化工进展 ›› 2022, Vol. 41 ›› Issue (10): 5588-5598.DOI: 10.16085/j.issn.1000-6613.2021-2498
氮掺杂生物炭材料的制备及其在环境中的应用
鞠梦灿( ), 严丽丽(
), 严丽丽( ), 简铃, 江思雨, 饶品华, 李光辉
), 简铃, 江思雨, 饶品华, 李光辉
- 上海工程技术大学化学化工学院, 上海 201620
-
收稿日期:2021-12-07修回日期:2022-02-28出版日期:2022-10-20发布日期:2022-10-21 -
通讯作者:严丽丽 -
作者简介:鞠梦灿(1998—),女,硕士研究生,研究方向为环境功能材料制备与应用。E-mail:mengcan_ju@163.com。 -
基金资助:地方院校能力建设项目(21010501400)
Preparation of nitrogen-doped biochar and its environmental applications
JU Mengcan( ), YAN Lili(
), YAN Lili( ), JIAN Ling, JIANG Siyu, RAO Pinhua, LI Guanghui
), JIAN Ling, JIANG Siyu, RAO Pinhua, LI Guanghui
- School of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, Shanghai 201620, China
-
Received:2021-12-07Revised:2022-02-28Online:2022-10-20Published:2022-10-21 -
Contact:YAN Lili
摘要:
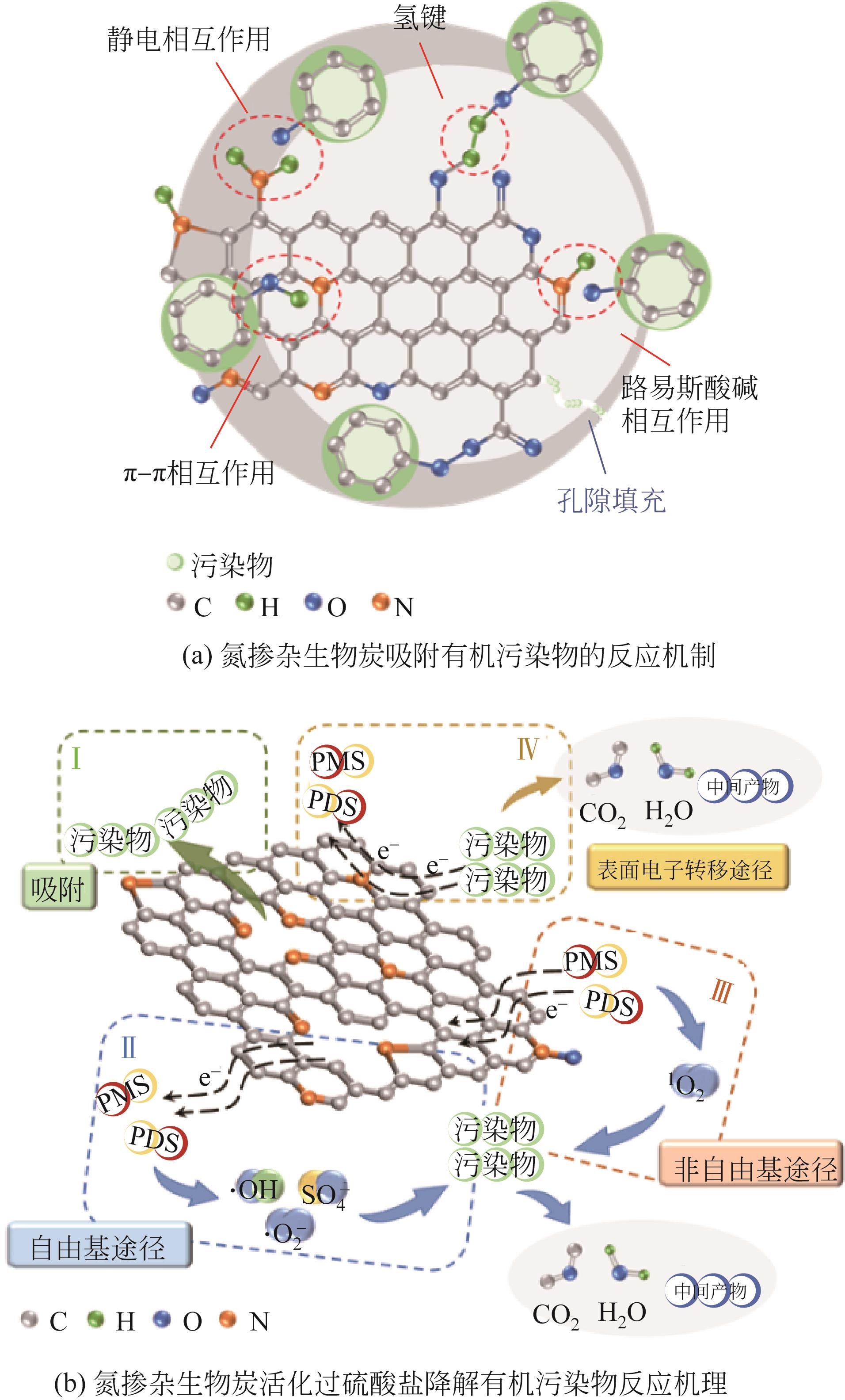
由生物质转化得到的生物炭材料因其成本低且环境友好被广泛用于环境领域,且对我国实现碳达峰与碳中和有积极的促进作用。非金属氮掺杂生物炭由于氮元素的引入,呈现表面碱度以及多吸附位点的特性,提高了其对污染物的去除性能,然而对氮掺杂生物炭材料的绿色可控合成及掺杂机理的关注不够。本文综述了近几年来国内外氮掺杂生物炭材料的制备及其在环境中的研究应用,梳理了氮掺杂生物炭材料中含氮官能团的类型和不同制备方法,含氮官能团包括吡啶N、吡咯N和石墨N等,其含量和类型受氮源、热解温度和时间的影响,阐明了其中的氮掺杂机理由氮源分解的中间产物、生物炭表面官能团和掺杂过程中的活化剂等因素决定。最后,对氮掺杂生物炭在环境方面的应用及作用机理进行探讨,并在此基础上提出未来研究高效氮掺杂生物炭的重点和研究方向,以期为氮掺杂生物炭在环境中的实际应用提供参考。
中图分类号:
引用本文
鞠梦灿, 严丽丽, 简铃, 江思雨, 饶品华, 李光辉. 氮掺杂生物炭材料的制备及其在环境中的应用[J]. 化工进展, 2022, 41(10): 5588-5598.
JU Mengcan, YAN Lili, JIAN Ling, JIANG Siyu, RAO Pinhua, LI Guanghui. Preparation of nitrogen-doped biochar and its environmental applications[J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5588-5598.
| 碳源 | 氮源 | 活化剂 | 处理工艺 | 含N量 /% | N分布 | 应用机理 | 数据来源 |
|---|---|---|---|---|---|---|---|
| 莲藕 | 莲藕 | — | N2氛围中,600~900℃下热解4h,升温速率为4℃/min;酸洗/水洗至中性 | 3.15① | 吡啶N、吡咯N和石墨N | 通过表面与孔隙的物理吸附作用吸附甲基橙 | [ |
| 蓝藻 | 蓝藻 | KOH | 蓝藻与KOH粉末混合;N2氛围中,500~800℃下热解2h,升温速率为10℃/min;水洗 | 5.55① | 吡啶N、吡咯N和石墨N | 孔隙与石墨N提供CO2吸附活性位点 | [ |
| 活性污泥 | 活性污泥 | — | N2氛围中,400~1000℃下热解2h,升温速率为5℃/min | — | 吡啶N和石墨N | 基于活性氢和表面电子转移途径催化降解硝基酚 | [ |
| 豆渣 | 豆渣 | K2CO3 | 豆渣与K2CO3溶液反应后干燥;N2氛围中,400~900℃下热解2h,升温速率为5℃/min;酸洗/水洗至中性 | — | 吡啶N、吡咯N和石墨N | 基于表面自由基和电子转移途径活化PDS降解双酚A | [ |
| 酵母 | 酵母 | NaCl/KCl | 酵母与NaCl/KCl溶液混合后冷冻干燥,Ar氛围中,700℃下热解2h,升温速率为5℃/min;水洗 | 5.91① 4.66② | 吡啶N、吡咯N和石墨N | 基于SO | [ |
| 海带 | 海带 | KOH | N2氛围中,500℃下热解1.5h;与KOH混合,N2氛围中,600~900℃下热解2h,升温速率为5℃/min;水洗 | 2.74① | 吡啶N、吡咯N和石墨N | 以1O2非自由基途径和O | [ |
| 琼脂 | NH3 | FeCl3 | 琼脂与FeCl3溶液反应2h后干燥;Ar氛 围中升温至600℃和800℃,升温速率为10℃/min;然后Ar转换成NH3,热解1h | — | 通过静电相互作用和氧化还原反应吸附Cr(Ⅵ) | [ | |
| 玉米秸秆 | NH3 | — | N2氛围中,600℃下热解2h;N2转换成NH3,600℃下热解1h后,再升温至700℃和800℃下热解1~3h,升温速率为20℃/min | 5.69② | 吡啶N、吡咯N和石墨N | 通过静电相互作用、π-π相互作用和路易斯酸碱相互作用吸附亚甲基蓝和酸性橙 | [ |
| 通过阳离子-π相互作用、生物炭表面羟基基团与石墨N的络合作用吸附Cu(Ⅱ)和Cd(Ⅱ) | [ | ||||||
| 芦苇 | 硝酸铵(NH4NO3) | — | 芦苇分散在乙醇溶液中,然后加入NH4NO3反应;N2氛围中,400~1000℃下热解1.5h,升温速率为15℃/min;水洗3次 | 1.76① | 吡啶N、吡咯N、石墨N和氮氧化物 | 基于1O2和自由基途径活化PDS降解有机污染物 | [ |
| 釜馏物 | 聚磷酸铵 磷酸脲[(NH4)3PO4] | — | 釜馏物与含氮磷酸盐混合,N2氛围中,600℃和900℃下热解1h,升温速率为10℃/min; 酸洗/水洗至中性 | 1.60① | 吡啶N、吡咯N、石墨N和氮氧化物 | 吸附甲苯 | [ |
| 废咖啡渣 | 尿素 | — | 咖啡渣与尿素混合后,N2氛围中,300~1000℃下热解1h,升温速率为10℃/min;水洗 | 2.80① | 吡啶N、吡咯N和石墨N | 基于1O2非自由基途径和SO | [ |
| 玉米秸秆 | 尿素 | — | 玉米秸秆与尿素溶液反应后干燥;N2氛围中,700℃下热解2h,升温速率为5℃/min | 11.16① | 吡啶N、吡咯N和石墨N | 通过表面电子转移途径活化PMS降解磺胺嘧啶 | [ |
| 木屑 | 尿素 | — | 木屑与尿素混合,无氧条件下,800℃下热解2h,升温速率为10℃/min;水洗 | 12.10② | 吡啶N、吡咯N和石墨N | 基于非自由基途径以及自由基途径活化PMS降解酸性橙 | [ |
| 芦竹 | 尿素 | KOH/ZnCl2 | 芦竹、尿素和KOH/ZnCl2混合,N2氛围中,600℃下热解2h,升温速率为3℃/min | 15.88① | 吡啶N和吡咯N | 吸收CO2 | [ |
表1 不同氮掺杂生物炭的基本性质及应用
| 碳源 | 氮源 | 活化剂 | 处理工艺 | 含N量 /% | N分布 | 应用机理 | 数据来源 |
|---|---|---|---|---|---|---|---|
| 莲藕 | 莲藕 | — | N2氛围中,600~900℃下热解4h,升温速率为4℃/min;酸洗/水洗至中性 | 3.15① | 吡啶N、吡咯N和石墨N | 通过表面与孔隙的物理吸附作用吸附甲基橙 | [ |
| 蓝藻 | 蓝藻 | KOH | 蓝藻与KOH粉末混合;N2氛围中,500~800℃下热解2h,升温速率为10℃/min;水洗 | 5.55① | 吡啶N、吡咯N和石墨N | 孔隙与石墨N提供CO2吸附活性位点 | [ |
| 活性污泥 | 活性污泥 | — | N2氛围中,400~1000℃下热解2h,升温速率为5℃/min | — | 吡啶N和石墨N | 基于活性氢和表面电子转移途径催化降解硝基酚 | [ |
| 豆渣 | 豆渣 | K2CO3 | 豆渣与K2CO3溶液反应后干燥;N2氛围中,400~900℃下热解2h,升温速率为5℃/min;酸洗/水洗至中性 | — | 吡啶N、吡咯N和石墨N | 基于表面自由基和电子转移途径活化PDS降解双酚A | [ |
| 酵母 | 酵母 | NaCl/KCl | 酵母与NaCl/KCl溶液混合后冷冻干燥,Ar氛围中,700℃下热解2h,升温速率为5℃/min;水洗 | 5.91① 4.66② | 吡啶N、吡咯N和石墨N | 基于SO | [ |
| 海带 | 海带 | KOH | N2氛围中,500℃下热解1.5h;与KOH混合,N2氛围中,600~900℃下热解2h,升温速率为5℃/min;水洗 | 2.74① | 吡啶N、吡咯N和石墨N | 以1O2非自由基途径和O | [ |
| 琼脂 | NH3 | FeCl3 | 琼脂与FeCl3溶液反应2h后干燥;Ar氛 围中升温至600℃和800℃,升温速率为10℃/min;然后Ar转换成NH3,热解1h | — | 通过静电相互作用和氧化还原反应吸附Cr(Ⅵ) | [ | |
| 玉米秸秆 | NH3 | — | N2氛围中,600℃下热解2h;N2转换成NH3,600℃下热解1h后,再升温至700℃和800℃下热解1~3h,升温速率为20℃/min | 5.69② | 吡啶N、吡咯N和石墨N | 通过静电相互作用、π-π相互作用和路易斯酸碱相互作用吸附亚甲基蓝和酸性橙 | [ |
| 通过阳离子-π相互作用、生物炭表面羟基基团与石墨N的络合作用吸附Cu(Ⅱ)和Cd(Ⅱ) | [ | ||||||
| 芦苇 | 硝酸铵(NH4NO3) | — | 芦苇分散在乙醇溶液中,然后加入NH4NO3反应;N2氛围中,400~1000℃下热解1.5h,升温速率为15℃/min;水洗3次 | 1.76① | 吡啶N、吡咯N、石墨N和氮氧化物 | 基于1O2和自由基途径活化PDS降解有机污染物 | [ |
| 釜馏物 | 聚磷酸铵 磷酸脲[(NH4)3PO4] | — | 釜馏物与含氮磷酸盐混合,N2氛围中,600℃和900℃下热解1h,升温速率为10℃/min; 酸洗/水洗至中性 | 1.60① | 吡啶N、吡咯N、石墨N和氮氧化物 | 吸附甲苯 | [ |
| 废咖啡渣 | 尿素 | — | 咖啡渣与尿素混合后,N2氛围中,300~1000℃下热解1h,升温速率为10℃/min;水洗 | 2.80① | 吡啶N、吡咯N和石墨N | 基于1O2非自由基途径和SO | [ |
| 玉米秸秆 | 尿素 | — | 玉米秸秆与尿素溶液反应后干燥;N2氛围中,700℃下热解2h,升温速率为5℃/min | 11.16① | 吡啶N、吡咯N和石墨N | 通过表面电子转移途径活化PMS降解磺胺嘧啶 | [ |
| 木屑 | 尿素 | — | 木屑与尿素混合,无氧条件下,800℃下热解2h,升温速率为10℃/min;水洗 | 12.10② | 吡啶N、吡咯N和石墨N | 基于非自由基途径以及自由基途径活化PMS降解酸性橙 | [ |
| 芦竹 | 尿素 | KOH/ZnCl2 | 芦竹、尿素和KOH/ZnCl2混合,N2氛围中,600℃下热解2h,升温速率为3℃/min | 15.88① | 吡啶N和吡咯N | 吸收CO2 | [ |
| 79 | ALHOKBANY N S, NAUSHAD M, KUMAR V, et al. Self-nitrogen doped carbons aerogel derived from waste cigarette butts (cellulose acetate) for the adsorption of BPA: kinetics and adsorption mechanisms[J]. Journal of King Saud University-Science, 2020, 32(8): 3351-3358. |
| 80 | CHENG Long, JI Yuanhui, LIU Xiaomin, et al. Sorption mechanism of organic dyes on a novel self-nitrogen-doped porous graphite biochar: coupling DFT calculations with experiments[J]. Chemical Engineering Science, 2021, 242: 116739. |
| 81 | KASERA Nitesh, HALL Steven, KOLAR Praveen. Effect of surface modification by nitrogen-containing chemicals on morphology and surface characteristics of N-doped pine bark biochars[J]. Journal of Environmental Chemical Engineering, 2021, 9(2): 105161. |
| 82 | MA Wenjie, WANG Na, DU Yunchen, et al. Human-hair-derived N,S-doped porous carbon: an enrichment and degradation system for wastewater remediation in the presence of peroxymonosulfate[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(2): 2718-2727. |
| 83 | QI Yuanfeng, GE Baoxin, ZHANG Yanqing, et al. Three-dimensional porous graphene-like biochar derived from Enteromorpha as a persulfate activator for sulfamethoxazole degradation: role of graphitic N and radicals transformation[J]. Journal of Hazardous Materials, 2020, 399: 123039. |
| 84 | LI Shengnan, Shih Hsin HO, HUA Tao, et al. Sustainable biochar as an electrocatalysts for the oxygen reduction reaction in microbial fuel cells[J]. Green Energy & Environment, 2021, 6(5): 644-659. |
| 85 | ALHASHIMI H A, AKTAS C B. Life cycle environmental and economic performance of biochar compared with activated carbon: a meta-analysis[J]. Resources, Conservation and Recycling, 2017, 118: 13-26. |
| 86 | AHMED M B, ZHOU J L, NGO H H, et al. Insight into biochar properties and its cost analysis[J]. Biomass and Bioenergy, 2016, 84: 76-86. |
| 1 | LIANG Xiaodu, LIU Ruonan, WU Xiaoliang. Biomass waste derived functionalized hierarchical porous carbon with high gravimetric and volumetric capacitances for supercapacitors[J]. Microporous and Mesoporous Materials, 2021, 310: 110659. |
| 2 | CHO Eun Jun, TRINH Ly Thi Phi, SONG Younho, et al. Bioconversion of biomass waste into high value chemicals[J]. Bioresource Technology, 2020, 298: 122386. |
| 3 | 洪亚军, 徐祖信, 冯承莲, 等. 水葫芦/污泥共热解法制备生物炭粒及其对Cr3+的吸附特性[J]. 环境科学研究, 2020, 33(4): 1052-1061. |
| HONG Yajun, XU Zuxin, FENG Chenglian, et al. Co-pyrolysis of water hyacinth and sewage sludge for preparation of biochar particles and its adsorption properties for Cr3+ [J]. Research of Environmental Sciences, 2020, 33(4): 1052-1061. | |
| 4 | LENG Lijian, XU Siyu, LIU Renfeng, et al. Nitrogen containing functional groups of biochar: an overview[J]. Bioresource Technology, 2020, 298: 122286. |
| 5 | FOWLES Malcolm. Black carbon sequestration as an alternative to bioenergy[J]. Biomass and Bioenergy, 2007, 31: 426-432. |
| 6 | WANG Jianlong, WANG Shizong. Preparation, modification and environmental application of biochar: a review[J]. Journal of Cleaner Production, 2019, 227: 1002-1022. |
| 7 | 王芳平, 马婧, 李小亚, 等. 板栗壳生物炭高性能对称性超级电容器电极材料的制备及性能[J]. 化工进展, 2021, 40(8): 4381-4387. |
| WANG Fangping, MA Jing, LI Xiaoya, et al. Preparation and properties of chestnut shell-based biochar electrode material for high-performance symmetrical supercapacitor[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4381-4387. | |
| 8 | WAN Zhonghao, SUN Yuqing, TSANG D C W, et al. Customised fabrication of nitrogen-doped biochar for environmental and energy applications[J]. Chemical Engineering Journal, 2020, 401: 126136. |
| 9 | YE Shujing, ZENG Guangming, TAN Xiaofei, et al. Nitrogen-doped biochar fiber with graphitization from boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer[J]. Applied Catalysis B: Environmental, 2020, 269: 118850. |
| 10 | 孙金龙, 张宇, 刘福跃, 等. 基于碳基催化剂活化过二硫酸盐降解有机污染物的研究进展[J]. 化工进展, 2021, 40(3): 1653-1666. |
| SUN Jinlong, ZHANG Yu, LIU Fuyue, et al. Research progress in degradation of organic pollutants by activation of persulfates with carbon-based catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1653-1666. | |
| 11 | INAGAKI Michio, TOYODA Masahiro, SONEDA Yasushi, et al. Nitrogen-doped carbon materials[J]. Carbon, 2018, 132: 104-140. |
| 12 | CHEN Xiao, Wen Da OH, HU Zhongting, et al. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes[J]. Applied Catalysis B: Environmental, 2018, 225: 243-257. |
| 13 | DING Dahu, YANG Shengjiong, QIAN Xiaoyong, et al. Nitrogen-doping positively whilst sulfur-doping negatively affect the catalytic activity of biochar for the degradation of organic contaminant[J]. Applied Catalysis B: Environmental, 2020, 263: 118348. |
| 14 | ShihHsin HO, CHEN Yidi, LI Ruixiang, et al. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation[J]. Water Research, 2019, 159: 77-86. |
| 15 | LI Dan, CHEN Wenhua, WU Jianping, et al. The preparation of waste biomass-derived N-doped carbons and their application in acid gas removal: focus on N functional groups[J]. Journal of Materials Chemistry A, 2020, 8(47): 24977-24995. |
| 16 | LIU Liyun, TAN Zhongxin, YE Zhixiong. Transformation and transport mechanism of nitrogenous compounds in a biochar “preparation–returning to the field” process studied by employing an isotope tracer method[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(2): 1780-1791. |
| 17 | CHEN Wei, YANG Haiping, CHEN Yingquan, et al. Transformation of nitrogen and evolution of N-containing species during algae pyrolysis[J]. Environmental Science & Technology, 2017, 51(11): 6570-6579. |
| 18 | LI Boyu, LI Kunquan. Effect of nitric acid pre-oxidation concentration on pore structure and nitrogen/oxygen active decoration sites of ethylenediamine-modified biochar for mercury(Ⅱ) adsorption and the possible mechanism[J]. Chemosphere, 2019, 220: 28-39. |
| 19 | CHEN Wei, CHEN Yingquan, YANG Haiping, et al. Co-pyrolysis of lignocellulosic biomass and microalgae: products characteristics and interaction effect[J]. Bioresource Technology, 2017, 245: 860-868. |
| 20 | CHEN Wei, CHEN Yingquan, YANG Haiping, et al. Investigation on biomass nitrogen-enriched pyrolysis: influence of temperature[J]. Bioresource Technology, 2018, 249: 247-253. |
| 21 | TIAN Yu, ZHANG Jun, ZUO Wei, et al. Nitrogen conversion in relation to NH3 and HCN during microwave pyrolysis of sewage sludge[J]. Environmental Science & Technology, 2013, 47(7): 3498-3505. |
| 22 | WANG Ani, FAN Ruiqing, PI Xinxin, et al. Nitrogen-doped microporous carbons derived from pyridine ligand-based metal-organic complexes as high-performance SO2 adsorption sorbents[J]. ACS Applied Materials & Interfaces, 2018, 10(43): 37407-37416. |
| 23 | JI Rongting, WU Yarui, BIAN Yongrong, et al. Nitrogen-doped porous biochar derived from marine algae for efficient solid-phase microextraction of chlorobenzenes from aqueous solution[J]. Journal of Hazardous Materials, 2021, 407: 124785. |
| 24 | MA Qiuxu, CHEN Wenhua, JIN Ziheng, et al. One-step synthesis of microporous nitrogen-doped biochar for efficient removal of CO2 and H2S[J]. Fuel, 2021, 289: 119932. |
| 25 | HE Jiawei, LONG Yuan, WANG Yiyan, et al. Aerosol-assisted self-assembly of reticulated N-doped carbonaceous submicron spheres for effective removal of hexavalent chromium[J]. ACS Applied Materials & Interfaces, 2016, 8(26): 16699-16707. |
| 26 | WANG Tengfei, ZHAI Yunbo, ZHU Yun, et al. Influence of temperature on nitrogen fate during hydrothermal carbonization of food waste[J]. Bioresource Technology, 2018, 247: 182-189. |
| 27 | WANG Tengfei, ZHAI Yunbo, ZHU Yun, et al. What is the influence of the nitrogen-containing composition during hydrothermal carbonization of biomass? A new perspective from mimic feedstock[J]. Bioresource Technology Reports, 2019, 5: 343-350. |
| 28 | GHASEMY Ebrahim, MOTEJADDED Hosein Banna, RASHIDI Alimorad, et al. N-doped CNT nanocatalyst prepared from camphor and urea for gas phase desulfurization: experimental and DFT study[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 85: 121-131. |
| 29 | LI Yunchao, XING Bo, WANG Xiaoliu, et al. Nitrogen-doped hierarchical porous biochar derived from corn stalks for phenol-enhanced adsorption[J]. Energy & Fuels, 2019, 33(12): 12459-12468. |
| 30 | WANG Li, YAN Wei, HE Chi, et al. Microwave-assisted preparation of nitrogen-doped biochars by ammonium acetate activation for adsorption of acid red 18[J]. Applied Surface Science, 2018, 433: 222-231. |
| 31 | LI Zhenhao, XING Bo, DING Yan, et al. A high-performance biochar produced from bamboo pyrolysis with in-situ nitrogen doping and activation for adsorption of phenol and methylene blue[J]. Chinese Journal of Chemical Engineering, 2020, 28(11): 2872-2880. |
| 32 | ZHU Q, MONEY S L, RUSSELL A E, et al. Determination of the fate of nitrogen functionality in carbonaceous materials during pyrolysis and combustion using X-ray absorption near edge structure spectroscopy[J]. Langmuir, 1997, 13(7): 2149-2157. |
| 33 | AN Xuefei, ZHAO Kun, ZHANG Weiping, et al. Tailoring the pore structure modified with functional groups for superior CO2 adsorption capacity and the selectivity of separation[J]. Fuel, 2022, 309: 122175. |
| 34 | ZHOU Qiying, JIANG Xia, LI Xi, et al. Preparation of high-yield N-doped biochar from nitrogen-containing phosphate and its effective adsorption for toluene[J]. RSC Advances, 2018, 8(53): 30171-30179. |
| 35 | HOU Yanrui, LIANG Ye, HU Hongbo, et al. Facile preparation of multi-porous biochar from Lotus biomass for methyl orange removal: kinetics, isotherms, and regeneration studies[J]. Bioresource Technology, 2021, 329: 124877. |
| 36 | WANG He, WANG Han, LIU Guoshuai, et al. In-situ pyrolysis of Taihu blue algae biomass as appealing porous carbon adsorbent for CO2 capture: role of the intrinsic N[J]. Science of the Total Environment, 2021, 771: 145424. |
| 37 | REN Xiaoya, TANG Lin, WANG Jiajia, et al. Highly efficient catalytic hydrogenation of nitrophenols by sewage sludge derived biochar[J]. Water Research, 2021, 201: 117360. |
| 38 | CAI Shu, ZHANG Qi, WANG Ziqian, et al. Pyrrolic N-rich biochar without exogenous nitrogen doping as a functional material for bisphenol A removal: performance and mechanism[J]. Applied Catalysis B: Environmental, 2021, 291: 120093. |
| 39 | XIE Yi, HU Wanrong, WANG Xuqian, et al. Molten salt induced nitrogen-doped biochar nanosheets as highly efficient peroxymonosulfate catalyst for organic pollutant degradation[J]. Environmental Pollution, 2020, 260: 114053. |
| 40 | HUANG Yimeng, LI Guang, LI Mingzhen, et al. Kelp-derived N-doped biochar activated peroxymonosulfate for ofloxacin degradation[J]. Science of the Total Environment, 2021, 754: 141999. |
| 41 | MIAN Md Manik, LIU Guijian, YOUSAF Balal, et al. Simultaneous functionalization and magnetization of biochar via NH3 ambiance pyrolysis for efficient removal of Cr(Ⅵ)[J]. Chemosphere, 2018, 208: 712-721. |
| 42 | LIAN Fei, CUI Guannan, LIU Zhongqi, et al. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity[J]. Journal of Environmental Management, 2016, 176(7): 61-68. |
| 43 | YU Wenchao, LIAN Fei, CUI Guannan, et al. N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution[J]. Chemosphere, 2018, 193: 8-16. |
| 44 | ZHU Shishu, HUANG Xiaochen, MA Fang, et al. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: inherent roles of adsorption and nonradical mechanisms[J]. Environmental Science & Technology, 2018, 52(15): 8649-8658. |
| 45 | OH W D, LISAK G, WEBSTER R D, et al. Insights into the thermolytic transformation of lignocellulosic biomass waste to redox-active carbocatalyst: durability of surface active sites[J]. Applied Catalysis B: Environmental, 2018, 233: 120-129. |
| 46 | WANG Huazhe, GUO Wanqian, LIU Banghai, et al. Edge-nitrogenated biochar for efficient peroxydisulfate activation: an electron transfer mechanism[J]. Water Research, 2019, 160: 405-414. |
| 47 | ZAENI Julia Raudlatul Jannah, Junwei LIM, WANG Zhaohui, et al. In situ nitrogen functionalization of biochar via one-pot synthesis for catalytic peroxymonosulfate activation: characteristics and performance studies[J]. Separation and Purification Technology, 2020, 241: 116702. |
| 48 | SINGH M G, LAKHI K S, PARK D H, et al. Facile one‐pot synthesis of activated porous biocarbons with a high nitrogen content for CO2 capture[J]. ChemNanoMat, 2018, 4(3): 281-290. |
| 49 | XU Lu, WU Chenxi, LIU Peihua, et al. Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants[J]. Chemical Engineering Journal, 2020, 387: 124065. |
| 50 | CHEN Wei, YANG Haiping, CHEN Yingquan, et al. Biomass pyrolysis for nitrogen-containing liquid chemicals and nitrogen-doped carbon materials[J]. Journal of Analytical and Applied Pyrolysis, 2016, 120: 186-193. |
| 51 | ZHANG Xiong, WU Jing, YANG Haiping, et al. Preparation of nitrogen-doped microporous modified biochar by high temperature CO2–NH3 treatment for CO2 adsorption: effects of temperature[J]. RSC Advances, 2016, 6(100): 98157-98166. |
| 52 | JIANG Siyu, YAN Lili, WANG Runkai, et al. Recyclable nitrogen-doped biochar via low-temperature pyrolysis for enhanced lead(Ⅱ) removal[J]. Chemosphere, 2022, 286: 131666. |
| 53 | JIN Ziheng, WANG Bangda, MA Liang, et al. Air pre-oxidation induced high yield N-doped porous biochar for improving toluene adsorption[J]. Chemical Engineering Journal, 2020, 385: 123843. |
| 54 | ANFAR Zakaria, FAKIR Abdellah Ait EL, ZBAIR Mohamed, et al. New functionalization approach synthesis of sulfur doped, nitrogen doped and co-doped porous carbon: superior metal-free carbocatalyst for the catalytic oxidation of aqueous organics pollutants[J]. Chemical Engineering Journal, 2021, 405: 126660. |
| 55 | YU Hongdi, WANG Wenjun, LIN Fawei, et al. A facile and green strategy to synthesize N/P co-doped bio-porous carbon with high yield from fungi residue for efficient VOC adsorption[J]. Separation and Purification Technology, 2021, 276: 119291. |
| 56 | DUAN Xiaoguang, INDRAWIRAWAN Stacey, SUN Hongqi, et al. Effects of nitrogen-, boron-, and phosphorus-doping or codoping on metal-free graphene catalysis[J]. Catalysis Today, 2015, 249: 184-191. |
| 57 | GUO Ruishui, YAN Lili, RAO Pinhua, et al. Nitrogen and sulfur co-doped biochar derived from peanut shell with enhanced adsorption capacity for diethyl phthalate[J]. Environmental Pollution, 2020, 258: 113674. |
| 58 | LI Jianqiu, HE Feifei, SHEN Xiaoyang, et al. Pyrolyzed fabrication of N/P co-doped biochars from (NH4)3PO4-pretreated coffee shells and appraisement for remedying aqueous Cr(Ⅵ) contaminants[J]. Bioresource Technology, 2020, 315: 123840. |
| 59 | PARK H, SCHLEKER P P M, LIU Zigeng, et al. Insights into water interaction at the interface of nitrogen-functionalized hydrothermal carbons[J]. The Journal of Physical Chemistry C, 2019, 123(41): 25146-25156. |
| 60 | HOU Yanrui, HUANG Gege, LI Junhui, et al. Hydrothermal conversion of bamboo shoot shell to biochar: preliminary studies of adsorption equilibrium and kinetics for rhodamine B removal[J]. Journal of Analytical and Applied Pyrolysis, 2019, 143: 104694. |
| 61 | LIANG Hongxu, SUN Ruru, SONG Bin, et al. Preparation of nitrogen-doped porous carbon material by a hydrothermal-activation two-step method and its high-efficiency adsorption of Cr(Ⅵ)[J]. Journal of Hazardous Materials, 2020, 387: 121987. |
| 62 | LENG Lijian, YANG Lihong, LENG Songqi, et al. A review on nitrogen transformation in hydrochar during hydrothermal carbonization of biomass containing nitrogen[J]. Science of the Total Environment, 2021, 756: 143679. |
| 63 | LIU Changhao, LIU Xiaochen, HE Yanhui, et al. Microwave-assisted catalytic pyrolysis of apple wood to produce biochar: co-pyrolysis behavior, pyrolysis kinetics analysis and evaluation of microbial carriers[J]. Bioresource Technology, 2021, 320: 124345. |
| 64 | SHI Shuo, LIU Yangxian. Nitrogen-doped activated carbons derived from microalgae pyrolysis by-products by microwave/KOH activation for CO2 adsorption[J]. Fuel, 2021, 306: 121762. |
| 65 | XU Xiaoyun, ZHENG Yulin, GAO Bin, et al. N-doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red[J]. Chemical Engineering Journal, 2019, 368: 564-572. |
| 66 | WU Dawei, YANG Yingju, LIU Jing, et al. Plasma-modified N/O-doped porous carbon for CO2 capture: an experimental and theoretical study[J]. Energy & Fuels, 2020, 34(5): 6077-6084. |
| 67 | ZHANG Junjie, SHAO Jingai, HUANG Danru, et al. Influence of different precursors on the characteristic of nitrogen-enriched biochar and SO2 adsorption properties[J]. Chemical Engineering Journal, 2020, 385: 123932. |
| 68 | CHEN Binling, YANG Zhuxian, MA Guiping, et al. Heteroatom-doped porous carbons with enhanced carbon dioxide uptake and excellent methylene blue adsorption capacities[J]. Microporous and Mesoporous Materials, 2018, 257: 1-8. |
| 69 | CHEN Huayi, YANG Xingjian, LIU Yonglin, et al. KOH modification effectively enhances the Cd and Pb adsorption performance of N-enriched biochar derived from waste chicken feathers[J]. Waste Management, 2021, 130: 82-92. |
| 70 | CHANG Binbin, SHI Weiwei, YIN Hang, et al. Poplar catkin-derived self-templated synthesis of N-doped hierarchical porous carbon microtubes for effective CO2 capture[J]. Chemical Engineering Journal, 2019, 358: 1507-1518. |
| 71 | ZHANG Xiong, ZHANG Shihong, YANG Haiping, et al. Generalized two-dimensional correlation infrared spectroscopy to reveal mechanisms of CO2 capture in nitrogen enriched biochar[J]. Proceedings of the Combustion Institute, 2017, 36(3): 3933-3940. |
| 72 | SHAO Jingai, ZHANG Junjie, ZHANG Xiong, et al. Enhance SO2 adsorption performance of biochar modified by CO2 activation and amine impregnation[J]. Fuel, 2018, 224: 138-146. |
| 73 | WAN Zeqing, LI Kunquan. Effect of pre-pyrolysis mode on simultaneous introduction of nitrogen/oxygen-containing functional groups into the structure of bagasse-based mesoporous carbon and its influence on Cu(Ⅱ) adsorption[J]. Chemosphere, 2018, 194: 370-380. |
| 74 | LUO Mingke, LIN Hai, LI Bing, et al. A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water[J]. Bioresource Technology, 2018, 259: 312-318. |
| 75 | WAN Shunli, WU Jiayu, ZHOU Shanshan, et al. Enhanced lead and cadmium removal using biochar-supported hydrated manganese oxide (HMO) nanoparticles: behavior and mechanism[J]. Science of the Total Environment, 2018, 616-617: 1298-1306. |
| 76 | LI Ronghua, LIANG Wen, WANG J J, et al. Facilitative capture of As(Ⅴ), Pb(Ⅱ) and methylene blue from aqueous solutions with MgO hybrid sponge-like carbonaceous composite derived from sugarcane leafy trash[J]. Journal of Environmental Management, 2018, 212: 77-87. |
| 77 | LING Lili, LIU Wujun, ZHANG Shun, et al. Magnesium oxide embedded nitrogen self-doped biochar composites: fast and high-efficiency adsorption of heavy metals in an aqueous solution[J]. Environmental Science & Technology, 2017, 51(17): 10081-10089. |
| 78 | MOOD Sohrab Haghighi, AYIANIA Michael, Yaime JEFFERSON-MILAN, et al. Nitrogen doped char from anaerobically digested fiber for phosphate removal in aqueous solutions[J]. Chemosphere, 2020, 240: 124889. |
| [1] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [8] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [9] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [10] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [11] | 王浩然, 殷全玉, 方明, 侯建林, 李军, 何斌, 张明月. 近临界水处理废弃烟梗工艺优化[J]. 化工进展, 2023, 42(9): 5019-5027. |
| [12] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [13] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [14] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [15] | 储甜甜, 刘润竹, 杜高华, 马嘉浩, 张孝阿, 王成忠, 张军营. 有机胍催化脱氢型RTV硅橡胶的制备和可降解性能[J]. 化工进展, 2023, 42(7): 3664-3673. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||