化工进展 ›› 2022, Vol. 41 ›› Issue (9): 4673-4681.DOI: 10.16085/j.issn.1000-6613.2021-2388
空气纳米气泡的稳定性及理化特性
- 华北电力大学环境科学与工程系,河北 保定 071003
-
收稿日期:2021-11-22修回日期:2022-01-29出版日期:2022-09-25发布日期:2022-09-27 -
通讯作者:张玉玲 -
作者简介:陈二军(1994—),男,硕士研究生,研究方向为工业水处理技术。E-mail:chenerjun94@163.com。 -
基金资助:国家自然科学基金(52170074)
Stability and physicochemical properties of air nanobubbles
CHEN Erjun( ), ZHANG Yuling(
), ZHANG Yuling( ), LU Shaolei, DUAN Haiyang, JIN Wenzhang
), LU Shaolei, DUAN Haiyang, JIN Wenzhang
- Department of Environmental Science and Engineering, North China Electric Power University, Baoding 071003, Hebei, China
-
Received:2021-11-22Revised:2022-01-29Online:2022-09-25Published:2022-09-27 -
Contact:ZHANG Yuling
摘要:
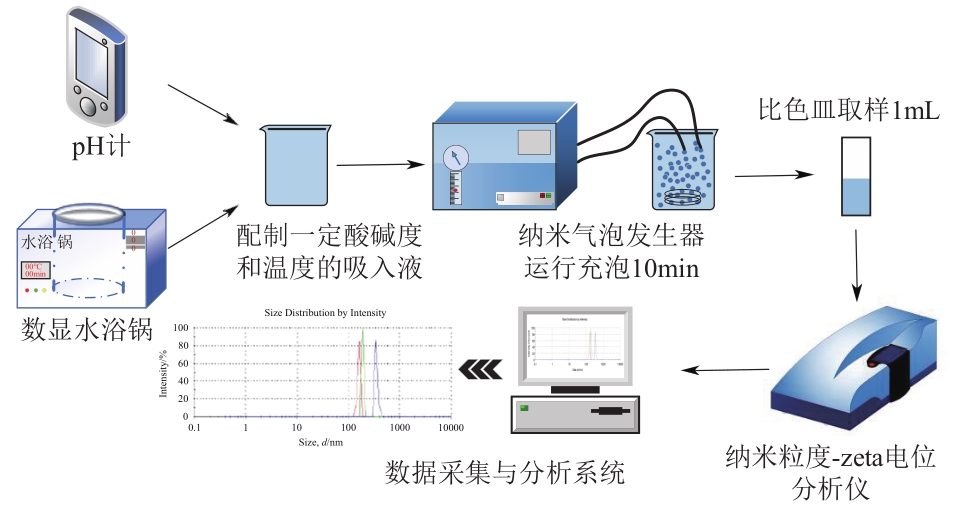
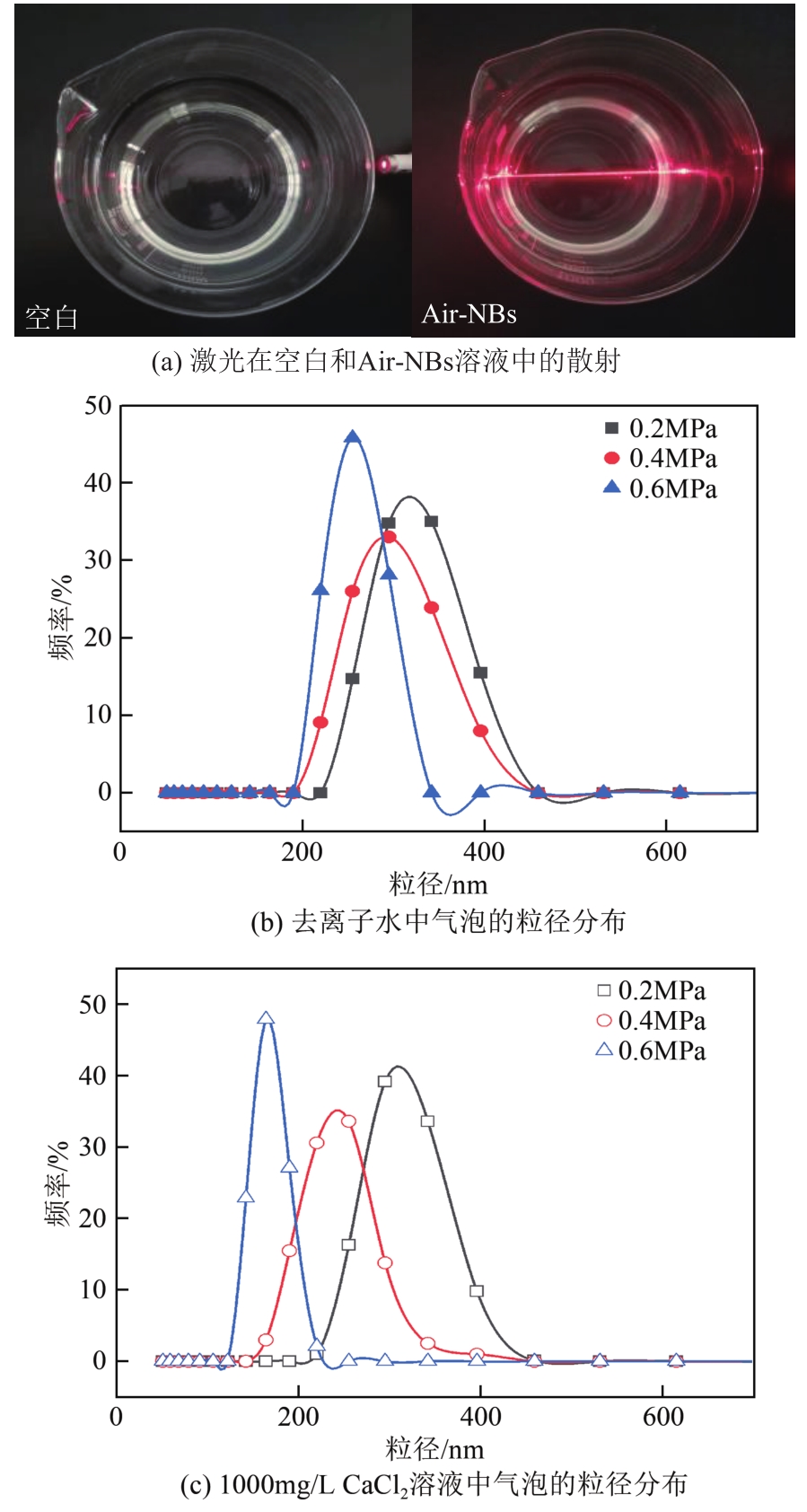
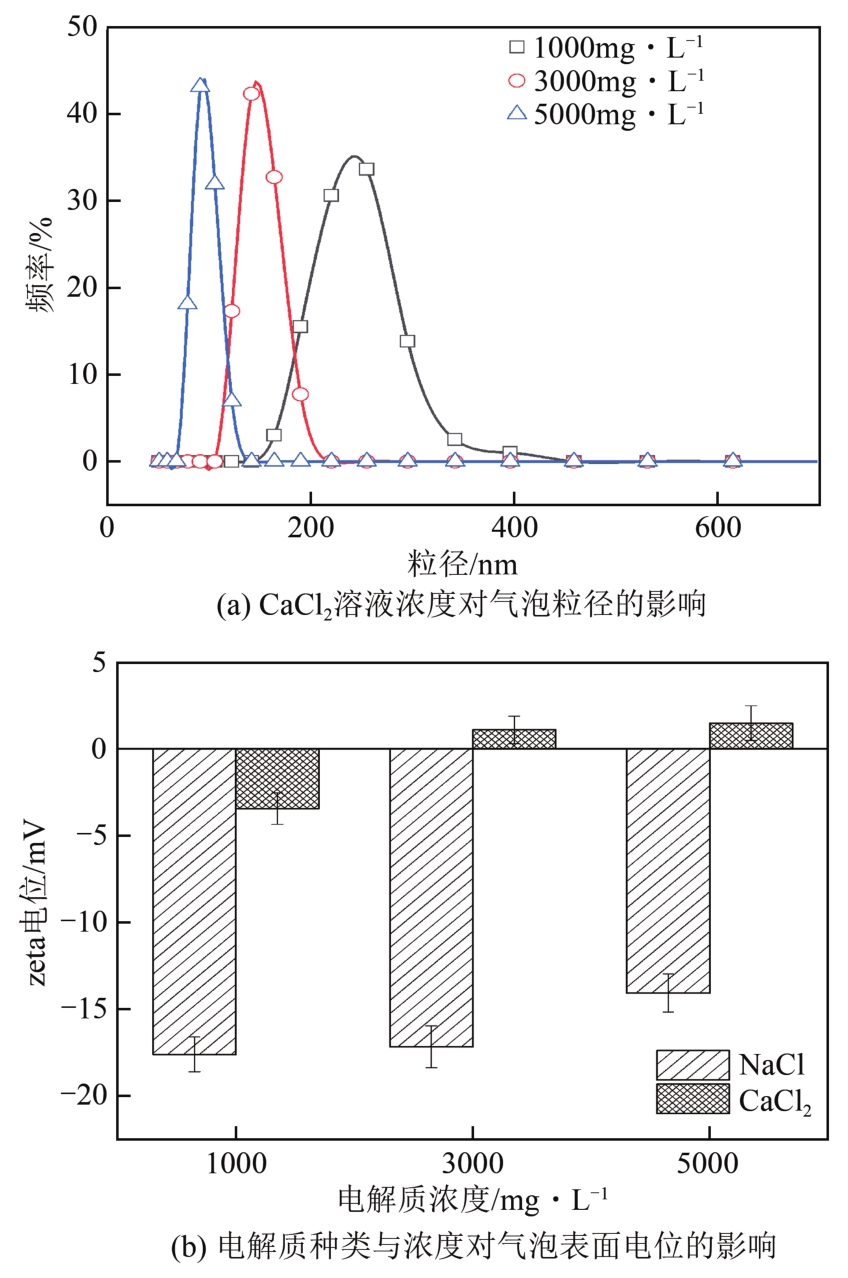
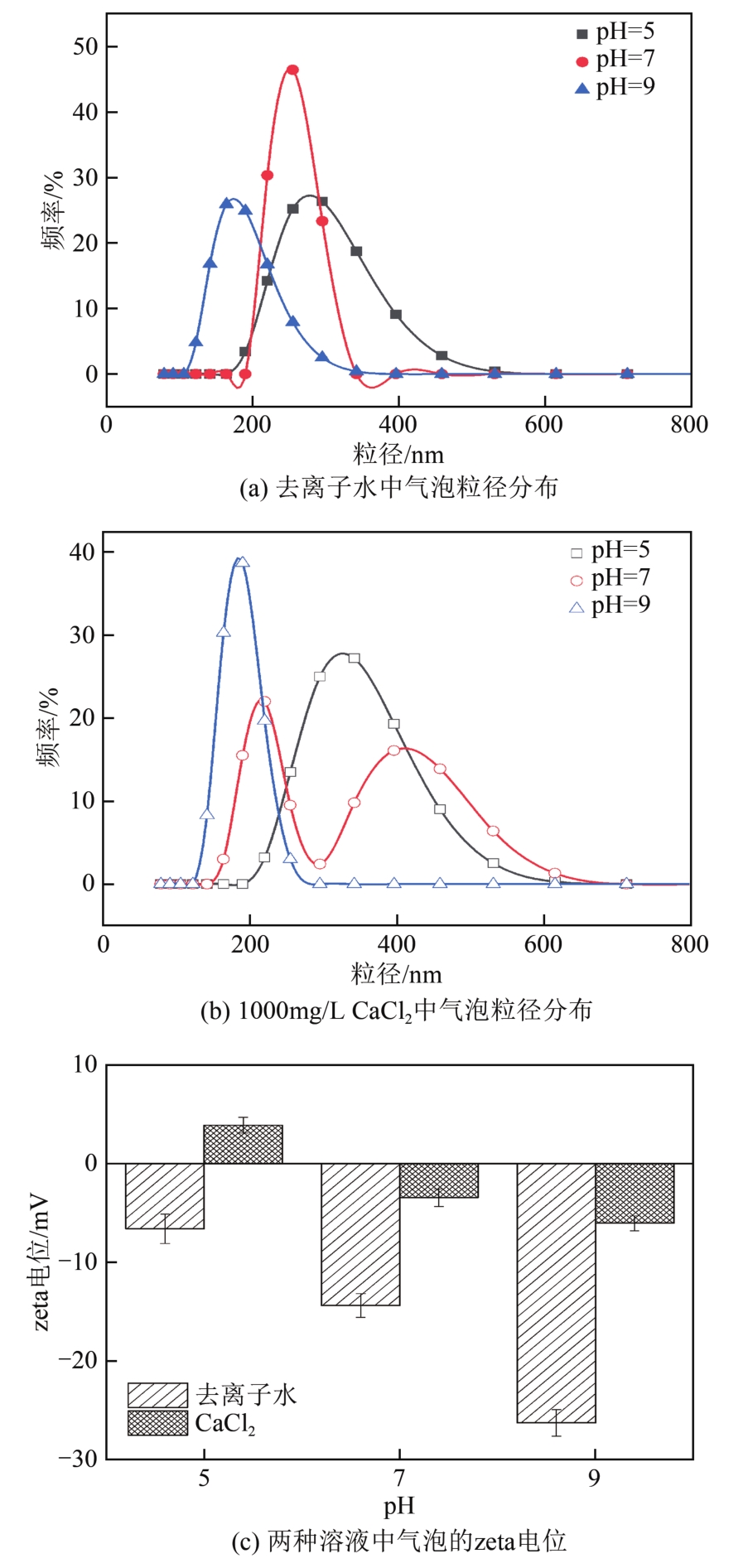
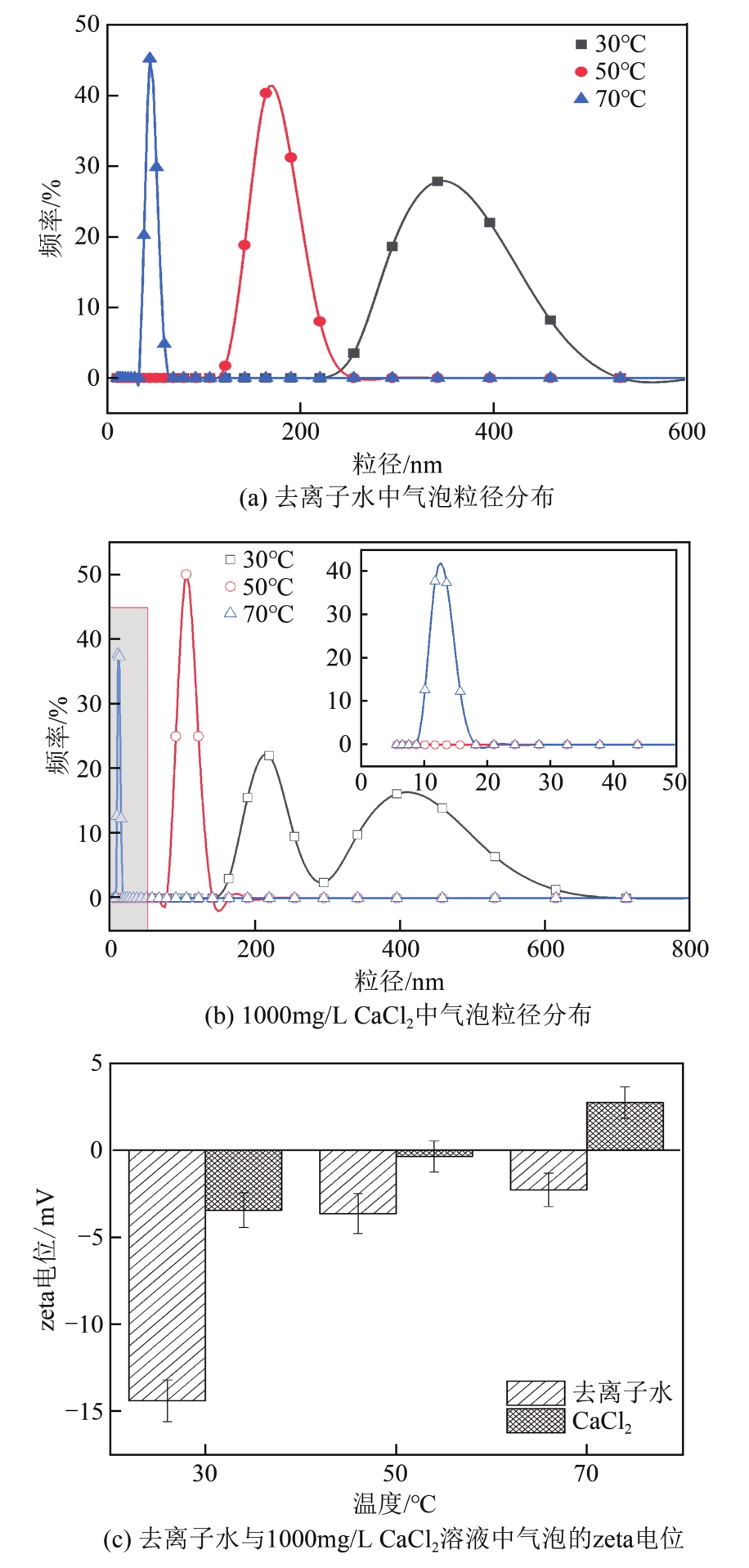
空气纳米气泡(Air-NBs)的理化特性是其广泛应用的基础。本文利用纳米粒度-zeta电位分析仪研究了水力空化原理产生的Air-NBs的理化特性,即粒径分布和zeta电位,考察了装置运行压力、电解质浓度、pH和温度对Air-NBs理化特性的影响;同时考察了去离子水和CaCl2溶液中Air-NBs稳定性。结果表明:纳米气泡发生器的压力越大,其产生的Air-NBs的小粒径比例更大,且电解质的加入有助于气泡粒径的缩小。随着溶液中电解质浓度、pH和温度的升高,溶液中Air-NBs的平均粒径逐渐减小,但气泡间的聚结作用会导致其粒径随时间逐渐增大;通过对两种溶液中Air-NBs粒径的实时监测发现其能在溶液中存在5天之久。胶体稳定(DLVO)理论和zeta电位效应可以合理阐释溶液中Air-NBs的稳定性。
中图分类号:
引用本文
陈二军, 张玉玲, 路少磊, 段海洋, 靳文章. 空气纳米气泡的稳定性及理化特性[J]. 化工进展, 2022, 41(9): 4673-4681.
CHEN Erjun, ZHANG Yuling, LU Shaolei, DUAN Haiyang, JIN Wenzhang. Stability and physicochemical properties of air nanobubbles[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4673-4681.
| 参数 | 数值 | 参数 | 数值 |
|---|---|---|---|
| 型号 | LF-1500 | 液体管或气体管/mm | ϕ6×2 |
| 电压/V | 220(AC) | 最大使用压力/MPa | 0.8 |
| 功率/W | 90 | 自吸高度/m | 0.8 |
| 温度范围/℃ | 0~75 | 工况流量/mL·min-1 | 1500 |
表1 纳米气泡发生器型号参数
| 参数 | 数值 | 参数 | 数值 |
|---|---|---|---|
| 型号 | LF-1500 | 液体管或气体管/mm | ϕ6×2 |
| 电压/V | 220(AC) | 最大使用压力/MPa | 0.8 |
| 功率/W | 90 | 自吸高度/m | 0.8 |
| 温度范围/℃ | 0~75 | 工况流量/mL·min-1 | 1500 |
| 1 | QUINN J J, KRACHT W, GOMEZ C O, et al. Comparing the effect of salts and frother (MIBC) on gas dispersion and froth properties[J]. Minerals Engineering, 2007, 20(14): 1296-1302. |
| 2 | QUINN J J, SOVECHLES J M, FINCH J A, et al. Critical coalescence concentration of inorganic salt solutions[J]. Minerals Engineering, 2014, 58: 1-6. |
| 3 | DAYARATHNE H N P, JEONG S, JANG A. Chemical-free scale inhibition method for seawater reverse osmosis membrane process: air micro-nano bubbles[J]. Desalination, 2019, 461: 1-9. |
| 4 | 马艳, 张鑫, 韩小蒙, 等. 臭氧微纳米气泡技术在水处理中的应用进展[J]. 净水技术, 2019, 38(8): 64-67. |
| MA Yan, ZHANG Xin, HAN Xiaomeng, et al. Application of micro-nano ozone bubble technology in water treatment: a review[J]. Water Purification Technology, 2019, 38(8): 64-67. | |
| 5 | LUKIANOVA-HLEB E Y, BELYANIN A, KASHINATH S, et al. Plasmonic nanobubble-enhanced endosomal escape processes for selective and guided intracellular delivery of chemotherapy to drug-resistant cancer cells[J]. Biomaterials, 2012, 33(6): 1821-1826. |
| 6 | WEN D S. Intracellular hyperthermia: nanobubbles and their biomedical applications[J]. International Journal of Hyperthermia, 2009, 25(7): 533-541. |
| 7 | GREENBERG M R, PIEROG J E. Evaluation of race and gender sensitivity in the American Heart Association materials for Advanced Cardiac Life Support[J]. Gender Medicine, 2009, 6(4): 604-613. |
| 8 | KHALED ABDELLA AHMED A, SUN C Z, HUA L K, et al. Colloidal properties of air, oxygen, and nitrogen nanobubbles in water: effects of ionic strength, natural organic matters, and surfactants[J]. Environmental Engineering Science, 2018, 35(7): 720-727. |
| 9 | AGARWAL A, NG W J, LIU Y. Principle and applications of microbubble and nanobubble technology for water treatment[J]. Chemosphere, 2011, 84(9): 1175-1180. |
| 10 | DAYARATHNE H N P, CHOI J, JANG A. Enhancement of cleaning-in-place (CIP) of a reverse osmosis desalination process with air micro-nano bubbles[J]. Desalination, 2017, 422: 1-4. |
| 11 | KHUNTIA S, MAJUMDER S K, GHOSH P. Microbubble-aided water and wastewater purification: a review[J]. Reviews in Chemical Engineering, 2012, 28(4/5/6): 191-221. |
| 12 | FUJITA T, KUROKAWA H, HAN Z Y, et al. Free radical degradation in aqueous solution by blowing hydrogen and carbon dioxide nanobubbles[J]. Scientific Reports, 2021, 11(1): 3068-3079. |
| 13 | DATTA S, PILLAI R, BORG M K, et al. Acoustothermal nucleation of surface nanobubbles[J]. Nano Letters, 2021, 21(3): 1267-1273. |
| 14 | 杨丽, 廖传华, 朱跃钊, 等. 微纳米气泡特性及在环境污染控制中的应用[J]. 化工进展, 2012, 31(6): 1333-1337. |
| YANG Li, LIAO Chuanhua, ZHU Yuezhao, et al. Characteristics of micro-bubble and nano-bubble and their application in environmental pollution control[J]. Chemical Industry and Engineering Progress, 2012, 31(6): 1333-1337. | |
| 15 | 张小波, 李琰君, 沈绍传, 等. 微泡吸收技术处理丙酮废气[J]. 化工进展, 2015, 34(7): 2075-2079. |
| ZHANG Xiaobo, LI Yanjun, SHEN Shaochun, et al. Experimental investigation on the microbubble absorption of acetone in waste gases[J]. Chemical Industry and Engineering Progress, 2015, 34(7): 2075-2079. | |
| 16 | AZEVEDO A, ETCHEPARE R, CALGAROTO S, et al. Aqueous dispersions of nanobubbles: generation, properties and features[J]. Minerals Engineering, 2016, 94: 29-37. |
| 17 | 孙逊, 赵越, 玄晓旭, 等. 基于水力空化的化工过程强化研究进展[J]. 化工进展, 2022, 41(5): 2243-2255. |
| SUN Xun, ZHAO Yue, XUAN Xiaoxu, et al. Advances in process intensification based on hydrodynamic cavitation[J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2243-2255. | |
| 18 | EKLUND F, SWENSON J. Stable air nanobubbles in water: the importance of organic contaminants[J]. Langmuir, 2018, 34(37): 11003-11009. |
| 19 | CHO S H, KIM J Y, CHUN J H, et al. Ultrasonic formation of nanobubbles and their zeta-potentials in aqueous electrolyte and surfactant solutions[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 269(1/2/3): 28-34. |
| 20 | CHEN Q J, WIEDENROTH H S, GERMAN S R, et al. Electrochemical nucleation of stable N2 nanobubbles at Pt nanoelectrodes[J]. Journal of the American Chemical Society, 2015, 137(37): 12064-12069. |
| 21 | NIRMALKAR N, PACEK A W, BARIGOU M. On the existence and stability of bulk nanobubbles[J]. Langmuir, 2018, 34(37): 10964-10973. |
| 22 | SOVECHLES J M, LEPAGE M R, JOHNSON B, et al. Effect of gas rate and impeller speed on bubble size in frother-electrolyte solutions[J]. Minerals Engineering, 2016, 99: 133-141. |
| 23 | CHERCHIL C, KESAANO M, BADRUZZAMAN M, et al. Municipal reclaimed water for multi-purpose applications in the power sector: a review[J]. Journal of Environmental Management, 2019, 236: 561-570. |
| 24 | CHAUSSEMIER M, POURMOHTASHAM E, GELUS D, et al. State of art of natural inhibitors of calcium carbonate scaling[J]. Desalination, 2015, 356: 47-55. |
| 25 | OKUDA T, MATSUI K, HASHIMOTO K, et al. Effect of ultrafine bubble onto accumulation and structure of urinary calculus[J]. Japanese Journal of Multiphase Flow, 2018, 32(1): 12-18. |
| 26 | YABE A, MORIMATSU T. Cleaning effect of small particle contaminated plate by utilizing nano-bubbles contained water[J]. Journal of the Heat Transfar Society of Japan, 2004, 43(183): 16-18. |
| 27 | ISHIZAKI T, MATSUDA Y, MORITA T, et al. Cleaning effect by fine bubbles generated with gas-liquid share method[J]. Journal of Chemical Engineering of Japan, 2018, 51(2): 170-174. |
| 28 | LIU G M, CRAIG V S J. Improved cleaning of hydrophilic protein-coated surfaces using the combination of nanobubbles and SDS[J]. ACS Applied Materials & Interfaces, 2009, 1(2): 481-487. |
| 29 | WU Z H, CHEN H B, DONG Y M, et al. Cleaning using nanobubbles: defouling by electrochemical generation of bubbles[J]. Journal of Colloid and Interface Science, 2008, 328(1): 10-14. |
| 30 | FANG Z, WANG L, WANG X Y, et al. Formation and stability of surface/bulk nanobubbles produced by decompression at lower gas concentration[J]. The Journal of Physical Chemistry C, 2018, 122(39): 22418-22423. |
| 31 | TUZIUTI T, YASUI K, KANEMATSU W. Influence of increase in static pressure on bulk nanobubbles[J]. Ultrasonics Sonochemistry, 2017, 38: 347-350. |
| 32 | TIAN Y L, XIAO Y F, ZHU H X, et al. Interfacial tensions between water and non-polar fluids at high pressures and high temperatures[J]. Acta Physico-Chimica Sinica, 1997, 13(1): 89-95. |
| 33 | YOO D H, TERASAKA K, TSUGE H. Behavior of bubble formation at elevated pressure[J]. Journal of Chemical Engineering of Japan, 1998, 31(1): 76-82. |
| 34 | O’CONNOR C T, RANDALL E W, GOODALL C M. Measurement of the effects of physical and chemical variables on bubble size[J]. International Journal of Mineral Processing, 1990, 28(1/2): 139-149. |
| 35 | SOVECHLES J M, WATERS K E. Effect of ionic strength on bubble coalescence in inorganic salt and seawater solutions[J]. AIChE Journal, 2015, 61(8): 2489-2496. |
| 36 | HENRY C L, DALTON C N, SCRUTON L, et al. Ion-specific coalescence of bubbles in mixed electrolyte solutions[J]. The Journal of Physical Chemistry C, 2007, 111(2): 1015-1023. |
| 37 | YE Y B, YU S L, HOU L A, et al. Microbubble aeration enhances performance of vacuum membrane distillation desalination by alleviating membrane scaling[J]. Water Research, 2019, 149: 588-595. |
| 38 | TAKAHASHI M. Zeta potential of microbubbles in aqueous solutions: electrical properties of the gas-water interface[J]. The Journal of Physical Chemistry B, 2005, 109(46): 21858-21864. |
| 39 | WU C D, NESSET K, MASLIYAH J, et al. Generation and characterization of submicron size bubbles[J]. Advances in Colloid and Interface Science, 2012, 179/180/181/182: 123-132. |
| 40 | CALGAROTO S, WILBERG K Q, RUBIO J. On the nanobubbles interfacial properties and future applications in flotation[J]. Minerals Engineering, 2014, 60: 33-40. |
| 41 | NAJAFI A S, DRELICH J, YEUNG A, et al. A novel method of measuring electrophoretic mobility of gas bubbles[J]. Journal of Colloid and Interface Science, 2007, 308(2): 344-350. |
| 42 | KIM J Y, SONG M G, KIM J D. Zeta potential of nanobubbles generated by ultrasonication in aqueous alkyl polyglycoside solutions[J]. Journal of Colloid and Interface Science, 2000, 223(2): 285-291. |
| 43 | SCHÄFER R, MERTEN C, EIGENBERGER G. Bubble size distributions in a bubble column reactor under industrial conditions[J]. Experimental Thermal and Fluid Science, 2002, 26(6/7): 595-604. |
| 44 | LIN T J, TSUCHIYA K, FAN L S. Bubble flow characteristics in bubble columns at elevated pressure and temperature[J]. AIChE Journal, 1998, 44(3): 545-560. |
| 45 | 田震, 成有为, 王丽军, 等. 温度与压力对单孔气泡形成过程的影响[J]. 化工学报, 2019, 70(9): 3337-3345. |
| TIAN Zheng, CHENG Youwei, WANG Lijun, et al. Effect of temperature and pressure on formation process of single-hole bubbles[J]. CIESC Journal, 2019, 70(9): 3337-3345. | |
| 46 | 李赟, 白博峰, 阎晓, 等. 微气泡形成过程及温度影响[J]. 工程热物理学报, 2008, 29(9): 1511-1514. |
| LI Yun, BAI Bofeng, YAN Xiao, et al. Microbubble generation process and temperature influence[J]. Journal of Engineering Thermophysics, 2008, 29(9): 1511-1514. | |
| 47 | USHIKUBO F Y, FURUKAWA T, NAKAGAWA R, et al. Evidence of the existence and the stability of nano-bubbles in water[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 361(1/2/3): 31-37. |
| 48 | AHMADI R, KHODADADI D A, ABDOLLAHY M, et al. Nano-microbubble flotation of fine and ultrafine chalcopyrite particles[J]. International Journal of Mining Science and Technology, 2014, 24(4): 559-566. |
| 49 | SHIN D, PARK J B, KIM Y J, et al. Growth dynamics and gas transport mechanism of nanobubbles in graphene liquid cells[J]. Nature Communications, 2015, 6(2): 6068-6073. |
| 50 | 廖世双, 欧乐明, 周伟光. 空化过程微纳米气泡性质及其对细粒矿物浮选的影响[J]. 中国有色金属学报, 2019, 29(7): 1567-1574. |
| LIAO Shishuang, Leming OU, ZHOU Weiguang. Micro-nano bubbles properties induced by hydrodynamic cavitation and their influences on fine mineral flotation[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(7): 1567-1574. | |
| 51 | ZHANG X H, LI G, MAEDA N, et al. Removal of induced nanobubbles from water/graphite interfaces by partial degassing[J]. Langmuir, 2006, 22(22): 9238-9243. |
| 52 | ANTONIO CERRÓN-CALLE G, LUNA MAGDALENO A, GRAF J C, et al. Elucidating CO2 nanobubble interfacial reactivity and impacts on water chemistry[J]. Journal of Colloid and Interface Science, 2022, 607: 720-728. |
| 53 | OH S H, KIM J M. Generation and stability of bulk nanobubbles[J]. Langmuir, 2017, 33(15): 3818-3823. |
| 54 | ALHESHIBRI M, CRAIG V S J. Differentiating between nanoparticles and nanobubbles by evaluation of the compressibility and density of nanoparticles[J]. The Journal of Physical Chemistry C, 2018, 122(38): 21998-22007. |
| 55 | LJUNGGREN S, ERIKSSON J C. The lifetime of a colloid-sized gas bubble in water and the cause of the hydrophobic attraction[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1997, 129/130: 151-155. |
| 56 | LIU S, KAWAGOE Y, MAKINO Y, et al. Effects of nanobubbles on the physicochemical properties of water: the basis for peculiar properties of water containing nanobubbles[J]. Chemical Engineering Science, 2013, 93: 250-256. |
| 57 | ULATOWSKI K, SOBIESZUK P, MRÓZ A, et al. Stability of nanobubbles generated in water using porous membrane system[J]. Chemical Engineering and Processing: Process Intensification, 2019, 136: 62-71. |
| 58 | DAS S, SNOEIJER J H, LOHSE D. Effect of impurities in description of surface nanobubbles[J]. Physical Review E, 2010, 82(5): 056310. |
| 59 | BUNKIN N F, SUYAZOV N V, SHKIRIN A V, et al. Cluster structure of stable dissolved gas nanobubbles in highly purified water[J]. Journal of Experimental and Theoretical Physics, 2009, 108(5): 800-816. |
| 60 | PALUCH M. Electrical properties of free surface of water and aqueous solutions[J]. Advances in Colloid and Interface Science, 2000, 84(1/2/3): 27-45. |
| 61 | HAMPTON M A, NGUYEN A V. Nanobubbles and the nanobubble bridging capillary force[J]. Advances in Colloid and Interface Science, 2010, 154(1/2): 30-55. |
| 62 | HEWAGE S A, KEWALRAMANI J, MEEGODA J N. Stability of nanobubbles in different salts solutions[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 609: 125669. |
| 63 | GUO Z T, WANG X Z, WANG H X, et al. Effects of nanobubble water on the growth of Lactobacillus acidophilus 1028 and its lactic acid production[J]. RSC Advances, 2019, 9(53): 30760-30767. |
| 64 | CHEN C S, LI J, ZHANG X R. The existence and stability of bulk nanobubbles: a long-standing dispute on the experimentally observed mesoscopic inhomogeneities in aqueous solutions[J]. Communications in Theoretical Physics, 2020, 72(3): 037601. |
| [1] | 王鑫, 王兵兵, 杨威, 徐志明. 金属表面PDA/PTFE超疏水涂层抑垢与耐腐蚀性能[J]. 化工进展, 2023, 42(8): 4315-4321. |
| [2] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [3] | 吴展华, 盛敏. 绝热加速量热仪在反应安全风险评估应用中的常见问题[J]. 化工进展, 2023, 42(7): 3374-3382. |
| [4] | 谢志伟, 吴张永, 朱启晨, 蒋佳骏, 梁天祥, 刘振阳. 植物油基Ni0.5Zn0.5Fe2O4磁流体的黏度特性及磁黏特性[J]. 化工进展, 2023, 42(7): 3623-3633. |
| [5] | 杨竞莹, 施万胜, 黄振兴, 谢利娟, 赵明星, 阮文权. 改性纳米零价铁材料制备的研究进展[J]. 化工进展, 2023, 42(6): 2975-2986. |
| [6] | 杨扬, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂OP-13促进HCFC-141b水合物生成[J]. 化工进展, 2023, 42(6): 2854-2859. |
| [7] | 李玲, 马超峰, 卢春山, 于万金, 石能富, 金佳敏, 张建君, 刘武灿, 李小年. 新型含氟替代品1,1,2-三氟乙烯的合成工艺与催化剂研究进展[J]. 化工进展, 2023, 42(4): 1822-1831. |
| [8] | 殷铭, 郭晋, 庞纪峰, 吴鹏飞, 郑明远. 铜催化剂在涉氢反应中的失活机制和稳定策略[J]. 化工进展, 2023, 42(4): 1860-1868. |
| [9] | 李云闯, 谢方明, 席亚男, 万新月, 孙玉虎, 赵永峰, 李根, 刘宏海, 高雄厚, 刘洪涛. 高水热稳定性介孔分子筛的低成本合成研究进展[J]. 化工进展, 2023, 42(4): 1877-1884. |
| [10] | 王钰琢, 李刚. 硫、氮共掺杂三维石墨烯的全固态超级电容器[J]. 化工进展, 2023, 42(4): 1974-1982. |
| [11] | 谭德新, 曾佳欣, 梁莉敏, 申思慧, 曾子倩, 王艳丽. 取代烷基变化对芳炔单体及其聚合物性能影响[J]. 化工进展, 2023, 42(4): 2031-2037. |
| [12] | 宗悦, 张瑞君, 高珊珊, 田家宇. “特殊稳定型”压力驱动薄膜复合(TFC)脱盐膜的研究进展[J]. 化工进展, 2023, 42(4): 2058-2067. |
| [13] | 李光文, 华渠成, 黄作鑫, 达志坚. 聚甲基丙烯酸酯类黏度指数改进剂的研究进展[J]. 化工进展, 2023, 42(3): 1562-1571. |
| [14] | 张晨光, 封硕, 邢玉烨, 沈伯雄, 苏立超. 柴油车用NH3-SCR铜基分子筛催化剂孤立态Cu2+研究进展[J]. 化工进展, 2023, 42(3): 1321-1331. |
| [15] | 张艺璇, 胡伟, 刘梦瑶, 鞠敬鸽, 赵义侠, 康卫民. 聚合物电解质在锌离子电池中的研究进展[J]. 化工进展, 2023, 42(3): 1397-1410. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||