化工进展 ›› 2022, Vol. 41 ›› Issue (12): 6364-6376.DOI: 10.16085/j.issn.1000-6613.2022-0363
纳米ZSM-5在高硅铝比料液中的再生长机制及其甲醇制芳烃性能
- 太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
-
收稿日期:2022-03-10修回日期:2022-05-14出版日期:2022-12-20发布日期:2022-12-29 -
通讯作者:付廷俊,李忠 -
作者简介:惠燕(1996—),女,硕士研究生,研究方向为分子筛合成与应用。E-mail:2837675038@qq.com。 -
基金资助:国家自然科学基金(21978191);国家重点研发计划(2018YFB0604901);山西省重点研发计划(国际科技合作)(201803D421011)
Secondary growth mechanism of nano-ZSM-5 in solution of high SiO2/Al2O3 ratio and its performance of methanol to aromatics
HUI Yan( ), FU Tingjun(
), FU Tingjun( ), MA Qian, LI Zhong(
), MA Qian, LI Zhong( )
)
- State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2022-03-10Revised:2022-05-14Online:2022-12-20Published:2022-12-29 -
Contact:FU Tingjun, LI Zhong
摘要:
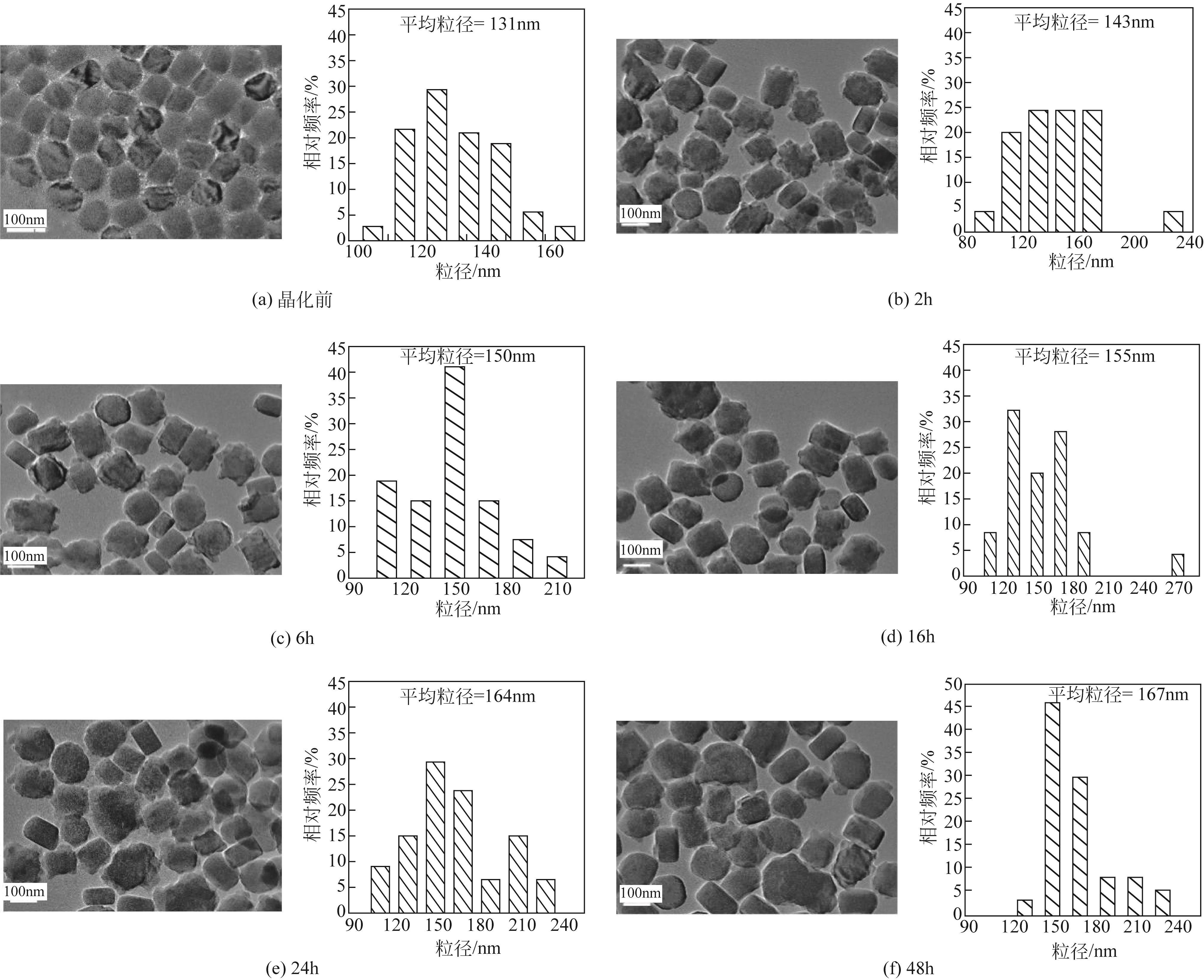
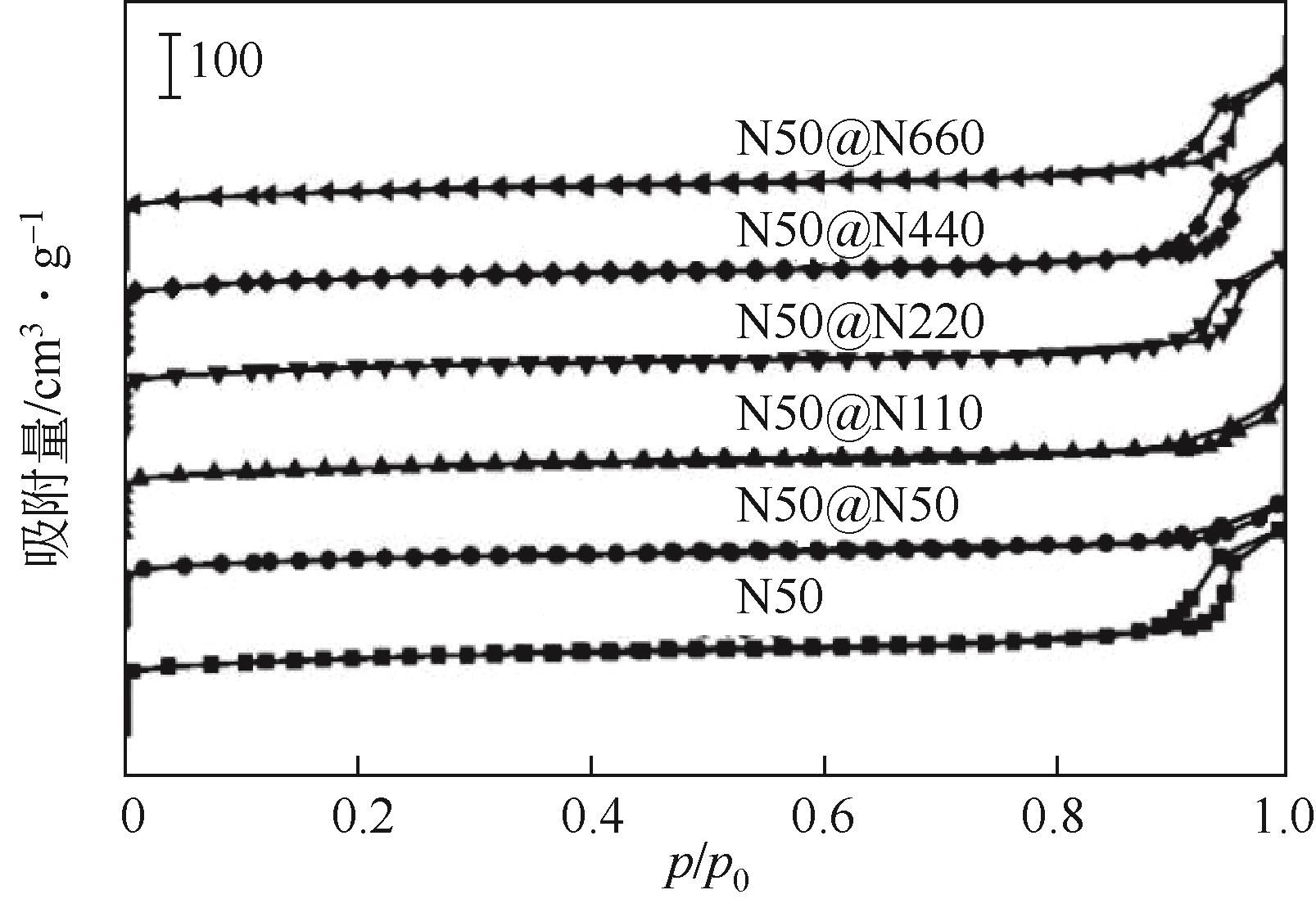
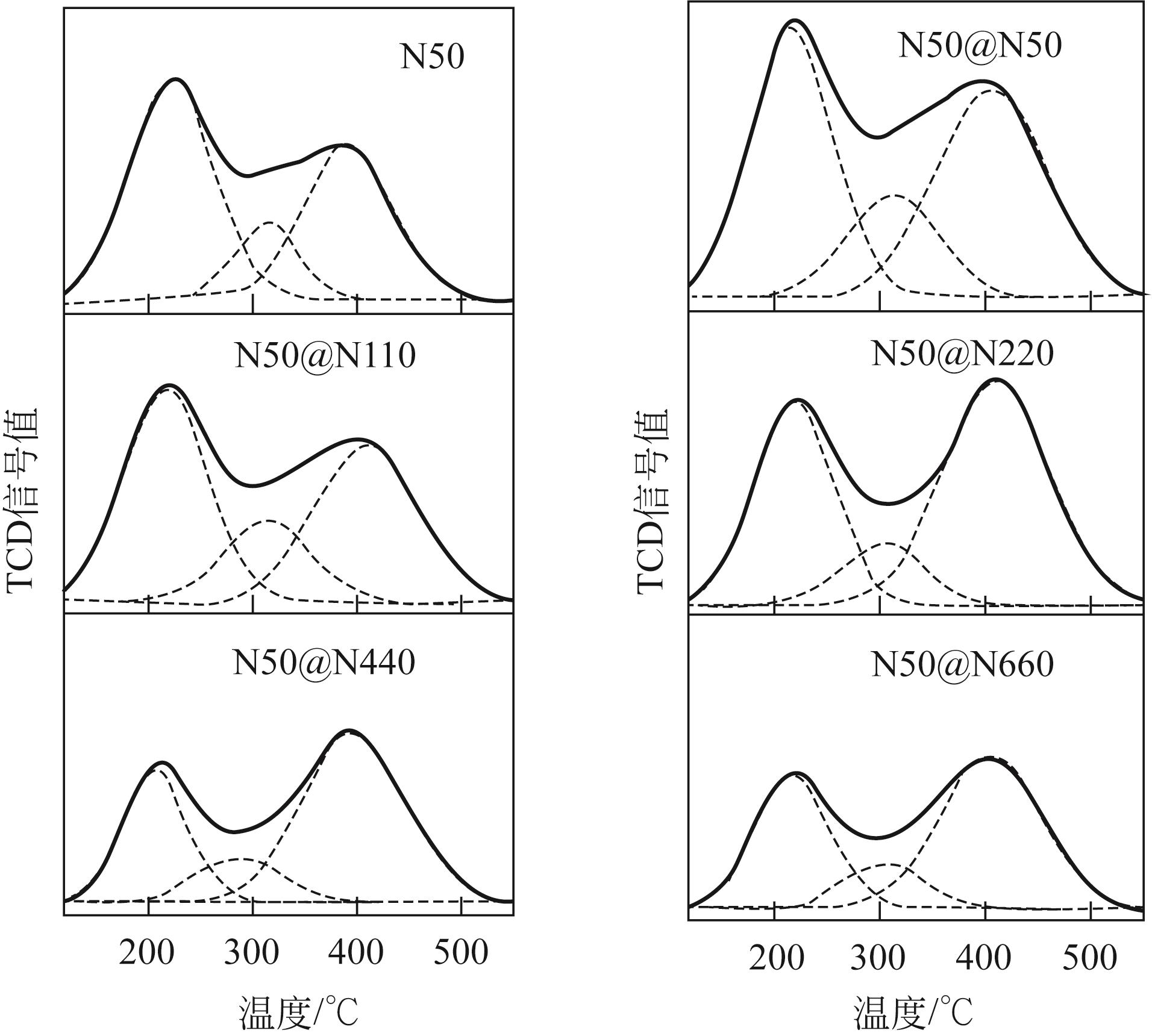
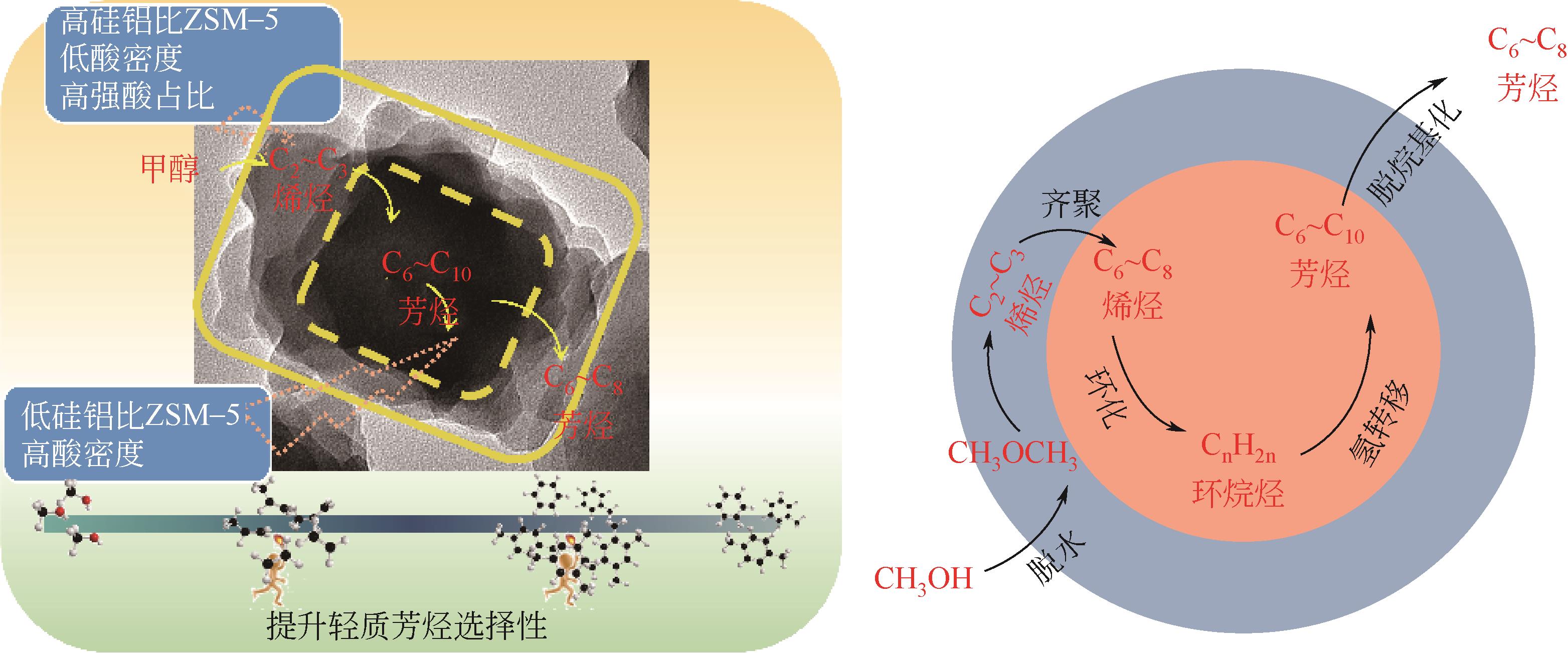
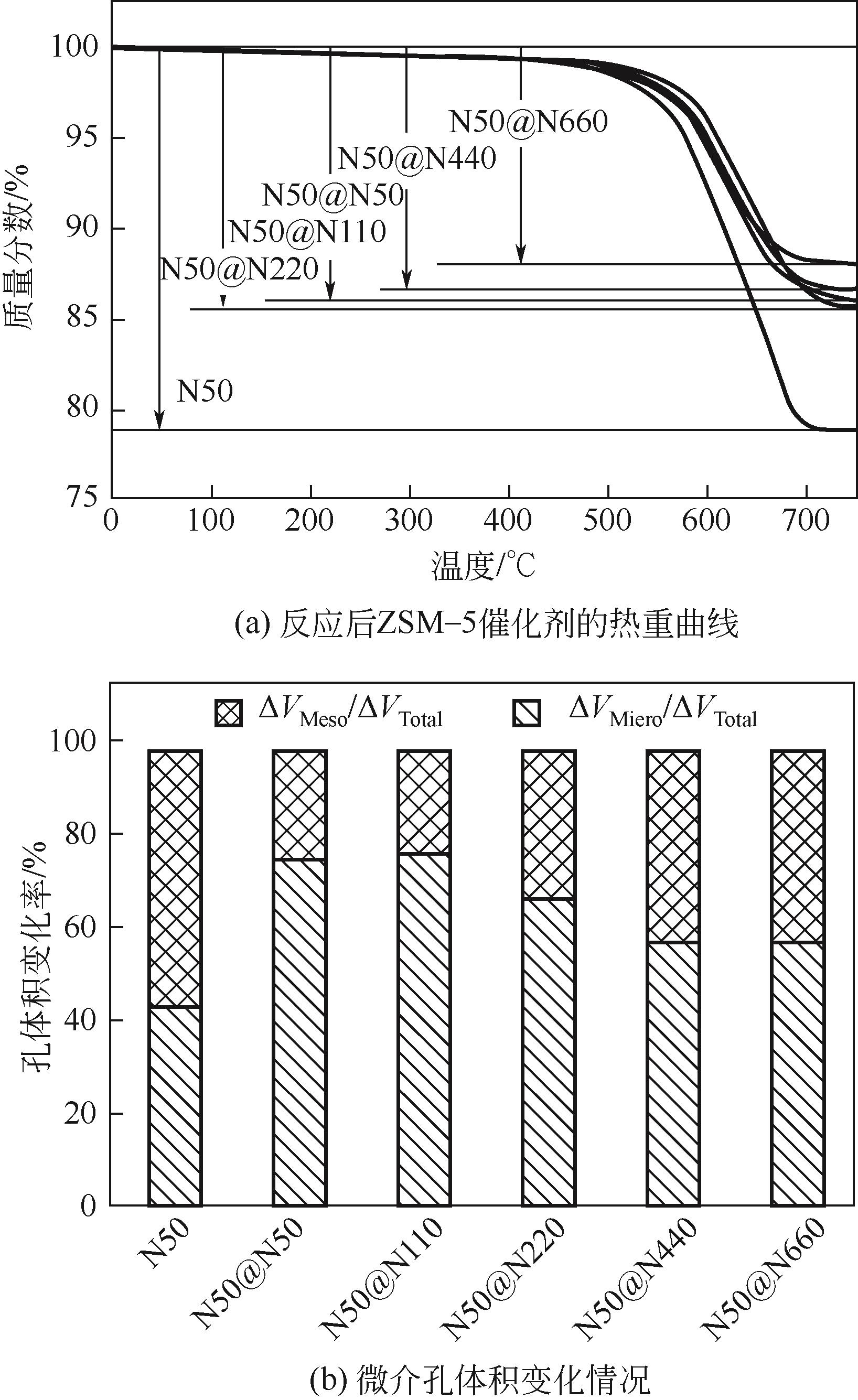
芳烃选择性低是ZSM-5催化甲醇制芳烃反应的难点问题,调变ZSM-5酸性质是提升选择性的重要方法。本研究将SiO2/Al2O3为50的纳米ZSM-5分别置于硅铝比为50、110、220、440和660的料液中继续水热生长,优化其表面酸性,以提高轻质芳烃选择性。采用X射线衍射(XRD)、透射电子显微镜(TEM)、X射线荧光光谱(XRF)、吡啶红外(Py-IR)、氨气程序升温脱附(NH3-TPD)等手段分析所得ZSM-5形貌、织构和酸性质,研究其水热再生长规律。发现生长过程中原粉部分溶解,包围在其表面的料液先形成小晶粒,经逐步堆积完成对原粉的包覆,最终形成孔道贯通性良好的单晶。在较低硅铝比料液中样品粒径分布不均匀,然而随着料液硅铝比的增加,包覆趋向均匀且表面呈现凸起结构。二次生长显著改变了表面酸性质及芳构化性能,在硅铝比为220的料液中生长后总酸量增加到194.9μmol/g,高于原粉的169.7μmol/g。值得注意的是,催化剂表面的强酸占比由原粉的37%显著增加至53%,B/L值也由原粉的0.56增加至3.19。该酸性的变化在促进甲醇芳构化的同时,还能强化芳烃的脱烷基化而提高BTX选择性,使总芳烃选择性和BTX选择性分别由16.1%和8.2%提高到23.8%和13.5%。
中图分类号:
引用本文
惠燕, 付廷俊, 马倩, 李忠. 纳米ZSM-5在高硅铝比料液中的再生长机制及其甲醇制芳烃性能[J]. 化工进展, 2022, 41(12): 6364-6376.
HUI Yan, FU Tingjun, MA Qian, LI Zhong. Secondary growth mechanism of nano-ZSM-5 in solution of high SiO2/Al2O3 ratio and its performance of methanol to aromatics[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6364-6376.
| 样品 | 比表面积/m2·g-1 | 体积/cm3·g-1 | ||||
|---|---|---|---|---|---|---|
| 总比表面积(SBET①) | 外比表面积(SExter②) | 微孔比表面积(SMicro②) | 总体积(VTotal③) | 介孔体积(VMeso④) | 微孔体积(VMicro②) | |
| N50 | 408 | 53 | 355 | 0.49 | 0.31 | 0.18 |
| N50@N50 | 383 | 31 | 352 | 0.30 | 0.13 | 0.17 |
| N50@N110 | 394 | 32 | 362 | 0.32 | 0.14 | 0.18 |
| N50@N220 | 401 | 37 | 364 | 0.42 | 0.25 | 0.17 |
| N50@N440 | 431 | 44 | 387 | 0.48 | 0.29 | 0.19 |
| N50@N660 | 434 | 44 | 390 | 0.46 | 0.28 | 0.18 |
表1 ZSM-5的结构性质和相对结晶度
| 样品 | 比表面积/m2·g-1 | 体积/cm3·g-1 | ||||
|---|---|---|---|---|---|---|
| 总比表面积(SBET①) | 外比表面积(SExter②) | 微孔比表面积(SMicro②) | 总体积(VTotal③) | 介孔体积(VMeso④) | 微孔体积(VMicro②) | |
| N50 | 408 | 53 | 355 | 0.49 | 0.31 | 0.18 |
| N50@N50 | 383 | 31 | 352 | 0.30 | 0.13 | 0.17 |
| N50@N110 | 394 | 32 | 362 | 0.32 | 0.14 | 0.18 |
| N50@N220 | 401 | 37 | 364 | 0.42 | 0.25 | 0.17 |
| N50@N440 | 431 | 44 | 387 | 0.48 | 0.29 | 0.19 |
| N50@N660 | 434 | 44 | 390 | 0.46 | 0.28 | 0.18 |
| 样品 | SiO2/Al2O3① | SiO2/Al2O3② | 酸量③/μmol·g-1 | 强酸/弱酸 | 酸量④/μmol·g-1 | B/L | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 总量 | 弱酸 | 中强酸 | 强酸 | B酸 | L酸 | |||||
| N50 | 72 | 21 | 169.7 | 84.0 | 22.5 | 63.2 | 0.75 | 26.8 | 47.6 | 0.56 |
| N50@N50 | 70 | — | 247.5 | 100.1 | 41.8 | 105.6 | 1.06 | — | — | — |
| N50@N110 | 77 | 27 | 194.9 | 85.2 | 33.8 | 75.9 | 0.89 | 37.1 | 19.8 | 1.87 |
| N50@N220 | 87 | 46 | 194.9 | 70.4 | 21.0 | 103.4 | 1.47 | 38.2 | 12.0 | 3.19 |
| N50@N440 | 94 | — | 142.6 | 41.9 | 19.6 | 81.1 | 1.93 | — | — | — |
| N50@N660 | 106 | 50 | 125.6 | 42.3 | 15.4 | 67.9 | 1.61 | 4.0 | 15.6 | 0.25 |
表2 不同ZSM-5样品的硅铝比和酸性质
| 样品 | SiO2/Al2O3① | SiO2/Al2O3② | 酸量③/μmol·g-1 | 强酸/弱酸 | 酸量④/μmol·g-1 | B/L | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 总量 | 弱酸 | 中强酸 | 强酸 | B酸 | L酸 | |||||
| N50 | 72 | 21 | 169.7 | 84.0 | 22.5 | 63.2 | 0.75 | 26.8 | 47.6 | 0.56 |
| N50@N50 | 70 | — | 247.5 | 100.1 | 41.8 | 105.6 | 1.06 | — | — | — |
| N50@N110 | 77 | 27 | 194.9 | 85.2 | 33.8 | 75.9 | 0.89 | 37.1 | 19.8 | 1.87 |
| N50@N220 | 87 | 46 | 194.9 | 70.4 | 21.0 | 103.4 | 1.47 | 38.2 | 12.0 | 3.19 |
| N50@N440 | 94 | — | 142.6 | 41.9 | 19.6 | 81.1 | 1.93 | — | — | — |
| N50@N660 | 106 | 50 | 125.6 | 42.3 | 15.4 | 67.9 | 1.61 | 4.0 | 15.6 | 0.25 |
| 样品 | 选择性/% | 氢转移指数① | 丙烯/乙烯(P/E) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 甲烷 | 乙烷 | 丙烷 | 丁烷 | 乙烯 | 丙烯 | 丁烯 | |||
| N50 | 0.7 | 0.4 | 4.4 | 17.1 | 8.5 | 21.5 | 16.3 | 0.17 | 2.53 |
| N50@N50 | 1.8 | 0.3 | 6.0 | 17.4 | 9.5 | 19.9 | 13.5 | 0.23 | 2.09 |
| N50@N110 | 1.3 | 0.2 | 5.3 | 16.9 | 8.8 | 19.9 | 14.0 | 0.21 | 2.27 |
| N50@N220 | 1.7 | 0.3 | 6.9 | 15.6 | 8.4 | 18.8 | 13.1 | 0.27 | 2.23 |
| N50@N440 | 1.4 | 0.3 | 5.6 | 13.4 | 8.2 | 22.3 | 16.7 | 0.20 | 2.72 |
| N50@N660 | 1.7 | 0.3 | 5.2 | 12.8 | 9.0 | 24.3 | 18.3 | 0.18 | 2.71 |
表3 MTH反应中低碳烷烃和烯烃在不同催化剂上的选择性
| 样品 | 选择性/% | 氢转移指数① | 丙烯/乙烯(P/E) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 甲烷 | 乙烷 | 丙烷 | 丁烷 | 乙烯 | 丙烯 | 丁烯 | |||
| N50 | 0.7 | 0.4 | 4.4 | 17.1 | 8.5 | 21.5 | 16.3 | 0.17 | 2.53 |
| N50@N50 | 1.8 | 0.3 | 6.0 | 17.4 | 9.5 | 19.9 | 13.5 | 0.23 | 2.09 |
| N50@N110 | 1.3 | 0.2 | 5.3 | 16.9 | 8.8 | 19.9 | 14.0 | 0.21 | 2.27 |
| N50@N220 | 1.7 | 0.3 | 6.9 | 15.6 | 8.4 | 18.8 | 13.1 | 0.27 | 2.23 |
| N50@N440 | 1.4 | 0.3 | 5.6 | 13.4 | 8.2 | 22.3 | 16.7 | 0.20 | 2.72 |
| N50@N660 | 1.7 | 0.3 | 5.2 | 12.8 | 9.0 | 24.3 | 18.3 | 0.18 | 2.71 |
| 样品 | 积炭量/g·gcat-1 | 积炭速率/g·gcat-1·h-1 |
|---|---|---|
| N50 | 0.21 | 2.8×10-3 |
| N50@N50 | 0.13 | 2.6×10-3 |
| N50@N110 | 0.14 | 2.1×10-3 |
| N50@N220 | 0.14 | 2.1×10-3 |
| N50@N440 | 0.13 | 1.8×10-3 |
| N50@N660 | 0.12 | 2.4×10-3 |
表4 失活催化剂的积炭量和积炭速率
| 样品 | 积炭量/g·gcat-1 | 积炭速率/g·gcat-1·h-1 |
|---|---|---|
| N50 | 0.21 | 2.8×10-3 |
| N50@N50 | 0.13 | 2.6×10-3 |
| N50@N110 | 0.14 | 2.1×10-3 |
| N50@N220 | 0.14 | 2.1×10-3 |
| N50@N440 | 0.13 | 1.8×10-3 |
| N50@N660 | 0.12 | 2.4×10-3 |
| 1 | LI Teng, SHOINKHOROVA Tuiana, GASCON Jorge, et al. Aromatics production via methanol-mediated transformation routes[J]. ACS Catalysis, 2021, 11(13): 7780-7819. |
| 2 | 陈嵩嵩, 张国帅, 霍锋, 等. 煤基大宗化学品市场及产业发展趋势[J]. 化工进展, 2020, 39(12): 5009-5020. |
| CHEN Songsong, ZHANG Guoshuai, HUO Feng, et al. Market and technology development trends of coal-based bulk chemicals[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 5009-5020. | |
| 3 | 米多, 王涛, 田佰和. 国内外芳烃主要产品市场分析[J]. 化学工业, 2020, 38(4): 57-67. |
| MI Duo, WANG Tao, TIAN Baihe. Analysis of the main aromatics market at home and abroad[J]. Chemical Industry, 2020, 38(4): 57-67. | |
| 4 | ZHANG Dan, YANG Minbo, FENG Xiao, et al. Integration of methanol aromatization with light hydrocarbon aromatization toward increasing aromatic yields: conceptual process designs and comparative analysis[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(30): 11376-11388. |
| 5 | ZHANG Jingui, QIAN Weizhong, KONG Chuiyan, et al. Increasing para-xylene selectivity in making aromatics from methanol with a surface-modified Zn/P/ZSM-5 catalyst[J]. ACS Catalysis, 2015, 5(5): 2982-2988. |
| 6 | CHANG Clarence D, SILVESTRI Anthony J. The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts[J]. Journal of Catalysis, 1977, 47(2): 249-259. |
| 7 | MA Yunhai, CAI Dali, LI Yiru, et al. The influence of straight pore blockage on the selectivity of methanol to aromatics in nanosized Zn/ZSM-5: an atomic Cs-corrected STEM analysis study[J]. RSC Advances, 2016, 6(78): 74797-74801. |
| 8 | YARULINA Irina, CHOWDHURY Abhishek Dutta, MEIRER Florian, et al. Recent trends and fundamental insights in the methanol-to-hydrocarbons process[J]. Nature Catalysis, 2018, 1(6): 398-411. |
| 9 | METZGER Kara E, MOYER Megan M, TREWYN Brian G. Tandem catalytic systems integrating biocatalysts and inorganic catalysts using functionalized porous materials[J]. ACS Catalysis, 2021, 11(1): 110-122. |
| 10 | YARULINA Irina, DE WISPELAERE Kristof, BAILLEUL Simon, et al. Structure-performance descriptors and the role of Lewis acidity in the methanol-to-propylene process[J]. Nature Chemistry, 2018, 10(8): 804-812. |
| 11 | GAO Yan, ZHENG Binghui, WU Guang, et al. Effect of the Si/Al ratio on the performance of hierarchical ZSM-5 zeolites for methanol aromatization[J]. RSC Advances, 2016, 6(87): 83581-83588. |
| 12 | MA Hao, SUN Yuan, YU Junping, et al. Theoretical study on the influence of ZSM-5 zeolite with different structures for methanol to aromatics[J]. Microporous and Mesoporous Materials, 2020, 294: 109838-109847. |
| 13 | MANDERSLOOT W G B, NICOLAIDES C P, SCURRELL M S. Comments on the effect of the acidic strength of silica-alumina in the conversion of methanol to hydrocarbons[J]. Applied Catalysis, 1986, 27(2): 393-396. |
| 14 | BI Yi, WANG Yingli, CHEN Xin, et al. Methanol aromatization over HZSM-5 catalysts modified with different zinc salts[J]. Chinese Journal of Catalysis, 2014, 35(10): 1740-1751. |
| 15 | LI Junhua, WANG Lina, ZHANG Dan, et al. Effect of ZSM-5 acid modification on aromatization performance of methanol[J]. Journal of Fuel Chemistry and Technology, 2019, 47(8): 957-963. |
| 16 | MIYAKE Koji, HIROTA Yuichiro, Kaito ONO, et al. Selective production of benzene, toluene and p-xylene (BTpX) from various C1-3 feedstocks over ZSM-5/silicalite-1 core-shell zeolite catalyst[J]. ChemistrySelect, 2016, 1(5): 967-969. |
| 17 | YANG Junhao, GONG Ke, MIAO Dengyun, et al. Enhanced aromatic selectivity by the sheet-like ZSM-5 in syngas conversion[J]. Journal of Energy Chemistry, 2019, 35: 44-48. |
| 18 | WANG Kai, DONG Mei, NIU Xianjun, et al. Highly active and stable Zn/ZSM-5 zeolite catalyst for the conversion of methanol to aromatics: effect of support morphology[J]. Catalysis Science & Technology, 2018, 8(21): 5646-5656. |
| 19 | 董道敏, 刘宾, 柴永明, 等. 动态水热法制备silicalite-1分子筛膜包覆多孔缺陷Al2O3微球[J]. 化工进展, 2018, 37(10): 3943-3948. |
| DONG Daomin, LIU Bin, CHAI Yongming, et al. Dynamic hydrothermal synthesis of silicalite-1 zeolite membrane to encapsulate defective porous alumina spheres[J]. Chemical Industry and Engineering Progress, 2018, 37(10): 3943-3948. | |
| 20 | XIE Chenlu, CHEN Chen, YU Yi, et al. Tandem catalysis for CO2 hydrogenation to C2—C4 hydrocarbons[J]. Nano Letters, 2017, 17(6): 3798-3802. |
| 21 | XU Yanfei, MA Guangyuan, BAI Jingyang, et al. Yolk@shell FeMn@hollow HZSM-5 nanoreactor for directly converting syngas to aromatics[J]. ACS Catalysis, 2021, 11(8): 4476-4485. |
| 22 | XU Guohao, ZHU Xuedong. A core-shell structured Zn/SiO2@ZSM-5 catalyst: preparation and enhanced catalytic properties in methane co-aromatization with propane[J]. Applied Catalysis B: Environmental, 2021, 293: 120241-120249. |
| 23 | WANG Xiaoxing, ZHANG Junfeng, ZHANG Tao, et al. Mesoporous ZnZSM-5 zeolites synthesized by one-step desilication and reassembly: a durable catalyst for methanol aromatization[J]. RSC Advances, 2016, 6(28): 23428-23437. |
| 24 | CUI Tianlu, LI Xinhao, Libing LYU, et al. Nanoscale Kirkendall growth of silicalite-1 zeolite mesocrystals with controlled mesoporosity and size[J]. Chemical Communications, 2015, 51(63): 12563-12566. |
| 25 | JIA Yanming, WANG Junwen, ZHANG Kan, et al. Hierarchical ZSM-5 zeolite synthesized via dry gel conversion-steam assisted crystallization process and its application in aromatization of methanol[J]. Powder Technology, 2018, 328: 415-429. |
| 26 | SUN Minghui, ZHOU Jian, HU Zhiyi, et al. Hierarchical zeolite single-crystal reactor for excellent catalytic efficiency[J]. Matter, 2020, 3(4): 1226-1245. |
| 27 | TAO Shuo, LI Xiaolei, WANG Xiaoge, et al. Facile synthesis of hierarchical nanosized single-crystal aluminophosphate molecular sieves from highly homogeneous and concentrated precursors[J]. Angewandte Chemie International Edition, 2020, 59(9): 3455-3459. |
| 28 | ZHU Jie, ZHU Yihan, ZHU Liangkui, et al. Highly mesoporous single-crystalline zeolite beta synthesized using a nonsurfactant cationic polymer as a dual-function template[J]. Journal of the American Chemical Society, 2014, 136(6): 2503-2510. |
| 29 | JAMIL Anas Karrar, MURAZA Oki, AL-AMER Adnan M. The role of alcohols and diols as co-solvents in fabrication of TON zeolite[J]. Journal of Industrial and Engineering Chemistry, 2015, 29: 112-119. |
| 30 | CHEN Xiaoxin, YAN Wenfu, CAO Xuejing, et al. Fabrication of silicalite-1 crystals with tunable aspect ratios by microwave-assisted solvothermal synthesis[J]. Microporous and Mesoporous Materials, 2009, 119(1/2/3): 217-222. |
| 31 | THOMMES Matthias, KANEKO Katsumi, NEIMARK Alexander V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report)[J]. Pure and Applied Chemistry, 2015, 87(9/10): 1051-1069. |
| 32 | GOODARZI Farnoosh, HERRERO Irene Pinilla, KALANTZOPOULOS Georgios N, et al. Synthesis of mesoporous ZSM-5 zeolite encapsulated in an ultrathin protective shell of silicalite-1 for MTH conversion[J]. Microporous and Mesoporous Materials, 2020, 292: 109730-109739. |
| 33 | JONES Andrew J, IGLESIA Enrique. The strength of Brønsted acid sites in microporous aluminosilicates[J]. ACS Catalysis, 2015, 5(10): 5741-5755. |
| 34 | JONES Andrew J, CARR Robert T, ZONES Stacey I, et al. Acid strength and solvation in catalysis by MFI zeolites and effects of the identity, concentration and location of framework heteroatoms[J]. Journal of Catalysis, 2014, 312: 58-68. |
| 35 | KATADA Naonobu, Hirofumi IGI, KIM Jong Ho. Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium[J]. The Journal of Physical Chemistry B, 1997, 101(31): 5969-5977. |
| 36 | KALITA Pranjal, GUPTA Narendra M, KUMAR Rajiv. Synergistic role of acid sites in the Ce-enhanced activity of mesoporous Ce-Al-MCM-41 catalysts in alkylation reactions: FTIR and TPD-ammonia studies[J]. Journal of Catalysis, 2007, 245(2): 338-347. |
| 37 | WANG Chao, CHU Yueying, ZHENG Anmin, et al. New insight into the hydrocarbon-pool chemistry of the methanol-to-olefins conversion over zeolite H-ZSM-5 from GC-MS, solid-state NMR spectroscopy, and DFT calculations[J]. Chemistry—a European Journal, 2014, 20(39): 12432-12443. |
| 38 | Róbert BARTHOS, Tamás BÁNSÁGI, SÜLI ZAKAR Tímea, et al. Aromatization of methanol and methylation of benzene over Mo2C/ZSM-5 catalysts[J]. Journal of Catalysis, 2007, 247(2): 368-378. |
| 39 | FENG Chaoqun, SU Xiaofang, WANG Wei, et al. Facile synthesis of ultrafine nanosized ZSM-5 zeolite using a hydroxyl radical initiator for enhanced catalytic performance in the MTG reaction[J]. Microporous and Mesoporous Materials, 2021, 312: 110780-110790. |
| 40 | FU Tingjun, SHAO Juan, LI Zhong. Catalytic synergy between the low Si/Al ratio Zn/ZSM-5 and high Si/Al ratio HZSM-5 for high-performance methanol conversion to aromatics[J]. Applied Catalysis B: Environmental, 2021, 291: 120098-120113. |
| 41 | SCHULZ Hans. “Coking” of zeolites during methanol conversion: basic reactions of the MTO-, MTP- and MTG processes[J]. Catalysis Today, 2010, 154(3/4): 183-194. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [10] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [11] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [12] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [13] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [14] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [15] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||