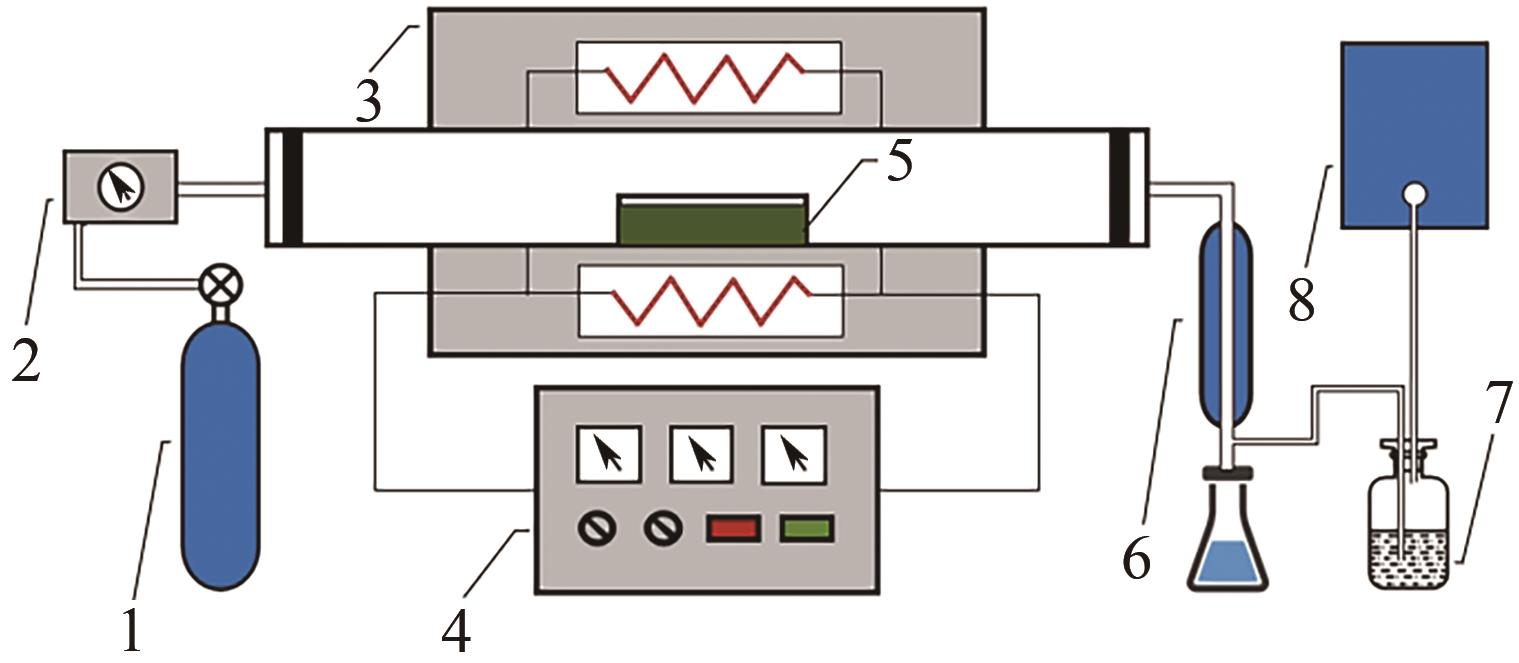
化工进展 ›› 2025, Vol. 44 ›› Issue (2): 1064-1075.DOI: 10.16085/j.issn.1000-6613.2024-0121
• 资源与环境化工 • 上一篇
污泥同农林废弃物共热解重金属迁移转化特性
赵佳琪( ), 黄亚继(
), 黄亚继( ), 李志远, 朱志成, 祁帅杰, 高嘉炜, 刘俊, 张煜尧
), 李志远, 朱志成, 祁帅杰, 高嘉炜, 刘俊, 张煜尧
- 东南大学能源热转换及其过程测控教育部重点实验室,江苏 南京 210096
-
收稿日期:2024-01-16修回日期:2024-05-25出版日期:2025-02-25发布日期:2025-03-10 -
通讯作者:黄亚继 -
作者简介:赵佳琪(1998—),男,硕士,研究方向为固体废弃物处理。E-mail:220210442@seu.edu.cn。 -
基金资助:江苏省科技计划(BE2022604)
Characteristics of heavy metal migration and transformation during co-pyrolysis of sludge with agroforestry wastes
ZHAO Jiaqi( ), HUANG Yaji(
), HUANG Yaji( ), LI Zhiyuan, ZHU Zhicheng, QI Shuaijie, GAO Jiawei, LIU Jun, ZHANG Yuyao
), LI Zhiyuan, ZHU Zhicheng, QI Shuaijie, GAO Jiawei, LIU Jun, ZHANG Yuyao
- Key Laboratory of Energy Thermal Conversion and Control of the Ministry of Education, Southeast University, Nanjing 210096, Jiangsu, China
-
Received:2024-01-16Revised:2024-05-25Online:2025-02-25Published:2025-03-10 -
Contact:HUANG Yaji
摘要:
城镇化的加速推进导致污泥处置压力不断增大。热解法作为资源化处置污泥的有效手段,重金属是处置过程中需要格外关注的一种污染物。本文在水平固定床反应器中开展污泥和农林废弃物的共热解实验,探究不同热解温度下(500℃、700℃)农林废弃物种类(稻壳、木屑、玉米秸秆)以及掺混比例(25%、50%)对重金属元素Co、Cr、Cu、Mn、Ni、Pb、Zn在生物炭中迁移转化特性的影响。研究发现,随着热解温度由500℃升至700℃,重金属在生物炭中的残余率呈现下降趋势,而掺混农林废弃物共热解则会使生物炭中Co、Cr、Mn的残余率上升。对重金属赋存形态的研究发现,热解温度的升高以及掺混农林废弃物共热解在大部分情况下可以使重金属以更稳定的形态赋存在生物炭中,但也应注意700℃时掺混农林废弃物共热解不利于生物炭中Ni向稳定形态转化。总体上看,掺混农林废弃物共热解会使生物炭的潜在生态风险系数呈下降趋势。本文期望为实际工业生产中污泥热处置重金属排放控制方面提供有价值的参考。
中图分类号:
引用本文
赵佳琪, 黄亚继, 李志远, 朱志成, 祁帅杰, 高嘉炜, 刘俊, 张煜尧. 污泥同农林废弃物共热解重金属迁移转化特性[J]. 化工进展, 2025, 44(2): 1064-1075.
ZHAO Jiaqi, HUANG Yaji, LI Zhiyuan, ZHU Zhicheng, QI Shuaijie, GAO Jiawei, LIU Jun, ZHANG Yuyao. Characteristics of heavy metal migration and transformation during co-pyrolysis of sludge with agroforestry wastes[J]. Chemical Industry and Engineering Progress, 2025, 44(2): 1064-1075.
| 样品 | 挥发分/% | 固定碳/% | 灰分/% |
|---|---|---|---|
| SS | 48.71 | 5.56 | 45.73 |
| RH | 67.18 | 19.57 | 13.25 |
| WC | 72.83 | 15.59 | 11.58 |
| CS | 82.26 | 13.28 | 4.46 |
表1 实验样品工业分析结果
| 样品 | 挥发分/% | 固定碳/% | 灰分/% |
|---|---|---|---|
| SS | 48.71 | 5.56 | 45.73 |
| RH | 67.18 | 19.57 | 13.25 |
| WC | 72.83 | 15.59 | 11.58 |
| CS | 82.26 | 13.28 | 4.46 |
| 样品 | C/% | H/% | O①/% | N/% | S/% |
|---|---|---|---|---|---|
| SS | 19.23 | 3.57 | 27.65 | 2.92 | 0.81 |
| RH | 37.68 | 5.23 | 43.61 | 0.23 | 0 |
| WC | 40.11 | 5.46 | 41.93 | 0.92 | 0 |
| CS | 45.77 | 6.06 | 43.50 | 0.21 | 0 |
表2 实验样品元素分析结果
| 样品 | C/% | H/% | O①/% | N/% | S/% |
|---|---|---|---|---|---|
| SS | 19.23 | 3.57 | 27.65 | 2.92 | 0.81 |
| RH | 37.68 | 5.23 | 43.61 | 0.23 | 0 |
| WC | 40.11 | 5.46 | 41.93 | 0.92 | 0 |
| CS | 45.77 | 6.06 | 43.50 | 0.21 | 0 |
| 样品 | Co /mg∙kg-1 | Cr /mg∙kg-1 | Cu /mg∙kg-1 | Mn /mg∙kg-1 | Ni /mg∙kg-1 | Pb /mg∙kg-1 | Zn /mg∙kg-1 |
|---|---|---|---|---|---|---|---|
| SS | 43 | 195 | 245 | 1951 | 96 | 102 | 1452 |
| RH | 未检出 | 未检出 | 未检出 | 3.7 | 未检出 | 未检出 | 3.5 |
| WC | 未检出 | 未检出 | 未检出 | 4.5 | 未检出 | 未检出 | 5.5 |
| CS | 未检出 | 未检出 | 未检出 | 2.3 | 未检出 | 未检出 | 1.7 |
表3 实验样品的重金属含量
| 样品 | Co /mg∙kg-1 | Cr /mg∙kg-1 | Cu /mg∙kg-1 | Mn /mg∙kg-1 | Ni /mg∙kg-1 | Pb /mg∙kg-1 | Zn /mg∙kg-1 |
|---|---|---|---|---|---|---|---|
| SS | 43 | 195 | 245 | 1951 | 96 | 102 | 1452 |
| RH | 未检出 | 未检出 | 未检出 | 3.7 | 未检出 | 未检出 | 3.5 |
| WC | 未检出 | 未检出 | 未检出 | 4.5 | 未检出 | 未检出 | 5.5 |
| CS | 未检出 | 未检出 | 未检出 | 2.3 | 未检出 | 未检出 | 1.7 |
| 提取形态 | 样品 | 提取试剂及条件 |
|---|---|---|
| F1:酸溶态和可交换态 | 0.5g | 20mL、0.1mol/L冰醋酸,振荡16h后离心,取上清液 |
| F2:可还原态 | F1固态残渣 | 20mL、0.1mol/L盐酸羟胺,振荡16h后离心,取上清液 |
| F3:可氧化态 | F2固态残渣 | 先加入30%(体积分数)过氧化氢溶液5mL,室温放置1h;随后加入30%(体积分数)过氧化氢溶液5mL,85℃水浴1h;最后加入25mL、1.0mol/L乙酸铵,振荡16h后离心,取上清液 |
| F4:残渣态 | F3固态残渣 | 消解方法如1.4.1节中所述 |
表4 BCR逐步提取重金属操作流程
| 提取形态 | 样品 | 提取试剂及条件 |
|---|---|---|
| F1:酸溶态和可交换态 | 0.5g | 20mL、0.1mol/L冰醋酸,振荡16h后离心,取上清液 |
| F2:可还原态 | F1固态残渣 | 20mL、0.1mol/L盐酸羟胺,振荡16h后离心,取上清液 |
| F3:可氧化态 | F2固态残渣 | 先加入30%(体积分数)过氧化氢溶液5mL,室温放置1h;随后加入30%(体积分数)过氧化氢溶液5mL,85℃水浴1h;最后加入25mL、1.0mol/L乙酸铵,振荡16h后离心,取上清液 |
| F4:残渣态 | F3固态残渣 | 消解方法如1.4.1节中所述 |
| 样品 | 产率/% | Co/mg∙kg-1 | Cr/mg∙kg-1 | Cu/mg∙kg-1 | Mn/mg∙kg-1 | Ni/mg∙kg-1 | Pb/mg∙kg-1 | Zn/mg∙kg-1 |
|---|---|---|---|---|---|---|---|---|
| SS | — | 43±1.5 | 195±2.3 | 245±1.3 | 1951±13.3 | 96±2.1 | 102±1.9 | 1452±17.5 |
| 500SS | 68.3±0.1 | 55.3±0.9 | 255.5±2.7 | 338.4±3.3 | 2561.1±37.5 | 129.1±2.4 | 147±7.2 | 2015.1±27.1 |
| 500SR1 | 61.7±0.1 | 46.6±1.1 | 216.1±9.8 | 279.5±1.3 | 2202±38.5 | 103.3±0.3 | 119.3±3.5 | 1658±33.5 |
| 500SW1 | 60.1±0 | 48.5±2.2 | 220.8±8.5 | 285.9±14.6 | 2362.8±42 | 107.3±5.3 | 119.5±5 | 1692±32 |
| 500SC1 | 59.1±0 | 48.8±0.7 | 225±11.5 | 290.5±2.2 | 2279±43.3 | 107.8±0.2 | 121.3±3.8 | 1728±31.5 |
| 500SR2 | 55.5±0.1 | 34.9±0.4 | 170±2 | 205±4 | 1664.5±12.5 | 76±3 | 88±3.5 | 1202±6 |
| 500SW2 | 52.4±0 | 37.4±1.5 | 173.5±15 | 216±14 | 1795±35.7 | 81±3.3 | 88±8 | 1260±72 |
| 500SC2 | 48.4±0 | 39.9±0.7 | 189±0.5 | 234±7.5 | 1861.5±58 | 86.7±0.9 | 93.5±1.5 | 1372±32 |
| 700SS | 62.7±0 | 57.1±2.1 | 261.8±5.2 | 365.4±8 | 2619.5±60 | 138.3±4 | 156.6±6.4 | 2157.4±72 |
| 700SR1 | 56.8±0 | 48.1±0.9 | 223.8±1.3 | 299±2.2 | 2352±21.5 | 110±1 | 126.9±0.1 | 1769±25 |
| 700SW1 | 54.6±0 | 50.4±1.1 | 228±5 | 309.4±4.5 | 2549±22 | 115.5±2 | 128.3±2 | 1801.5±31 |
| 700SC1 | 53.3±0 | 51.5±0.5 | 236±0.5 | 318±0.3 | 2408±30 | 117±3 | 132.5±2.5 | 1864±5 |
| 700SR2 | 50.8±0.1 | 37.8±1.1 | 180.5±3 | 220.5±1.5 | 1781±15 | 77±2.5 | 91.5±1.5 | 1285±25 |
| 700SW2 | 47.6±0 | 40.9±1 | 185.5±11 | 232.5±4.2 | 1962±63 | 84.5±4 | 93±0.5 | 1362±1.5 |
| 700SC2 | 44.6±0.1 | 40.5±1.9 | 203.8±8.8 | 249.5±13.5 | 1946±103 | 88.5±6 | 103±6.5 | 1461±76 |
表5 生物炭产率与样品中重金属含量
| 样品 | 产率/% | Co/mg∙kg-1 | Cr/mg∙kg-1 | Cu/mg∙kg-1 | Mn/mg∙kg-1 | Ni/mg∙kg-1 | Pb/mg∙kg-1 | Zn/mg∙kg-1 |
|---|---|---|---|---|---|---|---|---|
| SS | — | 43±1.5 | 195±2.3 | 245±1.3 | 1951±13.3 | 96±2.1 | 102±1.9 | 1452±17.5 |
| 500SS | 68.3±0.1 | 55.3±0.9 | 255.5±2.7 | 338.4±3.3 | 2561.1±37.5 | 129.1±2.4 | 147±7.2 | 2015.1±27.1 |
| 500SR1 | 61.7±0.1 | 46.6±1.1 | 216.1±9.8 | 279.5±1.3 | 2202±38.5 | 103.3±0.3 | 119.3±3.5 | 1658±33.5 |
| 500SW1 | 60.1±0 | 48.5±2.2 | 220.8±8.5 | 285.9±14.6 | 2362.8±42 | 107.3±5.3 | 119.5±5 | 1692±32 |
| 500SC1 | 59.1±0 | 48.8±0.7 | 225±11.5 | 290.5±2.2 | 2279±43.3 | 107.8±0.2 | 121.3±3.8 | 1728±31.5 |
| 500SR2 | 55.5±0.1 | 34.9±0.4 | 170±2 | 205±4 | 1664.5±12.5 | 76±3 | 88±3.5 | 1202±6 |
| 500SW2 | 52.4±0 | 37.4±1.5 | 173.5±15 | 216±14 | 1795±35.7 | 81±3.3 | 88±8 | 1260±72 |
| 500SC2 | 48.4±0 | 39.9±0.7 | 189±0.5 | 234±7.5 | 1861.5±58 | 86.7±0.9 | 93.5±1.5 | 1372±32 |
| 700SS | 62.7±0 | 57.1±2.1 | 261.8±5.2 | 365.4±8 | 2619.5±60 | 138.3±4 | 156.6±6.4 | 2157.4±72 |
| 700SR1 | 56.8±0 | 48.1±0.9 | 223.8±1.3 | 299±2.2 | 2352±21.5 | 110±1 | 126.9±0.1 | 1769±25 |
| 700SW1 | 54.6±0 | 50.4±1.1 | 228±5 | 309.4±4.5 | 2549±22 | 115.5±2 | 128.3±2 | 1801.5±31 |
| 700SC1 | 53.3±0 | 51.5±0.5 | 236±0.5 | 318±0.3 | 2408±30 | 117±3 | 132.5±2.5 | 1864±5 |
| 700SR2 | 50.8±0.1 | 37.8±1.1 | 180.5±3 | 220.5±1.5 | 1781±15 | 77±2.5 | 91.5±1.5 | 1285±25 |
| 700SW2 | 47.6±0 | 40.9±1 | 185.5±11 | 232.5±4.2 | 1962±63 | 84.5±4 | 93±0.5 | 1362±1.5 |
| 700SC2 | 44.6±0.1 | 40.5±1.9 | 203.8±8.8 | 249.5±13.5 | 1946±103 | 88.5±6 | 103±6.5 | 1461±76 |
| 样品 | Cf | Er | RI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co | Cr | Cu | Mn | Ni | Pb | Zn | Co | Cr | Cu | Mn | Ni | Pb | Zn | ||
| SS | 10.49 | 1.40 | 6.46 | 11.50 | 5.41 | 0.52 | 5.76 | 52.47 | 2.81 | 32.31 | 11.50 | 32.46 | 2.60 | 5.76 | 139.91 |
| 500SS | 3.33 | 1.08 | 3.61 | 8.09 | 3.67 | 0.12 | 6.81 | 16.65 | 2.17 | 18.04 | 8.09 | 22.04 | 0.61 | 6.81 | 74.41 |
| 500SR1 | 2.17 | 1.15 | 1.90 | 5.58 | 3.00 | 0.13 | 6.09 | 10.87 | 2.30 | 9.48 | 5.58 | 18.00 | 0.63 | 6.09 | 52.96 |
| 500SW1 | 2.13 | 1.20 | 1.72 | 6.30 | 2.92 | 0.10 | 6.14 | 10.67 | 2.40 | 8.62 | 6.30 | 17.53 | 0.51 | 6.14 | 52.18 |
| 500SC1 | 2.28 | 1.26 | 2.47 | 4.92 | 3.02 | 0.14 | 6.52 | 11.39 | 2.52 | 12.36 | 4.92 | 18.10 | 0.68 | 6.52 | 56.48 |
| 500SR2 | 1.99 | 1.28 | 1.06 | 4.59 | 1.28 | 0.22 | 5.41 | 9.97 | 2.57 | 5.31 | 4.59 | 7.67 | 1.08 | 5.41 | 36.59 |
| 500SW2 | 2.08 | 1.26 | 1.27 | 5.02 | 1.20 | 0.15 | 5.41 | 10.38 | 2.52 | 6.34 | 5.02 | 7.19 | 0.73 | 5.41 | 37.60 |
| 500SC2 | 1.99 | 1.30 | 1.35 | 4.65 | 1.23 | 0.15 | 5.37 | 9.97 | 2.60 | 6.74 | 4.65 | 7.39 | 0.73 | 5.37 | 37.44 |
| 700SS | 1.88 | 0.45 | 0.57 | 3.50 | 2.09 | 0.09 | 2.64 | 9.41 | 0.89 | 2.84 | 3.50 | 12.52 | 0.46 | 2.64 | 32.26 |
| 700SR1 | 1.96 | 0.43 | 0.84 | 2.97 | 2.42 | 0.08 | 2.28 | 9.79 | 0.86 | 4.21 | 2.97 | 14.55 | 0.39 | 2.28 | 35.05 |
| 700SW1 | 1.87 | 0.37 | 0.67 | 3.78 | 2.41 | 0.04 | 2.16 | 9.33 | 0.73 | 3.33 | 3.78 | 14.48 | 0.18 | 2.16 | 34.00 |
| 700SC1 | 1.69 | 0.43 | 0.79 | 3.02 | 2.65 | 0.06 | 1.92 | 8.44 | 0.87 | 3.94 | 3.02 | 15.90 | 0.29 | 1.92 | 34.37 |
| 700SR2 | 1.63 | 0.42 | 0.91 | 2.97 | 3.12 | 0.09 | 2.03 | 8.16 | 0.84 | 4.54 | 2.97 | 18.69 | 0.45 | 2.03 | 37.68 |
| 700SW2 | 1.72 | 0.24 | 0.73 | 3.65 | 3.08 | 0.06 | 1.99 | 8.59 | 0.48 | 3.67 | 3.65 | 18.49 | 0.29 | 1.99 | 37.15 |
| 700SC2 | 1.60 | 0.41 | 0.94 | 3.39 | 3.41 | 0.04 | 1.94 | 7.99 | 0.82 | 4.71 | 3.39 | 20.43 | 0.20 | 1.94 | 39.48 |
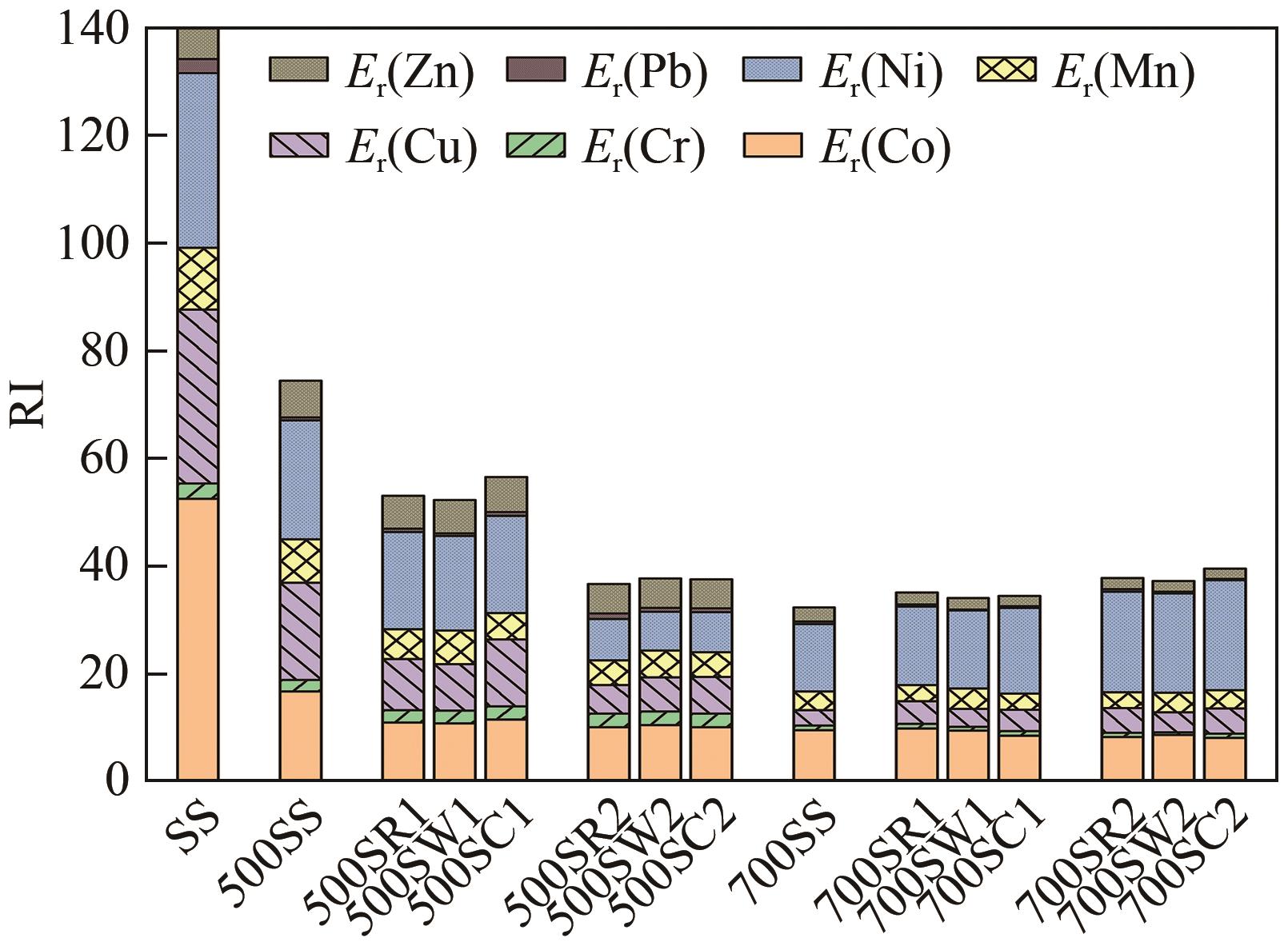
表6 污泥与生物炭中重金属Cf、Er和RI
| 样品 | Cf | Er | RI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co | Cr | Cu | Mn | Ni | Pb | Zn | Co | Cr | Cu | Mn | Ni | Pb | Zn | ||
| SS | 10.49 | 1.40 | 6.46 | 11.50 | 5.41 | 0.52 | 5.76 | 52.47 | 2.81 | 32.31 | 11.50 | 32.46 | 2.60 | 5.76 | 139.91 |
| 500SS | 3.33 | 1.08 | 3.61 | 8.09 | 3.67 | 0.12 | 6.81 | 16.65 | 2.17 | 18.04 | 8.09 | 22.04 | 0.61 | 6.81 | 74.41 |
| 500SR1 | 2.17 | 1.15 | 1.90 | 5.58 | 3.00 | 0.13 | 6.09 | 10.87 | 2.30 | 9.48 | 5.58 | 18.00 | 0.63 | 6.09 | 52.96 |
| 500SW1 | 2.13 | 1.20 | 1.72 | 6.30 | 2.92 | 0.10 | 6.14 | 10.67 | 2.40 | 8.62 | 6.30 | 17.53 | 0.51 | 6.14 | 52.18 |
| 500SC1 | 2.28 | 1.26 | 2.47 | 4.92 | 3.02 | 0.14 | 6.52 | 11.39 | 2.52 | 12.36 | 4.92 | 18.10 | 0.68 | 6.52 | 56.48 |
| 500SR2 | 1.99 | 1.28 | 1.06 | 4.59 | 1.28 | 0.22 | 5.41 | 9.97 | 2.57 | 5.31 | 4.59 | 7.67 | 1.08 | 5.41 | 36.59 |
| 500SW2 | 2.08 | 1.26 | 1.27 | 5.02 | 1.20 | 0.15 | 5.41 | 10.38 | 2.52 | 6.34 | 5.02 | 7.19 | 0.73 | 5.41 | 37.60 |
| 500SC2 | 1.99 | 1.30 | 1.35 | 4.65 | 1.23 | 0.15 | 5.37 | 9.97 | 2.60 | 6.74 | 4.65 | 7.39 | 0.73 | 5.37 | 37.44 |
| 700SS | 1.88 | 0.45 | 0.57 | 3.50 | 2.09 | 0.09 | 2.64 | 9.41 | 0.89 | 2.84 | 3.50 | 12.52 | 0.46 | 2.64 | 32.26 |
| 700SR1 | 1.96 | 0.43 | 0.84 | 2.97 | 2.42 | 0.08 | 2.28 | 9.79 | 0.86 | 4.21 | 2.97 | 14.55 | 0.39 | 2.28 | 35.05 |
| 700SW1 | 1.87 | 0.37 | 0.67 | 3.78 | 2.41 | 0.04 | 2.16 | 9.33 | 0.73 | 3.33 | 3.78 | 14.48 | 0.18 | 2.16 | 34.00 |
| 700SC1 | 1.69 | 0.43 | 0.79 | 3.02 | 2.65 | 0.06 | 1.92 | 8.44 | 0.87 | 3.94 | 3.02 | 15.90 | 0.29 | 1.92 | 34.37 |
| 700SR2 | 1.63 | 0.42 | 0.91 | 2.97 | 3.12 | 0.09 | 2.03 | 8.16 | 0.84 | 4.54 | 2.97 | 18.69 | 0.45 | 2.03 | 37.68 |
| 700SW2 | 1.72 | 0.24 | 0.73 | 3.65 | 3.08 | 0.06 | 1.99 | 8.59 | 0.48 | 3.67 | 3.65 | 18.49 | 0.29 | 1.99 | 37.15 |
| 700SC2 | 1.60 | 0.41 | 0.94 | 3.39 | 3.41 | 0.04 | 1.94 | 7.99 | 0.82 | 4.71 | 3.39 | 20.43 | 0.20 | 1.94 | 39.48 |
| 特征峰波长范围/cm-1 | 归属 |
|---|---|
| 4000~3862 | C—H弯曲振动 |
| 3600~3200 | O—H伸缩振动 |
| 3200~3000 | 芳香族C—H伸缩振动 |
| 2970~2850 | 脂肪族C—H伸缩振动 |
| 1600~1400 | 多烯/芳香族C |
| 1490~1420,1390~1360 | 脂肪族C—H弯曲振动 |
| 1300~1020 | C—O—C弯曲振动 |
| 1280~1270 | C |
| 1142~1130 | C—H在平面上的变形振动 |
| 1100~1050 | C—O变形振动 |
| 1050~1046 | Si—O—C或Si—O—Si振动 |
| 880~850 | 芳香环平面外C—H振动 |
| 800~796 | SiO和硅酸盐 |
| 665 | C—H弯曲振动 |
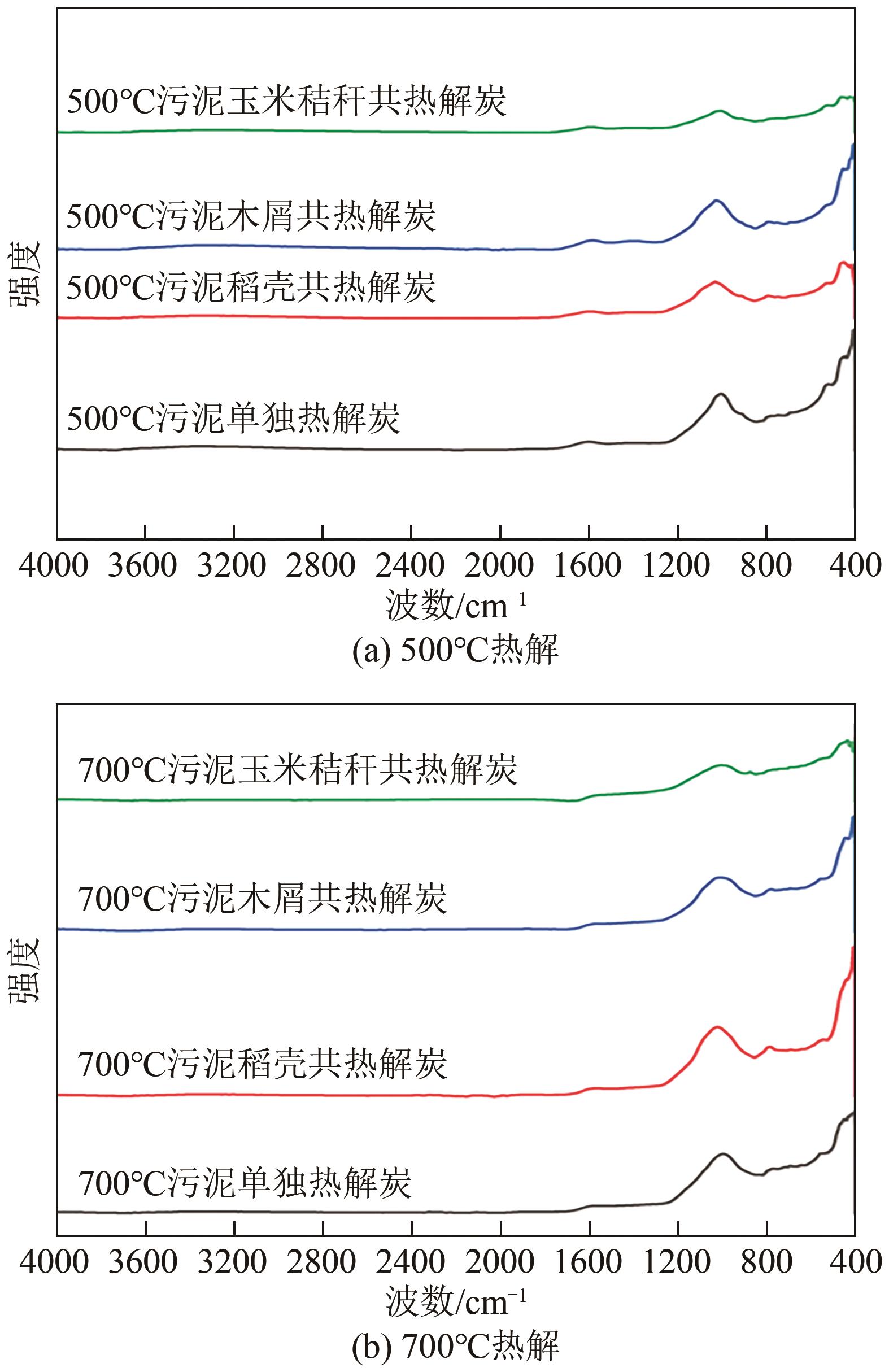
表7 热解炭特征峰波长范围及其归属
| 特征峰波长范围/cm-1 | 归属 |
|---|---|
| 4000~3862 | C—H弯曲振动 |
| 3600~3200 | O—H伸缩振动 |
| 3200~3000 | 芳香族C—H伸缩振动 |
| 2970~2850 | 脂肪族C—H伸缩振动 |
| 1600~1400 | 多烯/芳香族C |
| 1490~1420,1390~1360 | 脂肪族C—H弯曲振动 |
| 1300~1020 | C—O—C弯曲振动 |
| 1280~1270 | C |
| 1142~1130 | C—H在平面上的变形振动 |
| 1100~1050 | C—O变形振动 |
| 1050~1046 | Si—O—C或Si—O—Si振动 |
| 880~850 | 芳香环平面外C—H振动 |
| 800~796 | SiO和硅酸盐 |
| 665 | C—H弯曲振动 |
| 1 | WEI Liangliang, ZHU Fengyi, LI Qiaoyang, et al. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis[J]. Environment International, 2020, 144: 106093. |
| 2 | TAKDASTAN Afshin, RAHMANI Ali Reza, ALMASI Halime. A review of the effects of ozonation process on biological sludge reduction[J]. Desalination and Water Treatment, 2019, 162: 125-133. |
| 3 | Gokce KOR-BICAKCI, ESKICIOGLU Cigdem. Recent developments on thermal municipal sludge pretreatment technologies for enhanced anaerobic digestion[J]. Renewable and Sustainable Energy Reviews, 2019, 110: 423-443. |
| 4 | LI Yuanling, YU Han, LIU Lina, et al. Application of co-pyrolysis biochar for the adsorption and immobilization of heavy metals in contaminated environmental substrates[J]. Journal of Hazardous Materials, 2021, 420: 126655. |
| 5 | SHENTU Jiali, LI Xiaoxiao, HAN Ruifang, et al. Effect of site hydrological conditions and soil aggregate sizes on the stabilization of heavy metals (Cu, Ni, Pb, Zn) by biochar[J]. Science of the Total Environment, 2022, 802: 149949. |
| 6 | 李志远, 黄亚继, 赵佳琪, 等. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| LI Zhiyuan, HUANG Yaji, ZHAO Jiaqi, et al. Characterization of heavy metals during co-pyrolysis of sludge with PVC[J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4947-4956. | |
| 7 | WANG Xingdong, CHI Qiaoqiao, LIU Xuejiao, et al. Influence of pyrolysis temperature on characteristics and environmental risk of heavy metals in pyrolyzed biochar made from hydrothermally treated sewage sludge[J]. Chemosphere, 2019, 216: 698-706. |
| 8 | LI Chunxing, XIE Shengyu, YOU Futian, et al. Heavy metal stabilization and improved biochar generation via pyrolysis of hydrothermally treated sewage sludge with antibiotic mycelial residue[J]. Waste Management, 2021, 119: 152-161. |
| 9 | HAN Hengda, HU Song, SYED-HASSAN Syed Shatir A, et al. Effects of reaction conditions on the emission behaviors of arsenic, cadmium and lead during sewage sludge pyrolysis[J]. Bioresource Technology, 2017, 236: 138-145. |
| 10 | CHEN Xing, MA Rui, LUO Juan, et al. Co-microwave pyrolysis of electroplating sludge and municipal sewage sludge to synergistically improve the immobilization of high-concentration heavy metals and an analysis of the mechanism[J]. Journal of Hazardous Materials, 2021, 417: 126099. |
| 11 | WEN Yanjun, XIE Yingshen, JIANG Chi, et al. Products distribution and interaction mechanism during co-pyrolysis of rice husk and oily sludge by experiments and reaction force field simulation[J]. Bioresource Technology, 2021, 329: 124822. |
| 12 | HOU Jinyu, ZHONG Daoxu, LIU Wuxing. Catalytic co-pyrolysis of oil sludge and biomass over ZSM-5 for production of aromatic platform chemicals[J]. Chemosphere, 2022, 291: 132912. |
| 13 | GODLEWSKA Paulina, OLESZCZUK Patryk. Effect of biomass addition before sewage sludge pyrolysis on the persistence and bioavailability of polycyclic aromatic hydrocarbons in biochar-amended soil[J]. Chemical Engineering Journal, 2022, 429: 132143. |
| 14 | DONG Qing, ZHANG Shuping, WU Bo, et al. Co-pyrolysis of sewage sludge and rice straw: Thermal behavior and char characteristic evaluations[J]. Energy & Fuels, 2020, 34(1): 607-615. |
| 15 | LIU Tingting, LIU Zhengang, ZHENG Qingfu, et al. Effect of hydrothermal carbonization on migration and environmental risk of heavy metals in sewage sludge during pyrolysis[J]. Bioresource Technology, 2018, 247: 282-290. |
| 16 | 赵晓亮, 李响, 卢洪斌, 等. 东江湖表层沉积物重金属污染特征与潜在生态风险评价[J]. 环境科学, 2022, 43(6): 3048-3057. |
| ZHAO Xiaoliang, LI Xiang, LU Hongbin, et al. Analysis of heavy metal pollution characteristics and potential ecological risks of surface sediments in Dongjiang Lake[J]. Environmental Science, 2022, 43(6): 3048-3057. | |
| 17 | 赵佳琪, 黄亚继, 李志远, 等. 污泥和聚氯乙烯共热解三相产物特性[J]. 化工进展, 2023, 42(4): 2122-2129. |
| ZHAO Jiaqi, HUANG Yaji, LI Zhiyuan, et al. Characteristics of three-phase products from co-pyrolysis of sewage sludge and PVC[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2122-2129. | |
| 18 | JI Guozhao, YAO Joseph G, CLOUGH Peter T, et al. Enhanced hydrogen production from thermochemical processes[J]. Energy & Environmental Science, 2018, 11(10): 2647-2672. |
| 19 | 郑发, 李浩文, 林法伟, 等. 大庆罐底油泥热解特性及污染物释放特性[J]. 化工进展, 2022, 41(1): 476-484. |
| ZHENG Fa, LI Haowen, LIN Fawei, et al. Pyrolysis characteristics and pollutant release characteristics of Daqing oil sludge[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 476-484. | |
| 20 | CHEN Yiwei, LIU Guijian, WANG Lei, et al. Occurrence and fate of some trace elements during pyrolysis of Yima coal, China[J]. Energy & Fuels, 2008, 22(6): 3877-3882. |
| 21 | WANG Xingdong, LI Chunxing, LI Zhiwei, et al. Effect of pyrolysis temperature on characteristics, chemical speciation and risk evaluation of heavy metals in biochar derived from textile dyeing sludge[J]. Ecotoxicology and Environmental Safety, 2019, 168: 45-52. |
| 22 | LI Danni, SHAN Rui, JIANG Lixia, et al. A review on the migration and transformation of heavy metals in the process of sludge pyrolysis[J]. Resources, Conservation and Recycling, 2022, 185: 106452. |
| 23 | YUAN Xingzhong, LENG Lijian, HUANG Huajun, et al. Speciation and environmental risk assessment of heavy metal in bio-oil from liquefaction/pyrolysis of sewage sludge[J]. Chemosphere, 2015, 120: 645-652. |
| 24 | ZHANG Zhiyuan, JU Rui, ZHOU Hengtao, et al. Migration characteristics of heavy metals during sludge pyrolysis[J]. Waste Management, 2021, 120: 25-32. |
| 25 | CHANAKA UDAYANGA W D, VEKSHA Andrei, GIANNIS Apostolos, et al. Fate and distribution of heavy metals during thermal processing of sewage sludge[J]. Fuel, 2018, 226: 721-744. |
| 26 | 张双全, 武娜, 董明建, 等. 城市污泥与玉米秸秆共热解制备吸附剂的研究[J]. 中国矿业大学学报, 2011, 40(5): 799-803. |
| ZHANG Shuangquan, WU Na, DONG Mingjian, et al. Research on preparation of adsorbents by co-pyrolysis of sewage sludge with corn straw[J]. Journal of China University of Mining & Technology, 2011, 40(5): 799-803. | |
| 27 | MENG Jun, TAO Mengming, WANG Lili, et al. Changes in heavy metal bioavailability and speciation from a Pb-Zn mining soil amended with biochars from co-pyrolysis of rice straw and swine manure[J]. Science of the Total Environment, 2018, 633: 300-307. |
| 28 | ABANADES S, FLAMANT G, GAUTHIER D. Modelling of heavy metal vaporisation from a mineral matrix[J]. Journal of Hazardous Materials, 2001, 88(1): 75-94. |
| 29 | YUAN Xingzhong, HUANG Huajun, ZENG Guangming, et al. Total concentrations and chemical speciation of heavy metals in liquefaction residues of sewage sludge[J]. Bioresource Technology, 2011, 102(5): 4104-4110. |
| 30 | CHEN Ming, LI Xiaoming, YANG Qi, et al. Total concentrations and speciation of heavy metals in municipal sludge from Changsha, Zhuzhou and Xiangtan in middle-south region of China[J]. Journal of Hazardous Materials, 2008, 160(2/3): 324-329. |
| 31 | JIN Junwei, LI Yanan, ZHANG Jianyun, et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge[J]. Journal of Hazardous Materials, 2016, 320: 417-426. |
| 32 | HUANG Huajun, YANG Ting, LAI Faying, et al. Co-pyrolysis of sewage sludge and sawdust/rice straw for the production of biochar[J]. Journal of Analytical and Applied Pyrolysis, 2017, 125: 61-68. |
| 33 | 彭勃. 工业污泥与生物质共热解制炭及其重金属固化特性研究[D]. 南京: 东南大学, 2020. |
| PENG Bo. Study on the characteristics of char production and heavy metals solidification during co-pyrolysis of industrial sludge and biomass[D]. Nanjing: Southeast University, 2020. | |
| 34 | PARIHAR Anurag, SRIPADA Pramod, BAMBERY Keith, et al. Investigation of functional group changes in biomass during slow pyrolysis using synchrotron based infra-red microspectroscopy and thermogravimetry-infra-red spectroscopy[J]. Journal of Analytical and Applied Pyrolysis, 2017, 127: 394-401. |
| 35 | 朱赫男, 王志朴, 邢文龙, 等. 污泥与生物质共热解制备生物质炭工艺优化及吸附性能[J]. 化工进展, 2018, 37(S1): 199-204. |
| ZHU Henan, WANG Zhipu, XING Wenlong, et al. Process optimization and adsorption performance of co-pyrolysis of sludge and biomass to prepare biomass carbon[J]. Chemical Industry and Engineering Progress, 2018, 37(S1): 199-204. | |
| 36 | 任福民, 周玉松, 牛牧晨, 等. 污泥中的重金属特性分析和生态风险评价[J]. 北京交通大学学报, 2007, 31(1): 102-105. |
| REN Fumin, ZHOU Yusong, NIU Muchen, et al. Characteristics analysis and environmental assessment on heavy metals in the sludge of sewage[J]. Journal of Beijing Jiaotong University, 2007, 31(1): 102-105. | |
| 37 | WANG Xingdong, CHANG Victor Wei-Chung, LI Zhiwei, et al. Co-pyrolysis of sewage sludge and organic fractions of municipal solid waste: Synergistic effects on biochar properties and the environmental risk of heavy metals[J]. Journal of Hazardous Materials, 2021, 412: 125200. |
| 38 | SHAO Shanshan, ZHANG Huiyan, XIAO Rui, et al. Evolution of coke in the catalytic conversion of biomass-derivates by combined in situ DRIFTS and ex-situ approach: Effect of functional structure[J]. Fuel Processing Technology, 2018, 178: 88-97. |
| 39 | ZHANG Huiyan, SHAO Shanshan, RYABOV Georgy, et al. Functional group in situ evolution principles of produced solid and product distribution in biomass torrefaction process[J]. Energy & Fuels, 2017, 31(12): 13639-13646. |
| 40 | 江茂生, 黄彪, 陈学榕, 等. 木材炭化机理的FT-IR光谱分析研究[J]. 林产化学与工业, 2005, 25(2): 16-20. |
| JIANG Maosheng, HUANG Biao, CHEN Xuerong, et al. Ft-IR spectroscopic analysis on wood carbonization mechanism[J]. Chemistry & Industry of Forest Products, 2005, 25(2): 16-20. | |
| 41 | 简敏菲, 高凯芳, 余厚平. 不同裂解温度对水稻秸秆制备生物炭及其特性影响[J]. 环境科学学报, 2016, 36(5): 1757-1765. |
| JIAN Minfei, GAO Kaifang, YU Houping. Effects of different pyrolysis temperatures on the preparation and characteristics of bio-char from rice straw[J]. Huanjing Kexue Xuebao/Acta Scientiae Circumstantiae, 2016, 36(5):1757-1765. |
| [1] | 赵丽阳, 李倩, 何佩熹, 潘鸿辉, 刘艳, 刘细祥. 磷钼酸-Fe3O4球磨共改性污泥基生物炭对四环素的吸附特性[J]. 化工进展, 2025, 44(1): 583-595. |
| [2] | 李松亚, 陈炳桦, 刘彪, 王林裴, 王乐, 谷得明, 周一鸣, 王笑艳. 污泥颗粒化过程中信号分子AI-2调控作用研究进展[J]. 化工进展, 2024, 43(9): 5217-5225. |
| [3] | 全翠, 高宁博, 张广涛, 索浩杰. 含油污泥热解残渣制备渗水砖的重金属和多环芳烃浸出特性[J]. 化工进展, 2024, 43(9): 5226-5233. |
| [4] | 刘玉灿, 高中鲁, 徐心怡, 纪现国, 张岩, 孙洪伟, 王港. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 2024, 43(8): 4630-4641. |
| [5] | 毛华恺, 余洋, 张悦, 夏广坤, 吴赟韬, 楼乐瑶, 牛文娟, 刘念. 生物炭光催化氧化-吸附协同降解亚硝酸盐[J]. 化工进展, 2024, 43(8): 4757-4765. |
| [6] | 黄军, 张应娟, 林茵童, 韦雪纯, 吴雨桐, 毋高博, 莫钧麟, 赵祯霞, 赵钟兴. 蚕沙基生物多孔炭的制备及对杀虫单/呋虫胺的协同吸附与缓释性能[J]. 化工进展, 2024, 43(7): 3964-3971. |
| [7] | 于立爽, 李青云, 刘兆明, 张淑茹, 刘幽燕, 唐爱星. 油菜花粉生物炭固定化脂肪酶催化蒎烯环氧化[J]. 化工进展, 2024, 43(7): 3996-4004. |
| [8] | 孔祥蕊, 董玥岑, 张蒙雨, 王彪, 尹水娥, 陈冰, 陆家纬, 张媛, 冯乐乐, 王洪涛, 徐海云. 生活垃圾焚烧飞灰处理技术研究进展[J]. 化工进展, 2024, 43(7): 4102-4117. |
| [9] | 姚雪, 武淑慧, 杨阳, 王晓, 冯雷, 冯雪冬, 马艳飞. 含油污泥基生物炭处理含油废水[J]. 化工进展, 2024, 43(6): 3398-3409. |
| [10] | 潘伟亮, 张汛, 李姣妮, 古励, 何强, 敖良根. 次氯酸盐氧化耦合FeCl3絮凝调节改善污泥脱水[J]. 化工进展, 2024, 43(6): 3450-3458. |
| [11] | 莎莉, 苏莹嘉, 凌子琛, 于晓艳, 李书鹏, 郭丽莉, 熊静, 房连虎, 张冉, 张书廷. 烟煤掺混对污泥电脱水性能的影响[J]. 化工进展, 2024, 43(4): 2144-2152. |
| [12] | 赵瑞强, 周鑫, 牛冰心. 废水处理硝酸盐异化还原与厌氧氨氧化/反硝化耦合工艺构建[J]. 化工进展, 2024, 43(3): 1593-1605. |
| [13] | 巩志强, 刘雷, 王少华, 韩悦, 郭俊山, 商攀峰, 祝令凯, 郑威. 矿物质化合物对含油污泥焚烧过程中重金属迁移转化的影响[J]. 化工进展, 2024, 43(3): 1614-1620. |
| [14] | 郑钰, 李靖杰, 张宇峰, 赵梦琦, 张娜, 周澳, 于伟, 谭厚章, 王学斌. 典型炉排炉和流化床垃圾焚烧飞灰及螯合产物的重金属浸出毒性[J]. 化工进展, 2024, 43(3): 1630-1636. |
| [15] | 彭程, 徐漪琳, 石钰婧, 张玟, 李宇涛, 王皓冉, 张卫, 占绣萍. 生物炭改性及其对除草剂污染水体和土壤修复的研究进展[J]. 化工进展, 2024, 43(2): 1069-1081. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||