化工进展 ›› 2023, Vol. 42 ›› Issue (5): 2353-2370.DOI: 10.16085/j.issn.1000-6613.2022-1322
析氧助催化剂增强光阳极光电催化分解水性能研究进展
符淑瑢1( ), 王丽娜1, 王东伟2, 刘蕊2, 张晓慧2, 马占伟2(
), 王丽娜1, 王东伟2, 刘蕊2, 张晓慧2, 马占伟2( )
)
- 1.兰州城市学院培黎机械工程学院,甘肃 兰州 730070
2.中国科学院兰州化学物理研究所,甘肃 兰州 730000
-
收稿日期:2022-07-14修回日期:2022-09-07出版日期:2023-05-10发布日期:2023-06-02 -
通讯作者:符淑瑢,马占伟 -
作者简介:符淑瑢(1990—),女,博士,副教授,研究方向为光电催化。E-mail:shurongfu@126.com。 -
基金资助:甘肃省科技计划(21JR7RA542);甘肃省教育厅创新基金(2022A-142);国家自然科学基金(22102194)
Oxygen evolution cocatalyst enhancing the photoanode performances for photoelectrochemical water splitting
FU Shurong1( ), WANG Lina1, WANG Dongwei2, LIU Rui2, ZHANG Xiaohui2, MA Zhanwei2(
), WANG Lina1, WANG Dongwei2, LIU Rui2, ZHANG Xiaohui2, MA Zhanwei2( )
)
- 1.School of Bailie Mechanical Engineering, Lanzhou City University, Lanzhou 730070, Gansu, China
2.Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, Gansu, China
-
Received:2022-07-14Revised:2022-09-07Online:2023-05-10Published:2023-06-02 -
Contact:FU Shurong, MA Zhanwei
摘要:
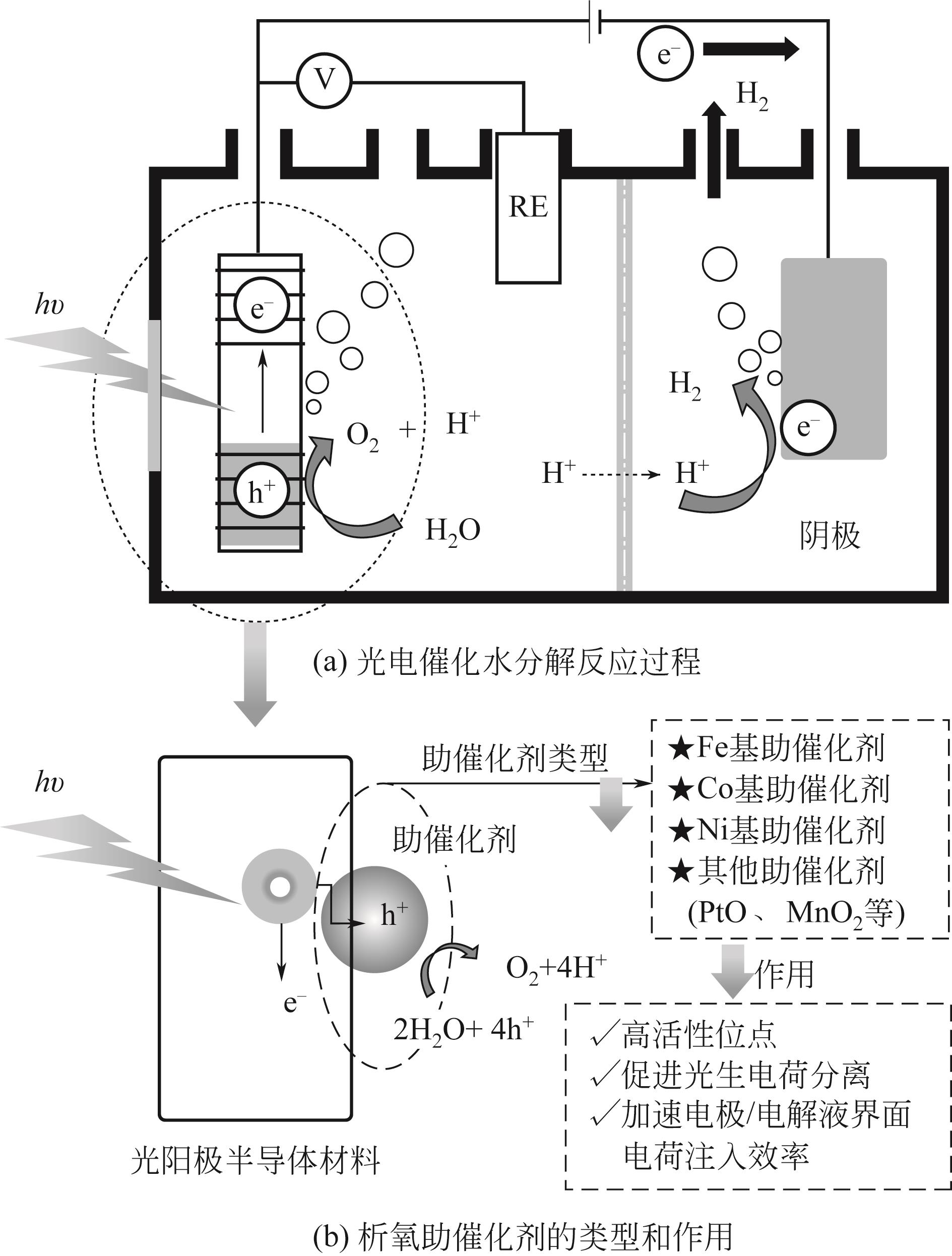
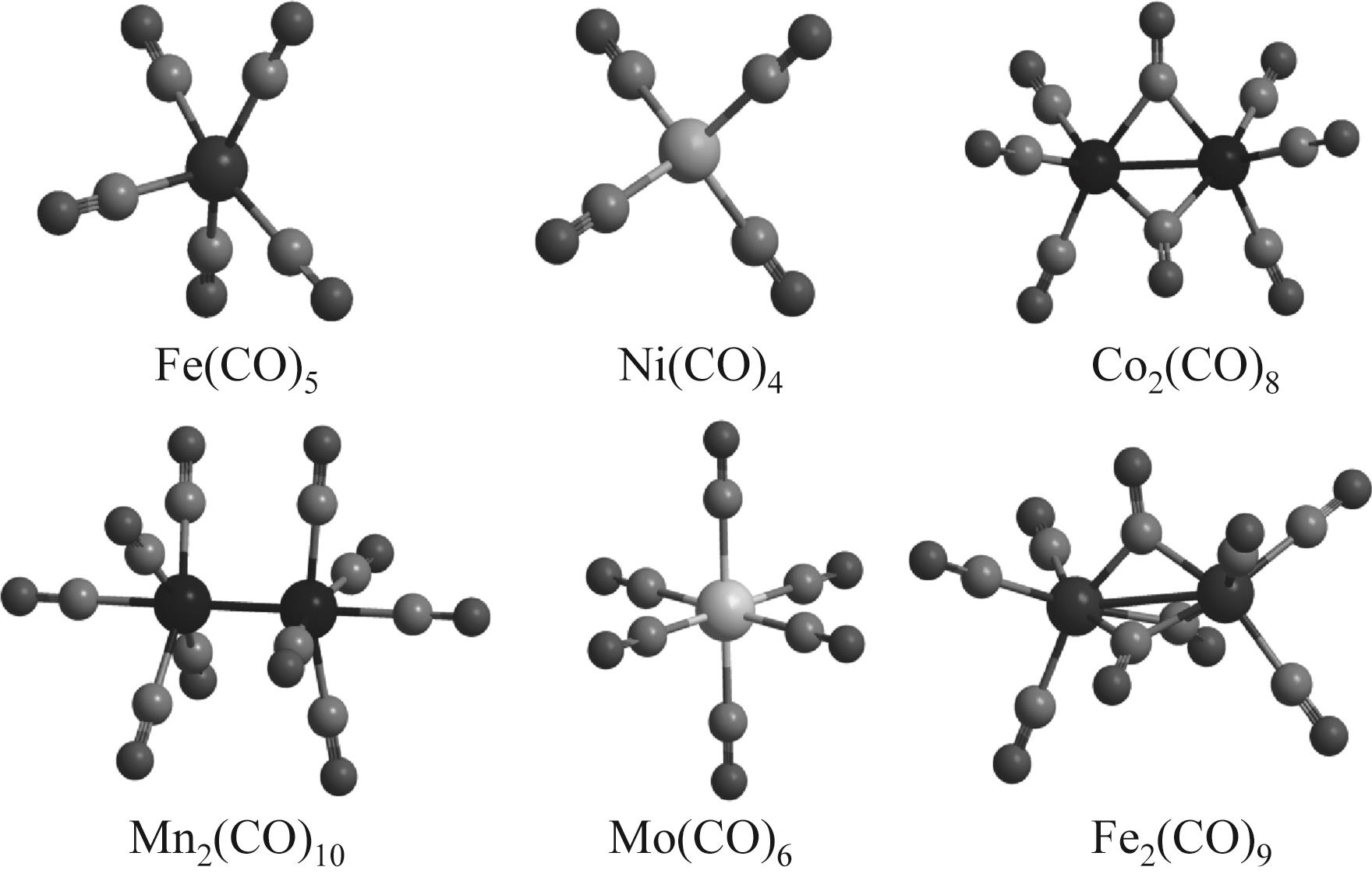
氢气因其热值高、质轻、可存储且无碳排放,被认为是最佳的碳中和能源载体。光电催化分解水制氢技术是目前生产绿氢最理想的技术途径之一,但光阳极表面水氧化速率极为缓慢,限制了阴极产氢效率。析氧助催化剂在水氧化反应过程中能够为其提供高效的活性中心,使反应易于发生,进而促进阴极产氢效率。本文从光阳极表面助催化剂的起源和作用出发,综述了近年来半导体光电极表面的助催化剂类型、微纳结构对水氧化活性的影响、界面构筑策略和光电性能研究进展。首先,阐述了电催化剂作为光阳极助催化剂在水氧化反应中所扮演的角色,不同助催化剂对半导体光电极的电荷分离和转移及稳定性的影响;然后,总结了助催化剂对半导体光电极光电催化活性的影响因素(尺寸效应、表面缺陷和氟化)。进一步归纳了助催化剂/半导体之间的界面优化策略(载流子传输通道、空穴储存层和界面化学键)。最后对析氧助催化剂所面临的问题和未来的发展方向进行了探讨和展望,认为通过调控析氧助催化剂的晶体结构、簇、单原子和界面化学键能够增强光阳极光电催化分解水的性能,并提出羰基金属是一种独特的构筑析氧助催化剂前体。
中图分类号:
引用本文
符淑瑢, 王丽娜, 王东伟, 刘蕊, 张晓慧, 马占伟. 析氧助催化剂增强光阳极光电催化分解水性能研究进展[J]. 化工进展, 2023, 42(5): 2353-2370.
FU Shurong, WANG Lina, WANG Dongwei, LIU Rui, ZHANG Xiaohui, MA Zhanwei. Oxygen evolution cocatalyst enhancing the photoanode performances for photoelectrochemical water splitting[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2353-2370.
| 序号 | 光阳极 | 电解液 | 光源 | 偏压 | 光电流密度/mA·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|
| 1 | FeOOH/BiVO4 | 0.1mol/L KH2PO4 | AM 1.5G,100mW/cm2 | 1.2VRHE | 1.7 | [ |
| 2 | FeOOH QDs/ZnO | 0.1mol/L磷酸盐缓冲液(pH=7) | AM 1.5G,100mW/cm2 | 1.23VRHE | 0.44 | [ |
| 3 | FeOOH/H-TiO2 | 1mol/L NaOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 0.6 | [ |
| 4 | α-FeOOH晶体/BiVO4 | 0.2mol/L Na2SO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 2.64 | [ |
| 5 | β-FeOOH/BiVO4 | 0.2mol/L Na2SO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 4.3 | [ |
| 6 | FeP/Ti-Fe2O3 | 1mol/L KOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 3.9 | [ |
| 7 | FeF x /Fe2O3 | 1mol/L KOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 2.4 | [ |
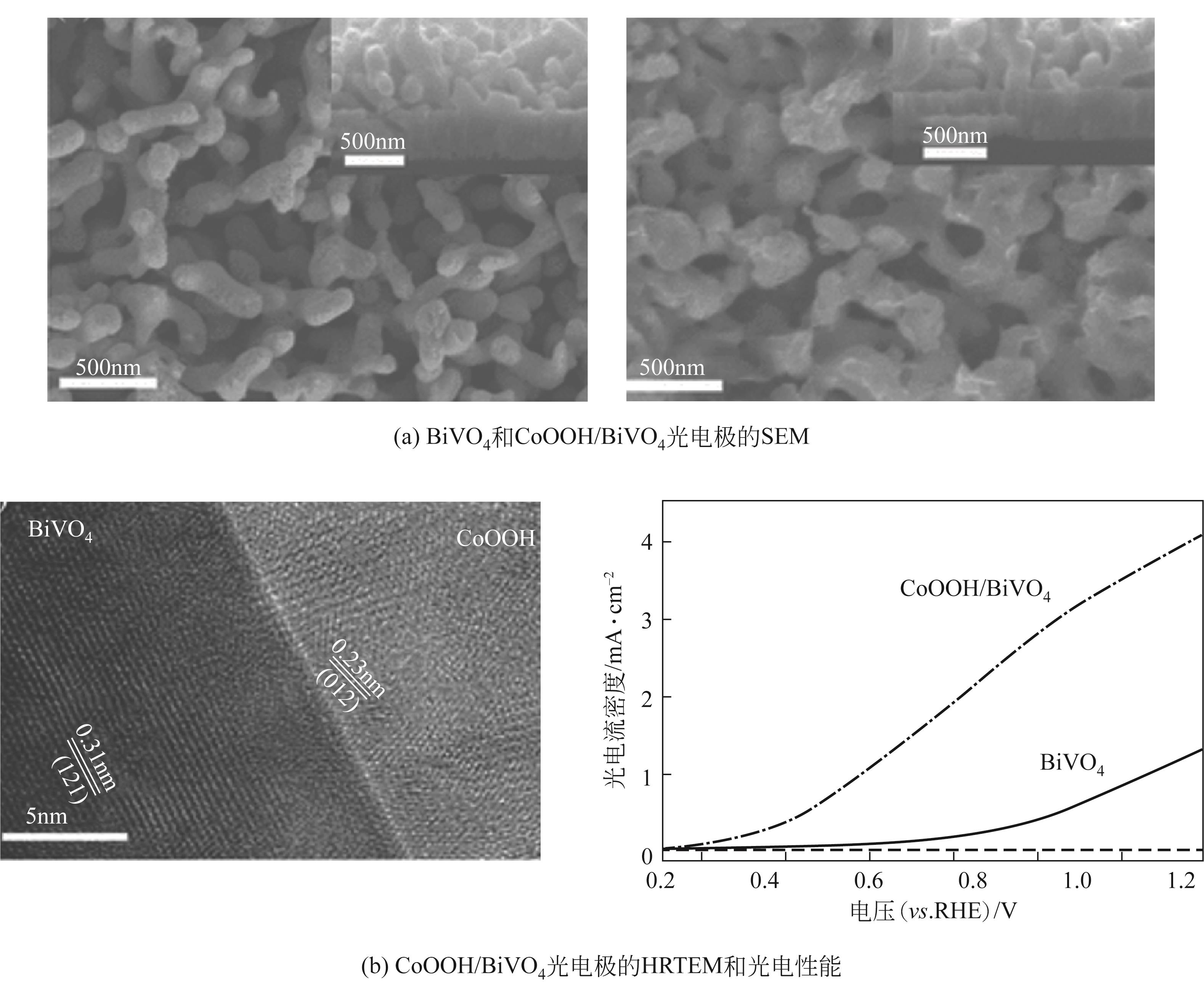
| 8 | CoOOH/BiVO4 | 0.5mol/L Na2SO4+0.2mol/L K3PO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 4.0 | [ |
| 9 | CoOOH/TiO2 | 1mol/L KOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 1.3 | [ |
| 10 | CoP/BiVO4 | 硼酸盐缓冲液 | AM 1.5G,100mW/cm2 | 1.23VRHE | 4.0 | [ |
| 11 | CoP/Fe2O3 | 1mol/L NaOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 3.54 | [ |
| 12 | MnO2/TiO2 | 1mol/L NaOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 1.95 | [ |
| 13 | PtO/ZnO | 0.2mol/L Na2SO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 2.3 | [ |
| 14 | CeO x /Fe2O3 | 1mol/LNaOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 0.6 | [ |
| 15 | NiCo2O4/Mo:BiVO4 | 0.5mol/L KH2PO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 4.5 | [ |
| 16 | CoNiO2/BiVO4 | 0.5mol/L Na2SO4 | 500W Xenon lamps | 1.23VRHE | 1.16 | [ |
| 17 | NiFeOOH/BiVO4 | 0.5mol/L K3BO3 | AM 1.5G,100mW/cm2 | 1.23VRHE | 5.8 | [ |
| 18 | FeCoW/W:BiVO4 | 1mol/L NaOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 0.49 | [ |
| 19 | CoFePi/Ti-Fe2O3 | 0.1mol/L KOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 1.75 | [ |
| 20 | FeOOH/Ag/BiVO4 | 0.1mol/L磷酸盐缓冲液(pH=7) | AM 1.5G,100mW/cm2 | 1.23VRHE | 3.19 | [ |
| 21 | NiOOH/FeOOH/CQD/BiVO4 | KH2PO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 5.99 | [ |
| 22 | NiOOH/ZnWO4/ZnO | 0.02mol/L KOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 1.7 | [ |
| 23 | NiOOH/FeOOH/BiVO4/rGO/V2O5 | 0.5mol/L Na2SO4 | AM 1.5G,100mW/cm2 | 1.5VAg/AgCl | 3.06 | [ |
表1 已报道助催化剂修饰光电极的光电性能
| 序号 | 光阳极 | 电解液 | 光源 | 偏压 | 光电流密度/mA·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|
| 1 | FeOOH/BiVO4 | 0.1mol/L KH2PO4 | AM 1.5G,100mW/cm2 | 1.2VRHE | 1.7 | [ |
| 2 | FeOOH QDs/ZnO | 0.1mol/L磷酸盐缓冲液(pH=7) | AM 1.5G,100mW/cm2 | 1.23VRHE | 0.44 | [ |
| 3 | FeOOH/H-TiO2 | 1mol/L NaOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 0.6 | [ |
| 4 | α-FeOOH晶体/BiVO4 | 0.2mol/L Na2SO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 2.64 | [ |
| 5 | β-FeOOH/BiVO4 | 0.2mol/L Na2SO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 4.3 | [ |
| 6 | FeP/Ti-Fe2O3 | 1mol/L KOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 3.9 | [ |
| 7 | FeF x /Fe2O3 | 1mol/L KOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 2.4 | [ |
| 8 | CoOOH/BiVO4 | 0.5mol/L Na2SO4+0.2mol/L K3PO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 4.0 | [ |
| 9 | CoOOH/TiO2 | 1mol/L KOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 1.3 | [ |
| 10 | CoP/BiVO4 | 硼酸盐缓冲液 | AM 1.5G,100mW/cm2 | 1.23VRHE | 4.0 | [ |
| 11 | CoP/Fe2O3 | 1mol/L NaOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 3.54 | [ |
| 12 | MnO2/TiO2 | 1mol/L NaOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 1.95 | [ |
| 13 | PtO/ZnO | 0.2mol/L Na2SO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 2.3 | [ |
| 14 | CeO x /Fe2O3 | 1mol/LNaOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 0.6 | [ |
| 15 | NiCo2O4/Mo:BiVO4 | 0.5mol/L KH2PO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 4.5 | [ |
| 16 | CoNiO2/BiVO4 | 0.5mol/L Na2SO4 | 500W Xenon lamps | 1.23VRHE | 1.16 | [ |
| 17 | NiFeOOH/BiVO4 | 0.5mol/L K3BO3 | AM 1.5G,100mW/cm2 | 1.23VRHE | 5.8 | [ |
| 18 | FeCoW/W:BiVO4 | 1mol/L NaOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 0.49 | [ |
| 19 | CoFePi/Ti-Fe2O3 | 0.1mol/L KOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 1.75 | [ |
| 20 | FeOOH/Ag/BiVO4 | 0.1mol/L磷酸盐缓冲液(pH=7) | AM 1.5G,100mW/cm2 | 1.23VRHE | 3.19 | [ |
| 21 | NiOOH/FeOOH/CQD/BiVO4 | KH2PO4 | AM 1.5G,100mW/cm2 | 1.23VRHE | 5.99 | [ |
| 22 | NiOOH/ZnWO4/ZnO | 0.02mol/L KOH | AM 1.5G,100mW/cm2 | 1.23VRHE | 1.7 | [ |
| 23 | NiOOH/FeOOH/BiVO4/rGO/V2O5 | 0.5mol/L Na2SO4 | AM 1.5G,100mW/cm2 | 1.5VAg/AgCl | 3.06 | [ |
| 1 | FUJISHIMA AKIRA, HONDA KENICHI. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38. |
| 2 | 符淑瑢, 张勤生, 鲁金芝, 等. ZnO基光电极的构筑及其光电催化水分解性能研究进展[J]. 化工进展, 2021, 40(3): 1413-1424. |
| FU Shurong, ZHANG Qinsheng, LU Jinzhi, et al. Research progress of fabrication of ZnO-based photoanode and photoelectrocatalytic water splitting performances[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1413-1424. | |
| 3 | LEE Joo-Won, CHO Ki-Hyun, YOON Joon-Soo, et al. Photoelectrochemical water splitting using one-dimensional nanostructures[J]. Journal of Materials Chemistry A, 2021, 9(38): 21576-21606. |
| 4 | 张文华, 佃丽雯, 陈海燕, 等. 氧化钨(WO3)薄膜光电催化性能的改善及应用[J]. 化工进展, 2020, 39(2): 521-532. |
| ZHANG Wenhua, DIAN Liwen, CHEN Haiyan, et al. Improvement on the photoelectrocatalytic performance of tungsten oxide (WO3) thin film and its application prospects[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 521-532. | |
| 5 | GRIMAUD Alexis, Oscar DIAZ-MORALES, HAN Binghong, et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution[J]. Nature Chemistry, 2017, 9(5): 457-465. |
| 6 | DING Chunmei, SHI Jingying, WANG Zhiliang, et al. Photoelectrocatalytic water splitting: Significance of cocatalysts, electrolyte, and interfaces[J]. ACS Catalysis, 2017, 7(1): 675-688. |
| 7 | ZHONG Miao, HISATOMI Takashi, KUANG Yongbo, et al. Surface modification of CoO x loaded BiVO4 photoanodes with ultrathin p-type NiO layers for improved solar water oxidation[J]. Journal of the American Chemical Society, 2015, 137(15): 5053-5060. |
| 8 | David TILLEY S, CORNUZ Maurin, SIVULA Kevin, et al. Light-induced water splitting with hematite: Improved nanostructure and iridium oxide catalysis[J]. Angewandte Chemie, 2010, 122(36): 6549-6552. |
| 9 | YANG Jinhui, Walczak Karl, Anzenberg Eitan, et al. Efficient and sustained photoelectrochemical water oxidation by cobalt oxide/silicon photoanodes with nanotextured interfaces[J]. Journal of the American Chemical Society, 2014, 136(17): 6191-6194. |
| 10 | David TILLEY S, SCHREIER Marcel, AZEVEDO João, et al. Ruthenium oxide hydrogen evolution catalysis on composite cuprous oxide water-splitting photocathodes[J]. Advanced Functional Materials, 2014, 24(3): 303-311. |
| 11 | CHEMELEWSKI William D, LEE Heung-Chan, LIN Jung-Fu, et al. Amorphous FeOOH oxygen evolution reaction catalyst for photoelectrochemical water splitting[J]. Journal of the American Chemical Society, 2014, 136(7): 2843-2850. |
| 12 | SEABOLD Jason A, CHOI Kyoung-Shin. Efficient and stable photo-oxidation of water by a bismuth vanadate photoanode coupled with an iron oxyhydroxide oxygen evolution catalyst[J]. Journal of the American Chemical Society, 2012, 134(4): 2186-2192. |
| 13 | ZHAN Faqi, YANG Yahui, LIU Wenhua, et al. Facile synthesis of FeOOH quantum dots modified ZnO nanorods films via a metal-solating process[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(6): 7789-7798. |
| 14 | PANZERI G, DELL’ORO R, PANZERI A, et al. FeOOH modified H-TiO2 nanorods array (NRA) for stable and improved low-bias photoelectrochemical water splitting[J]. Journal of the Electrochemical Society, 2021, 168(8): 086505. |
| 15 | ZHANG Wen, MA Jiani, XIONG Lunqiao, et al. Well-crystallized α-FeOOH cocatalysts modified BiVO4 photoanodes for efficient and stable photoelectrochemical water splitting[J]. ACS Applied Energy Materials, 2020, 3(6): 5927-5936. |
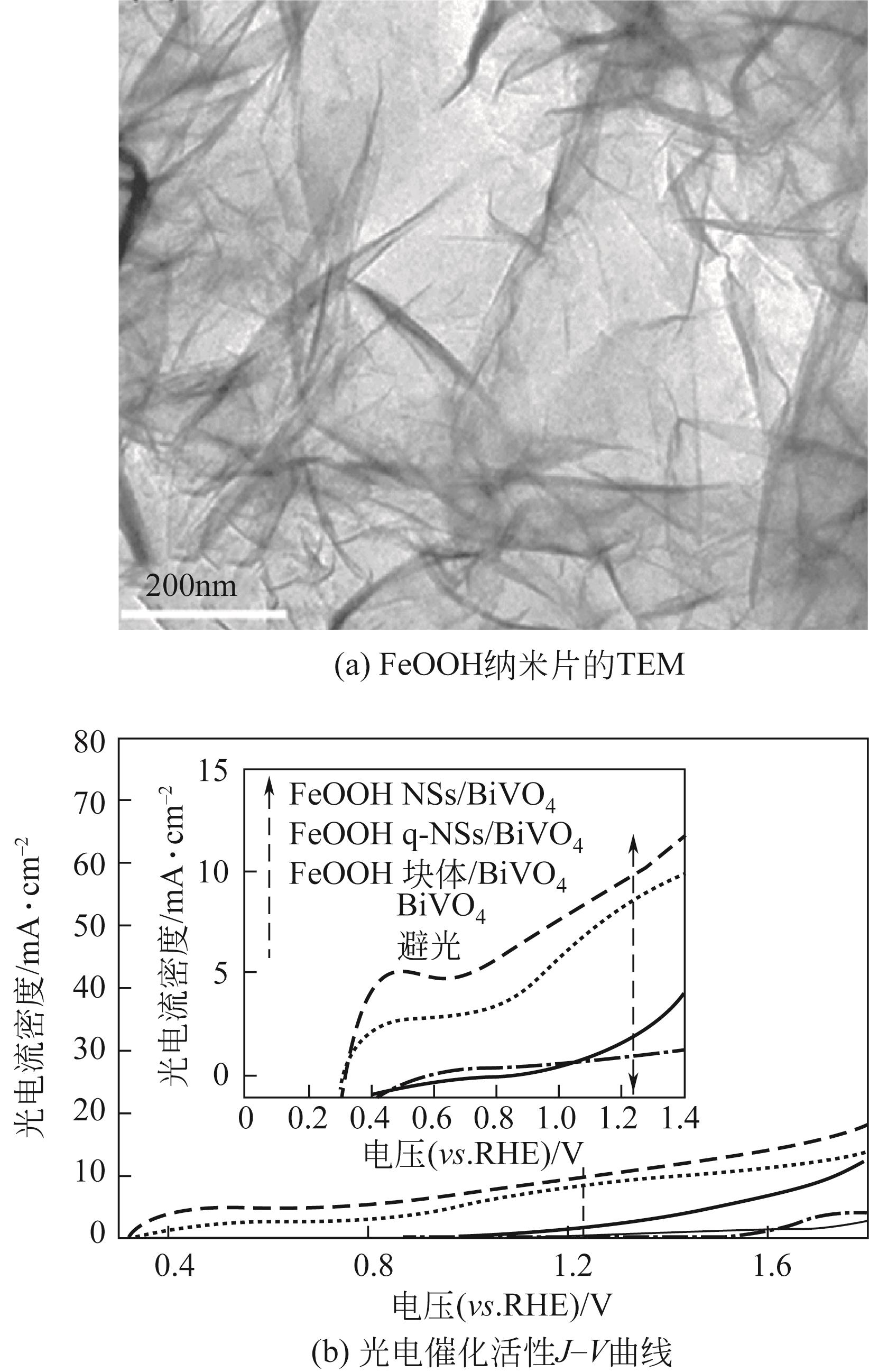
| 16 | ZHANG Beibei, WANG Lei, ZHANG Yajun, et al. Ultrathin FeOOH nanolayers with abundant oxygen vacancies on BiVO4 photoanodes for efficient water oxidation[J]. Angewandte Chemie International Edition, 2018, 57(8): 2248-2252. |
| 17 | LUO Wenjun, JIANG Chaoran, LI Yaomin, et al. Highly crystallized α-FeOOH for a stable and efficient oxygen evolution reaction[J]. Journal of Materials Chemistry A, 2017, 5(5): 2021-2028. |
| 18 | XIONG Dehua, LI Wei, WANG Xiaoguang, et al. Passivation of hematite nanorod photoanodes with a phosphorus overlayer for enhanced photoelectrochemical water oxidation[J]. Nanotechnology, 2016, 27(37): 375401. |
| 19 | BU Qijing, LI Shuo, WU Qiannan, et al. In situ synthesis of FeP-decorated Ti-Fe2O3: An effective strategy to improve the interfacial charge transfer in the photoelectrochemical water oxidation reaction[J]. Catalysis Science & Technology, 2019, 9(20): 5812-5818. |
| 20 | FENG Chenchen, WANG Lei, FU Shurong, et al. Ultrathin FeF x nanolayers accelerating hole transfer for enhanced photoelectrochemical water oxidation[J]. Journal of Materials Chemistry A, 2018, 6(40): 19342-19346. |
| 21 | WANG Youwei, QIU Wujie, SONG Erhong, et al. Adsorption-energy-based activity descriptors for electrocatalysts in energy storage applications[J]. National Science Review, 2018, 5(3): 327-341. |
| 22 | ZHONG Diane K, SUN Jianwei, INUMARU Hiroki, et al. Solar water oxidation by composite catalyst/α-Fe2O3 photoanodes[J]. Journal of the American Chemical Society, 2009, 131(17): 6086-6087. |
| 23 | ZHONG Diane K, CHOI Sujung, GAMELIN Daniel R. Near-complete suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W: BiVO4 [J]. Journal of the American Chemical Society, 2011, 133(45): 18370-18377. |
| 24 | NELLIST Michael, QIU Jingjing, LASKOWSKI Forrest A L, et al. Potential-sensing electrochemical AFM shows CoPi as a hole collector and oxygen evolution catalyst on BiVO4 water-splitting photoanodes[J]. ACS Energy Letters, 2018, 3(9): 2286-2291. |
| 25 | DING Chunmei, SHI Jingying, WANG Donge, et al. Visible light driven overall water splitting using cocatalyst/BiVO4 photoanode with minimized bias[J]. Physical Chemistry Chemical Physics, 2013, 15(13): 4589-4595. |
| 26 | LIAO Maijia, FENG Jianyong, LUO Wenjun, et al. Co3O4 nanoparticles as robust water oxidation catalysts towards remarkably enhanced photostability of a Ta3N5 photoanode[J]. Advanced Functional Materials, 2012, 22(14): 3066-3074. |
| 27 | BERGMANN Arno, Elias MARTINEZ-MORENO, TESCHNER Detre, et al. Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution[J]. Nature Communications, 2015, 6(1): 8625. |
| 28 | YANG Jinhui, COOPER Jason K, TOMA Francesca M, et al. A multifunctional biphasic water splitting catalyst tailored for integration with high-performance semiconductor photoanodes[J]. Nature Materials, 2017, 16(3): 335-341. |
| 29 | WANG Pengpeng, FU Ping, MA Jiangping, et al. Ultrathin cobalt oxide interlayer facilitated hole storage for sustained water oxidation over composited tantalum nitride photoanodes[J]. ACS Catalysis, 2021, 11(20): 12736-12744. |
| 30 | WANG Hsin-Yi, HUNG Sung-Fu, CHEN Hanyi, et al. In operando identification of geometrical-site-dependent water oxidation activity of spinel Co3O4 [J]. Journal of the American Chemical Society, 2016, 138(1): 36-39. |
| 31 | TANG Fumin, CHENG Weiren, SU Hui, et al. Smoothing surface trapping states in 3D coral-like CoOOH-wrapped-BiVO4 for efficient photoelectrochemical water oxidation[J]. ACS Applied Materials & Interfaces, 2018, 10(7): 6228-6234. |
| 32 | REN Xiangrong, JI Yujin, ZHAI Yiyue, et al. Self-assembled CoOOH on TiO2 for enhanced photoelectrochemical water oxidation[J]. Journal of Energy Chemistry, 2021, 60: 512-521. |
| 33 | TONG Haili, JIANG Yi, ZHANG Qian, et al. Boosting photoelectrochemical water oxidation with cobalt phosphide nanosheets on porous BiVO4 [J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 769-778. |
| 34 | KIM Jin Hyun, HAN Suenghoon, Yim Hyun JO, et al. A precious metal-free solar water splitting cell with a bifunctional cobalt phosphide electrocatalyst and doubly promoted bismuth vanadate photoanode[J]. Journal of Materials Chemistry A, 2018, 6(3): 1266-1274. |
| 35 | JIANG Daochuan, ZHANG Lei, YUE Qiudi, et al. Efficient suppression of surface charge recombination by CoP-modified nanoporous BiVO4 for photoelectrochemical water splitting[J]. International Journal of Hydrogen Energy, 2021, 46(29): 15517-15525. |
| 36 | QUANG Nguyen Duc, HU Weiguang, CHANG Hyo Sik, et al. Fe2O3 hierarchical tubular structure decorated with cobalt phosphide (CoP) nanoparticles for efficient photoelectrochemical water splitting[J]. Chemical Engineering Journal, 2021, 417: 129278. |
| 37 | MA Yuli, HU Yun hang. Efficient Ni(OH)2/WO3 photoanode for photoelectrocatalytic water splitting at low bias[J]. The Journal of Physical Chemistry C, 2020, 124(36): 19447-19456. |
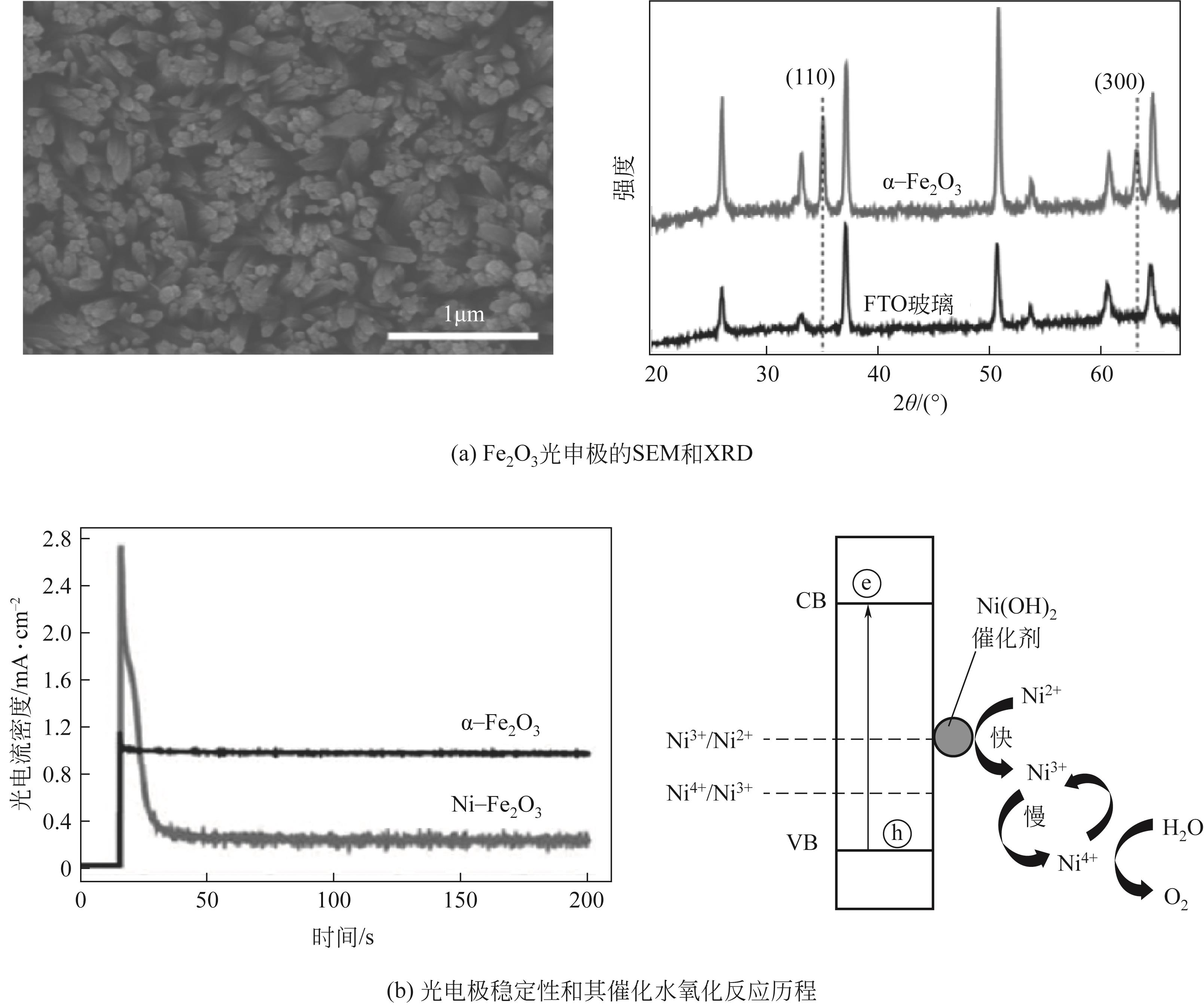
| 38 | XU Dandan, FU Zewen, WANG Dejun, et al. A Ni(OH)2-modified Ti-doped α-Fe2O3 photoanode for improved photoelectrochemical oxidation of urea: The role of Ni(OH)2 as a cocatalyst[J]. Physical Chemistry Chemical Physics, 2015, 17(37): 23924-23930. |
| 39 | WANG Gongming, LING Yichuan, LU Xihong, et al. A mechanistic study into the catalytic effect of Ni(OH)2 on hematite for photoelectrochemical water oxidation[J]. Nanoscale, 2013, 5(10): 4129-4133. |
| 40 | MALARA Francesco, MINGUZZI Alessandro, MARELLI Marcello, et al. α-Fe2O3/NiOOH: An effective heterostructure for photoelectrochemical water oxidation[J]. ACS Catalysis, 2015, 5(9): 5292-5300. |
| 41 | RONG Jiayue, WANG Zhenzhen, LV Jiaqi, et al. Ni(OH)2 quantum dots as a stable cocatalyst modified α-Fe2O3 for enhanced photoelectrochemical water-splitting[J]. Chinese Journal of Catalysis, 2021, 42(11): 1999-2009. |
| 42 | QIU Ping, LI Fei, ZHANG Hongda, et al. Photoelectrochemical performance of α-Fe2O3@NiOOH fabricated with facile photo-assisted electrodeposition method[J]. Electrochimica Acta, 2020, 358: 136847. |
| 43 | ZHUANG Changwan, SONG Zhiyuan, YU Zhuobin, et al. Photoelectrochemical performance of TiO2 nanotube arrays modified with Ni2P co-catalyst[J]. International Journal of Hydrogen Energy, 2021, 46(7): 4981-4991. |
| 44 | WEN Peng, SU Feijing, LI Hui, et al. A Ni2P nanocrystal cocatalyst enhanced TiO2 photoanode towards highly efficient photoelectrochemical water splitting[J]. Chemical Engineering Journal, 2020, 385: 123878. |
| 45 | CHENG Xiang, DONG Guojun, ZHANG Yajun, et al. Dual-bonding interactions between MnO2 cocatalyst and TiO2 photoanodes for efficient solar water splitting[J]. Applied Catalysis B: Environmental, 2020, 267: 118723. |
| 46 | FU Shurong, ZHANG Beibei, HU Hongyan, et al. ZnO nanowire arrays decorated with PtO nanowires for efficient solar water splitting[J]. Catalysis Science & Technology, 2018, 8(11): 2789-2793. |
| 47 | AHMED Mahmoud G, ZHANG Mengyuan, Ying Fan TAY, et al. Surface modification of hematite photoanodes with CeO x cocatalyst for improved photoelectrochemical water oxidation kinetics[J]. ChemSusChem, 2020, 13(20): 5489-5496. |
| 48 | FENG Chenchen, ZHOU Qi, ZHENG Bin, et al. Ultrathin NiCo2O4 nanosheets with dual-metal active sites for enhanced solar water splitting of a BiVO4 photoanode[J]. Journal of Materials Chemistry A, 2019, 7(39): 22274-22278. |
| 49 | FANG Guozhen, LIU Zhifeng, HAN Changcun, et al. CoNiO2 as a novel water oxidation cocatalyst to enhance PEC water splitting performance of BiVO4 [J]. Chemical Communications, 2020, 56(64): 9158-9161. |
| 50 | LU Yumeng, SU Jinzhan, SHI Jinwen, et al. Surface recombination passivation of the BiVO4 photoanode by the synergistic effect of the cobalt/nickel sulfide cocatalyst[J]. ACS Applied Energy Materials, 2020, 3(9): 9089-9097. |
| 51 | KUANG Yongbo, JIA Qingxin, NISHIYAMA Hiroshi, et al. A front-illuminated nanostructured transparent BiVO4 photoanode for >2% efficient water splitting[J]. Advanced Energy Materials, 2016, 6(2): 1501645. |
| 52 | ZHANG Beibei, HUANG Xiaojuan, ZHANG Yan, et al. Unveiling the activity and stability origin of BiVO4 photoanodes with FeNi oxyhydroxides for oxygen evolution[J]. Angewandte Chemie International Edition, 2020, 59(43): 18990-18995. |
| 53 | ZHAO Fei, LI Na, WU Yun, et al. BiVO4 photoanode decorated with cobalt-manganese layered double hydroxides for enhanced photoelectrochemical water oxidation[J]. International Journal of Hydrogen Energy, 2020, 45: 31902-31912. |
| 54 | FANG Guozhen, LIU Zhifeng, HAN Changcun, et al. Promising CoFe-NiOOH ternary polymetallic cocatalyst for BiVO4-based photoanodes in photoelectrochemical water splitting[J]. ACS Applied Energy Materials, 2021, 4(4): 3842-3850. |
| 55 | ZHANG Bo, ZHENG Xueli, VOZNYY Oleksandr, et al. Homogeneously dispersed multimetal oxygen-evolving catalysts[J]. Science, 2016, 352(6283): 333-337. |
| 56 | LIANG Xiaorong, XIE Jiale, XIONG Jinyun, et al. FeCoW multimetal oxide-coated W: BiVO4 photoanode for efficient oxygen evolution[J]. Sustainable Energy & Fuels, 2018, 2(9): 2053-2059. |
| 57 | GE Ge, LIU Min, LIU Chao, et al. Ultrathin FeOOH nanosheets as an efficient cocatalyst for photocatalytic water oxidation[J]. Journal of Materials Chemistry A, 2019, 7(15): 9222-9229. |
| 58 | DU Chun, WANG Jun, LIU Xiao, et al. Ultrathin CoO x -modified hematite with low onset potential for solar water oxidation[J]. Physical Chemistry Chemical Physics, 2017, 19(21): 14178-14184. |
| 59 | CHOI Min-Ju, KIM Taemin L, CHOI Kyoung Soon, et al. Controlled band offsets in ultrathin hematite for enhancing the photoelectrochemical water splitting performance of heterostructured photoanodes[J]. ACS Applied Materials & Interfaces, 2022, 14(6): 7788-7795. |
| 60 | LIU Guang, ZHAO Yong, YAO Rui, et al. Realizing high performance solar water oxidation for Ti-doped hematite nanoarrays by synergistic decoration with ultrathin cobalt-iron phosphate nanolayers[J]. Chemical Engineering Journal, 2019, 355: 49-57. |
| 61 | LIU Qiong, CAO Fengren, WU Fangli, et al. Ultrathin amorphous Ni(OH)2 nanosheets on ultrathin α-Fe2O3 films for improved photoelectrochemical water oxidation[J]. Advanced Materials Interfaces, 2016, 3(21): 1600256. |
| 62 | YANG Gaoliang, LI Yunxiang, PANG Hong, et al. Ultrathin cobalt-manganese nanosheets: An efficient platform for enhanced photoelectrochemical water oxidation with electron-donating effect[J]. Advanced Functional Materials, 2019, 29(46): 1904622. |
| 63 | YU Xuelian, LIU Jianqiao, YIN Wenchao, et al. Ultrathin NiMn-layered double hydroxide nanosheets coupled with α-Fe2O3 nanorod arrays for photoelectrochemical water splitting[J]. Applied Surface Science, 2019, 492: 264-271. |
| 64 | YI Yunan, WU Qianbao, WANG Wei, et al. In situ depositing an ultrathin CoO x H y layer on hematite in alkaline media for photoelectrochemical water oxidation[J]. Applied Catalysis B: Environmental, 2020, 263: 118334. |
| 65 | LUO Heng, LIU Changhai, XU Yu, et al. An ultra-thin NiOOH layer loading on BiVO4 photoanode for highly efficient photoelectrochemical water oxidation[J]. International Journal of Hydrogen Energy, 2019, 44(57): 30160-30170. |
| 66 | BAO Jian, ZHANG Xiaodong, FAN Bo, et al. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation[J]. Angewandte Chemie International Edition, 2015, 54(25): 7399-7404. |
| 67 | ZHANG Beibei, HUANG Xiaojuan, HU Hongyan, et al. Defect-rich and ultrathin CoOOH nanolayers as highly efficient oxygen evolution catalysts for photoelectrochemical water splitting[J]. Journal of Materials Chemistry A, 2019, 7(9): 4415-4419. |
| 68 | SHE Houde, YUE Pengfei, HUANG Jingwei, et al. One-step hydrothermal deposition of F:FeOOH onto BiVO4 photoanode for enhanced water oxidation[J]. Chemical Engineering Journal, 2020, 392: 123703. |
| 69 | LI Yan, MEI Qiong, LIU Zejun, et al. Fluorine-doped iron oxyhydroxide cocatalyst: Promotion on the WO3 photoanode conducted photoelectrochemical water splitting[J]. Applied Catalysis B: Environmental, 2022, 304: 120995. |
| 70 | ALAM Suhaib, SAHU Tushar Kanta, QURESHI Mohammad. One-dimensional Co(OH)F as a noble metal-free redox mediator and hole extractor for boosted photoelectrochemical water oxidation in worm-like bismuth vanadate[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(14): 5155-5165. |
| 71 | GU Xinning, ZHANG Jialing, HOU Liqiong, et al. Dual modification with Ag and FeOOH significantly increased the photoelectrochemical water splitting activity of BiVO4 photoanodes[J]. Surfaces and Interfaces, 2021, 25: 101224. |
| 72 | YE Kaihang, WANG Zilong, GU Jiuwang, et al. Carbon quantum dots as a visible light sensitizer to significantly increase the solar water splitting performance of bismuth vanadate photoanodes[J]. Energy & Environmental Science, 2017, 10(3): 772-779. |
| 73 | NING Fanyu, SHAO Mingfei, XU Simin, et al. TiO2/graphene/NiFe-layered double hydroxide nanorod array photoanodes for efficient photoelectrochemical water splitting[J]. Energy & Environmental Science, 2016, 9(8): 2633-2643. |
| 74 | SUN Lixia, SUN Jianhua, YANG Xiaojun, et al. An integrating photoanode consisting of BiVO4, rGO and LDH for photoelectrochemical water splitting[J]. Dalton Transactions, 2019, 48(42): 16091-16098. |
| 75 | ZENG Guihua, HOU Liqiong, ZHANG Jialing, et al. FeOOH/rGO/BiVO4 photoanode for highly enhanced photoelectrochemical water splitting performance[J]. ChemCatChem, 2020, 12(14): 3769-3775. |
| 76 | FENG Chenchen, FU Han, JIA Henan, et al. Ultrathin Ti3C2 nanosheets served as a highly efficient hole transport layer on a Fe2O3 photoanode for photoelectrochemical water oxidation[J]. New Journal of Chemistry, 2021, 45(44): 20537-20541. |
| 77 | FU Shurong, HU Hongyan, FENG Chenchen, et al. Epitaxial growth of ZnWO4 hole-storage nanolayers on ZnO photoanodes for efficient solar water splitting[J]. Journal of Materials Chemistry A, 2019, 7(6): 2513-2517. |
| 78 | Chong Siang YAW, TANG Junwang, Ai Kah SOH, et al. Synergistic effects of dual-electrocatalyst FeOOH/NiOOH thin films as effective surface photogenerated hole extractors on a novel hierarchical heterojunction photoanode structure for solar-driven photoelectrochemical water splitting[J]. Chemical Engineering Journal, 2020, 380: 122501. |
| 79 | WANG Tong, LONG Xuefeng, WEI Shenqi, et al. Boosting hole transfer in the fluorine-doped hematite photoanode by depositing ultrathin amorphous FeOOH/CoOOH cocatalysts[J]. ACS Applied Materials & Interfaces, 2020, 12(44): 49705-49712. |
| 80 | ISMAIL Ahmed S M, Ivan GARCIA-TORREGROSA, VOLLENBROEK Jeroen C, et al. Detection of spontaneous FeOOH formation at the hematite/Ni(Fe)OOH interface during photoelectrochemical water splitting by operando X-ray absorption spectroscopy[J]. ACS Catalysis, 2021, 11(19): 12324-12335. |
| 81 | DUBALE Amare Aregahegn, SU Wei-Nien, TAMIRAT Andebet Gedamu, et al. The synergetic effect of graphene on Cu2O nanowire arrays as a highly efficient hydrogen evolution photocathode in water splitting[J]. Journal of Materials Chemistry A, 2014, 2(43): 18383-18397. |
| 82 | XU Weiwei, TIAN Wei, MENG Linxing, et al. Interfacial chemical bond-modulated Z-scheme charge transfer for efficient photoelectrochemical water splitting[J]. Advanced Energy Materials, 2021, 11(8): 2003500. |
| 83 | LI Sijie, ZHANG Limin, ZHAO Wenqing, et al. Designing interfacial chemical bonds towards advanced metal-based energy-storage/conversion materials[J]. Energy Storage Materials, 2020, 32: 477-496. |
| [1] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [4] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [5] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [6] | 叶振东, 刘涵, 吕静, 张亚宁, 刘洪芝. 基于钙镁二元盐的热化学储能反应器的性能优化[J]. 化工进展, 2023, 42(8): 4307-4314. |
| [7] | 王蕴青, 杨国锐, 延卫. 过渡金属磷化物的改性方法及其在电化学析氢中的应用[J]. 化工进展, 2023, 42(7): 3532-3549. |
| [8] | 李吉焱, 景艳菊, 邢郭宇, 刘美辰, 龙永, 朱照琪. 耐盐型太阳能驱动界面光热材料及蒸发器的研究进展[J]. 化工进展, 2023, 42(7): 3611-3622. |
| [9] | 杜保宁, 赵珊, 刘向卿, 张毅, 肖雅茹, 张少飞, 李田田, 孙金峰. 纳米多孔CuMn基氧化物电极的制备及性能[J]. 化工进展, 2023, 42(3): 1484-1492. |
| [10] | 杜涛, 马进伟, 陈茜茜, 方浩, 陈秉章, 陈厚仁. PV/T模块复合冷却模式性能对比测试与数值模拟分析[J]. 化工进展, 2023, 42(2): 722-730. |
| [11] | 张赫, 李小可, 熊颖, 文劲. 基于水凝胶界面光蒸发的压裂返排液脱盐降污处理[J]. 化工进展, 2023, 42(2): 1073-1079. |
| [12] | 张会霞, 周立山, 张程蕾, 钱光磊, 谢陈鑫, 朱令之. Bi2S3/TiO2纳米锥光阳极的制备及其光电催化降解土霉素[J]. 化工进展, 2023, 42(10): 5548-5557. |
| [13] | 许春树, 姚庆达, 梁永贤, 周华龙. 金属-有机框架材料结构设计及其对合成染料的吸附性能[J]. 化工进展, 2023, 42(10): 5322-5338. |
| [14] | 韩丽, 李望良, 李艳香, 安高军, 鲁长波. 纤维状钙钛矿太阳能电池研究进展[J]. 化工进展, 2023, 42(10): 5135-5146. |
| [15] | 白浩良, 王晨, 卢静, 康雪. 聚光光伏系统太阳能电池散热技术及发展现状[J]. 化工进展, 2023, 42(1): 159-177. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||