 |
佟云艳. CeO2基固溶体负载Ni和Ru催化剂CO2甲烷化反应机理研究[D]. 南昌, 南昌大学, 2020.
|
|
TONG Yunyan. Study on CO2 methanation mechanism on CeO2-based solid solution supported Ni and Ru catalysts[D]. Nanchang: Nanchang University, 2020.
|
| 27 |
GUO Xinpeng, HE Hongyan, TRAITANGWONG Atsadang, et al. Ceria imparts superior low temperature activity to nickel catalysts for CO2 methanation[J]. Catalysis Science & Technology, 2019, 9: 5636-5650.
|
| 28 |
SUN C, BEAUNIER P, LA PAROLA V, et al. Ni/CeO2 nanoparticles promoted by yttrium doping as catalysts for CO2 methanation[J]. ACS Applied Nano Materials, 2020, 3: 12355-12368.
|
 ), 肖晴月1,3, 岳君容1, 崔彦斌1, 刘姣1(
), 肖晴月1,3, 岳君容1, 崔彦斌1, 刘姣1( ), 许光文3
), 许光文3
 ), XIAO Qingyue1,3, YUE Junrong1, CUI Yanbin1, LIU Jiao1(
), XIAO Qingyue1,3, YUE Junrong1, CUI Yanbin1, LIU Jiao1( ), XU Guangwen3
), XU Guangwen3