化工进展 ›› 2022, Vol. 41 ›› Issue (12): 6586-6605.DOI: 10.16085/j.issn.1000-6613.2022-0369
磁性生物质炭的制备及在污染水体中的应用
- 1.西安建筑科技大学华清学院,陕西 西安 710043
2.西安建筑科技大学环境与市政工程学院,陕西 西安 710055
-
收稿日期:2022-03-11修回日期:2022-07-02出版日期:2022-12-20发布日期:2022-12-29 -
通讯作者:徐金兰 -
作者简介:宋少花(1988—),女,讲师,硕士,研究方向为石油污染土壤修复技术及水污染控制。E-mail:915998273@qq.com。 -
基金资助:国家自然科学基金(51778524);陕西省教育厅专项科研计划(21JK0738);2021年西安建筑科技大学华清学院大学生创新训练项目(S202113679014)
Preparation of magnetic biochar and its application in polluted water
SONG Shaohua1( ), XU Jinlan2, SONG Xiaoqiao1, YU Yuan1
), XU Jinlan2, SONG Xiaoqiao1, YU Yuan1
- 1.Huaqing College, Xi’an University of Architecture and Technology, Xi’an 710043, Shaanxi, China
2.School of Environmental and Municipal Engineering, Xi’an University of Architecture & Technology, Xi’an 710055, Shaanxi, China
-
Received:2022-03-11Revised:2022-07-02Online:2022-12-20Published:2022-12-29 -
Contact:XU Jinlan
摘要:
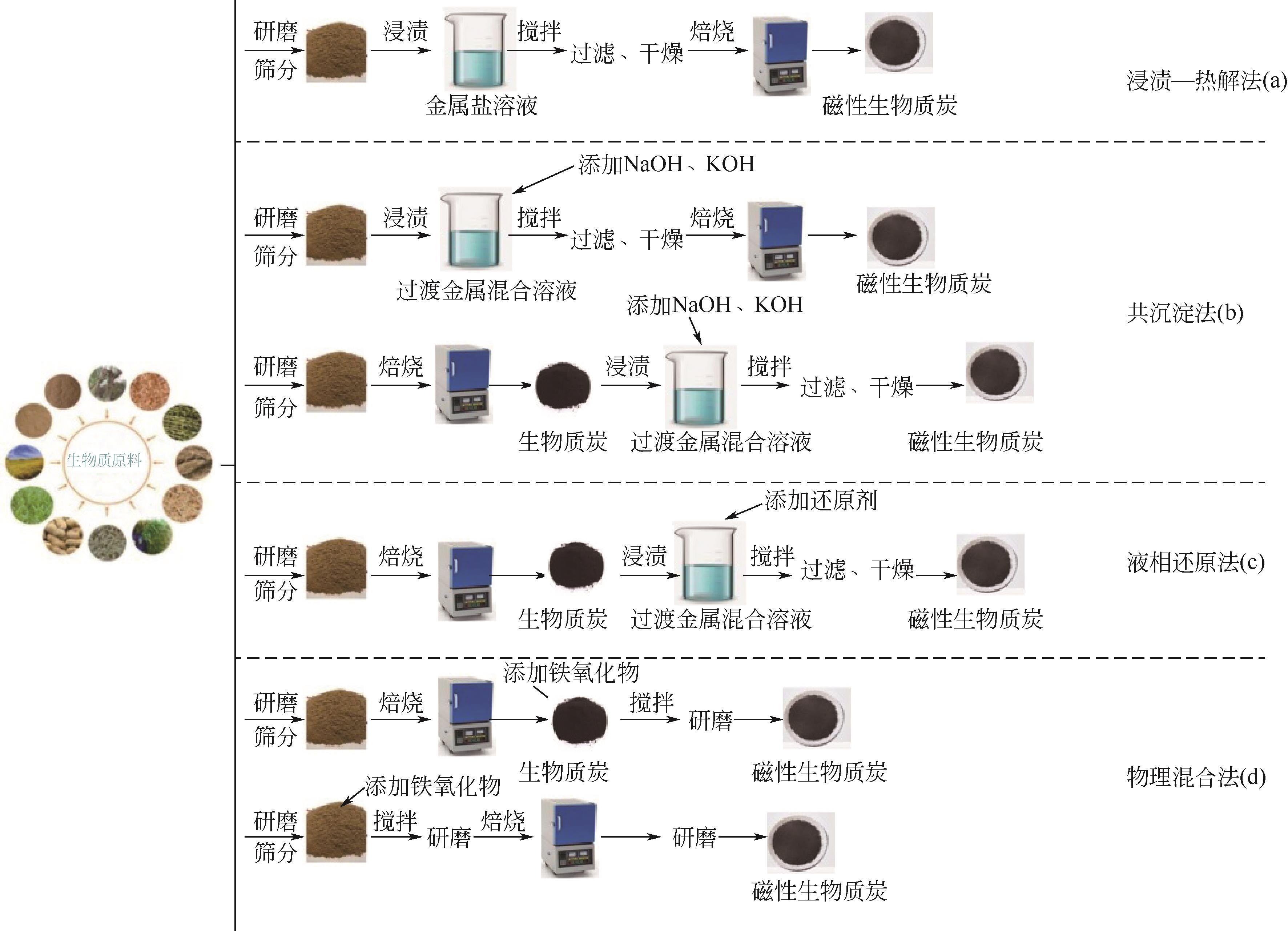
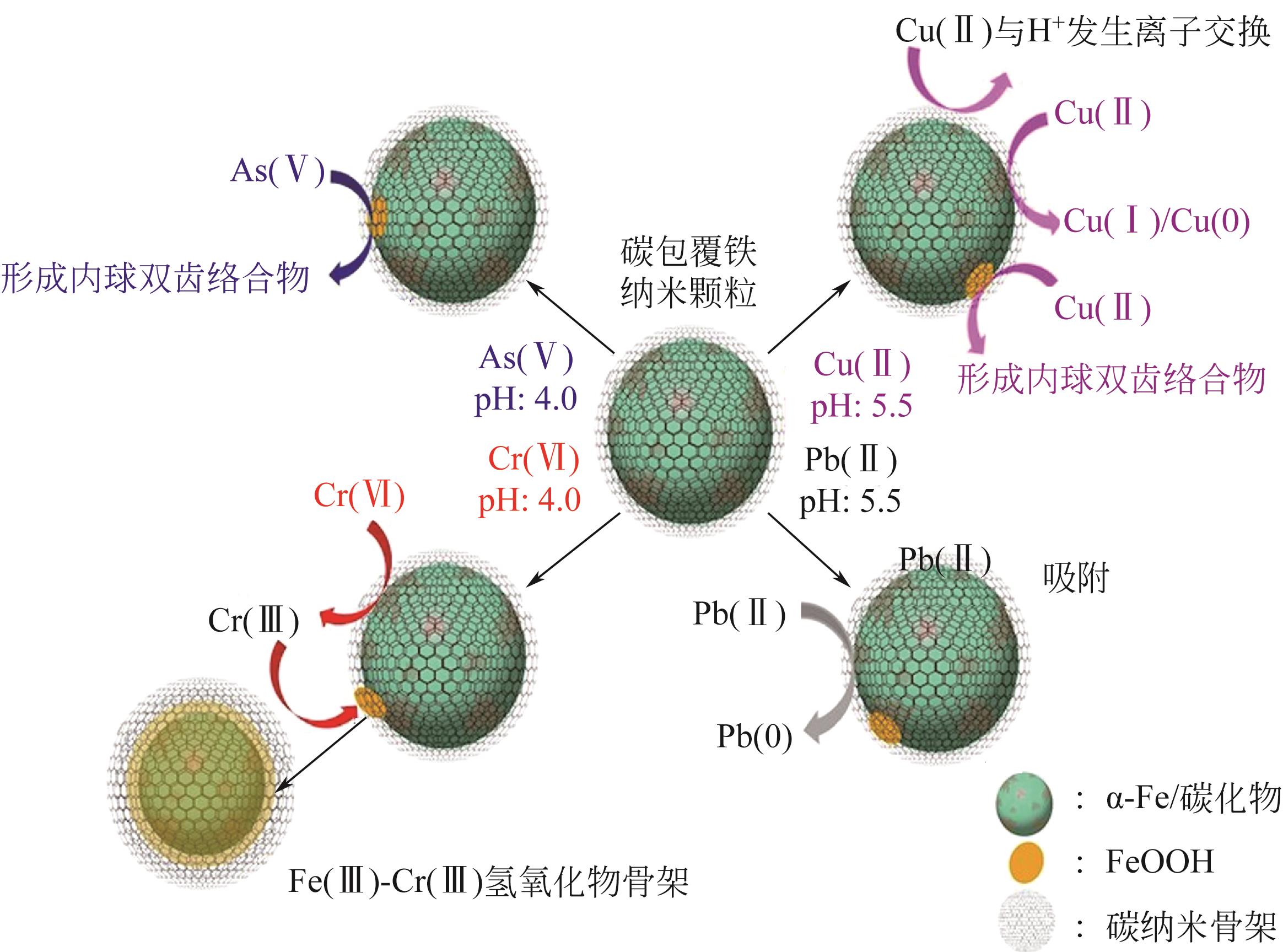
介绍了磁性生物质炭的制备方法及所需的原料,尤其是在污染水体中的应用。阐述了磁性生物质炭的制备方法,包括浸渍-热解法、液相还原法、共沉淀法和物理混合法,并指出各种制备方法的优缺点。重点分析了磁性生物质炭吸附污染物的影响因素及机理,发现磁性生物质炭的热解温度及投加量、溶液的pH、反应时间、污染物的初始浓度、吸附温度和溶液中其他竞争离子等对污染物吸附效果都有一定的影响,而且其吸附机理比较复杂,关键的吸附机理包括物理吸附、离子交换、静电吸附、共沉淀、表面络合等。详细总结磁性生物质炭在污染水体中的应用,磁性生物质炭已广泛用于去除水体中的各种污染物,包括重金属、无机阴离子、抗生素、农药、有机染料及核污染物。此外,还对磁性生物质炭的再生和回收进行了评价。
中图分类号:
引用本文
宋少花, 徐金兰, 宋晓乔, 于媛. 磁性生物质炭的制备及在污染水体中的应用[J]. 化工进展, 2022, 41(12): 6586-6605.
SONG Shaohua, XU Jinlan, SONG Xiaoqiao, YU Yuan. Preparation of magnetic biochar and its application in polluted water[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6586-6605.
| 名称 | 原材料 | 污染物 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| 磁性玉米秸秆生物质炭 | 玉米秸秆 | Cd(Ⅱ) | 33.45 | [ |
| 磁性小麦秸秆生物质炭 | 小麦秸秆 | Cd(Ⅱ) | 23. 44 | [ |
| 磁性谷壳生物质炭 | 谷壳 | Pb(Ⅱ) | 45. 7 | [ |
| NH2磁性米糠生物质炭复合材料 | 米糠 | Ni(Ⅱ) | 201.62 | [ |
| 乙二胺四乙酸(EDTA)磁性落叶生物质炭纳米复合材料 | 落叶 | Pb(Ⅱ) | 146.84 | [ |
| 磁性生物质炭负载Mg-Fe水滑石复合材料 | 油茶树果壳 | Cd(Ⅱ) Ni(Ⅱ) | 263.156 43.291 | [ |
| 磁性铁酸盐/生物质炭复合材料 | 松木屑 | Pb(Ⅱ) | 99.5 | [ |
| 磁性黑藻生物质炭复合材料 | 黑藻 | Cu(Ⅱ) | 24.28 | [ |
| 磁性生物质炭矿物复合材料 | 养猪场污泥 | Pb(Ⅱ) | 450.58 | [ |
| 磁性生物质炭 | 活性污泥 | Cd(Ⅱ) Pb(Ⅱ) | 8.5 48.05 | [ |
磁性废骨粉生物质炭 | 废骨粉 | Cd(Ⅱ) Cu(Ⅱ) Pb(Ⅱ) | 151.3 219.8 271.9 | [ |
| 磁性蘑菇渣生物质炭 | 蘑菇渣 | Cr(Ⅵ) | 47.62 | [ |
表1 不同生物质原料制备的磁性生物质炭的吸附性能
| 名称 | 原材料 | 污染物 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| 磁性玉米秸秆生物质炭 | 玉米秸秆 | Cd(Ⅱ) | 33.45 | [ |
| 磁性小麦秸秆生物质炭 | 小麦秸秆 | Cd(Ⅱ) | 23. 44 | [ |
| 磁性谷壳生物质炭 | 谷壳 | Pb(Ⅱ) | 45. 7 | [ |
| NH2磁性米糠生物质炭复合材料 | 米糠 | Ni(Ⅱ) | 201.62 | [ |
| 乙二胺四乙酸(EDTA)磁性落叶生物质炭纳米复合材料 | 落叶 | Pb(Ⅱ) | 146.84 | [ |
| 磁性生物质炭负载Mg-Fe水滑石复合材料 | 油茶树果壳 | Cd(Ⅱ) Ni(Ⅱ) | 263.156 43.291 | [ |
| 磁性铁酸盐/生物质炭复合材料 | 松木屑 | Pb(Ⅱ) | 99.5 | [ |
| 磁性黑藻生物质炭复合材料 | 黑藻 | Cu(Ⅱ) | 24.28 | [ |
| 磁性生物质炭矿物复合材料 | 养猪场污泥 | Pb(Ⅱ) | 450.58 | [ |
| 磁性生物质炭 | 活性污泥 | Cd(Ⅱ) Pb(Ⅱ) | 8.5 48.05 | [ |
磁性废骨粉生物质炭 | 废骨粉 | Cd(Ⅱ) Cu(Ⅱ) Pb(Ⅱ) | 151.3 219.8 271.9 | [ |
| 磁性蘑菇渣生物质炭 | 蘑菇渣 | Cr(Ⅵ) | 47.62 | [ |
| 名称 | 磁性前体 | 污染物 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| 磁性海藻生物质炭复合材料 | FeCl3·6H2O、FeSO4·7H2O | 甲基橙 | 240.3 | [ |
| 磁性污泥生物质炭 | FeCl3·6H2O | Cd(Ⅱ) | 167.42 | [ |
| 磁性明胶改性生物质炭 | FeCl2、FeCl3 | 双氯芬酸钠 | 266 | [ |
| 磁性玉米生物质炭 | FeCl3 | As(Ⅲ) | 51.81 | [ |
| 磁性稻壳生物质炭 | FeSO4·7H2O | 菲 | 89. 64 | [ |
| 磁性花生壳生物质炭复合材料 | FeSO4·7H2O、Fe2(SO4)3 | 磷 | 22. 32 | [ |
| 磁性松木生物炭 | 赤铁矿 | As(Ⅲ) | 429 | [ |
| 天然铁矿石-生物质炭复合材料 | 赤铁矿 黄铁矿 | 诺氟沙星 | 1.676 1.978 | [ |
| 磁性生物炭复合材料 | 菱铁矿 | U(Ⅵ) | 52.63 | [ |
| 磁性生物质炭 | Fe2O3 | 磷酸盐 | 330.86 | [ |
| 磁性花生壳生物质炭 | Fe3O4 | 亚甲基蓝 | 666.67 | [ |
表2 不同磁性前体制备的磁性生物质炭的吸附性能
| 名称 | 磁性前体 | 污染物 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| 磁性海藻生物质炭复合材料 | FeCl3·6H2O、FeSO4·7H2O | 甲基橙 | 240.3 | [ |
| 磁性污泥生物质炭 | FeCl3·6H2O | Cd(Ⅱ) | 167.42 | [ |
| 磁性明胶改性生物质炭 | FeCl2、FeCl3 | 双氯芬酸钠 | 266 | [ |
| 磁性玉米生物质炭 | FeCl3 | As(Ⅲ) | 51.81 | [ |
| 磁性稻壳生物质炭 | FeSO4·7H2O | 菲 | 89. 64 | [ |
| 磁性花生壳生物质炭复合材料 | FeSO4·7H2O、Fe2(SO4)3 | 磷 | 22. 32 | [ |
| 磁性松木生物炭 | 赤铁矿 | As(Ⅲ) | 429 | [ |
| 天然铁矿石-生物质炭复合材料 | 赤铁矿 黄铁矿 | 诺氟沙星 | 1.676 1.978 | [ |
| 磁性生物炭复合材料 | 菱铁矿 | U(Ⅵ) | 52.63 | [ |
| 磁性生物质炭 | Fe2O3 | 磷酸盐 | 330.86 | [ |
| 磁性花生壳生物质炭 | Fe3O4 | 亚甲基蓝 | 666.67 | [ |
| 制备方法 | 优点 | 缺点 | 参考文献 |
|---|---|---|---|
| 浸渍-热解法 | 生物质炭的磁化和热解同时完成;操作简单;所得磁性生物质炭稳定性好;金属浸出少;制备过程中产生的废液较少 | 热解过程中会产生气体污染物和焦油,处理不当会造成二次污染;需要较高的热解温度(300~1000℃),导致能耗较高 | [ |
| 共沉淀法 | 所得磁性生物质炭稳定性好,金属浸出少;无需过高温度;高生产率;操作简单;可控性强 | 需要引入大量碱性试剂,增加了成本;碱性废水需要处理 | [ |
| 液相还原法 | 可以得到负载有零价金属的磁性生物质炭;所得磁性生物质炭还原性强,稳定性好;可控性强;生产效率高 | 添加的还原剂有毒,需要妥善储存和使用,并考虑后续处理;氢气通常在制备过程中产生,存在一定的安全隐患 | [ |
| 物理混合法 | 制备方法绿色且经济,而且可制备有效的低成本磁性吸附剂 | 混合不均匀;制备的磁性生物质炭有团聚的可能 | [ |
表3 不同磁性生物质炭制备方法的优缺点
| 制备方法 | 优点 | 缺点 | 参考文献 |
|---|---|---|---|
| 浸渍-热解法 | 生物质炭的磁化和热解同时完成;操作简单;所得磁性生物质炭稳定性好;金属浸出少;制备过程中产生的废液较少 | 热解过程中会产生气体污染物和焦油,处理不当会造成二次污染;需要较高的热解温度(300~1000℃),导致能耗较高 | [ |
| 共沉淀法 | 所得磁性生物质炭稳定性好,金属浸出少;无需过高温度;高生产率;操作简单;可控性强 | 需要引入大量碱性试剂,增加了成本;碱性废水需要处理 | [ |
| 液相还原法 | 可以得到负载有零价金属的磁性生物质炭;所得磁性生物质炭还原性强,稳定性好;可控性强;生产效率高 | 添加的还原剂有毒,需要妥善储存和使用,并考虑后续处理;氢气通常在制备过程中产生,存在一定的安全隐患 | [ |
| 物理混合法 | 制备方法绿色且经济,而且可制备有效的低成本磁性吸附剂 | 混合不均匀;制备的磁性生物质炭有团聚的可能 | [ |
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 重金属 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 玉米秸秆 | 500 | 3 | 23.58 | — | Cd(Ⅱ) | 33.45 | [ |
| 小麦秸秆 | 450 | 2 | 152.24 | 0.13 | Cd(Ⅱ) | 23.44 | [ |
| 城市污泥 | 500 | 3 | 82.39 | 0.18 | Cd(Ⅱ) | 167.42 | [ |
| 棕榈纤维 | 400 | 2 | 117.62 | — | Cd(Ⅱ) | 197.96 | [ |
| 谷壳 | 400 | 4 | 72.94 | — | Pb(Ⅱ) | 43.9 | [ |
| 落叶 | 450 | 1 | — | — | Pb(Ⅱ) | 146.84 | [ |
| 松木屑 | 200 | 8 | 138.5 | 0.288 | Pb(Ⅱ) | 99.5 | [ |
| 贝壳 | 200 | 8 | 57.5 | — | Pb(Ⅱ) | 129.31 | [ |
| 杉木屑 | 700 | 2 | 168 | 0.181 | Pb(Ⅱ) | 817.64 | [ |
| 玉米秸秆 | 600 | 3 | — | — | Pb(Ⅱ) | 210.85 | [ |
| 小麦秸秆 | 800 | 2 | 44.31 | 0.0652 | Pb(Ⅱ) | 196.91 | [ |
| 松木屑 | 200 | 8 | 138.5 | — | Pb(Ⅱ) | 99.5 | [ |
| 养猪场污泥 | 200 | 4 | 23.32 | — | Pb(Ⅱ) | 459.24 | [ |
| 300 | 39.99 | — | 355.89 | ||||
| 400 | 27.63 | — | 369.02 | ||||
| 500 | 18.59 | — | 450.58 | ||||
| 600 | 22.61 | — | 402.78 | ||||
| 赤泥 | 350 | 1 | 9.31 | 0.05 | Cr(Ⅵ) | 125.37 | [ |
| 650 | 75.01 | 0.10 | 325.35 | ||||
| 850 | 106.03 | 0.15 | 363.35 | ||||
| 浒苔 | 400 | 2 | 708.27 | — | Cr(Ⅵ) | 53.08 | [ |
| 800 | 780.14 | — | 95.23 | ||||
| 花生壳 | 650 | 1 | 2.40 | 0.02 | Cr(Ⅵ) | 182.32 | [ |
| 700 | 1.80 | 0.01 | 195.22 | ||||
| 750 | 7.74 | 0.03 | 208.33 | ||||
| 800 | 8.81 | 0.05 | 223.21 | ||||
| 小麦秸秆 | 500 | 1 | 33.73 | 0.128 | Cr(Ⅵ) | 23.94 | [ |
| 香菇废基质 | 600 | 4 | 92.78 | 0.112 | Cr(Ⅵ) | 47.62 | [ |
| 纳氏叶栅藻 | 200 | 8 | 142.49 | 0.38 | Cr(Ⅵ) | 56.79 | [ |
| 污水厂污泥 | 500 | 1 | — | — | Cr(Ⅵ) | 11.56 | [ |
| 竹子 | 700 | 2 | — | — | Cr(Ⅵ) | 127 | [ |
| 花生壳 | 220 | 12 | 62.4 | 0.34 | Cr(Ⅵ) | 142.86 | [ |
| 柚皮 | 300 | 1 | 35.88 | 0.024 | Cr(Ⅵ) | 209.64 | [ |
| 稻壳 | 500 | 2 | 17.6 | — | Cr(Ⅵ) | 23.25 | [ |
| 凤凰树叶 | 500 | 2 | 83.6 | — | Cr(Ⅵ) | 55.0 | [ |
| 马缨丹 | 800 | 2 | 607.53 | — | Cr(Ⅵ) | 161.23 | [ |
| 米糠 | 500 | 3 | — | — | Ni(Ⅱ) | 201.62 | [ |
| 黑藻 | 450 | 2 | — | — | Cu(Ⅱ) | 24.28 | [ |
| 丝瓜络 | 700 | 5 | — | — | Cu(Ⅱ) | 54.68 | [ |
| 马尾松锯末 | 240 | 8 | 63.75 | 0.35 | Cu(Ⅱ) | 9.58 | [ |
| 木屑 | 500 | 3 | 25.654 | 0.13 | Cu(Ⅱ) | 3.23 | [ |
| 苹果渣 | 600 | 1 | 102.18 | 0.09 | Ag(Ⅰ) | 818.4 | [ |
| 西瓜皮 | 500 | 1 | — | — | Tl(Ⅰ) | 1123 | [ |
| 蘑菇废料 | 750 | — | 71 | 0.19 | Sb(Ⅲ) | 56.49 | [ |
| 茶树果壳 | 600 | 1 | 257.795 | 1.141 | Cd(Ⅱ) | 263.156 | [ |
| Ni(Ⅱ) | 43.291 | ||||||
| 活性污泥 | 220 | 4 | 114.57 | 0.9 | Cd(Ⅱ) | 48.05 | [ |
| Pb(Ⅱ) | 8.5 | ||||||
| 废骨粉 | 450 | 2 | 288.0 | 0.117 | Cd(Ⅱ) | 151.31 | [ |
| Cu(Ⅱ) | 219.84 | ||||||
| Pb(Ⅱ) | 219.84 | ||||||
| 棕榈纤维粉 | 700 | 3 | 574 | — | Cd(Ⅱ) | 363 | [ |
| As(Ⅲ) | 92.8 | ||||||
| 棕榈仁蛋糕 | 350 | 2 | 89.39 | — | Cd(Ⅱ) | 18.60 | [ |
| Cr(Ⅲ) | 19.92 | ||||||
| Pb(Ⅱ) | 49.64 | ||||||
| Hg(Ⅱ) | 13.69 | ||||||
| 玉米秸秆 | 500 | 2 | — | — | Cd(Ⅱ) | 33.81 | [ |
| As(Ⅲ) | 148.5 | ||||||
| 稻草 | 400 | 2 | — | — | Cd(Ⅱ) | 6.34 | [ |
| As(Ⅲ) | 10.07 | ||||||
| 松木 | 300 | 8 | 1.676 | — | Cd(Ⅱ) | 173 | [ |
| Cu(Ⅱ) | 359 | ||||||
| 450 | 4 | 14.3 | — | Cd(Ⅱ) | 138 | ||
| Cu(Ⅱ) | 172 | ||||||
| 600 | 1 | 75.48 | — | Cd(Ⅱ) | 130 | ||
| Cu(Ⅱ) | 197 |
表4 磁性生物质炭对水体重金属污染物的吸附
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 重金属 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 玉米秸秆 | 500 | 3 | 23.58 | — | Cd(Ⅱ) | 33.45 | [ |
| 小麦秸秆 | 450 | 2 | 152.24 | 0.13 | Cd(Ⅱ) | 23.44 | [ |
| 城市污泥 | 500 | 3 | 82.39 | 0.18 | Cd(Ⅱ) | 167.42 | [ |
| 棕榈纤维 | 400 | 2 | 117.62 | — | Cd(Ⅱ) | 197.96 | [ |
| 谷壳 | 400 | 4 | 72.94 | — | Pb(Ⅱ) | 43.9 | [ |
| 落叶 | 450 | 1 | — | — | Pb(Ⅱ) | 146.84 | [ |
| 松木屑 | 200 | 8 | 138.5 | 0.288 | Pb(Ⅱ) | 99.5 | [ |
| 贝壳 | 200 | 8 | 57.5 | — | Pb(Ⅱ) | 129.31 | [ |
| 杉木屑 | 700 | 2 | 168 | 0.181 | Pb(Ⅱ) | 817.64 | [ |
| 玉米秸秆 | 600 | 3 | — | — | Pb(Ⅱ) | 210.85 | [ |
| 小麦秸秆 | 800 | 2 | 44.31 | 0.0652 | Pb(Ⅱ) | 196.91 | [ |
| 松木屑 | 200 | 8 | 138.5 | — | Pb(Ⅱ) | 99.5 | [ |
| 养猪场污泥 | 200 | 4 | 23.32 | — | Pb(Ⅱ) | 459.24 | [ |
| 300 | 39.99 | — | 355.89 | ||||
| 400 | 27.63 | — | 369.02 | ||||
| 500 | 18.59 | — | 450.58 | ||||
| 600 | 22.61 | — | 402.78 | ||||
| 赤泥 | 350 | 1 | 9.31 | 0.05 | Cr(Ⅵ) | 125.37 | [ |
| 650 | 75.01 | 0.10 | 325.35 | ||||
| 850 | 106.03 | 0.15 | 363.35 | ||||
| 浒苔 | 400 | 2 | 708.27 | — | Cr(Ⅵ) | 53.08 | [ |
| 800 | 780.14 | — | 95.23 | ||||
| 花生壳 | 650 | 1 | 2.40 | 0.02 | Cr(Ⅵ) | 182.32 | [ |
| 700 | 1.80 | 0.01 | 195.22 | ||||
| 750 | 7.74 | 0.03 | 208.33 | ||||
| 800 | 8.81 | 0.05 | 223.21 | ||||
| 小麦秸秆 | 500 | 1 | 33.73 | 0.128 | Cr(Ⅵ) | 23.94 | [ |
| 香菇废基质 | 600 | 4 | 92.78 | 0.112 | Cr(Ⅵ) | 47.62 | [ |
| 纳氏叶栅藻 | 200 | 8 | 142.49 | 0.38 | Cr(Ⅵ) | 56.79 | [ |
| 污水厂污泥 | 500 | 1 | — | — | Cr(Ⅵ) | 11.56 | [ |
| 竹子 | 700 | 2 | — | — | Cr(Ⅵ) | 127 | [ |
| 花生壳 | 220 | 12 | 62.4 | 0.34 | Cr(Ⅵ) | 142.86 | [ |
| 柚皮 | 300 | 1 | 35.88 | 0.024 | Cr(Ⅵ) | 209.64 | [ |
| 稻壳 | 500 | 2 | 17.6 | — | Cr(Ⅵ) | 23.25 | [ |
| 凤凰树叶 | 500 | 2 | 83.6 | — | Cr(Ⅵ) | 55.0 | [ |
| 马缨丹 | 800 | 2 | 607.53 | — | Cr(Ⅵ) | 161.23 | [ |
| 米糠 | 500 | 3 | — | — | Ni(Ⅱ) | 201.62 | [ |
| 黑藻 | 450 | 2 | — | — | Cu(Ⅱ) | 24.28 | [ |
| 丝瓜络 | 700 | 5 | — | — | Cu(Ⅱ) | 54.68 | [ |
| 马尾松锯末 | 240 | 8 | 63.75 | 0.35 | Cu(Ⅱ) | 9.58 | [ |
| 木屑 | 500 | 3 | 25.654 | 0.13 | Cu(Ⅱ) | 3.23 | [ |
| 苹果渣 | 600 | 1 | 102.18 | 0.09 | Ag(Ⅰ) | 818.4 | [ |
| 西瓜皮 | 500 | 1 | — | — | Tl(Ⅰ) | 1123 | [ |
| 蘑菇废料 | 750 | — | 71 | 0.19 | Sb(Ⅲ) | 56.49 | [ |
| 茶树果壳 | 600 | 1 | 257.795 | 1.141 | Cd(Ⅱ) | 263.156 | [ |
| Ni(Ⅱ) | 43.291 | ||||||
| 活性污泥 | 220 | 4 | 114.57 | 0.9 | Cd(Ⅱ) | 48.05 | [ |
| Pb(Ⅱ) | 8.5 | ||||||
| 废骨粉 | 450 | 2 | 288.0 | 0.117 | Cd(Ⅱ) | 151.31 | [ |
| Cu(Ⅱ) | 219.84 | ||||||
| Pb(Ⅱ) | 219.84 | ||||||
| 棕榈纤维粉 | 700 | 3 | 574 | — | Cd(Ⅱ) | 363 | [ |
| As(Ⅲ) | 92.8 | ||||||
| 棕榈仁蛋糕 | 350 | 2 | 89.39 | — | Cd(Ⅱ) | 18.60 | [ |
| Cr(Ⅲ) | 19.92 | ||||||
| Pb(Ⅱ) | 49.64 | ||||||
| Hg(Ⅱ) | 13.69 | ||||||
| 玉米秸秆 | 500 | 2 | — | — | Cd(Ⅱ) | 33.81 | [ |
| As(Ⅲ) | 148.5 | ||||||
| 稻草 | 400 | 2 | — | — | Cd(Ⅱ) | 6.34 | [ |
| As(Ⅲ) | 10.07 | ||||||
| 松木 | 300 | 8 | 1.676 | — | Cd(Ⅱ) | 173 | [ |
| Cu(Ⅱ) | 359 | ||||||
| 450 | 4 | 14.3 | — | Cd(Ⅱ) | 138 | ||
| Cu(Ⅱ) | 172 | ||||||
| 600 | 1 | 75.48 | — | Cd(Ⅱ) | 130 | ||
| Cu(Ⅱ) | 197 |
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 有机污染物 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 城市污泥 | 800 | 2 | — | — | 四环素 | 159.26 | [ |
| 甘蔗渣 | 400 | 2 | 25.45 | — | 四环素 | 48.35 | [ |
| 锯屑 | 800 | 1 | 1710.3 | 0.969 | 四环素 | 423.7 | [ |
| 椰子、松子和核桃壳 | 500 | 1.5 | 365 | 0.54 | 卡马西平 | 62.7 | [ |
| 四环素 | 94.2 | ||||||
| 鸡骨 | 500 | 2 | 328 | 0.285 | 四环素 | 98.89 | [ |
| 罗丹明B | 13.31 | ||||||
| 樟脑叶 | 650 | 2 | 915 | 0.552 | 环丙沙星 | 449.40 | [ |
| 柳木锯末 | 600 | 3 | 662.68 | 0.153 | 达托霉素 | 217.39 | [ |
| 松木锯末 | 634.22 | 0.126 | 212.77 | ||||
| 城市污泥 | 500 | 2 | 39.1 | 0.129 | 环丙沙星 | 83.7 | [ |
| 诺氟沙星 | 39.3 | ||||||
| 氧氟沙星 | 25.4 | ||||||
| 黄芪草药渣 | 700 | 3 | 203.70 | — | 环丙沙星 | 68.9 | [ |
| 玉米秸秆 | 500 | 1.5 | 760.7 | 0.3698 | 诺氟沙星 | 7.63 | [ |
| 松木屑 | — | — | 125.8 | — | 磺胺甲𫫇唑 | 111.2 | [ |
| 甘蔗渣 | 800 | 2 | 613.87 | 0.29 | 磺胺甲𫫇唑 | 187.31 | [ |
| 竹子 | 550 | 1 | 61.48 | 0.157 | 双酚A | 101.5 | [ |
| 磺胺甲𫫇唑 | 99.99 | ||||||
| 橙皮 | 500 | 4 | 857.42 | 0.12 | 布洛芬 | 58.12 | [ |
| 磺胺甲𫫇唑 | 60.90 | ||||||
| 海藻粉 | 700 | 1.5 | 622.88 | 0.733 | 甲基橙 | 622.88 | [ |
| 香蕉皮 | 600 | 1 | — | — | 甲基蓝 | 862 | [ |
| 花生壳 | 750 | 1.5 | 1219 | 0.5767 | 亚甲基蓝 | 666.67 | [ |
| 裙带菜 | 800 | 2 | 744.15 | 0.451 | 亚甲基蓝 | 479.49 | [ |
| 苹果树废枝 | 200 | 12 | 15.17 | 0.071 | 亚甲基蓝 | 46.32 | [ |
| 果树残枝 | 200 | 12 | 135.3 | 0.285 | 亚甲基蓝 | 81.9 | [ |
| 核桃壳 | 700 | 4 | — | — | 亚甲基蓝 | 710 | [ |
| 松仁壳 | 700 | 3 | — | — | 酸性铬蓝K | 79.81 | [ |
| 花生壳 | 800 | 1.5 | 722.34 | 0.336 | 孔雀石绿 | 1106.40 | [ |
| 玉米秸秆 | 500 | 3 | 80.1 | 0.127 | 孔雀石绿 | 515.77 | [ |
| 玉米秸秆 | 400 | 2 | — | — | 结晶紫 | 349.40 | [ |
| 稻草 | 600 | 1 | 145.96 | 0.06549 | 结晶紫 | 199 | [ |
| 褐藻 | 600 | 1 | 336.97 | 0.2323 | 酸性橙7 | 382.01 | [ |
| 油菜秸秆 | 600 | 2.5 | — | — | 双氯芬酸钠 | 266 | [ |
| 稻壳 | 500 | 2 | 108.53 | 0.05 | 菲 | 89.64 | [ |
| 棉花秸秆 | 500 | 6 | 41.91 | 0.076 | 对硝基苯酚 | 48.94 | [ |
| 芦苇 | 600 | 2 | 254.6 | 0.257 | 氟苯尼考 | 9.29 | [ |
| 柳枝稷 | 450 | 1 | 1.1 | — | 灭蚁灵 | 155 | [ |
| 琼脂 | 900 | 2 | 563.0 | 0.30 | 4-硝基苯酚 | 227.27 | [ |
| 稻壳 | 350 | 6 | 184.91 | — | 三氯乙烯 | 98.9 | [ |
| 道格拉斯冷杉 | 600 | 1 | 494 | — | 苯胺 | 338 | [ |
| 硝基苯 | 178 | ||||||
| 菌丝球团 | 400 | 2 | 1986 | 1.04 | 双氯芬酸 | 361.25 | [ |
| 甘蔗渣 | 400 | 1 | 166.87 | 0.14 | 17β-雌二醇 | 50.24 | [ |
| 600 | 1 | 339.12 | 0.14 | 41.71 | |||
| 800 | 1 | 321.68 | 0.13 | 34.06 | |||
| 稻草 | 500 | 2 | 190.28 | — | 17β-雌二醇 | 46.22 | [ |
| 道格拉斯冷杉 | 600~700 | 1.5 | 745.3 | 0.258 | 4-硝基苯胺水杨酸 | 114 | [ |
| 苯甲酸 | 109 | ||||||
| 90 | |||||||
| 邻苯二甲酸 | 86 | ||||||
| 玉米秸秆 | 600 | 1 | 14.26 | — | 阿特拉津 | 143.15 | [ |
表5 磁性生物质炭对水体有机污染物的吸附
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 有机污染物 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 城市污泥 | 800 | 2 | — | — | 四环素 | 159.26 | [ |
| 甘蔗渣 | 400 | 2 | 25.45 | — | 四环素 | 48.35 | [ |
| 锯屑 | 800 | 1 | 1710.3 | 0.969 | 四环素 | 423.7 | [ |
| 椰子、松子和核桃壳 | 500 | 1.5 | 365 | 0.54 | 卡马西平 | 62.7 | [ |
| 四环素 | 94.2 | ||||||
| 鸡骨 | 500 | 2 | 328 | 0.285 | 四环素 | 98.89 | [ |
| 罗丹明B | 13.31 | ||||||
| 樟脑叶 | 650 | 2 | 915 | 0.552 | 环丙沙星 | 449.40 | [ |
| 柳木锯末 | 600 | 3 | 662.68 | 0.153 | 达托霉素 | 217.39 | [ |
| 松木锯末 | 634.22 | 0.126 | 212.77 | ||||
| 城市污泥 | 500 | 2 | 39.1 | 0.129 | 环丙沙星 | 83.7 | [ |
| 诺氟沙星 | 39.3 | ||||||
| 氧氟沙星 | 25.4 | ||||||
| 黄芪草药渣 | 700 | 3 | 203.70 | — | 环丙沙星 | 68.9 | [ |
| 玉米秸秆 | 500 | 1.5 | 760.7 | 0.3698 | 诺氟沙星 | 7.63 | [ |
| 松木屑 | — | — | 125.8 | — | 磺胺甲𫫇唑 | 111.2 | [ |
| 甘蔗渣 | 800 | 2 | 613.87 | 0.29 | 磺胺甲𫫇唑 | 187.31 | [ |
| 竹子 | 550 | 1 | 61.48 | 0.157 | 双酚A | 101.5 | [ |
| 磺胺甲𫫇唑 | 99.99 | ||||||
| 橙皮 | 500 | 4 | 857.42 | 0.12 | 布洛芬 | 58.12 | [ |
| 磺胺甲𫫇唑 | 60.90 | ||||||
| 海藻粉 | 700 | 1.5 | 622.88 | 0.733 | 甲基橙 | 622.88 | [ |
| 香蕉皮 | 600 | 1 | — | — | 甲基蓝 | 862 | [ |
| 花生壳 | 750 | 1.5 | 1219 | 0.5767 | 亚甲基蓝 | 666.67 | [ |
| 裙带菜 | 800 | 2 | 744.15 | 0.451 | 亚甲基蓝 | 479.49 | [ |
| 苹果树废枝 | 200 | 12 | 15.17 | 0.071 | 亚甲基蓝 | 46.32 | [ |
| 果树残枝 | 200 | 12 | 135.3 | 0.285 | 亚甲基蓝 | 81.9 | [ |
| 核桃壳 | 700 | 4 | — | — | 亚甲基蓝 | 710 | [ |
| 松仁壳 | 700 | 3 | — | — | 酸性铬蓝K | 79.81 | [ |
| 花生壳 | 800 | 1.5 | 722.34 | 0.336 | 孔雀石绿 | 1106.40 | [ |
| 玉米秸秆 | 500 | 3 | 80.1 | 0.127 | 孔雀石绿 | 515.77 | [ |
| 玉米秸秆 | 400 | 2 | — | — | 结晶紫 | 349.40 | [ |
| 稻草 | 600 | 1 | 145.96 | 0.06549 | 结晶紫 | 199 | [ |
| 褐藻 | 600 | 1 | 336.97 | 0.2323 | 酸性橙7 | 382.01 | [ |
| 油菜秸秆 | 600 | 2.5 | — | — | 双氯芬酸钠 | 266 | [ |
| 稻壳 | 500 | 2 | 108.53 | 0.05 | 菲 | 89.64 | [ |
| 棉花秸秆 | 500 | 6 | 41.91 | 0.076 | 对硝基苯酚 | 48.94 | [ |
| 芦苇 | 600 | 2 | 254.6 | 0.257 | 氟苯尼考 | 9.29 | [ |
| 柳枝稷 | 450 | 1 | 1.1 | — | 灭蚁灵 | 155 | [ |
| 琼脂 | 900 | 2 | 563.0 | 0.30 | 4-硝基苯酚 | 227.27 | [ |
| 稻壳 | 350 | 6 | 184.91 | — | 三氯乙烯 | 98.9 | [ |
| 道格拉斯冷杉 | 600 | 1 | 494 | — | 苯胺 | 338 | [ |
| 硝基苯 | 178 | ||||||
| 菌丝球团 | 400 | 2 | 1986 | 1.04 | 双氯芬酸 | 361.25 | [ |
| 甘蔗渣 | 400 | 1 | 166.87 | 0.14 | 17β-雌二醇 | 50.24 | [ |
| 600 | 1 | 339.12 | 0.14 | 41.71 | |||
| 800 | 1 | 321.68 | 0.13 | 34.06 | |||
| 稻草 | 500 | 2 | 190.28 | — | 17β-雌二醇 | 46.22 | [ |
| 道格拉斯冷杉 | 600~700 | 1.5 | 745.3 | 0.258 | 4-硝基苯胺水杨酸 | 114 | [ |
| 苯甲酸 | 109 | ||||||
| 90 | |||||||
| 邻苯二甲酸 | 86 | ||||||
| 玉米秸秆 | 600 | 1 | 14.26 | — | 阿特拉津 | 143.15 | [ |
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 无机污染物 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 花生壳 | 600 | 1 | 178.15 | 0.15 | 磷酸盐 | 2.906 | [ |
| 裙带菜根 | 800 | 1 | 172.81 | 0.247 | 磷酸盐 | 428.87 | [ |
| 废催化剂 | 350 | 4 | — | — | 磷酸盐 | 6.32 | [ |
| 玉米秸秆 | 800 | 2 | 20.61 | — | 磷酸盐 | 330.86 | [ |
| 水葫芦 | 450 | — | — | — | 磷酸盐 | 5.07 | [ |
| 梭菌 | 600 | 1 | 233.29 | 0.13 | 磷酸盐 | 205.7 | [ |
| 稻壳 | 600 | — | 50.66 | 0.069 | 磷酸盐 | 11.52 | [ |
| 厌氧消化残渣 | 800 | 1 | 41.79 | 0.0690 | 磷酸盐 | 149.25 | [ |
| 烟秆 | 600 | 1 | 229.71 | 0.16 | 磷酸盐 | 106.52 | [ |
| 玉米芯 | 500 | 2 | 6.19 | — | 磷 | 1.99 | [ |
| 园林木屑 | 500 | 2 | 9.42 | — | 磷 | 2.75 | |
| 木屑 | 500 | 2 | 11.08 | — | 磷 | 3.20 | |
| 菠萝皮 | 300 | 1 | 84.89 | — | 磷 | 101.16 | [ |
| 芦苇秸秆 | 500 | 3 | 19.76 | 0.061 | 磷 | 9.05 | [ |
| 污泥 | 500 | 1 | 126.74 | 0.97 | 氨氮 | 3.18 | [ |
| 道格拉斯冷杉 | 600 | 1 | 494 | — | 硝酸盐 | 15.5 | [ |
| 氟化物 | 9.04 | ||||||
| 小麦秸秆 | 600 | 1 | 3.9 | — | 硝酸盐 | 24.8 | [ |
表6 磁性生物质炭对水体无机污染物的吸附
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 无机污染物 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 花生壳 | 600 | 1 | 178.15 | 0.15 | 磷酸盐 | 2.906 | [ |
| 裙带菜根 | 800 | 1 | 172.81 | 0.247 | 磷酸盐 | 428.87 | [ |
| 废催化剂 | 350 | 4 | — | — | 磷酸盐 | 6.32 | [ |
| 玉米秸秆 | 800 | 2 | 20.61 | — | 磷酸盐 | 330.86 | [ |
| 水葫芦 | 450 | — | — | — | 磷酸盐 | 5.07 | [ |
| 梭菌 | 600 | 1 | 233.29 | 0.13 | 磷酸盐 | 205.7 | [ |
| 稻壳 | 600 | — | 50.66 | 0.069 | 磷酸盐 | 11.52 | [ |
| 厌氧消化残渣 | 800 | 1 | 41.79 | 0.0690 | 磷酸盐 | 149.25 | [ |
| 烟秆 | 600 | 1 | 229.71 | 0.16 | 磷酸盐 | 106.52 | [ |
| 玉米芯 | 500 | 2 | 6.19 | — | 磷 | 1.99 | [ |
| 园林木屑 | 500 | 2 | 9.42 | — | 磷 | 2.75 | |
| 木屑 | 500 | 2 | 11.08 | — | 磷 | 3.20 | |
| 菠萝皮 | 300 | 1 | 84.89 | — | 磷 | 101.16 | [ |
| 芦苇秸秆 | 500 | 3 | 19.76 | 0.061 | 磷 | 9.05 | [ |
| 污泥 | 500 | 1 | 126.74 | 0.97 | 氨氮 | 3.18 | [ |
| 道格拉斯冷杉 | 600 | 1 | 494 | — | 硝酸盐 | 15.5 | [ |
| 氟化物 | 9.04 | ||||||
| 小麦秸秆 | 600 | 1 | 3.9 | — | 硝酸盐 | 24.8 | [ |
| 1 | LI Xiang, QIN Yang, JIA Yan, et al. Preparation and application of Fe/biochar (Fe-BC) catalysts in wastewater treatment: a review[J]. Chemosphere, 2021, 274: 129766. |
| 2 | WANG Qing, YANG Zhiming. Industrial water pollution, water environment treatment, and health risks in China[J]. Environmental Pollution, 2016, 218: 358-365. |
| 3 | LI Xiang, ZHOU Minghua, PAN Yuwei. Enhanced degradation of 2, 4-dichlorophenoxyacetic acid by pre-magnetization Fe-C activated persulfate: influential factors, mechanism and degradation pathway[J]. Journal of Hazardous Materials, 2018, 353: 454-465. |
| 4 | LI Xiang, ZHOU Minghua, PAN Yuwei. Degradation of diclofenac by H2O2 activated with pre-magnetization Fe0: influencing factors and degradation pathways[J]. Chemosphere, 2018, 212: 853-862. |
| 5 | CAI Ruquan, ZHANG Baogang, SHI Jiaxin, et al. Rapid photocatalytic decolorization of methyl orange under visible light using VS4/carbon powder nanocomposites[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 7690-7699. |
| 6 | WANG Yue, YANG Qixia, CHEN Jiacheng, et al. Adsorption behavior of Cr(VI) by magnetically modified Enteromorpha prolifera based biochar and the toxicity analysis[J]. Journal of Hazardous Materials, 2020, 395: 122658. |
| 7 | HAN Yanhe, LI Han, LIU Meili, et al. Purification treatment of dyes wastewater with a novel micro-electrolysis reactor[J]. Separation and Purification Technology, 2016, 170: 241-247. |
| 8 | CHEN Weiming, ZHANG Aiping, GU Zhepei, et al. Enhanced degradation of refractory organics in concentrated landfill leachate by Fe0/H2O2 coupled with microwave irradiation[J]. Chemical Engineering Journal, 2018, 354: 680-691. |
| 9 | YAO Bin, LUO Zirui, ZHI Dan, et al. Current progress in degradation and removal methods of polybrominated diphenyl ethers from water and soil: a review[J]. Journal of Hazardous Materials, 2021, 403: 123674. |
| 10 | NADEEM R, ZAFAR M N, AFZAL A, et al. Potential of NaOH pretreated Mangifera indica waste biomass for the mitigation of Ni(II) and Co(II) from aqueous solutions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(3): 967-972. |
| 11 | HADDAD M, OIE C, VO DUY S, et al. Adsorption of micropollutants present in surface waters onto polymeric resins: impact of resin type and water matrix on performance[J]. Science of the Total Environment, 2019, 660: 1449-1458. |
| 12 | KHATAEE A R, KASIRI M B. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: influence of the chemical structure of dyes[J]. Journal of Molecular Catalysis A: Chemical, 2010, 328(1/2): 8-26. |
| 13 | XU Huacheng, JI Li, KONG Ming, et al. Molecular weight-dependent adsorption fractionation of natural organic matter on ferrihydrite colloids in aquatic environment[J]. Chemical Engineering Journal, 2019, 363: 356-364. |
| 14 | XIONG X N, YU I K M, TSANG D C W, et al. Value-added chemicals from food supply chain wastes: state-of-the-art review and future prospects[J]. Chemical Engineering Journal, 2019, 375: 121983. |
| 15 | YI Yunqiang, TU Guoquan, ZHAO Dongye, et al. Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor[J]. Chemical Engineering Journal, 2019, 360: 212-220. |
| 16 | ZHANG Lianke, GUO Jinyue, HUANG Xuemin, et al. Functionalized biochar-supported magnetic MnFe2O4 nanocomposite for the removal of Pb(ii) and Cd(ii)[J]. RSC Advances, 2019, 9(1): 365-376. |
| 17 | YI Yunqiang, HUANG Zhexi, LU Baizhou, et al. Magnetic biochar for environmental remediation: a review[J]. Bioresource Technology, 2020, 298: 122468. |
| 18 | WANG Jianlong, WANG Shizong. Preparation, modification and environmental application of biochar: a review[J]. Journal of Cleaner Production, 2019, 227: 1002-1022. |
| 19 | JING Xiangrong, WANG Yuanying, LIU Wujun, et al. Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar[J]. Chemical Engineering Journal, 2014, 248: 168-174. |
| 20 | INYANG M, GAO B, ZIMMERMAN A, et al. Synthesis, characterization, and dye sorption ability of carbon nanotube-biochar nanocomposites[J]. Chemical Engineering Journal, 2014, 236: 39-46. |
| 21 | SHI Jingxin, HAN Hongjun, XU Chunyan. A novel enhanced anaerobic biodegradation method using biochar and Fe(OH)3@biochar for the removal of nitrogen heterocyclic compounds from coal gasification wastewater[J]. Science of the Total Environment, 2019, 697: 134052. |
| 22 | LIU Banghai, GUO Wanqian, WANG Huazhe, et al. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: the pivotal roles of persistent free radicals and ecotoxicity assessment[J]. Journal of Hazardous Materials, 2020, 398: 122768. |
| 23 | FENG Yu, LIU Peng, WANG Yanxin, et al. Distribution and speciation of iron in Fe-modified biochars and its application in removal of As(V), As(III), Cr(VI), and Hg(II): an X-ray absorption study[J]. Journal of Hazardous Materials, 2020, 384: 121342. |
| 24 | LEE T, NAM I H, JUNG S, et al. Synthesis of nickel/biochar composite from pyrolysis of Microcystis aeruginosa and its practical use for syngas production[J]. Bioresource Technology, 2020, 300: 122712. |
| 25 | LUO Junmei, BO Shufeng, QIN Yanan, et al. Transforming goat manure into surface-loaded cobalt/biochar as PMS activator for highly efficient ciprofloxacin degradation[J]. Chemical Engineering Journal, 2020, 395: 125063. |
| 26 | DAI Shijin, ZHAO Youcai, NIU Dongjie, et al. Preparation and reactivation of magnetic biochar by molten salt method: relevant performance for chlorine-containing pesticides abatement[J]. Journal of the Air & Waste Management Association, 2019, 69(1): 58-70. |
| 27 | FENG Zhuqing, YUAN Rongfang, WANG Fei, et al. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: a review[J]. Science of the Total Environment, 2021, 765: 142673. |
| 28 | 胡学玉, 陈窈君, 张沙沙, 等. 磁性玉米秸秆生物炭对水体中Cd的去除作用及回收利用[J]. 农业工程学报, 2018, 34(19): 208-218. |
| HU Xueyu, CHEN Yaojun, ZHANG Shasha, et al. Cd removal from aqueous solution using magnetic biochar derived from maize straw and its recycle[J]. Transactions of the Chinese Society of Agricultural Engineering, 2018, 34(19): 208-218. | |
| 29 | 崔志文, 任艳芳, 王伟, 等. 碱和磁复合改性小麦秸秆生物炭对水体中镉的吸附特性及机制[J]. 环境科学, 2020, 41(7): 3315-3325. |
| CUI Zhiwen, REN Yanfang, WANG Wei, et al. Adsorption characteristics and mechanism of cadmium in water by alkali and magnetic composite modified wheat straw biochar[J]. Environmental Science, 2020, 41(7): 3315-3325. | |
| 30 | 曹玮, 周航, 邓贵友, 等. 改性谷壳生物炭负载磁性Fe去除废水中Pb2+的效果及机制[J]. 环境工程学报, 2017, 11(3): 1437-1444. |
| CAO Wei, ZHOU Hang, DENG Guiyou, et al. Effects and mechanisms of magnetic iron supported on rice husk biochar removing Pb2+ in wastewater[J]. Chinese Journal of Environmental Engineering, 2017, 11(3): 1437-1444. | |
| 31 | GUO Zhiqiang, CHEN Rui, YANG Rongrong, et al. Synthesis of amino-functionalized biochar/spinel ferrite magnetic composites for low-cost and efficient elimination of Ni(II) from wastewater[J]. Science of the Total Environment, 2020, 722: 137822. |
| 32 | WANG Chongqing, WANG Hui. Pb(II) sorption from aqueous solution by novel biochar loaded with nano-particles[J]. Chemosphere, 2018, 192: 1-4. |
| 33 | 符剑刚, 贾阳, 李政, 等. 磁性生物炭负载Mg-Fe水滑石的制备及其吸附水中Cd(Ⅱ)和Ni(Ⅱ)的性能[J]. 化工环保, 2019, 39(5): 574-580. |
| FU Jiangang, JIA Yang, LI Zheng, et al. Preparation of Mg-Fe hydrotalcite supported on magnetic biochar and its adsorption capacity to Cd(Ⅱ) and Ni(Ⅱ) from water[J]. Environmental Protection of Chemical Industry, 2019, 39(5): 574-580. | |
| 34 | NIU Zhirui, FENG Wenli, HUANG Hua, et al. Green synthesis of a novel Mn-Zn ferrite/biochar composite from waste batteries and pine sawdust for Pb2+ removal[J]. Chemosphere, 2020, 252: 126529. |
| 35 | 高海荣, 姜明月, 黄振旭, 等. 磁性黑藻生物炭复合材料的制备及其对水体Cu2+的吸附[J]. 化工新型材料, 2021, 49(10): 186-190. |
| GAO Hairong, JIANG Mingyue, HUANG Zhenxu, et al. Preparation of magnetic black algae biochar composite material and its adsorption of Cu2+ in water[J]. New Chemical Materials, 2021, 49(10): 186-190. | |
| 36 | LUO Xuewen, SHEN Minxian, HUANG Zhujian, et al. Efficient removal of Pb(II) through recycled biochar-mineral composite from the coagulation sludge of swine wastewater[J]. Environmental Research, 2020, 190: 110014. |
| 37 | 袁健, 钱雅洁, 薛罡, 等. 活性污泥水热碳化法制备磁性炭及对水体Cd2+及Pb2+的去除[J]. 环境工程, 2020, 38(2): 55-62. |
| YUAN Jian, QIAN Yajie, XUE Gang, et al. Removal of cadmium and lead in water by magnetic carbon prepared from activated sludge with hydrothermal carbonization[J]. Environmental Engineering, 2020, 38(2): 55-62. | |
| 38 | AJMAL Z, MUHMOOD A, DONG Renjie, et al. Probing the efficiency of magnetically modified biomass-derived biochar for effective phosphate removal[J]. Journal of Environmental Management, 2020, 253: 109730. |
| 39 | XIAO Jiang, HU Rui, CHEN Guangcai, et al. Facile synthesis of multifunctional bone biochar composites decorated with Fe/Mn oxide micro-nanoparticles: physicochemical properties, heavy metals sorption behavior and mechanism[J]. Journal of Hazardous Materials, 2020, 399: 123067. |
| 40 | WANG Can, TAN Hang, LIU Huakang, et al. A nanoscale ferroferric oxide coated biochar derived from mushroom waste to rapidly remove Cr(VI) and mechanism study[J]. Bioresource Technology Reports, 2019, 7: 100253. |
| 41 | 王向辉, 石建军, 游诚航, 等. Fe3O4@ABc复合材料的制备及其对水中甲基橙的吸附[J]. 精细化工, 2020, 37(7): 1422-1428. |
| WANG Xianghui, SHI Jianjun, YOU Chenghang, et al. Preparation of Fe3O4@ABc composite and its adsorption for methyl orange in water[J]. Fine Chemicals, 2020, 37(7): 1422-1428. | |
| 42 | 周雅兰, 周冰. Fe浸渍污泥生物炭对含Cd(Ⅱ)废水的吸附性能研究[J]. 工业水处理, 2021, 41(5): 80-85. |
| ZHOU Yalan, ZHOU Bing. Adsorption performance of Fe-impregnated sludge biochar for removing Cd(Ⅱ)-containing wastewater[J]. Industrial Water Treatment, 2021, 41(5): 80-85. | |
| 43 | 杨期鑫, 俞璐军, 董余兵, 等. 磁功能化多孔生物质炭复合材料的制备及吸波性能[J]. 新型炭材料, 2019, 34(5): 455-463. |
| YANG Qixin, YU Lujun, DONG Yubing, et al. Preparation and microwave absorption properties of magnetic functional porous biomass carbon composites[J]. New Carbon Materials, 2019, 34(5): 455-463. | |
| 44 | 陈昌诚, 罗米娜, 黄超, 等. 磁性明胶改性秸秆生物炭对双氯芬酸钠的吸附研究[J]. 应用化工, 2021, 50(7): 1850-1854. |
| CHEN Changcheng, LUO Mina, HUANG Chao, et al. Study on sorption of diclofenac sodium using magnetic-gelatin supported on Brassica-straw biochar[J]. Applied Chemical Industry, 2021, 50(7): 1850-1854. | |
| 45 | 郑景华, 王宇, 王聪, 等. 磁性生物炭对As(Ⅲ)的吸附行为研究[J]. 离子交换与吸附, 2018, 34(2): 116-126. |
| ZHENG Jinghua, WANG Yu, WANG Cong, et al. Adsoption of As(ⅲ) on magnetic biochar[J]. Ion Exchange and Adsorption, 2018, 34(2): 116-126. | |
| 46 | 赵旭, 王淑娟, 郭伟, 等. 磁性稻壳生物炭对水体中菲的去除特性[J]. 水资源保护, 2019, 35(5): 70-77. |
| ZHAO Xu, WANG Shujuan, GUO Wei, et al. Characteristics of removing phenanthrene (PHE) from water by magnetic rice husk biochar[J]. Water Resources Protection, 2019, 35(5): 70-77. | |
| 47 | 万霞, 梅昌艮, 何俐臻, 等. 磁性生物炭的制备、表征及对磷的吸附特性[J]. 安全与环境学报, 2017, 17(3): 1069-1075. |
| WAN Xia, MEI Changgen, HE Lizhen, et al. On the synthesis, characterization and phosphate removal of the biocharbased magnetic composites[J]. Journal of Safety and Environment, 2017, 17(3): 1069-1075. | |
| 48 | WANG Shengsen, GAO Bin, ZIMMERMAN A R, et al. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite[J]. Bioresource Technology, 2015, 175: 391-395. |
| 49 | YANG Xing, ZHANG Xiaoli, WANG Zhaowei, et al. Mechanistic insights into removal of norfloxacin from water using different natural iron ore - biochar composites: more rich free radicals derived from natural pyrite-biochar composites than hematite-biochar composites[J]. Applied Catalysis B: Environmental, 2019, 255: 117752. |
| 50 | LU Jing, YANG Yaqian, LIU Pengxiao, et al. Iron-montmorillonite treated corn straw biochar: interfacial chemical behavior and stability[J]. Science of the Total Environment, 2020, 708: 134773. |
| 51 | LI Mengxue, LIU Haibo, CHEN Tianhu, et al. Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption[J]. Science of the Total Environment, 2019, 651: 1020-1028. |
| 52 | QU Jianhua, AKINDOLIE M S, FENG Yan, et al. One-pot hydrothermal synthesis of NaLa(CO3)2 decorated magnetic biochar for efficient phosphate removal from water: kinetics, isotherms, thermodynamics, mechanisms and reusability exploration[J]. Chemical Engineering Journal, 2020, 394: 124915. |
| 53 | 陶利春, 陈豪宇, 刘杰, 等. 磁性花生壳基活性炭对亚甲基蓝的吸附特性[J]. 环境污染与防治, 2018, 40(6): 639-644, 692. |
| TAO Lichun, CHEN Haoyu, LIU Jie, et al. Adsorption characteristics of methylene blue onto magnetic peanut shell based activated carbon[J]. Environmental Pollution & Control, 2018, 40(6): 639-644, 692. | |
| 54 | ZHANG Sijing, JI Yongliang, DANG Jing, et al. Magnetic apple pomace biochar: Simple preparation, characterization, and application for enriching Ag(I) in effluents[J]. Science of the Total Environment, 2019, 668: 115-123. |
| 55 | WANG He, WANG Han, ZHAO Hui, et al. Adsorption and Fenton-like removal of chelated nickel from Zn-Ni alloy electroplating wastewater using activated biochar composite derived from Taihu blue algae[J]. Chemical Engineering Journal, 2020, 379: 122372. |
| 56 | LIU Shaobo, LI Meifang, LIU Yunguo, Liu, et al. Removal of 17β-estradiol from aqueous solution by graphene oxide supported activated magnetic biochar: adsorption behavior and mechanism[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 102:330-339. |
| 57 | LIU Kai, LI Fangbai, CUI Jianghu, et al. Simultaneous removal of Cd(Ⅱ) and As(Ⅲ) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: synergistic effects and mechanisms[J]. Journal of Hazardous Materials, 2020, 395: 122623. |
| 58 | 肖芳芳, 张莹莹, 程建华, 等. 壳聚糖/磁性生物碳对重金属Cu(Ⅱ)的吸附性能[J]. 环境工程学报, 2019, 13(5): 1048-1055. |
| XIAO Fangfang, ZHANG Yingying, CHENG Jianhua, et al. Adsorption properties of chitosan/magnetic biochar for Cu(Ⅱ) removal from solution[J]. Chinese Journal of Environmental Engineering, 2019, 13(5): 1048-1055. | |
| 59 | ZHOU Xiaohui, ZHOU Jianjun, LIU Yaochi, et al. Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd(Ⅱ) removal in water[J]. Fuel, 2018, 233: 469-479. |
| 60 | LI Mingrun, WEI Dong, LIU Ting, et al. EDTA functionalized magnetic biochar for Pb( Ⅱ ) removal: adsorption performance, mechanism and SVM model prediction[J]. Separation and Purification Technology, 2019, 227: 115696. |
| 61 | MANEECHAKR P, MONGKOLLERTLOP S. Investigation on adsorption behaviors of heavy metal ions (Cd2+, Cr3+, Hg2+ and Pb2+) through low-cost/active manganese dioxide-modified magnetic biochar derived from palm kernel cake residue[J]. Journal of Environmental Chemical Engineering, 2020, 8(6): 104467. |
| 62 | WANG Huabin, CAI Jiayi, LIAO Zhuwei, et al. Black liquor as biomass feedstock to prepare zero-valent iron embedded biochar with red mud for Cr( Ⅵ ) removal: mechanisms insights and engineering practicality[J]. Bioresource Technology, 2020, 311: 123553. |
| 63 | YU Jiangfang, TANG Lin, PANG Ya, et al. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: internal electron transfer mechanism[J]. Chemical Engineering Journal, 2019, 364: 146-159. |
| 64 | GAN Quan, HOU Huijie, LIANG Sha, et al. Sludge-derived biochar with multivalent iron as an efficient Fenton catalyst for degradation of 4-chlorophenol[J]. Science of the Total Environment, 2020, 725: 138299. |
| 65 | WANG Jia, SHEN Min, GONG Qing, et al. One-step preparation of ZVI-sludge derived biochar without external source of iron and its application on persulfate activation[J]. Science of the Total Environment, 2020, 714: 136728. |
| 66 | LIU Y Y, SOHI S P, LIU S Y, et al. Adsorption and reductive degradation of Cr(Ⅵ) and TCE by a simply synthesized zero valent iron magnetic biochar[J]. Journal of Environmental Management, 2019, 235: 276-281. |
| 67 | ZHANG Xiaoying, SUN Peizhe, WEI Kajia, et al. Enhanced H2O2 activation and sulfamethoxazole degradation by Fe-impregnated biochar[J]. Chemical Engineering Journal, 2020, 385: 123921. |
| 68 | 吴卫蔚, 毛磊, 胡慧兰, 等. 不同铁改性剂对磁性麦秆生物炭吸附Cr(Ⅵ)的影响[J]. 有色金属(冶炼部分), 2022(2): 90-98. |
| WU Weiwei, MAO Lei, HU Huilan, et al. Effect of Fe-bearing modifying agents on adsorption performance of magnetic straw-derived biochars for Cr(Ⅵ)[J]. Nonferrous Metals (Extractive Metallurgy), 2022(2): 90-98. | |
| 69 | 沈玲芳, 董隽, 单胜道, 等. 磁性生物质炭制备方法及其对水体Pb2+吸附特性的影响[J]. 环境工程, 2021, 39(9): 48-55. |
| SHEN Lingfang, DONG Jun, SHAN Shengdao, et al. Influence of magnetic biochar preparation methods on adsorption characteristics of pb2+ in wastewater[J]. Environmental Engineering, 2021, 39(9): 48-55. | |
| 70 | ZHOU Jingyao, LIU Yuyan, HAN Yitong, et al. Bone-derived biochar and magnetic biochar for effective removal of fluoride in groundwater: effects of synthesis method and coexisting chromium[J]. Water Environment Research, 2019, 91(7): 588-597. |
| 71 | YANG Dong, WANG Lu, LI Zhangtao, et al. Simultaneous adsorption of Cd(Ⅱ) and As(Ⅲ) by a novel biochar-supported nanoscale zero-valent iron in aqueous systems[J]. Science of the Total Environment, 2020, 708: 134823. |
| 72 | QIAN Linbo, ZHANG Wenying, YAN Jingchun, et al. Nanoscale zero-valent iron supported by biochars produced at different temperatures: synthesis mechanism and effect on Cr(Ⅵ) removal[J]. Environmental Pollution, 2017, 223: 153-160. |
| 73 | YI Yan, WANG Xiangyu, MA Jun, et al. An efficient Egeria najas-derived biochar supported nZVI composite for Cr( Ⅵ ) removal: characterization and mechanism investigation based on visual MINTEQ model[J]. Environmental Research, 2020, 189: 109912. |
| 74 | WANG Kun, SUN Yuebing, TANG Jingchun, et al. Aqueous Cr(Ⅵ) removal by a novel ball milled Fe0-biochar composite: role of biochar electron transfer capacity under high pyrolysis temperature[J]. Chemosphere, 2020, 241: 125044. |
| 75 | HEO J, YOON Y, LEE G, et al. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4-biochar composite[J]. Bioresource Technology, 2019, 281: 179-187. |
| 76 | LI Yanfei, ZIMMERMAN A R, HE Feng, et al. Solvent-free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue[J]. Science of the Total Environment, 2020, 722: 137972. |
| 77 | ZHANG Han, XIAO Ran, LI Ronghua, et al. Enhanced aqueous Cr(Ⅵ) removal using chitosan-modified magnetic biochars derived from bamboo residues[J]. Chemosphere, 2020, 261: 127694. |
| 78 | FENG Zhuqing, CHEN Huilun, LI Haiqing, et al. Preparation, characterization, and application of magnetic activated carbon for treatment of biologically treated papermaking wastewater[J]. Science of the Total Environment, 2020, 713: 136423. |
| 79 | GAO Jie, HAN Dongqiang, XU Yun, et al. Persulfate activation by sulfide-modified nanoscale iron supported by biochar (S-nZVI/BC) for degradation of ciprofloxacin[J]. Separation and Purification Technology, 2020, 235: 116202. |
| 80 | IFTHIKAR J, WANG Jia, WANG Qiliang, et al. Highly efficient lead distribution by magnetic sewage sludge biochar: sorption mechanisms and bench applications[J]. Bioresource Technology, 2017, 238: 399-406. |
| 81 | MEI Jinfeng, ZHANG Hui, LI Zhongyu, et al. A novel tetraethylenepentamine crosslinked chitosan oligosaccharide hydrogel for total adsorption of Cr( Ⅵ )[J]. Carbohydrate Polymers, 2019, 224: 115154. |
| 82 | WANG Bo, JIANG Yansong, LI Fayun, et al. Preparation of biochar by simultaneous carbonization, magnetization and activation for norfloxacin removal in water[J]. Bioresource Technology, 2017, 233: 159-165. |
| 83 | ZHANG Yuting, LIU Na, YANG Yadong, et al. Novel carbothermal synthesis of Fe, N co-doped oak wood biochar (Fe/N-OB) for fast and effective Cr( Ⅵ ) removal[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 600: 124926. |
| 84 | LI Xiangping, WANG Chuanbin, ZHANG Jianguang, et al. Preparation and application of magnetic biochar in water treatment: a critical review[J]. Science of the Total Environment, 2020, 711: 134847. |
| 85 | JUNG K W, LEE S, LEE Y J. Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions[J]. Bioresource Technology, 2017, 245: 751-759. |
| 86 | 肖作义, 肖宇, 肖明慧, 等. 磁性水滑石/生物炭复合材料的制备及其对水溶液中磷的吸附性能[J]. 环境污染与防治, 2020, 42(9): 1090-1095, 1101. |
| XIAO Zuoyi, XIAO Yu, XIAO Minghui, et al. Preparation of magnetic hydrotalcite/biochar composite and its adsorption performance for phosphorus in aqueous solution[J]. Environmental Pollution & Control, 2020, 42(9): 1090-1095, 1101. | |
| 87 | LIU Liheng, LIU Xiu, WANG Dunqiu, et al. Removal and reduction of Cr(Ⅵ) in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge[J]. Journal of Cleaner Production, 2020, 257: 120562. |
| 88 | 王芳君, 桑倩倩, 邓颖, 等. 磁性铁基改性生物炭去除水中氨氮[J]. 环境科学, 2021, 42(4): 1913-1922. |
| WANG Fangjun, SANG Qianqian, DENG Ying, et al. Synthesis of magnetic iron modifying biochar for ammonia nitrogen removal from water[J]. Environmental Science, 2021, 42(4): 1913-1922. | |
| 89 | WU Jizi, HUANG Dan, LIU Xingmei, et al. Remediation of As(Ⅲ) and Cd(Ⅱ) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar[J]. Journal of Hazardous Materials, 2018, 348: 10-19. |
| 90 | CAI Weiquan, WEI Jiahao, LI Zhonglei, et al. Preparation of amino-functionalized magnetic biochar with excellent adsorption performance for Cr(Ⅵ) by a mild one-step hydrothermal method from peanut hull[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 563: 102-111. |
| 91 | NEELI S T, RAMSURN H, NG C Y, et al. Removal of Cr(Ⅵ), As (Ⅴ), Cu( Ⅱ ), and Pb( Ⅱ ) using cellulose biochar supported iron nanoparticles: a kinetic and mechanistic study[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 103886. |
| 92 | QIU Bingbing, TAO Xuedong, WANG Hao, et al. Biochar as a low-cost adsorbent for aqueous heavy metal removal: a review[J]. Journal of Analytical and Applied Pyrolysis, 2021, 155: 105081. |
| 93 | 马锋锋, 赵保卫, 刁静茹, 等. 磁性生物炭对水体中对硝基苯酚的吸附特性[J]. 中国环境科学, 2019, 39(1): 170-178. |
| MA Fengfeng, ZHAO Baowei, DIAO Jingru, et al. Adsorption characteristics of p-nitrophenol removal by magnetic biochar[J]. China Environmental Science, 2019, 39(1): 170-178. | |
| 94 | ZHANG P, O’CONNOR D, WANG Y N, et al. A green biochar/iron oxide composite for methylene blue removal[J]. Journal of Hazardous Materials, 2020, 384: 121286. |
| 95 | ZHAO Yueling, ZHANG Ruyu, LIU Haibo, et al. Green preparation of magnetic biochar for the effective accumulation of Pb(Ⅱ): performance and mechanism[J]. Chemical Engineering Journal, 2019, 375: 122011. |
| 96 | 张连科, 王洋, 王维大, 等. 磁性羟基磷灰石/生物炭复合材料的制备及对Pb2+的吸附性能[J]. 环境科学学报, 2018, 38(11): 4360-4370. |
| ZHANG Lianke, WANG Yang, WANG Weida, et al. Preparation of magnetic hydroxyapatite/biochar composite and its adsorption behavior of Pb2+ and recycling performance[J]. Acta Scientiae Circumstantiae, 2018, 38(11): 4360-4370. | |
| 97 | CAI Ru, WANG Xin, JI Xionghui, et al. Phosphate reclaim from simulated and real eutrophic water by magnetic biochar derived from water hyacinth[J]. Journal of Environmental Management, 2017, 187: 212-219. |
| 98 | MA Yongfei, LI Ming, LI Ping, et al. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal[J]. Bioresource Technology, 2021, 319: 124199. |
| 99 | AGRAFIOTI E, KALDERIS D, DIAMADOPOULOS E. Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions[J]. Journal of Environmental Management, 2014, 146: 444-450. |
| 100 | SU Chunli, WANG Sheng, ZHOU Ziyi, et al. Chemical processes of Cr( Ⅵ ) removal by Fe-modified biochar under aerobic and anaerobic conditions and mechanism characterization under aerobic conditions using synchrotron-related techniques[J]. Science of the Total Environment, 2021, 768: 144604. |
| 101 | LIAO Taiwan, LI Ting, SU Xiangde, et al. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal[J]. Bioresource Technology, 2018, 263: 207-213. |
| 102 | PENG Yaru, AZEEM M, LI Ronghua, et al. Zirconium hydroxide nanoparticle encapsulated magnetic biochar composite derived from rice residue: application for As( Ⅲ ) and As( Ⅴ ) polluted water purification[J]. Journal of Hazardous Materials, 2022, 423: 127081. |
| 103 | YU Yang, AN Qiang, JIN Lin, et al. Unraveling sorption of Cr(Ⅵ) from aqueous solution by FeCl3 and ZnCl2-modified corn stalks biochar: implicit mechanism and application[J]. Bioresource Technology, 2020, 297: 122466. |
| 104 | YIN Zhibing, XU Shuang, LIU Sen, et al. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium[J]. Bioresource Technology, 2020, 300: 122680. |
| 105 | WANG Xuedong, XU Jin, LIU Jia, et al. Mechanism of Cr(Ⅵ) removal by magnetic greigite/biochar composites[J]. Science of the Total Environment, 2020, 700: 134414. |
| 106 | LIANG Sha, SHI Shunquan, ZHANG Haohao, et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: formation mechanism and efficient adsorption of Cr(Ⅵ) in an aqueous solution[J]. Science of the Total Environment, 2019, 695: 133886. |
| 107 | NARZARI R, PODDAR M K, BORDOLOI N, et al. A comprehensive study to understand removal efficiency for Cr6+ using magnetic and activated biochar through response surface methodology[J]. Biomass Conversion and Biorefinery, 2021, DOI: 10.10071s13399-021-01448-3 . |
| 108 | 盖希坤, 马晓锋, 骆美宇, 等. 马尾松基磁性水热炭的制备及其吸附性能[J]. 化工进展, 2022, 41(4): 1994-1999. |
| GAI Xikun, MA Xiaofeng, LUO Meiyu, et al. Preparation and adsorption properties of magnetic hydrothermal carbon based on Pinus Massoniana[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1994-1999. | |
| 109 | 郭丰艳, 刘迎, 金昌磊. 果胶@生物炭-Fe3O4的制备及其对Cu2+的吸附性能[J]. 唐山学院学报, 2020, 33(6): 24-30. |
| GUO Fengyan, LIU Ying, JIN Changlei. Making of pectin @ biochar Fe3O4 and its adsorption property for Cu2+ [J]. Journal of Tangshan University, 2020, 33(6): 24-30. | |
| 110 | LI Huosheng, XIONG Jingfang, ZHANG Gaosheng, et al. Enhanced thallium(I) removal from wastewater using hypochlorite oxidation coupled with magnetite-based biochar adsorption[J]. Science of the Total Environment, 2020, 698: 134166. |
| 111 | ZHU Guocheng, LIN Jialin, YUAN Qian, et al. A biochar supported magnetic metal organic framework for the removal of trivalent antimony[J]. Chemosphere, 2021, 282: 131068. |
| 112 | WANG Shengsen, ZHAO Mingyue, ZHOU Min, et al. Biomass facilitated phase transformation of natural hematite at high temperatures and sorption of Cd2+ and Cu2+ [J]. Environment International, 2019, 124: 473-481. |
| 113 | GUO Feiqiang, LI Xiaolei, JIANG Xiaochen, et al. Characteristics and toxic dye adsorption of magnetic activated carbon prepared from biomass waste by modified one-step synthesis[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 555: 43-54. |
| 114 | ZHOU Yaoyu, HE Yangzhou, HE Yangzhuo, et al. Analyses of tetracycline adsorption on alkali-acid modified magnetic biochar: Site energy distribution consideration[J]. Science of the Total Environment, 2019, 650: 2260-2266. |
| 115 | RATTANACHUESKUL N, SANING A, KAOWPHONG S, et al. Magnetic carbon composites with a hierarchical structure for adsorption of tetracycline, prepared from sugarcane bagasse via hydrothermal carbonization coupled with simple heat treatment process[J]. Bioresource Technology, 2017, 226: 164-172. |
| 116 | CHEN Siqin, CHEN Yali, JIANG Hong. Slow pyrolysis magnetization of hydrochar for effective and highly stable removal of tetracycline from aqueous solution[J]. Industrial & Engineering Chemistry Research, 2017, 56(11): 3059-3066. |
| 117 | SHAN Danna, DENG Shubo, ZHAO Tianning, et al. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling[J]. Journal of Hazardous Materials, 2016, 305: 156-163. |
| 118 | OLADIPO A A, IFEBAJO A O. Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: two-stage adsorber analysis[J]. Journal of Environmental Management, 2018, 209: 9-16. |
| 119 | HU Yi, ZHU Yuan, ZHANG Yi, et al. An efficient adsorbent: Simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption[J]. Bioresource Technology, 2019, 288: 121511. |
| 120 | AI Tian, JIANG Xiaojun, LIU Qingyu, et al. Daptomycin adsorption on magnetic ultra-fine wood-based biochars from water: kinetics, isotherms, and mechanism studies[J]. Bioresource Technology, 2019, 273: 8-15. |
| 121 | MA Yongfei, LI Ping, YANG Lie, et al. Iron/zinc and phosphoric acid modified sludge biochar as an efficient adsorbent for fluoroquinolones antibiotics removal[J]. Ecotoxicology and Environmental Safety, 2020, 196: 110550. |
| 122 | KONG Xiangrui, LIU Yaoxuan, PI Jiachang, et al. Low-cost magnetic herbal biochar: characterization and application for antibiotic removal[J]. Environmental Science and Pollution Research International, 2017, 24(7): 6679-6687. |
| 123 | REGUYAL F, SARMAH A K. Adsorption of sulfamethoxazole by magnetic biochar: Effects of pH, ionic strength, natural organic matter and 17α-ethinylestradiol[J]. Science of the Total Environment, 2018, 628/629: 722-730. |
| 124 | ZHANG Runyuan, ZHENG Xiaoxian, CHEN Bohan, et al. Enhanced adsorption of sulfamethoxazole from aqueous solution by Fe-impregnated graphited biochar[J]. Journal of Cleaner Production, 2020, 256: 120662. |
| 125 | AI Tian, JIANG Xiaojun, ZHONG Zhenxia, et al. Methanol-modified ultra-fine magnetic orange peel powder biochar as an effective adsorbent for removal of ibuprofen and sulfamethoxazole from water[J]. Adsorption Science & Technology, 2020, 38: 304-321. |
| 126 | YAO Xinxin, JI Lili, GUO Jian, et al. Magnetic activated biochar nanocomposites derived from wakame and its application in methylene blue adsorption[J]. Bioresource Technology, 2020, 302: 122842. |
| 127 | 李茜, 封温俐, 牛志睿, 等. 绿色合成磁性水热炭及对亚甲基蓝的吸附研究[J]. 安全与环境学报, 2021, 21(4): 1769-1776. |
| LI Qian, FENG Wenli, NIU Zhirui, et al. Green synthesis of magnetic hydrothermal biochar formethylene blue removal[J]. Journal of Safety and Environment, 2021, 21(4): 1769-1776. | |
| 128 | 王博, 牛志睿, 黄华, 等. 绿色合成磁性生物炭及其对亚甲基蓝的吸附[J]. 环境污染与防治, 2021, 43(4): 427-431, 435. |
| WANG Bo, NIU Zhirui, HUANG Hua, et al. Green synthesis of magnetic biochar and its adsorption capacity for methylene blue[J]. Environmental Pollution & Control, 2021, 43(4): 427-431, 435. | |
| 129 | 黄超, 罗米娜, 陈馥, 等. 核桃壳基磁性生物炭对亚甲基蓝的吸附特性研究[J]. 应用化工, 2020, 49(8): 1956-1961, 1965. |
| HUANG Chao, LUO Mina, CHEN Fu, et al. Study on sorption characteristics of methylene blue by magnetic biochar derived from walnut shell[J]. Applied Chemical Industry, 2020, 49(8): 1956-1961, 1965. | |
| 130 | WANG Huan, WANG Shan, GAO Yihong. Cetyl trimethyl ammonium bromide modified magnetic biochar from pine nut shells for efficient removal of acid chrome blue K[J]. Bioresource Technology, 2020, 312: 123564. |
| 131 | ELTAWEIL A S, MOHAMED H A, EL-MONAEM E M ABD, et al. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: characterization, adsorption kinetics, thermodynamics and isotherms[J]. Advanced Powder Technology, 2020, 31(3): 1253-1263. |
| 132 | SUN Pengfei, HUI Cai, AZIM KHAN R, et al. Efficient removal of crystal violet using Fe3O4-coated biochar: the role of the Fe3O4 nanoparticles and modeling study their adsorption behavior[J]. Scientific Reports, 2015, 5: 12638. |
| 133 | DU Cong, SONG Yonghui, SHI Shengnan, et al. Preparation and characterization of a novel Fe3O4-graphene-biochar composite for crystal violet adsorption[J]. Science of the Total Environment, 2020, 711: 134662. |
| 134 | JUNG K W, CHOI B H, JEONG T U, et al. Facile synthesis of magnetic biochar/Fe3O4 nanocomposites using electro-magnetization technique and its application on the removal of acid orange 7 from aqueous media[J]. Bioresource Technology, 2016, 220: 672-676. |
| 135 | ZHAO Huaxuan, LANG Yinhai. Adsorption behaviors and mechanisms of florfenicol by magnetic functionalized biochar and reed biochar[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 88: 152-160. |
| 136 | ESSANDOH M, WOLGEMUTH D, PITTMAN C U, et al. Adsorption of metribuzin from aqueous solution using magnetic and nonmagnetic sustainable low-cost biochar adsorbents[J]. Environmental Science and Pollution Research International, 2017, 24(5): 4577-4590. |
| 137 | GAO Xiangyu, LIU Ruilin, MA Jin, et al. Combined dual-metal templates for fabrication of magnetic hierarchical porous carbon for highly efficient removal of 4-nitrophenol[J]. Journal of Porous Materials, 2016, 23(1): 157-164. |
| 138 | YAN Jingchun, QIAN Linbo, GAO Weiguo, et al. Enhanced Fenton-like degradation of trichloroethylene by hydrogen peroxide activated with nanoscale zero valent iron loaded on biochar[J]. Scientific Reports, 2017, 7: 43051. |
| 139 | BOMBUWALA DEWAGE N, LIYANAGE A S, SMITH Q, et al. Fast aniline and nitrobenzene remediation from water on magnetized and nonmagnetized Douglas fir biochar[J]. Chemosphere, 2019, 225: 943-953. |
| 140 | LUO Haiqiong, ZHANG Yongkui, XIE Yi, et al. Iron-rich microorganism-enabled synthesis of magnetic biocarbon for efficient adsorption of diclofenac from aqueous solution[J]. Bioresource Technology, 2019, 282: 310-317. |
| 141 | DONG Xinwei, HE Lingzhi, HU Hui, et al. Removal of 17β-estradiol by using highly adsorptive magnetic biochar nanoparticles from aqueous solution[J]. Chemical Engineering Journal, 2018, 352: 371-379. |
| 142 | KARUNANAYAKE A G, TODD O A, CROWLEY M L, et al. Rapid removal of salicylic acid, 4-nitroaniline, benzoic acid and phthalic acid from wastewater using magnetized fast pyrolysis biochar from waste Douglas fir[J]. Chemical Engineering Journal, 2017, 319: 75-88. |
| 143 | WANG Yifan, KANG Jiaming, JIANG Simeng, et al. A composite of Ni–Fe–Zn layered double hydroxides/biochar for atrazine removal from aqueous solution[J]. Biochar, 2020, 2(4): 455-464. |
| 144 | YUAN Ling, QIU Zhaofu, YUAN Lin, et al. Adsorption and mechanistic study for phosphate removal by magnetic Fe3O4-doped spent FCC catalysts adsorbent[J]. Chemosphere, 2019, 219: 183-190. |
| 145 | CUI Qingliang, XU Jinling, WANG Wei, et al. Phosphorus recovery by core-shell γ-Al2O3/Fe3O4 biochar composite from aqueous phosphate solutions[J]. Science of the Total Environment, 2020, 729: 138892. |
| 146 | LIU Jiwei, JIANG Jianguo, AIHEMAITI A, et al. Removal of phosphate from aqueous solution using MgO-modified magnetic biochar derived from anaerobic digestion residue[J]. Journal of Environmental Management, 2019, 250: 109438. |
| 147 | 余佳敏, 赵宇, 肖勇, 等. 烟秆基Fe/Mg磁性生物炭复合材料的制备、表征及吸附性能[J]. 江苏农业科学, 2019, 47(9): 257-262. |
| YU Jiamin, ZHAO Yu, XIAO Yong, et al. Study on preparation and characterization of tobacco stems magnetic biochar composites and its adsorbing capacity[J]. Jiangsu Agricultural Sciences, 2019, 47(9): 257-262. | |
| 148 | MICHÁLEKOVÁ-RICHVEISOVÁ B, FRIŠTÁK V, PIPÍŠKA M, et al. Iron-impregnated biochars as effective phosphate sorption materials[J]. Environmental Science and Pollution Research International, 2017, 24(1): 463-475. |
| 149 | BOMBUWALA DEWAGE N, LIYANAGE A S, PITTMAN C U, et al. Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar[J]. Bioresource Technology, 2018, 263: 258-265. |
| 150 | XUE Lihong, GAO Bin, WAN Yongshan, et al. High efficiency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 63: 312-317. |
| 151 | WANG Binliang, LI Yingying, ZHENG Junli, et al. Efficient removal of U(Ⅵ) from aqueous solutions using the magnetic biochar derived from the biomass of a bloom-forming cyanobacterium (Microcystis aeruginosa)[J]. Chemosphere, 2020, 254: 126898. |
| 152 | LI Mengxue, LIU Haibo, CHEN Tianhu, et al. Synthesis of magnetic biochar composites for enhanced uranium(Ⅵ) adsorption[J]. Science of the Total Environment, 2019, 651: 1020-1028. |
| 153 | ZHU Yuling, ZHENG Cong, WU Siying, et al. Interaction of Eu(III) on magnetic biochar investigated by batch, spectroscopic and modeling techniques[J]. Journal of Radioanalytical and Nuclear Chemistry, 2018, 316(3): 1337-1346. |
| 154 | HU Qingyuan, ZHU Yuling, HU Baowei, et al. Mechanistic insights into sequestration of U(Ⅵ) toward magnetic biochar: batch, XPS and EXAFS techniques[J]. Journal of Environmental Sciences, 2018, 70: 217-225. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [8] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [9] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [10] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [11] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [12] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [13] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [14] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [15] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||