化工进展 ›› 2022, Vol. 41 ›› Issue (7): 3660-3675.DOI: 10.16085/j.issn.1000-6613.2021-1678
多孔液体设计制备及性能分析研究进展
- 1.东南大学能源与环境学院,江苏 南京 210096
2.江苏省太阳能技术重点实验室,江苏 南京 210096
3.教育部能源热转化与控制重点实验室,江苏 南京 210096
-
收稿日期:2021-08-06修回日期:2021-10-20出版日期:2022-07-25发布日期:2022-07-23 -
通讯作者:陈振乾 -
作者简介:生丽莎(1993—),女,博士研究生,研究方向为可变形多孔介质中的传热传质。E-mail:lisa_sheng@seu.edu.cn 。 -
基金资助:国家自然科学基金(51676037)
Design and preparation of porous liquids and their applications in CO2 adsorption
SHENG Lisha1,2,3( ), CHEN Zhenqian1,2,3
), CHEN Zhenqian1,2,3
- 1.School of Energy and Environment, Southeast University, Nanjing 210096, Jiangsu, China
2.Jiangsu Province Key Laboratory of Solar Energy Science and Technology, Nanjing 210096, Jiangsu, China
3.Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, Nanjing 210096, Jiangsu, China
-
Received:2021-08-06Revised:2021-10-20Online:2022-07-25Published:2022-07-23 -
Contact:CHEN Zhenqian
摘要:
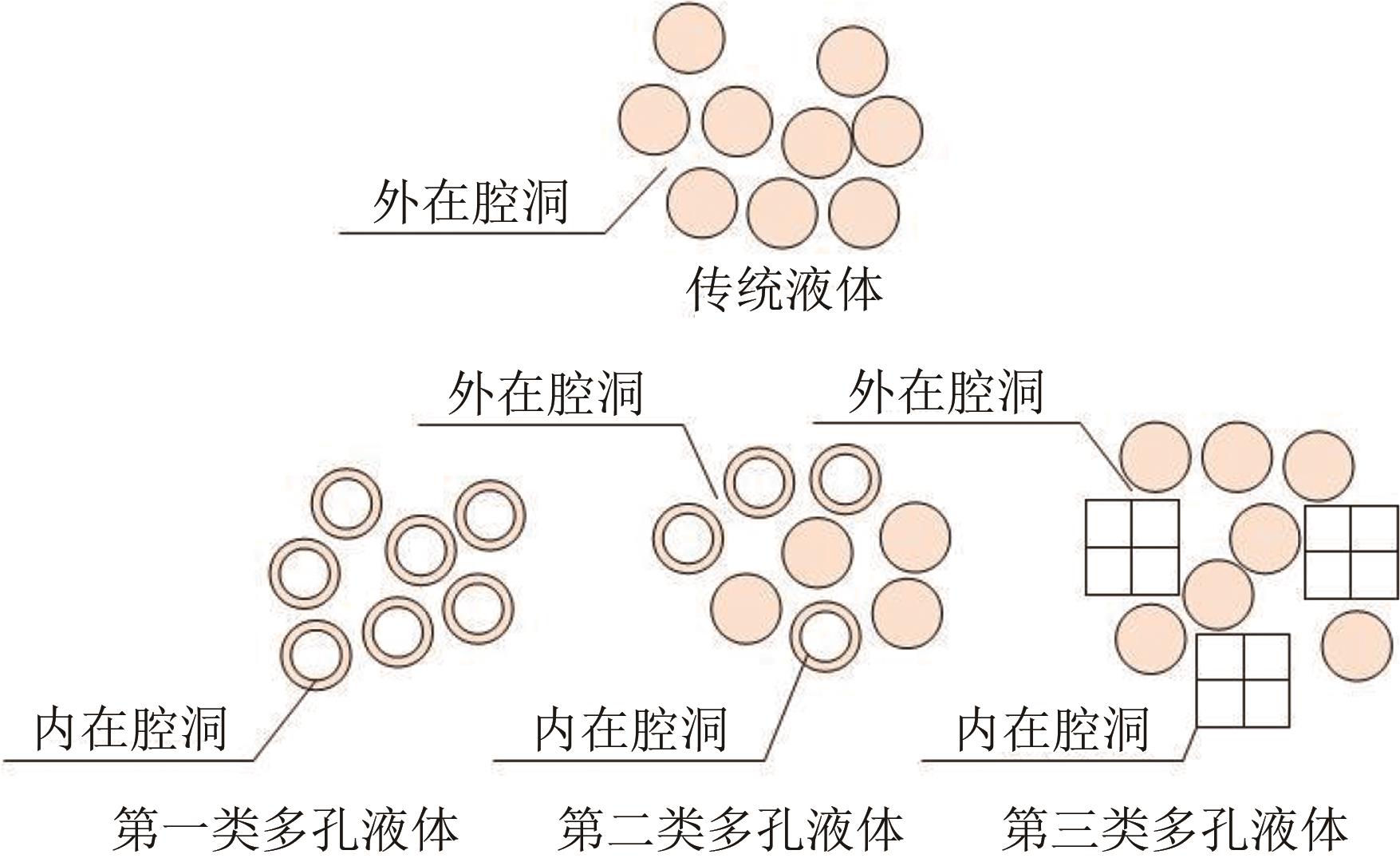
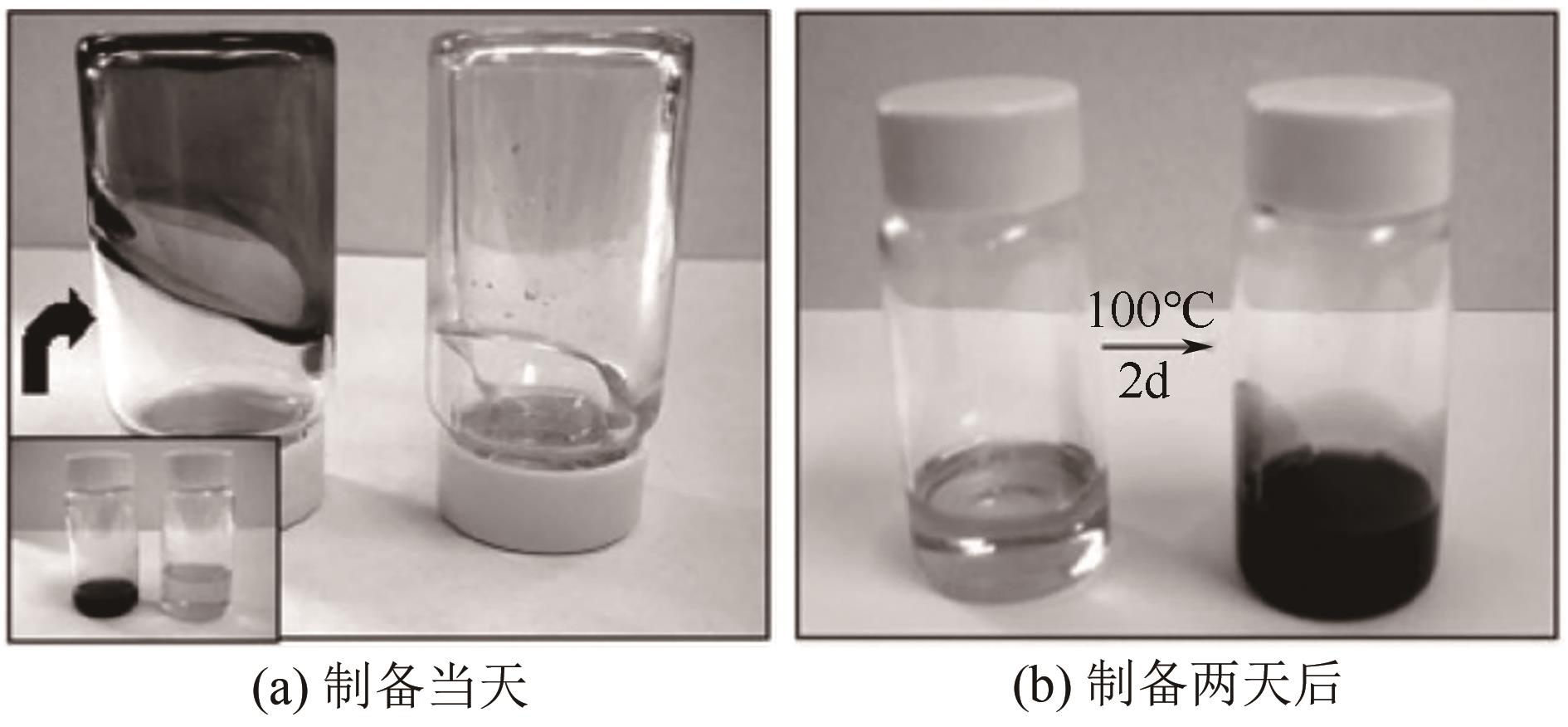
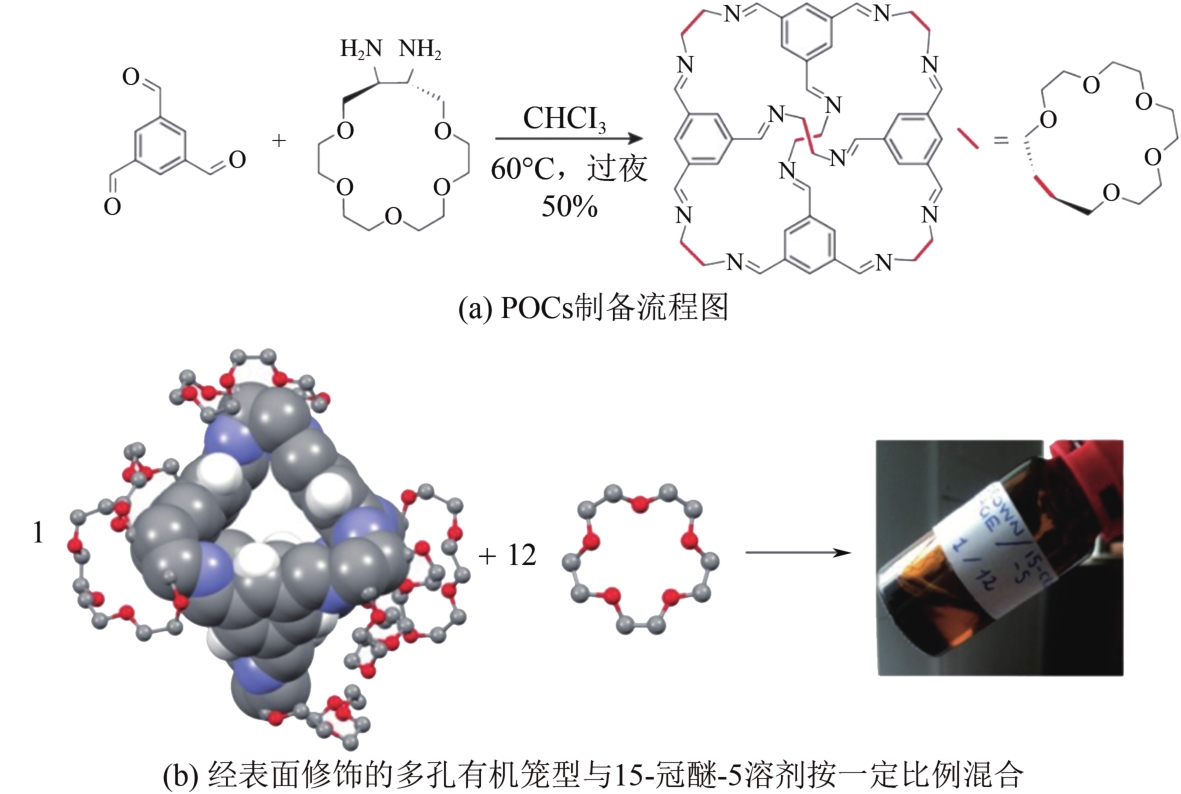
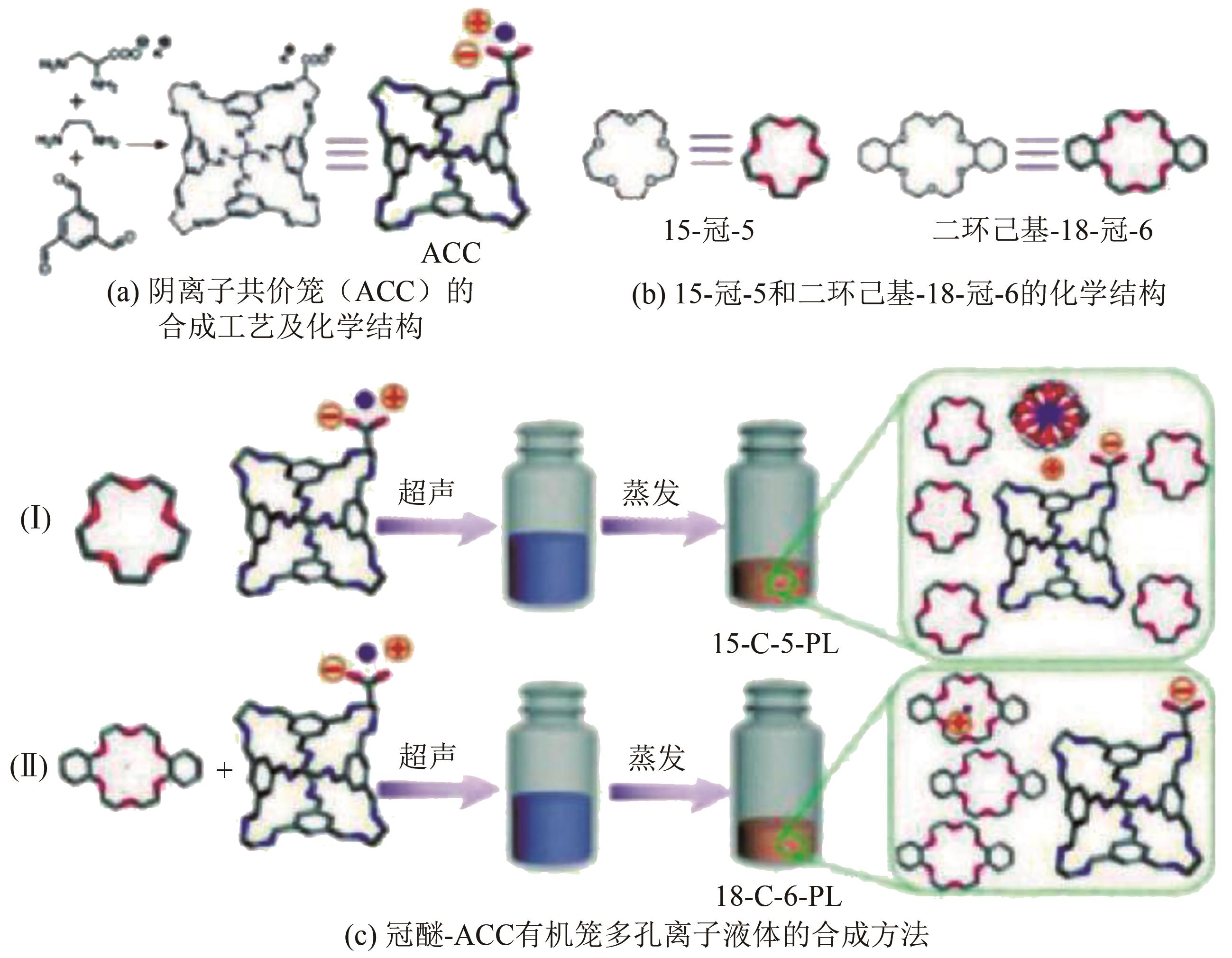
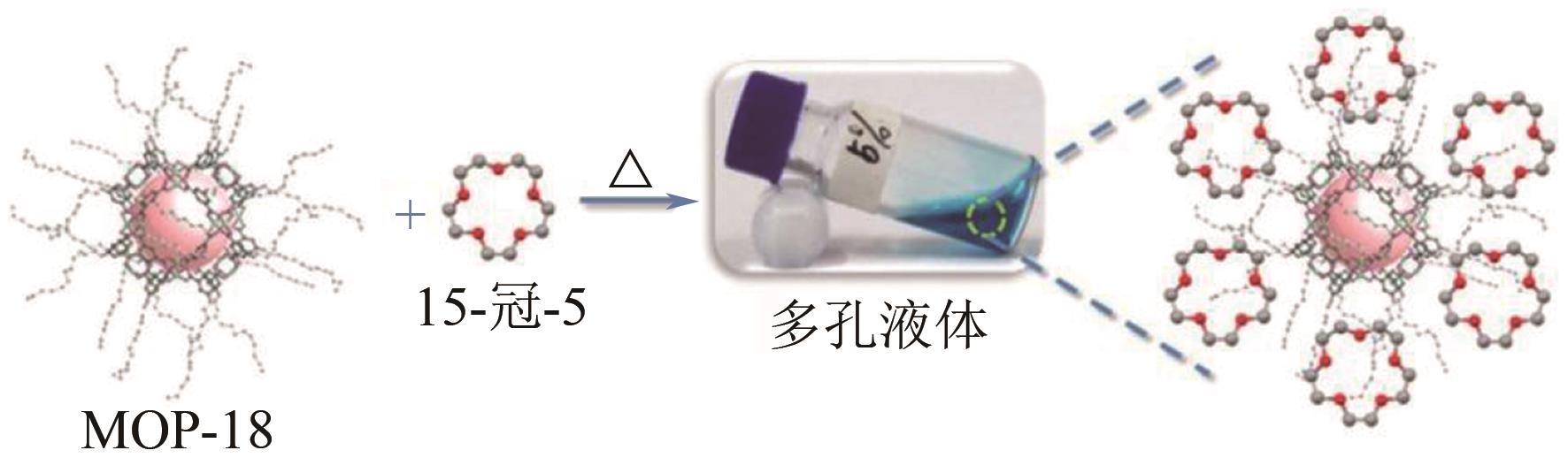
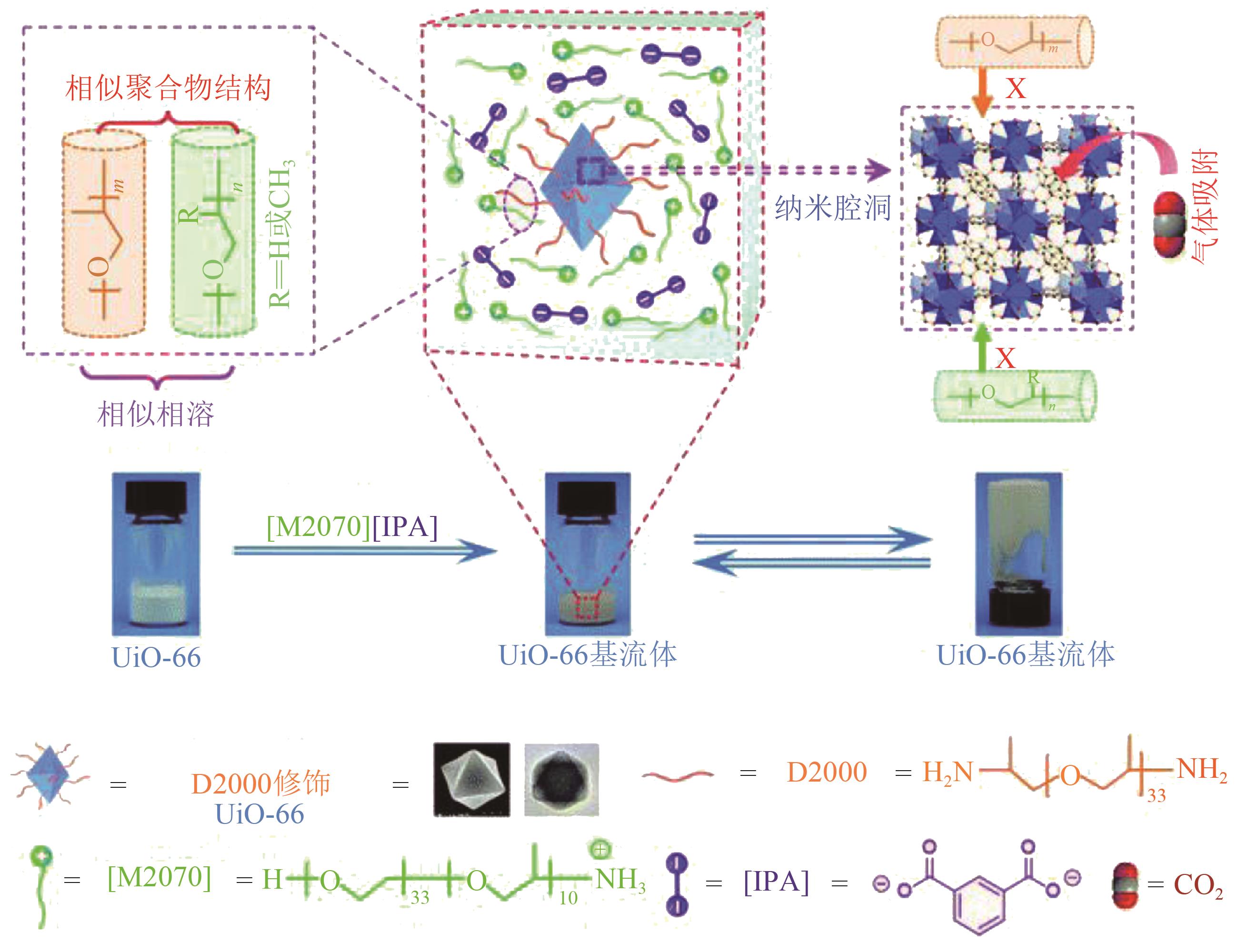
多孔液体(porous liquids,PLs)作为一种新型材料,由于兼具固体多孔性和液体流动性,在催化、储能、石油化工、光电材料、气体吸附分离、气体储运、生物医药等领域具有广泛的应用前景。但多孔液体制备过程中存在合成路线复杂、有机溶剂挥发、液体黏度大、久置沉淀等问题,制约了多孔液体的进一步发展与应用。本文围绕多孔液体的设计制备过程中存在的可行性、稳定性、流动性及碳捕集性能等问题,阐述了多孔液体的种类,综述了近年来多孔液体制备方法和流程以及多孔液体内核外冠结构对稳定性、流动性的影响,概述了目前多孔液体在碳捕集方面的研究进展。最后对多孔液体在制备合成方面的挑战进行了归纳总结,在气体吸附分离及其他方面的应用进行了展望。
中图分类号:
引用本文
生丽莎, 陈振乾. 多孔液体设计制备及性能分析研究进展[J]. 化工进展, 2022, 41(7): 3660-3675.
SHENG Lisha, CHEN Zhenqian. Design and preparation of porous liquids and their applications in CO2 adsorption[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3660-3675.
| 1 | NIAMH O, NICOLA G, JAMES S L. Porous liquids[J]. Chemistry, 2007, 13(11): 3020-3025. |
| 2 | 侯林慧, 曾祥平, 张建勇. 液体的多孔性[J]. 化学通报, 2008, 71(7): 518-521. |
| HOU Linhui, ZENG Xiangping, ZHANG Jianyong. Porosity in liquids[J]. Chemistry, 2008, 71(7): 518-521. | |
| 3 | 李彦霖, 段尊斌, 霍添, 等. 多孔液体新型材料研究及应用进展[J]. 化工进展, 2017, 36(4): 1342-1350. |
| LI Yanlin, DUAN Zunbin, HUO Tian, et al. Progresses in exploration and application of porous liquid materials[J]. Chemical Industry and Engineering Progress, 2017, 36(4): 1342-1350. | |
| 4 | JAMES S L. The dam bursts for porous liquids[J]. Advanced Materials, 2016, 28(27): 5712-5716. |
| 5 | BOURLINOS A B, CHOWDHURY S R, JIANG D D, et al. Layered organosilicate nanoparticles with liquidlike behavior[J]. Small, 2005, 1(1): 80-82. |
| 6 | BOURLINOS A B, CHOWDHURY S RAY, HERRERA R, et al. Functionalized nanostructures with liquid-like behavior: expanding the gallery of available nanostructures[J]. Advanced Functional Materials, 2005, 15(8): 1285-1290. |
| 7 | BOURLINOS A, STASSINOPOULOS A, ANGLOS D, et al. Functionalized ZnO nanoparticles with liquidlike behavior and their photoluminescence properties[J]. Small, 2006, 2(4): 513-516. |
| 8 | WANG D C, XIN Y Y, LI X Q, et al. Transforming metal-organic frameworks into porous liquids via a covalent linkage strategy for CO2 capture[J]. ACS Applied Materials & Interfaces, 2021, 13(2): 2600-2609. |
| 9 | WANG D C, NING H L, XIN Y Y, et al. Transforming Ti3C2T x MXenes into nanoscale ionic materials via an electronic interaction strategy[J]. Journal of Materials Chemistry A, 2021, 9(27): 15441-15451. |
| 10 | 王德超, 辛洋洋, 李晓倩, 等. 多孔液体在气体捕集与分离领域的应用[J]. 化学进展, 2021(10): 1874-1886. |
| WANG D C, XIN Y Y, LI X Q, et al. Porous liquids and their applications in gas capture and separation[J]. Progress in Chemistry, 2021(10): 1874-1886. | |
| 11 | WANG D C, XIN Y Y, WANG Y D, et al. A general way to transform Ti3C2T x MXene into solvent-free fluids for filler phase applications[J]. Chemical Engineering Journal, 2021, 409: 128082. |
| 12 | ATWOOD J L, BARBOUR L J, JERGA A. Storage of methane and Freon by interstitial van der Waals confinement[J]. Science, 2002, 296(5577): 2367-2369. |
| 13 | CAIRA M R, BOURNE S A, MHLONGO W T, et al. New crystalline forms of permethylated β-cyclodextrin[J]. Chemical Communications, 2004, 10(19): 2216-2217. |
| 14 | EHLERS J, KÖNIG W A, LUTZ S, et al. Gas chromatographic separation of enantiomeric olefins[J]. Angewandte Chemie International Edition, 1988, 27(11): 1556-1558. |
| 15 | BOURLINOS A B, SIMOPOULOS A, PETRIDIS D. Synthesis of capped ultrafine γ-Fe2O3 particles from iron(Ⅲ) hydroxide caprylate: a novel starting material for readily attainable organosols[J]. Chemistry of Materials, 2002, 14(2): 899-903. |
| 16 | BOURLINOS A B, RAMAN K, HERRERA R, et al. A liquid derivative of 12-tungstophosphoric acid with unusually high conductivity[J]. Journal of the American Chemical Society, 2004, 126(47): 15358-15359. |
| 17 | MAPESA E U, CANTILLO N M, HAMILTON S T, et al. Localized and collective dynamics in liquid-like polyethylenimine-based nanoparticle organic hybrid materials[J]. Macromolecules, 2021, 54(5): 2296-2305. |
| 18 | DU P X, LIU D, YUAN P, et al. Controlling the macroscopic liquid-like behaviour of halloysite-based solvent-free nanofluids via a facile core pretreatment[J]. Applied Clay Science, 2018, 156: 126-133. |
| 19 | SCHAEFER J L, MOGANTY S S, ARCHER L A. Nanoscale organic hybrid electrolytes[J]. Advanced Materials, 2010, 22(33):3677-3680. |
| 20 | YANG R L, FAN W D, ZHENG Y P, et al. Effects of the core of liquid-like SiO2 nanoparticle organic hybrid materials on CO2 capture[J]. Journal of Materials Science, 2018, 53(7): 5172-5182. |
| 21 | BOURLINOS A B, HERRERA R, CHALKIAS N, et al. Surface-functionalized nanoparticles with liquid-like behavior[J]. Advanced Materials, 2005, 17(2): 234-237. |
| 22 | PARK Y, SHIN D, JANG Y N, et al. CO2 capture capacity and swelling measurements of liquid-like nanoparticle organic hybrid materials via attenuated total reflectance Fourier transform infrared spectroscopy[J]. Journal of Chemical & Engineering Data, 2012, 57(1): 40-45. |
| 23 | LIN K Y A, PARK A H A. Effects of bonding types and functional groups on CO2 capture using novel multiphase systems of liquid-like nanoparticle organic hybrid materials[J]. Environmental Science & Technology, 2011, 45(15): 6633-6639. |
| 24 | GIRI N, DAVIDSON C E, MELAUGH G, et al. Alkylated organic cages: from porous crystals to neat liquids[J]. Chemical Science, 2012, 3(6): 2153. |
| 25 | MELAUGH G, GIRI N, DAVIDSON C E, et al. Designing and understanding permanent microporosity in liquids[J]. Physical Chemistry Chemical Physics, 2014, 16(20): 9422-9431. |
| 26 | ZHANG J S, CHAI S H, QIAO Z N, et al. Porous liquids: a promising class of media for gas separation[J]. Angewandte Chemie International Edition, 2015, 54(3): 932-936. |
| 27 | SHI T, ZHENG Y P, WANG T, et al. Effect of pore size on the carbon dioxide adsorption behavior of porous liquids based on hollow silica[J]. ChemPhysChem, 2018, 19(1): 130-137. |
| 28 | LI P P, SCHOTT J A, ZHANG J S, et al. Electrostatic-assisted liquefaction of porous carbons[J]. Angewandte Chemie International Edition, 2017, 56(47): 14958-14962. |
| 29 | TANG Z H, ZENG C F, LEI Y D, et al. Fluorescent whitening agent stabilized graphene and its composites with chitosan[J]. Journal of Materials Chemistry, 2011, 21(43): 17111. |
| 30 | NEEL A J, HILTON M J, SIGMAN M S, et al. Exploiting non-covalent π interactions for catalyst design[J]. Nature, 2017, 543(7647): 637-646. |
| 31 | 生丽莎, 陈振乾. 静电辅助多孔液体的制备及特性研究[J]. 化工学报, 2019, 70(3): 1163-1170. |
| SHENG Lisha, CHEN Zhenqian. Preparation and characterization of electrostatic-assisted porous liquid[J]. CIESC Journal, 2019, 70(3): 1163-1170. | |
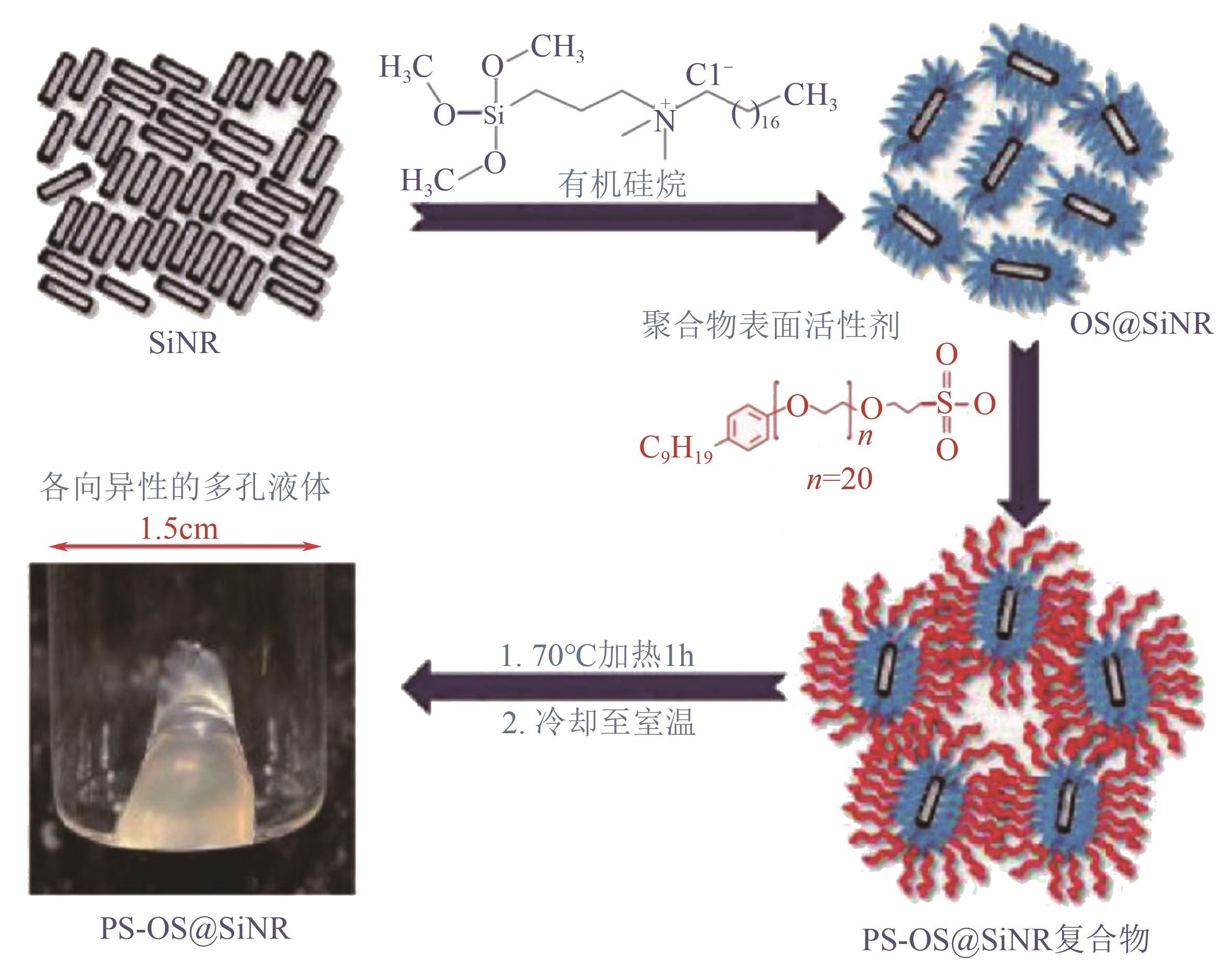
| 32 | KUMAR R, DHASAIYAN P, NAVEENKUMAR P M, et al. A solvent-free porous liquid comprising hollow nanorod-polymer surfactant conjugates[J]. Nanoscale Advances, 2019, 1(10): 4067-4075. |
| 33 | ZHAO X R, AN S H, DAI J L, et al. Transforming surface-modified metal organic framework powder into room temperature porous liquids via an electrical balance strategy[J]. New Journal of Chemistry, 2020, 44(29): 12715-12722. |
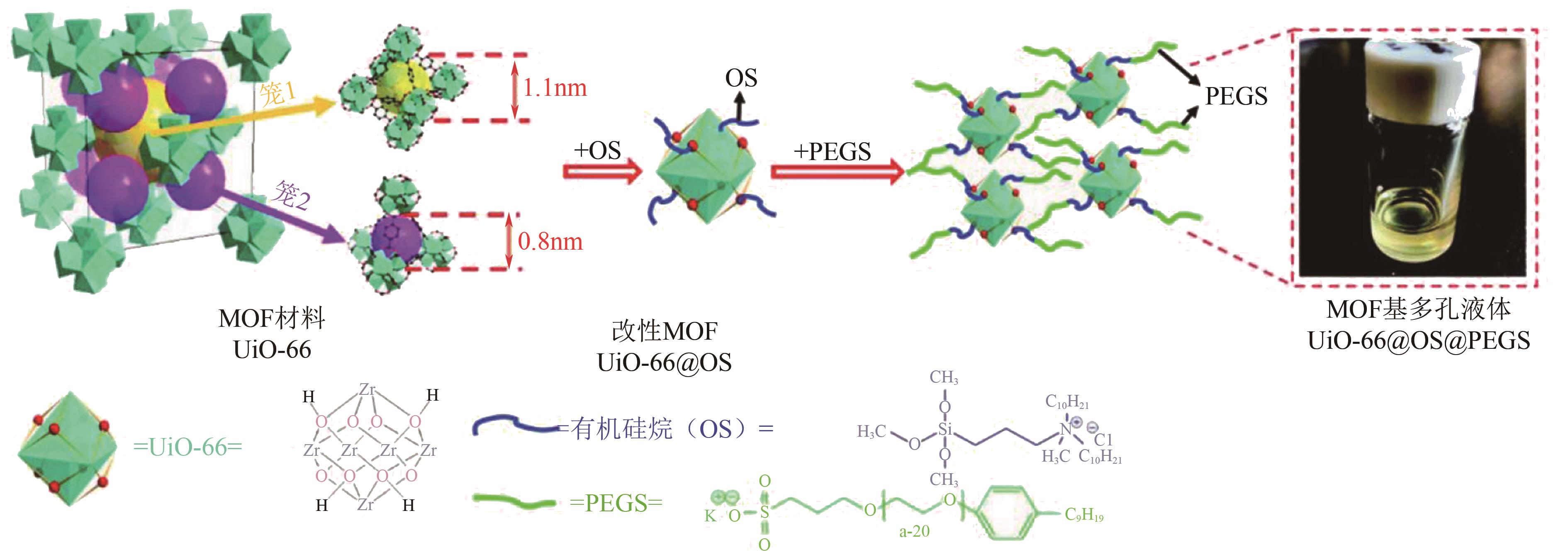
| 34 | WANG D C, XIN Y Y, LI X Q, et al. A universal approach to turn UiO-66 into type 1 porous liquids via post-synthetic modification with corona-canopy species for CO2 capture[J]. Chemical Engineering Journal, 2021, 416: 127625. |
| 35 | TOZAWA T, JONES J T A, SWAMY S I, et al. Porous organic cages[J]. Nature Materials, 2009, 8(12): 973-978. |
| 36 | LYDON D P, CAMPBELL N L, ADAMS D J, et al. Scalable synthesis for porous organic cages[J]. Synthetic Communications, 2011, 41(14): 2146-2151. |
| 37 | JIANG S, JONES J T A, HASELL T, et al. Porous organic molecular solids by dynamic covalent scrambling[J]. Nature Communications, 2011, 2: 207. |
| 38 | JONES J T A, HOLDEN D, MITRA T, et al. On-off porosity switching in a molecular organic solid[J]. Angewandte Chemie International Edition, 2011, 123(3): 775-779. |
| 39 | HERNÁNDEZ-IBÁÑEZ N, LEE J S M, INIESTA J, et al. pH effects on molecular hydrogen storage in porous organic cages deposited onto platinum electrodes[J]. Journal of Electroanalytical Chemistry, 2018, 819: 46-50. |
| 40 | GIRI N, DEL PÓPOLO M G, MELAUGH G, et al. Liquids with permanent porosity[J]. Nature, 2015, 527(7577): 216-220. |
| 41 | MASTALERZ M. Liquefied molecular holes[J]. Nature, 2015, 527(7577): 174-175. |
| 42 | KEARSEY R J, ALSTON B M, BRIGGS M E, et al. Accelerated robotic discovery of type Ⅱ porous liquids[J]. Chemical Science, 2019, 10(41): 9454-9465. |
| 43 | JIE K C, ONISHI N, SCHOTT J A, et al. Transforming porous organic cages into porous ionic liquids via a supramolecular complexation strategy[J]. Angewandte Chemie International Edition, 2020, 59(6): 2268-2272. |
| 44 | 熊鑫坤, 宋华, 苑彬彬, 等. 多孔液体:合成与应用[J]. 化工进展, 2021, 40(8): 4346-4359. |
| XIONG Xinkun, SONG Hua, YUAN Binbin, et al. Porous liquids: synthesis and application[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4346-4359. | |
| 45 | DENG Z, YING W, GONG K, et al. Facilitate gas transport through metal-organic polyhedra constructed porous liquid membrane[J]. Small, 2020, 16(11): 1907016. |
| 46 | EDDAOUDI M, KIM J, ROSI N, et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage[J]. Science, 2002, 295(5554): 469-472. |
| 47 | ZHAO X, WANG Y X, LI D S, et al. Metal-organic frameworks for separation[J]. Advanced Materials, 2018, 30(37): 1705189. |
| 48 | BANERJEE R, PHAN A, WANG B, et al. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture[J]. Science, 2008, 319(5865): 939-943. |
| 49 | WANG B, CÔTÉ A P, FURUKAWA H, et al. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs[J]. Nature, 2008, 453(7192): 207-211. |
| 50 | BANERJEE R, FURUKAWA H, BRITT D, et al. Control of pore size and functionality in isoreticular zeolitic imidazolate frameworks and their carbon dioxide selective capture properties[J]. Journal of the American Chemical Society, 2009, 131(11): 3875-3877. |
| 51 | MCKEOWN N B. Polymers of intrinsic microporosity (PIMs)[J]. Polymer, 2020, 202: 122736. |
| 52 | DING S Y, WANG W. Covalent organic frameworks (COFs): from design to applications[J]. Chemical Society Reviews, 2013, 42(2): 548-568. |
| 53 | LIU H, LIU B, LIN L C, et al. A hybrid absorption-adsorption method to efficiently capture carbon[J]. Nature Communications, 2014, 5: 5147. |
| 54 | LIU H, GUO P, REGUEIRA T, et al. Irreversible change of the pore structure of ZIF-8 in carbon dioxide capture with water coexistence[J]. The Journal of Physical Chemistry C, 2016, 120(24): 13287-13294. |
| 55 | SHAN W, FULVIO P F, KONG L, et al. New class of type Ⅲ porous liquids: a promising platform for rational adjustment of gas sorption behavior[J]. ACS Applied Materials & Interfaces, 2018, 10(1): 32-36. |
| 56 | LIU S, LIU J, HOU X, et al. Porous liquid: a stable ZIF-8 colloid in ionic liquid with permanent porosity[J]. Langmuir, 2018, 34(12): 3654-3660. |
| 57 | LI P P, CHEN H, SCHOTT J A, et al. Porous liquid zeolites: hydrogen bonding-stabilized H-ZSM-5 in branched ionic liquids[J]. Nanoscale, 2019, 11(4): 1515-1519. |
| 58 | ZHAO X M, YUAN Y H, LI P P, et al. A polyether amine modified metal organic framework enhanced the CO2 adsorption capacity of room temperature porous liquids[J]. Chemical Communications, 2019, 55(87): 13179-13182. |
| 59 | CAHIR J, TSANG M Y, LAI B B, et al. Type 3 porous liquids based on non-ionic liquid phases-broad and tailorable platform of selective, fluid gas sorbents[J]. Chemical Science, 2020, 11(8): 2077-2084. |
| 60 | HE S F, CHEN L H, CUI J, et al. General way to construct micro-and mesoporous metal-organic framework-based porous liquids[J]. Journal of the American Chemical Society, 2019, 141(50): 19708-19714. |
| 61 | MISSANA T, ADELL A. On the applicability of DLVO theory to the prediction of clay colloids stability[J]. Journal of Colloid and Interface Science, 2000, 230(1): 150-156. |
| 62 | POPA I, GILLIES G, PAPASTAVROU G, et al. Attractive and repulsive electrostatic forces between positively charged latex particles in the presence of anionic linear polyelectrolytes[J]. The Journal of Physical Chemistry B, 2010, 114(9): 3170-3177. |
| 63 | 白明洁, 刘金龙, 齐志娜, 等. 石墨烯纳米流体研究进展[J]. 材料工程, 2020, 48(4): 46-59. |
| BAI Mingjie, LIU Jinlong, QI Zhina, et al. Research progress in nanofluids with graphene addition[J]. Journal of Materials Engineering, 2020, 48(4): 46-59. | |
| 64 | 胡纪华, 杨兆禧, 郑忠, 等. 胶体与界面化学[M]. 广州: 华南理工大学出版社, 2002. |
| HU Jihua, YANG Zhaoxi, ZHENG Zhong, et al. Colloid and interface chemistry[M]. Guangzhou: South China University of Technology Press, 2002. | |
| 65 | TIARA A M, CHAKRABORTY S, SARKAR I, et al. Synthesis and characterization of Zn-Al layered double hydroxide nanofluid and its application as a coolant in metal quenching[J]. Applied Clay Science, 2017, 143: 241-249. |
| 66 | SHIRANI M, AKBARI-ADERGANI B, JAZI M B, et al. Green ultrasound assisted magnetic nanofluid-based liquid phase microextraction coupled with gas chromatography-mass spectrometry for determination of permethrin, deltamethrin, and cypermethrin residues[J]. Microchimica Acta, 2019, 186(10): 1-11. |
| 67 | YIN Q G, LI C H, ZHANG Y B, et al. Spectral analysis and power spectral density evaluation in Al2O3 nanofluid minimum quantity lubrication milling of 45 steel[J]. The International Journal of Advanced Manufacturing Technology, 2018, 97(1/2/3/4): 129-145. |
| 68 | AKOH H, TSUKASAKI Y, YATSUYA S, et al. Magnetic properties of ferromagnetic ultrafine particles prepared by vacuum evaporation on running oil substrate[J]. Journal of Crystal Growth, 1978, 45: 495-500. |
| 69 | APARNA Z, MICHAEL M, PABI S K, et al. Thermal conductivity of aqueous Al2O3/Ag hybrid nanofluid at different temperatures and volume concentrations: an experimental investigation and development of new correlation function[J]. Powder Technology, 2019, 343: 714-722. |
| 70 | AGNIHOTRI P, LAD V N. Magnetic nanofluid: synthesis and characterization[J]. Chemical Papers, 2020, 74(9): 3089-3100. |
| 71 | JABBARI F, RAJABPOUR A, SAEDODIN S. Thermal conductivity and viscosity of nanofluids: a review of recent molecular dynamics studies[J]. Chemical Engineering Science, 2017, 174: 67-81. |
| 72 | LU J, LIU D M, YANG X N, et al. Molecular dynamics simulations of interfacial interactions between small nanoparticles during diffusion-limited aggregation[J]. Applied Surface Science, 2015, 357: 1114-1121. |
| 73 | 李旭. APTS表面接枝纳米SiO2改性MPIA绝缘纸热稳定性的机理研究[D]. 重庆: 西南大学, 2018. |
| LI Xu. The thermal stability improvement mechanism of MPIA insulating paper modified by nano-SiO2 surface-grafted via APTS[D]. Chongqing: Southwest University, 2018. | |
| 74 | 生丽莎, 陈振乾. 纳米流体中纳米颗粒分散性能的分子动力学模拟[J]. 东南大学学报(自然科学版), 2021, 51(4): 700-706. |
| SHENG Lisha, CHEN Zhenqian. Molecular dynamics simulation of dispersion property of nanoparticles in nanofluids[J]. Journal of Southeast University (Natural Science Edition), 2021, 51(4): 700-706. | |
| 75 | SZEJTLI J. Introduction and general overview of cyclodextrin chemistry[J]. Chemical Reviews, 1998, 98(5): 1743-1754. |
| 76 | SHENG L S, CHEN Z Q, WANG Y. Molecular dynamics simulations of stability and fluidity of porous liquids[J]. Applied Surface Science, 2021, 536: 147951. |
| 77 | SHENG L S, CHEN Z Q. Molecular dynamics study of dispersion and fluidity of porous liquids with different pore sizes[J]. Journal of Molecular Liquids, 2021, 333: 115890. |
| 78 | SHENG L S, CHEN Z Q, XU B, et al. Molecular dynamics study of the dispersion stability and fluidity of porous liquids with different canopy structures[J]. The Journal of Physical Chemistry B, 2021, 125(20): 5387-5396. |
| 79 | EASTMAN J A, PHILLPOT S R, CHOI S U S, et al. Thermal transport in nanofluids[J]. Annual Review of Materials Research, 2004, 34(1): 219-246. |
| 80 | LIN K Y. Design, synthesis and evaluation of liquid-like nanoparticle organic hybrid materials (NOHMs) for carbon dioxide capture[D]. New York: Columbia University, 2012. |
| 81 | 王宝和, 程飞, 白麟, 等. 水基纳米流体传递性质的分子动力学模拟研究[J]. 河南化工, 2019, 36(3): 18-22. |
| WANG Baohe, CHENG Fei, BAI Lin, et al. Molecular dynamics simulation research of transport properties of water-based nanofluids[J]. Henan Chemical Industry, 2019, 36(3): 18-22. | |
| 82 | BAO L L, ZHONG C Y, JIE P F, et al. The effect of nanoparticle size and nanoparticle aggregation on the flow characteristics of nanofluids by molecular dynamics simulation[J]. Advances in Mechanical Engineering, 2019, 11(11): 168781401988948. |
| 83 | LI X Q, WANG D C, HE Z J, et al. Zeolitic imidazolate frameworks-based porous liquids with low viscosity for CO2 and toluene uptakes[J]. Chemical Engineering Journal, 2021, 417: 129239. |
| 84 | 周玉梅, 周宝晶, 聂雪玫, 等. MD/QM/CSM方法计算β-环糊精及其衍生物对二甲四氯的包合机理[J]. 化工进展, 2015, 34(12): 4185-4190. |
| ZHOU Yumei, ZHOU Baojing, NIE Xuemei, et al. A theoretical study on the microencapsulation of herbicide MCPA with native β-cyclodextrin and its derivatives by a molecular dynamics/quantum mechanics/continuum solvent model approach[J]. Chemical Industry and Engineering Progress, 2015, 34(12): 4185-4190. | |
| 85 | ZHOU H C, LONG J R, YAGHI O M. Introduction to metal-organic frameworks[J]. Chemical Reviews, 2012, 112(2): 673-674. |
| 86 | ZHANG W, XIONG R G. Ferroelectric metal-organic frameworks[J]. Chemical Reviews, 2012, 112(2): 1163-1195. |
| 87 | XUAN W M, ZHU C F, LIU Y, et al. Mesoporous metal-organic framework materials[J]. Chemical Society Reviews, 2012, 41(5): 1677-1695. |
| 88 | XIANG Z H, CAO D P. Porous covalent-organic materials: synthesis, clean energy application and design[J]. Journal of Materials Chemistry A, 2013, 1(8): 2691-2718. |
| 89 | FENG X, DING X S, JIANG D L. Covalent organic frameworks[J]. Chemical Society Reviews, 2012, 41(18): 6010. |
| 90 | HUANG Y, KE S H. Hydrogen storage in metal-organic frameworks[J]. Applied Mechanics and Materials, 2013, 316/317: 946-949. |
| 91 | SAMANTA A, ZHAO A, SHIMIZU G K H, et al. Post-combustion CO2 capture using solid sorbents: a review[J]. Industrial & Engineering Chemistry Research, 2012, 51(4): 1438-1463. |
| 92 | BEN T, REN H, MA S Q, et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area[J]. Angewandte Chemie International Edition, 2009, 121(50): 9621-9624. |
| 93 | BEN T, PEI C Y, ZHANG D L, et al. Gas storage in porous aromatic frameworks (PAFs)[J]. Energy & Environmental Science, 2011, 4(10): 3991. |
| 94 | LAN J H, CAO D P, WANG W C, et al. High-capacity hydrogen storage in porous aromatic frameworks with diamond-like structure[J]. The Journal of Physical Chemistry Letters, 2010, 1(6): 978-981. |
| 95 | FAVRE E, SVENDSEN H F. Membrane contactors for intensified post-combustion carbon dioxide capture by gas-liquid absorption processes[J]. Journal of Membrane Science, 2012, 407/408: 1-7. |
| 96 | ROCHELLE G T. Amine scrubbing for CO2 capture[J]. Science, 2009, 325(5948): 1652-1654. |
| 97 | HUTTENHUIS P J G, AGRAWAL N J, SOLBRAA E, et al. The solubility of carbon dioxide in aqueous N-methyldiethanolamine solutions[J]. Fluid Phase Equilibria, 2008, 264(1/2): 99-112. |
| 98 | 黄宽. 基于离子液体或多孔碳的酸性气体捕集介质的设计、合成及性能研究[D]. 南京: 南京大学, 2015. |
| HUANG Kuan. Design, synthesis and performance of ionic liquids or porous carbons-based absorbents/adsorbents for acidic gas capture[D]. Nanjing: Nanjing University, 2015. | |
| 99 | IM J, HONG S Y, CHEON Y, et al. Steric hindrance-induced zwitterionic carbonates from alkanolamines and CO2: highly efficient CO2 absorbents[J]. Energy & Environmental Science, 2011, 4(10): 4284-4289. |
| 100 | BARZAGLI F, LAI S, MANI F. A new class of single-component absorbents for reversible carbon dioxide capture under mild conditions[J]. ChemSusChem, 2015, 8(1): 184-191. |
| 101 | PETIT C, PARK Y, LIN K Y A, et al. Spectroscopic investigation of the canopy configurations in nanoparticle organic hybrid materials of various grafting densities during CO2 capture[J]. The Journal of Physical Chemistry C, 2012, 116(1): 516-525. |
| 102 | PARK Y, DECATUR J, LIN K Y A, et al. Investigation of CO2 capture mechanisms of liquid-like nanoparticle organic hybrid materials via structural characterization[J]. Physical Chemistry Chemical Physics, 2011, 13(40): 18115-18122. |
| 103 | LIN K Y A, PETIT C, PARK A H A. Effect of SO2 on CO2 capture using liquid-like nanoparticle organic hybrid materials[J]. Energy & Fuels, 2013, 27(8): 4167-4174. |
| 104 | PARK Y, PETIT C, HAN P, et al. Effect of canopy structures and their steric interactions on CO2 sorption behavior of liquid-like nanoparticle organic hybrid materials[J]. RSC Advances, 2014, 4(17): 8723. |
| 105 | EGLESTON B D, LUZYANIN K V, BRAND M C, et al. Controlling gas selectivity in molecular porous liquids by tuning the cage window size[J]. Angewandte Chemie International Edition, 2020, 59(19): 7362-7366. |
| 106 | ATILHAN M, CINCOTTI A, APARICIO S. Nanoscopic characterization of type Ⅱ porous liquid and its use for CO2 absorption from molecular simulation[J]. Journal of Molecular Liquids, 2021, 330: 115660. |
| 107 | ZHANG F, YANG F, HUANG J, et al. Thermodynamics and kinetics of gas storage in porous liquids[J]. The Journal of Physical Chemistry B, 2016, 120(29): 7195-7200. |
| 108 | GREENAWAY R L, HOLDEN D, EDEN E G B, et al. Understanding gas capacity, guest selectivity, and diffusion in porous liquids[J]. Chemical Science, 2017, 8(4): 2640-2651. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [3] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [4] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [5] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [6] | 赵晨, 苗天泽, 张朝阳, 洪芳军, 汪大海. 负压状态窄缝通道乙二醇水溶液传热特性[J]. 化工进展, 2023, 42(S1): 148-157. |
| [7] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [8] | 陈林, 徐培渊, 张晓慧, 陈杰, 徐振军, 陈嘉祥, 密晓光, 冯永昌, 梅德清. 液化天然气绕管式换热器壳侧混合工质流动及传热特性[J]. 化工进展, 2023, 42(9): 4496-4503. |
| [9] | 罗成, 范晓勇, 朱永红, 田丰, 崔楼伟, 杜崇鹏, 王飞利, 李冬, 郑化安. 中低温煤焦油加氢反应器不同分配器中液体分布的CFD模拟[J]. 化工进展, 2023, 42(9): 4538-4549. |
| [10] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [11] | 王尚彬, 欧红香, 薛洪来, 曹海珍, 王钧奇, 毕海普. 黄原胶和纳米二氧化硅对无氟泡沫性能的影响[J]. 化工进展, 2023, 42(9): 4856-4862. |
| [12] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [13] | 王鑫, 王兵兵, 杨威, 徐志明. 金属表面PDA/PTFE超疏水涂层抑垢与耐腐蚀性能[J]. 化工进展, 2023, 42(8): 4315-4321. |
| [14] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [15] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||