化工进展 ›› 2024, Vol. 43 ›› Issue (10): 5867-5880.DOI: 10.16085/j.issn.1000-6613.2023-1510
• 资源与环境化工 • 上一篇
钢渣构筑Fe-CSH吸附溶液中Pb(Ⅱ)、Cu(Ⅱ)、Zn(Ⅱ)性能及机理
单书月( ), 罗中秋(
), 罗中秋( ), 周新涛(
), 周新涛( ), 尚波, 田鑫聪, 阎崔蓉
), 尚波, 田鑫聪, 阎崔蓉
- 昆明理工大学化学工程学院,云南 昆明 650500
-
收稿日期:2023-08-30修回日期:2024-02-20出版日期:2024-10-15发布日期:2024-10-29 -
通讯作者:罗中秋,周新涛 -
作者简介:单书月(1999—),女,硕士研究生,研究方向为固体物资源化利用和危废安全处理处置。E-mail:1287965670@qq.com。 -
基金资助:国家自然科学基金(22366023);国家钒钛资源综合利用国家重点实验室开放课题(2022P4FZG03A);云南省“兴滇英才支持计划”青年人才专项(YNWR-QNBJ-2020-063);昆明理工大学分析测试基金(2022T20160009)
Adsorption performance and mechanism for Pb(Ⅱ), Cu(Ⅱ) and Zn(Ⅱ) removal from aqueous solutions by Fe-CSH derived from steel slag
SHAN Shuyue( ), LUO Zhongqiu(
), LUO Zhongqiu( ), ZHOU Xintao(
), ZHOU Xintao( ), SHANG Bo, TIAN Xincong, YAN Cuirong
), SHANG Bo, TIAN Xincong, YAN Cuirong
- College of Chemical Engineering, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
-
Received:2023-08-30Revised:2024-02-20Online:2024-10-15Published:2024-10-29 -
Contact:LUO Zhongqiu, ZHOU Xintao
摘要:
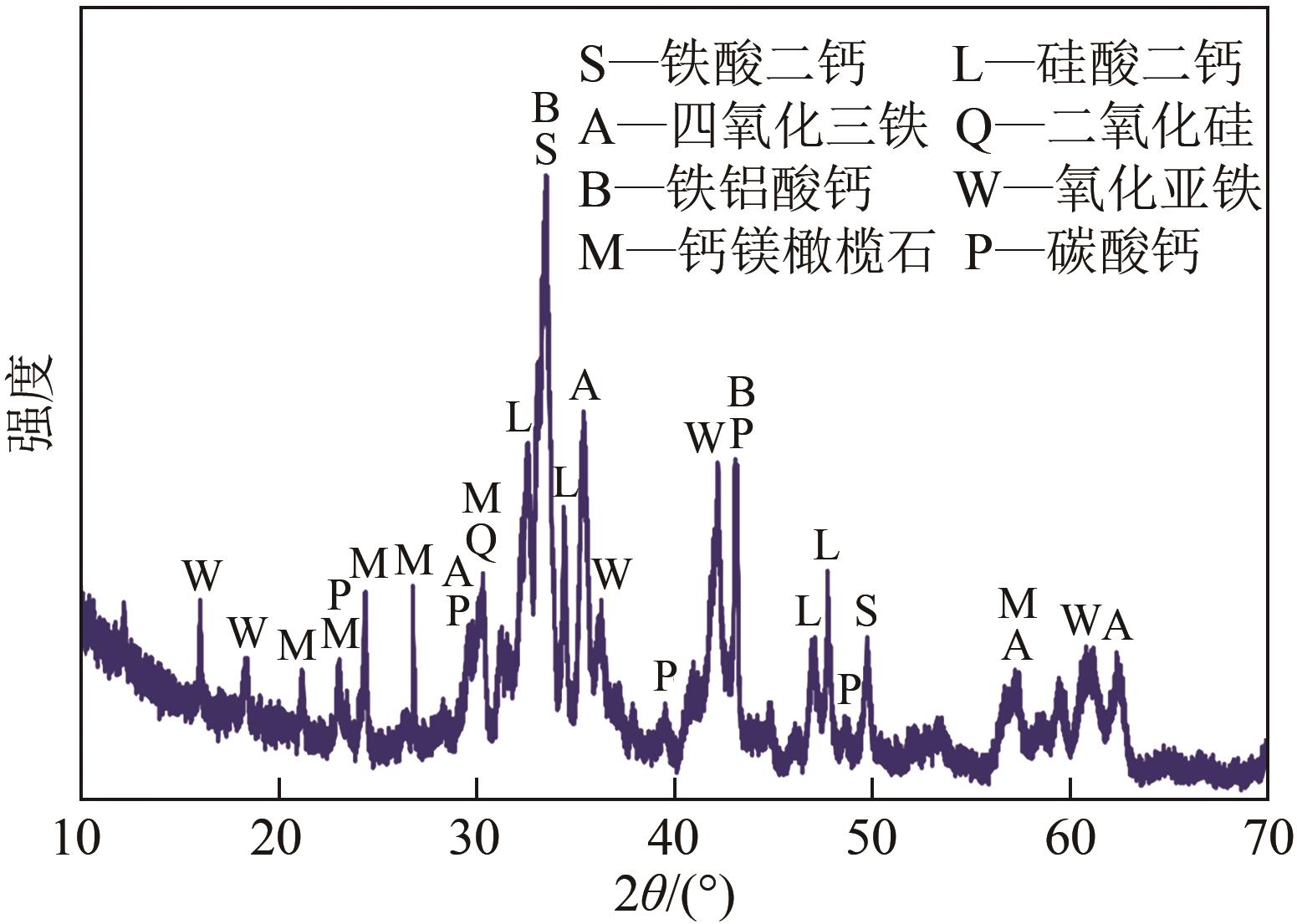
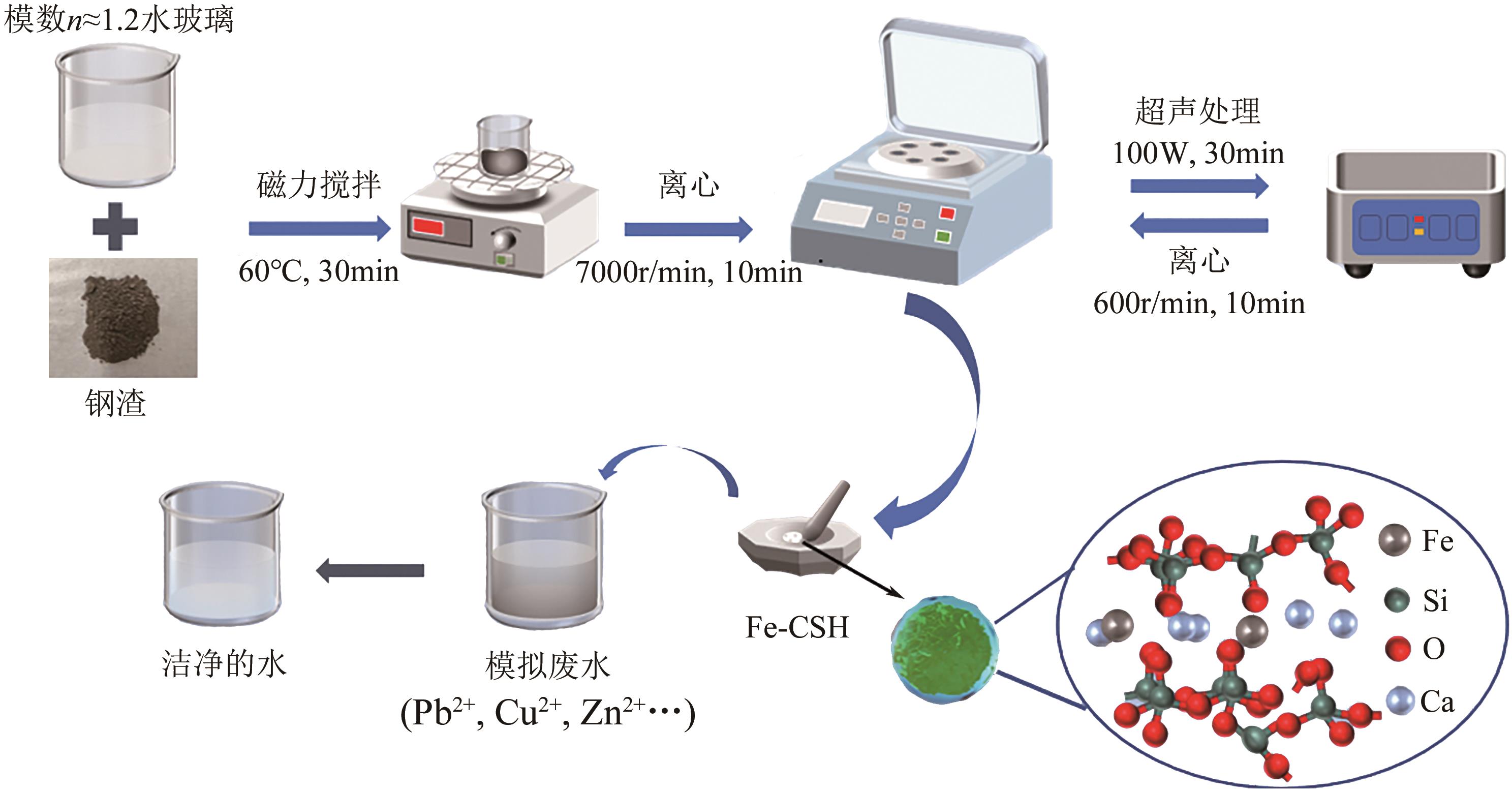
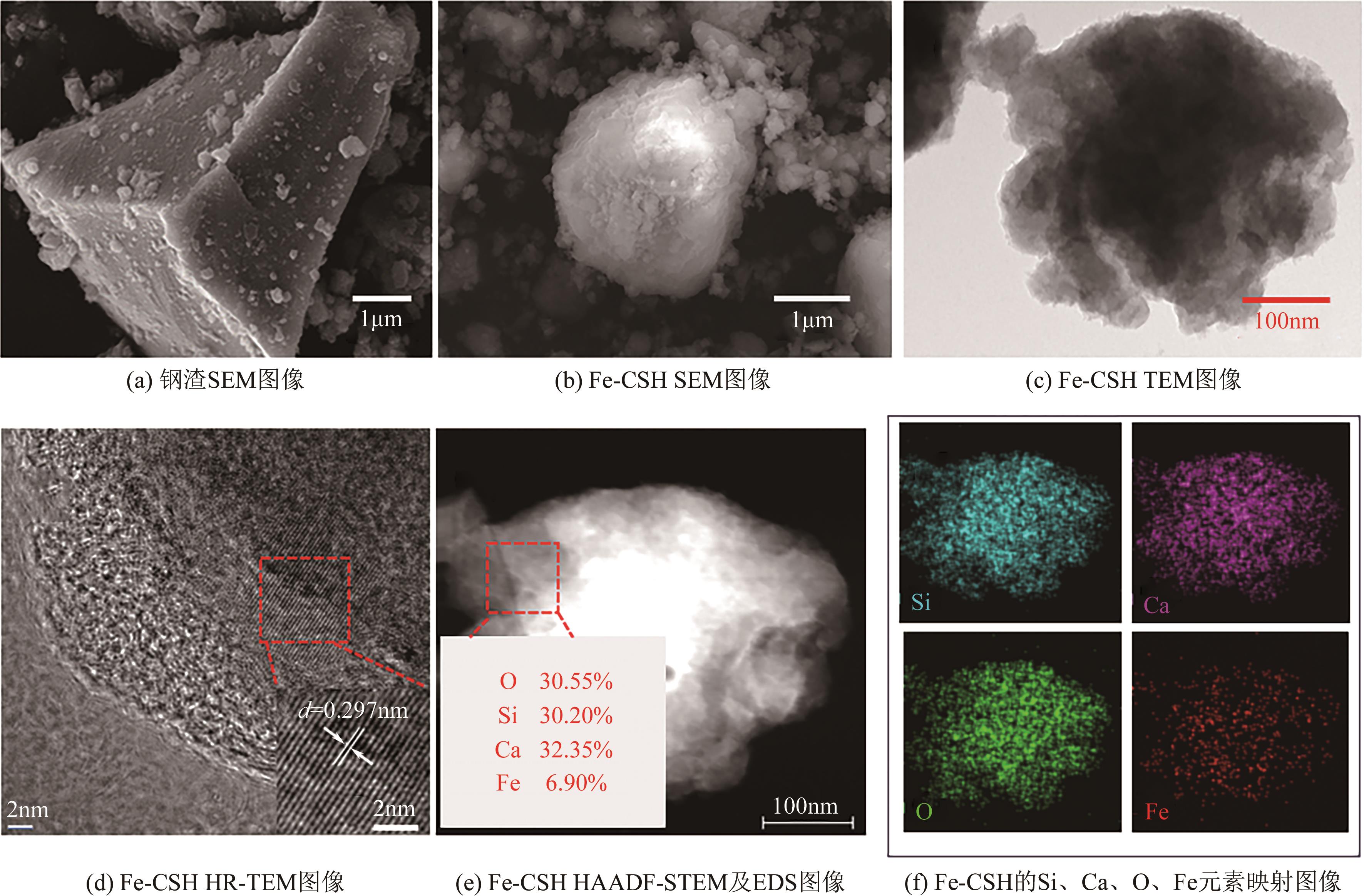
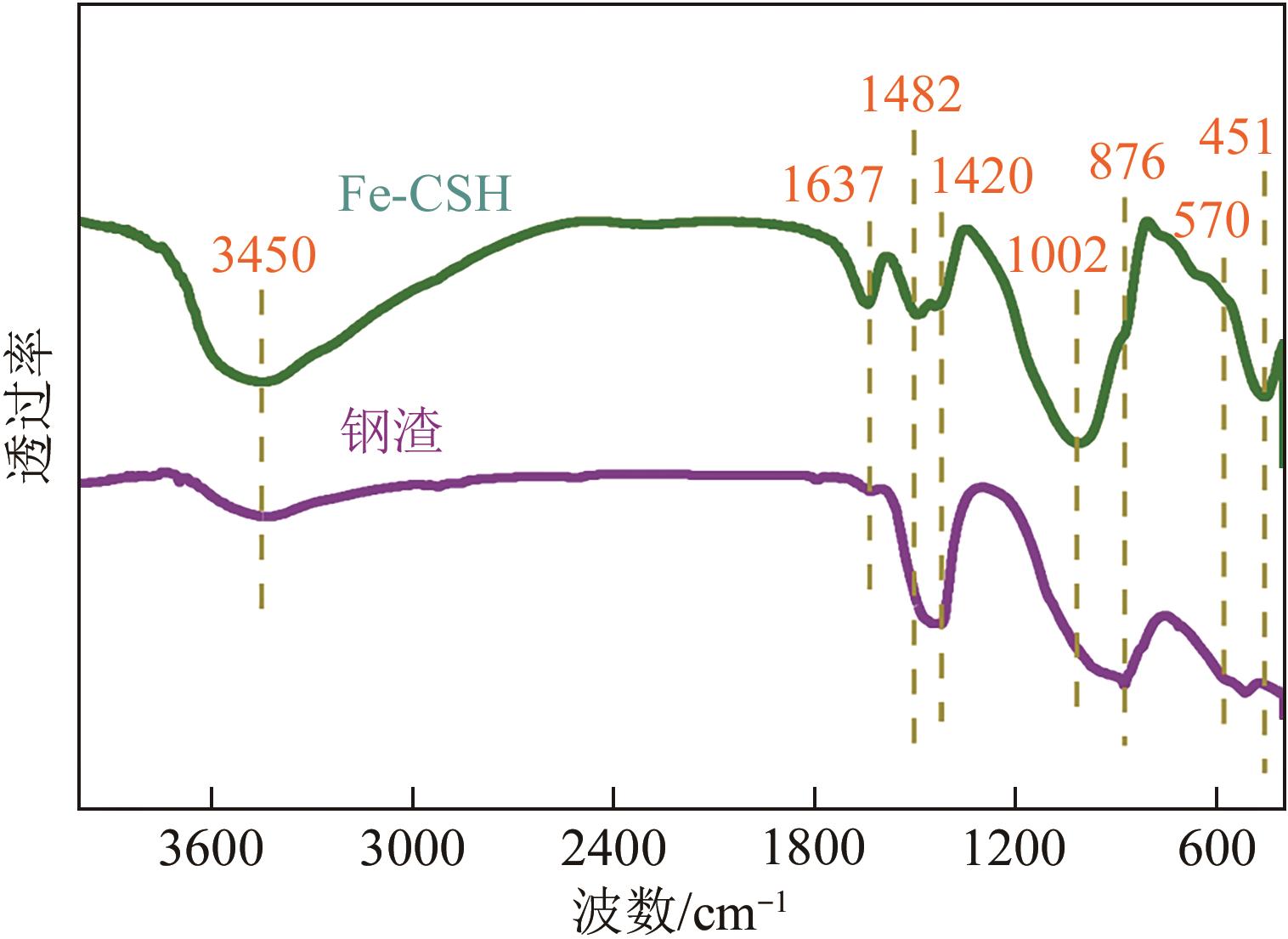
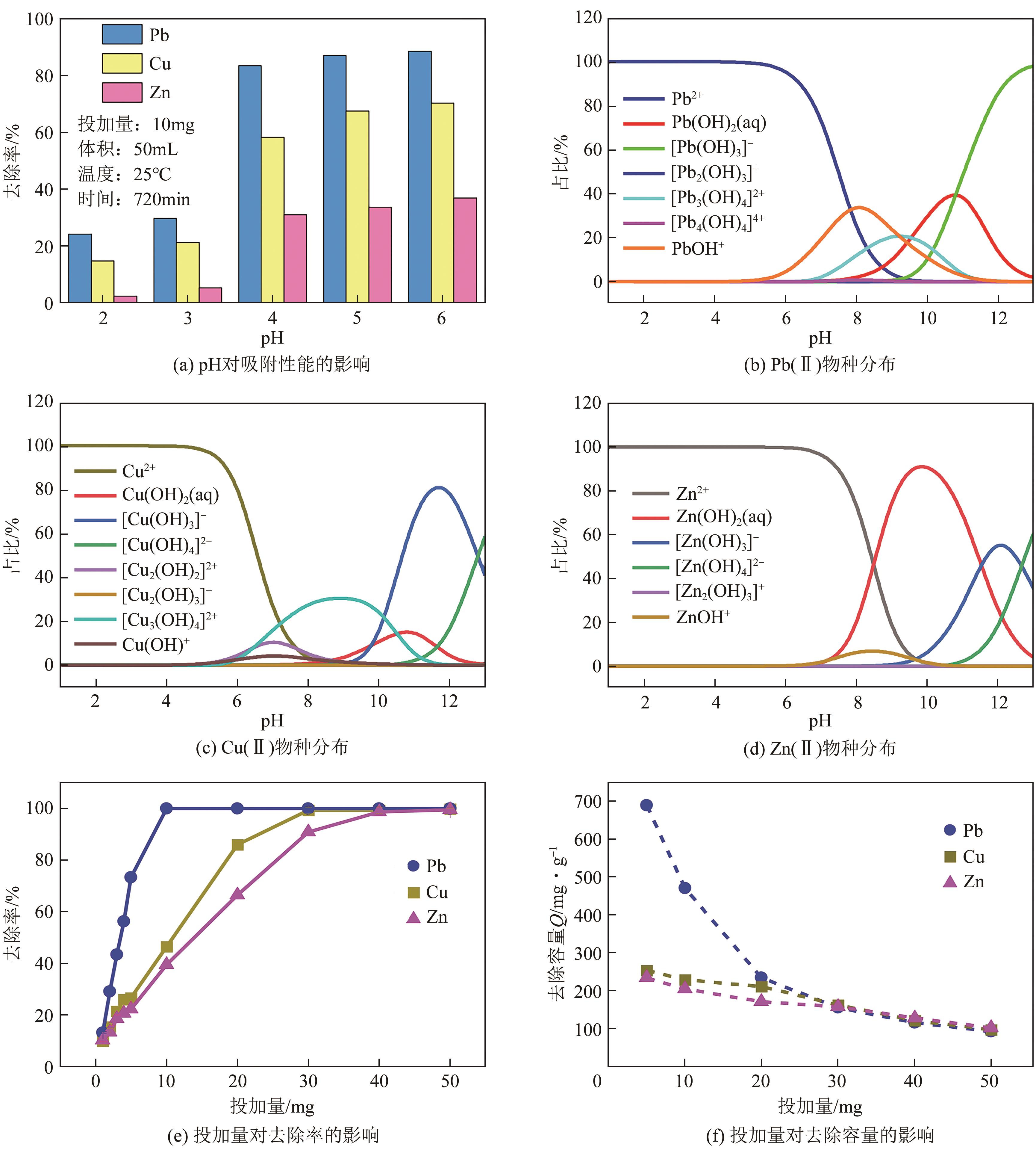
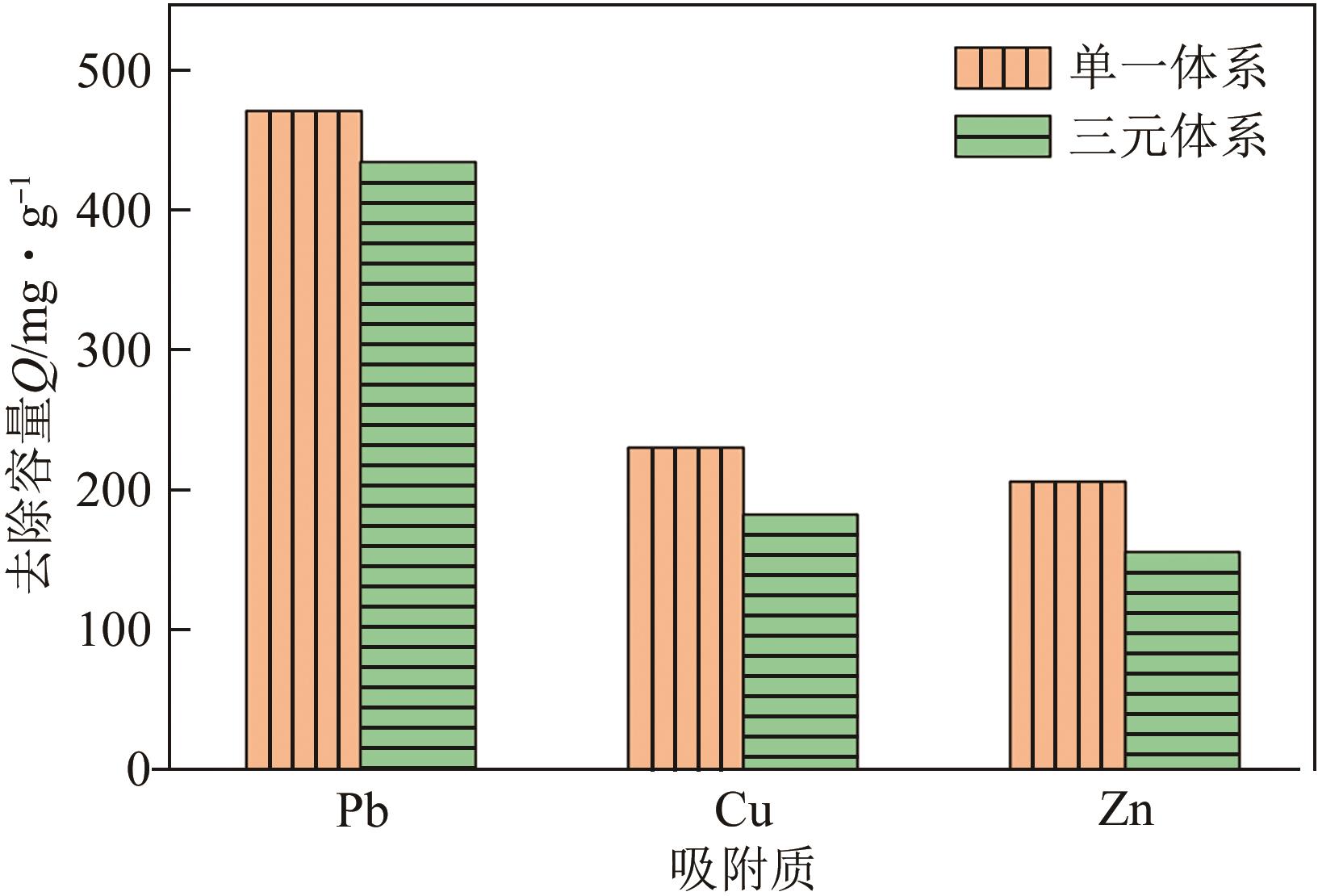
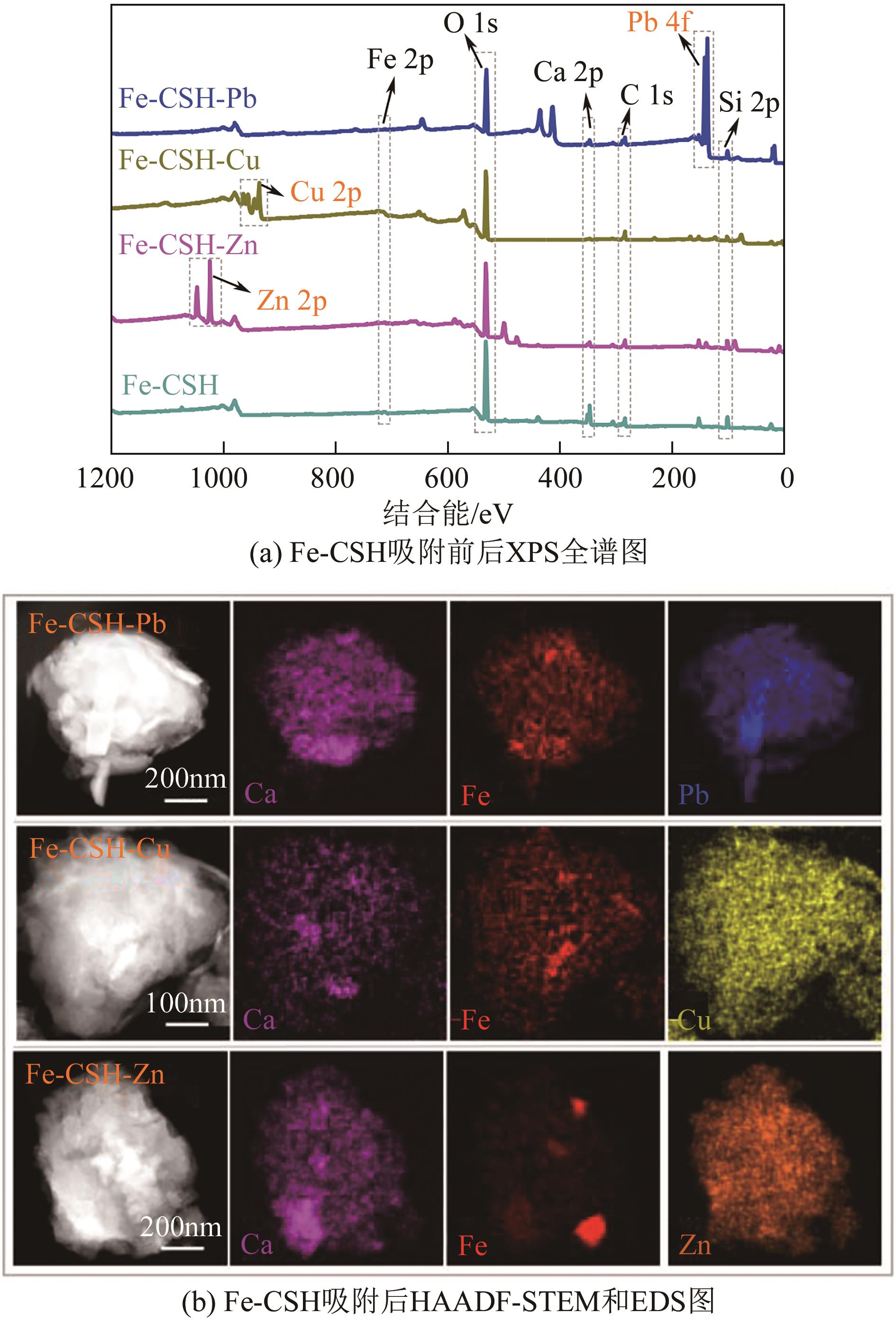
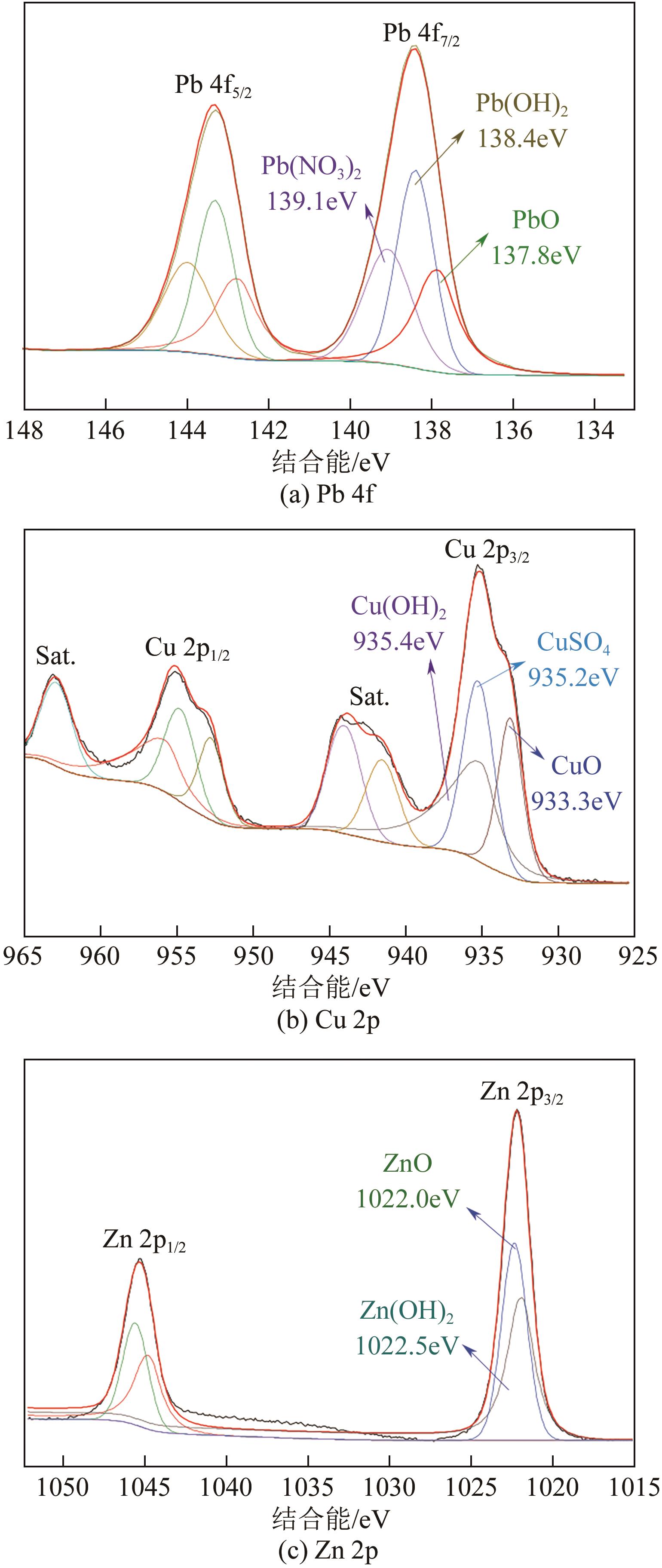
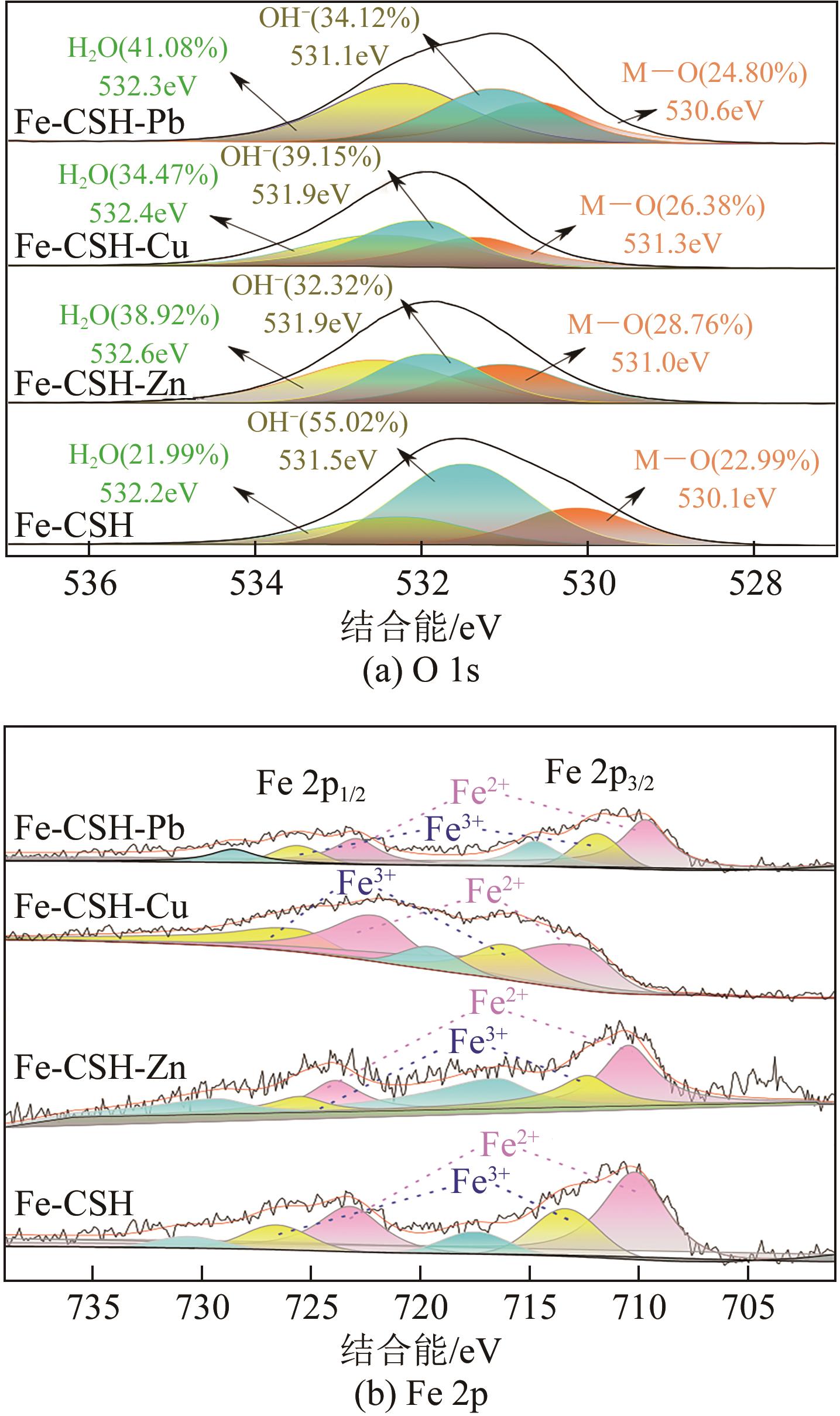
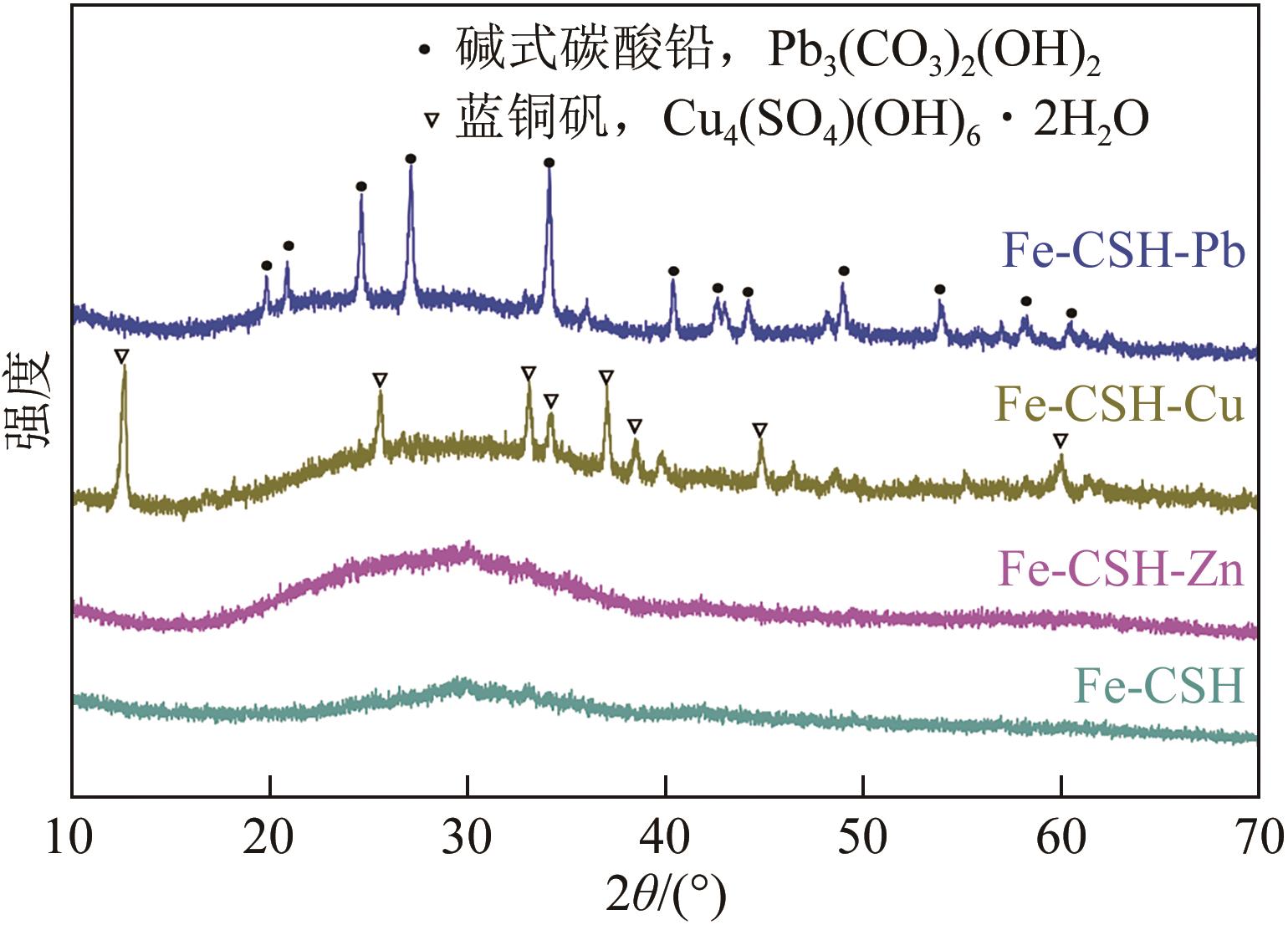
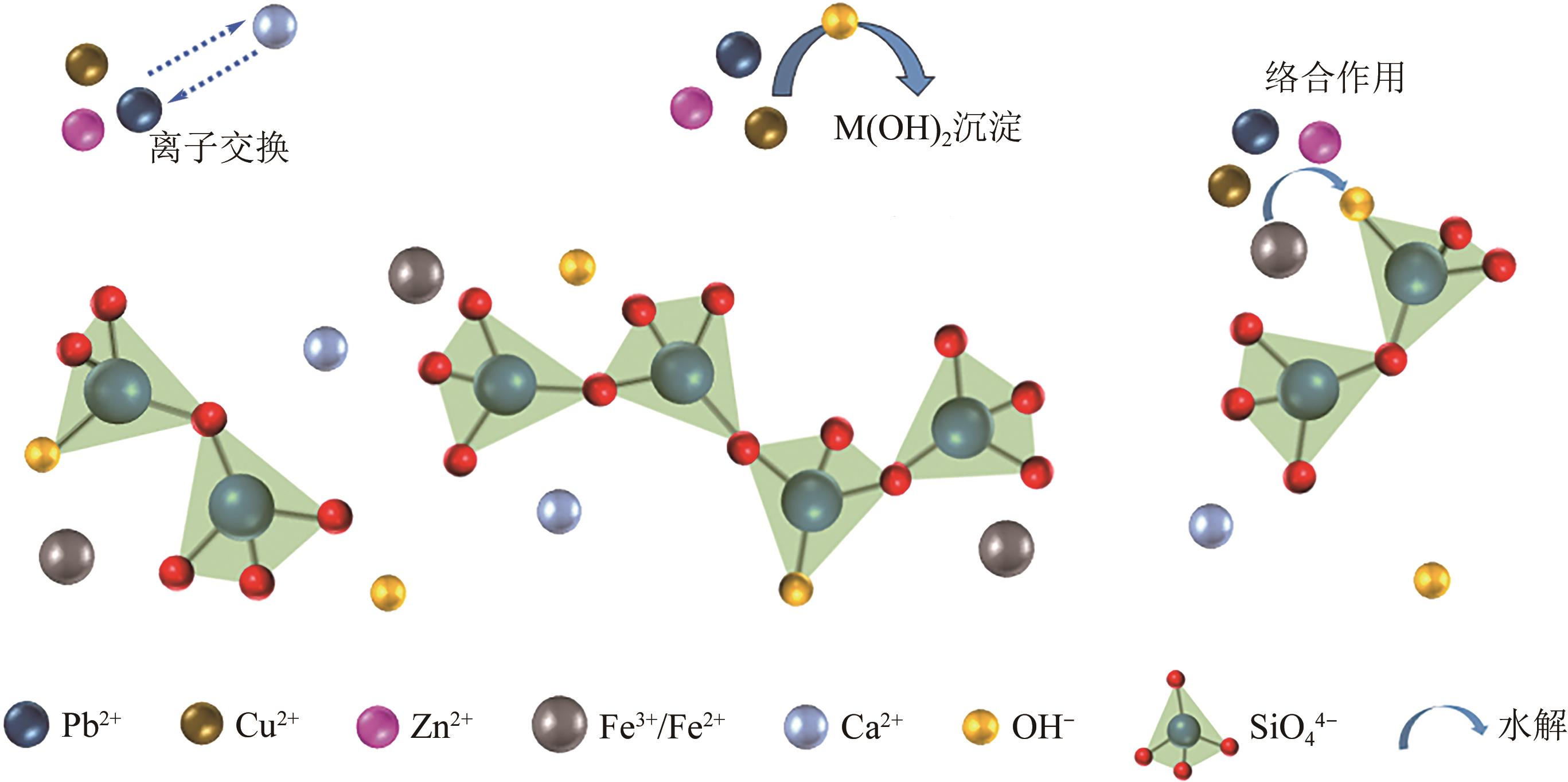
以钢渣为原位源、硅酸钠为活化剂合成多级孔结构的铁质水合硅酸钙(Fe-CSH),并将其作为吸附剂高效去除溶液中的Pb(Ⅱ)、Cu(Ⅱ)、Zn(Ⅱ)等重金属。重点探究初始溶液pH、Fe-CSH投加量和溶液初始浓度对Pb(Ⅱ)、Cu(Ⅱ)、Zn(Ⅱ)吸附性能的影响,并通过吸附动力学、热力学以及X射线衍射、傅里叶变换红外光谱、扫描电子显微镜、透射电子显微镜、比表面积分析、X射线光电子能谱等手段对其吸附机理进行解析。结果表明,Fe-CSH对Pb(Ⅱ)、Cu(Ⅱ)、Zn(Ⅱ)的吸附过程均符合拟二级动力学模型,对Pb(Ⅱ)、Cu(Ⅱ)的吸附符合Langmuir模型,而对Zn(Ⅱ)的吸附更符合Freundlich模型,去除容量分别为1546mg/g、483mg/g和369mg/g;通过物理吸附、离子交换、沉淀和配位络合等作用机制实现重金属Pb(Ⅱ)、Cu(Ⅱ)、Zn(Ⅱ)的高效去除。本研究遵循“以废治废”环保理念,对利用固体废弃物合成吸附剂的资源化利用和污水净化处理都具有重要意义。
中图分类号:
引用本文
单书月, 罗中秋, 周新涛, 尚波, 田鑫聪, 阎崔蓉. 钢渣构筑Fe-CSH吸附溶液中Pb(Ⅱ)、Cu(Ⅱ)、Zn(Ⅱ)性能及机理[J]. 化工进展, 2024, 43(10): 5867-5880.
SHAN Shuyue, LUO Zhongqiu, ZHOU Xintao, SHANG Bo, TIAN Xincong, YAN Cuirong. Adsorption performance and mechanism for Pb(Ⅱ), Cu(Ⅱ) and Zn(Ⅱ) removal from aqueous solutions by Fe-CSH derived from steel slag[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5867-5880.
| 组分 | 质量分数/% |
|---|---|
| CaO | 34.33 |
| Fe2O3 | 21.72 |
| SiO2 | 16.73 |
| MnO | 6.80 |
| MgO | 5.76 |
| Al2O3 | 4.24 |
| TiO2 | 3.26 |
| P2O5 | 2.14 |
| 其他 | 5.02 |
表1 钢渣主要化学组成
| 组分 | 质量分数/% |
|---|---|
| CaO | 34.33 |
| Fe2O3 | 21.72 |
| SiO2 | 16.73 |
| MnO | 6.80 |
| MgO | 5.76 |
| Al2O3 | 4.24 |
| TiO2 | 3.26 |
| P2O5 | 2.14 |
| 其他 | 5.02 |
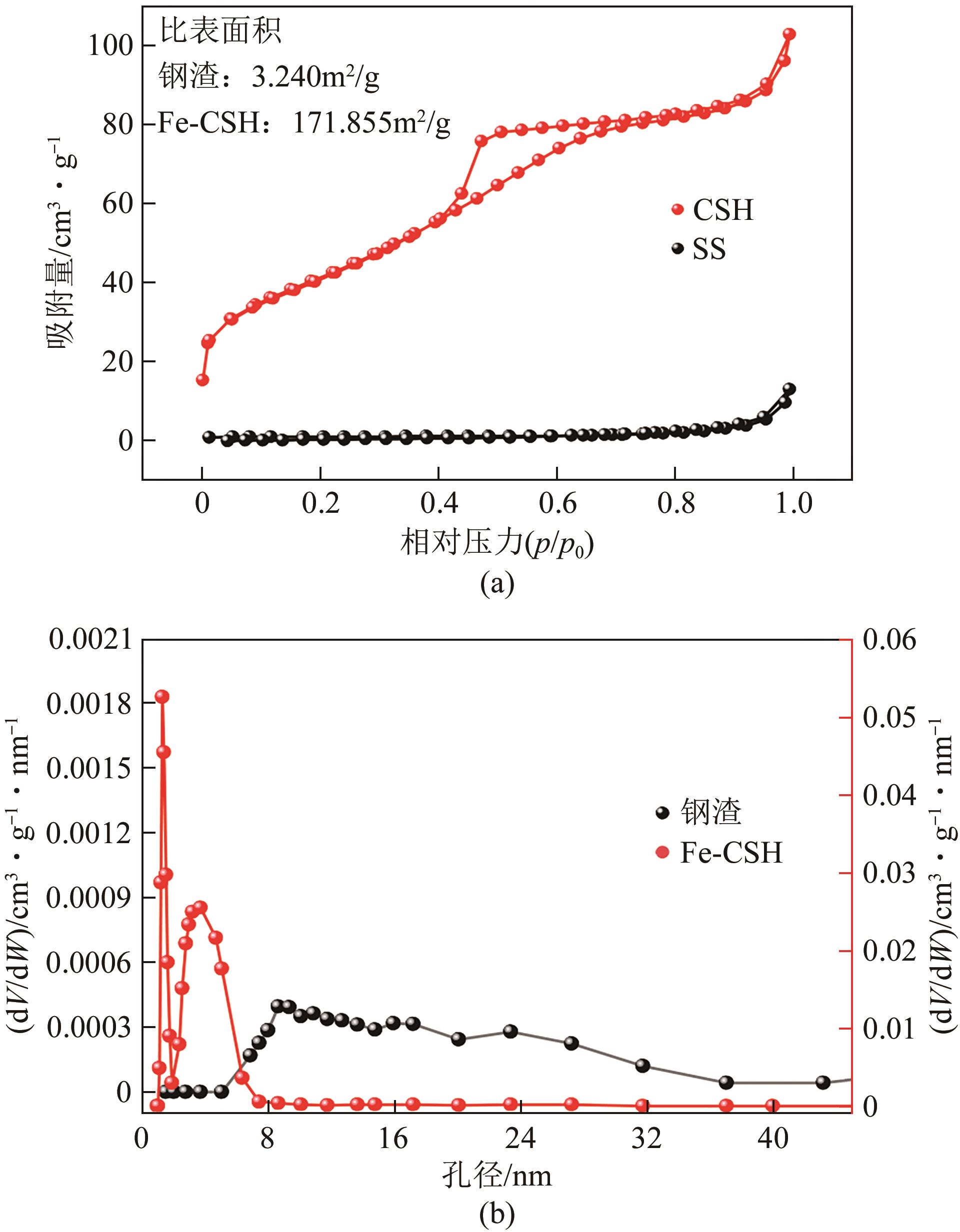
| 样本 | BET比表面积/m2·g-1 | 平均孔径/nm | 孔体积/cm3·g-1 |
|---|---|---|---|
| 钢渣 | 3.240 | 23.548 | 0.021 |
| Fe-CSH | 171.855 | 4.070 | 0.154 |
表2 钢渣处理前后BET及孔结构参数
| 样本 | BET比表面积/m2·g-1 | 平均孔径/nm | 孔体积/cm3·g-1 |
|---|---|---|---|
| 钢渣 | 3.240 | 23.548 | 0.021 |
| Fe-CSH | 171.855 | 4.070 | 0.154 |
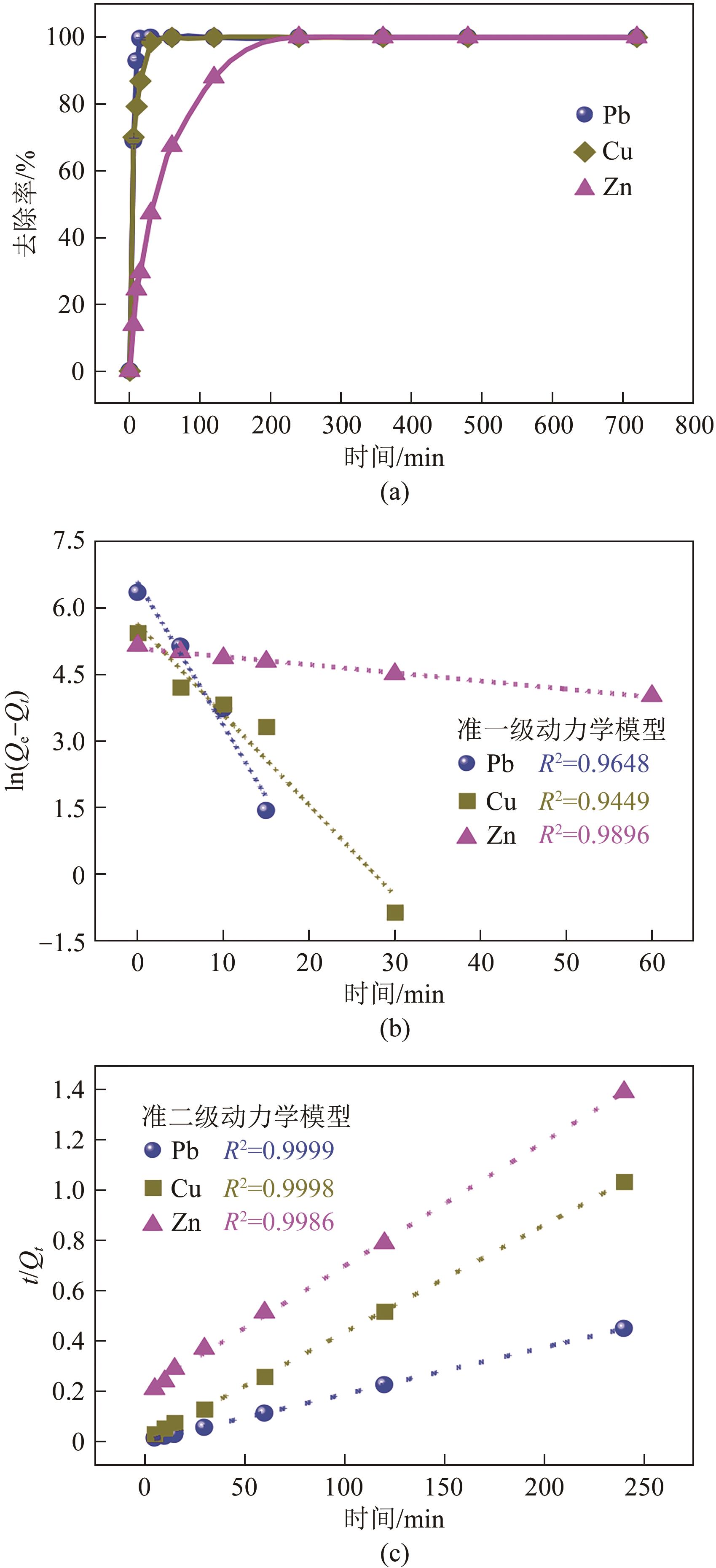
| 吸附质 | Qe, exp/mg·g-1 | 拟一级动力学模型 | 拟二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| Qe, the/mg·g-1 | K1/min-1 | R12 | Qe, the/mg·g-1 | K2/g·mg-1·min-1 | R22 | ||
| Pb(Ⅱ) | 532 | 719 | 0.322 | 0.9648 | 534 | 2.35×10-3 | 0.9999 |
| Cu(Ⅱ) | 225 | 280 | 0.203 | 0.9449 | 233 | 2.72×10-3 | 0.9998 |
| Zn(Ⅱ) | 172 | 162 | 0.018 | 0.9896 | 202 | 1.20×10-4 | 0.9986 |
表3 动力学模型拟合参数
| 吸附质 | Qe, exp/mg·g-1 | 拟一级动力学模型 | 拟二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| Qe, the/mg·g-1 | K1/min-1 | R12 | Qe, the/mg·g-1 | K2/g·mg-1·min-1 | R22 | ||
| Pb(Ⅱ) | 532 | 719 | 0.322 | 0.9648 | 534 | 2.35×10-3 | 0.9999 |
| Cu(Ⅱ) | 225 | 280 | 0.203 | 0.9449 | 233 | 2.72×10-3 | 0.9998 |
| Zn(Ⅱ) | 172 | 162 | 0.018 | 0.9896 | 202 | 1.20×10-4 | 0.9986 |
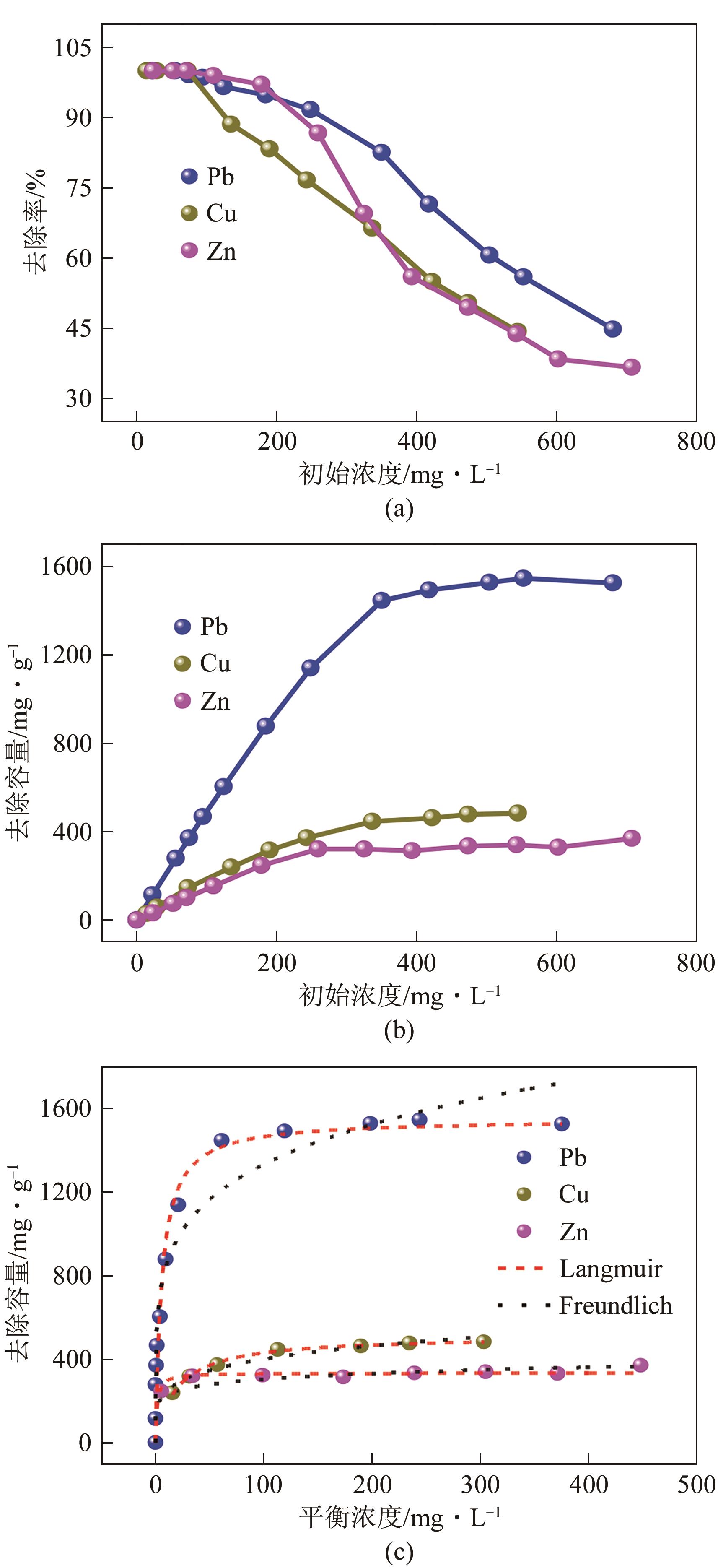
| 吸附质 | Qe, exp/mg·g-1 | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|---|
| Qmax/mg·g-1 | KL | R2 | KF | n | R2 | ||
| Pb2+ | 1546 | 1666 | 0.25 | 0.958 | 546.42 | 0.194 | 0.936 |
| Cu2+ | 483 | 509 | 0.06 | 0.994 | 152.21 | 0.211 | 0.942 |
| Zn2+ | 369 | 357 | 0.09 | 0.919 | 172.45 | 0.124 | 0.961 |
表4 等温线模型拟合参数
| 吸附质 | Qe, exp/mg·g-1 | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|---|
| Qmax/mg·g-1 | KL | R2 | KF | n | R2 | ||
| Pb2+ | 1546 | 1666 | 0.25 | 0.958 | 546.42 | 0.194 | 0.936 |
| Cu2+ | 483 | 509 | 0.06 | 0.994 | 152.21 | 0.211 | 0.942 |
| Zn2+ | 369 | 357 | 0.09 | 0.919 | 172.45 | 0.124 | 0.961 |
| 吸附剂 | 吸附量/mg·g-1 | 参考文献 | ||
|---|---|---|---|---|
| Pb | Cu | Zn | ||
| 改性磷石膏 | 2.627 | 3.937 | 3.994 | [ |
| 磁性壳聚糖 | 76.9 | 34.5 | 20.8 | [ |
| 改性橘子皮 | 209.8 | 70.73 | 56.18 | [ |
| 羟基磷灰石 | 1095 | 86.76 | 64.68 | [ |
| Fe-CSH | 1546 | 483 | 369 | 本研究 |
表5 其他吸附剂对Pb(Ⅱ)、Cu(Ⅱ)、Zn(Ⅱ)的最大吸附容量
| 吸附剂 | 吸附量/mg·g-1 | 参考文献 | ||
|---|---|---|---|---|
| Pb | Cu | Zn | ||
| 改性磷石膏 | 2.627 | 3.937 | 3.994 | [ |
| 磁性壳聚糖 | 76.9 | 34.5 | 20.8 | [ |
| 改性橘子皮 | 209.8 | 70.73 | 56.18 | [ |
| 羟基磷灰石 | 1095 | 86.76 | 64.68 | [ |
| Fe-CSH | 1546 | 483 | 369 | 本研究 |
| 27 | MIAO Fangfang, ZHANG Yimei, LI Yu, et al. Analysis of soil pollution and health risk assessment of chemical forms of heavy metal ions in a landfill site[J]. Environmental Engineering, 2022, 40(7): 94-100. |
| 28 | 靳强, 高鹏元, 陈宗元, 等. Visual MINTEQ软件在大学化学教学中的应用[J]. 大学化学, 2021, 36(12): 186-192. |
| JIN Qiang, GAO Pengyuan, CHEN Zongyuan, et al. Application of visual MINTEQ software in college chemistry teaching[J]. University Chemistry, 2021, 36(12): 186-192. | |
| 29 | 牛乙涛, 包国庆, 吴纯鑫, 等. 功能化纳米复合材料Fe3O4@SiO2-3-氨丙基三甲氧基硅烷的制备及其对Pb(Ⅱ)的吸附[J]. 复合材料学报, 2023, 40(6): 3350-3365. |
| NIU Yitao, BAO Guoqing, WU Chunxin, et al. Preparation of functionalized nanocomposites Fe3O4@SiO2-3-aminopropyltrimethoxysilane and its adsorption to Pb(Ⅱ)[J]. Acta Materiae Compositae Sinica, 2023, 40(6): 3350-3365. | |
| 30 | 姜昱聪, 夏天翔, 贾晓洋, 等. 铁铝吸附剂对起爆药污染土壤中锑的稳定化研究[J]. 中国环境科学, 2020, 40(8): 3520-3529. |
| JIANG Yucong, XIA Tianxiang, JIA Xiaoyang, et al. Study on stabilization of antimony (Sb) in contaminated soil by primary explosives using iron-based and aluminum-based adsorbents[J]. China Environmental Science, 2020, 40(8): 3520-3529. | |
| 31 | AKPOMIE Kovo Godfrey, DAWODU Folasegun Anthony. Efficient abstraction of nickel(Ⅱ) and manganese(Ⅱ) ions from solution onto an alkaline-modified montmorillonite[J]. Journal of Taibah University for Science, 2014, 8(4): 343-356. |
| 32 | 杨月红, 舒敦涛, 宁平. 微波场诱导改性磷石膏吸附Cu2+, Zn2+, Pb2+和Cd2+的动力学与热力学研究[J]. 中南大学学报(自然科学版), 2013, 44(5): 2157-2164. |
| YANG Yuehong, SHU Duntao, NING Ping. Kinetics and thermodynamics of Cu2+, Zn2+, Pb2+ and Cd2+ adsorption onto microwave-preconditioned phosphogypsum[J]. Journal of Central South University (Science and Technology), 2013, 44(5): 2157-2164. | |
| 33 | ZHU Yehua, HU Jun, WANG Jianlong. Competitive adsorption of Pb(Ⅱ), Cu(Ⅱ) and Zn(Ⅱ) onto xanthate-modified magnetic chitosan[J]. Journal of Hazardous Materials, 2012, 221/222: 155-161. |
| 34 | 冯宁川, 郭学益. 改性橘子皮对铜、铅和锌的吸附特性及吸附机制[J]. 中国有色金属学报, 2012, 22(5): 1224-1231. |
| FENG Ningchuan, GUO Xueyi. Characterization of adsorptive capacity and mechanisms on adsorption of copper, lead and zinc by modified orange peel[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(5): 1224-1231. | |
| 35 | 刘海弟, 李福志, 赵璇, 等. 工业石膏合成羟基磷灰石及其对Pb2+, Cu2+, Zn2+和Ni2+的吸附作用[J]. 过程工程学报, 2008, 8(1): 42-47. |
| LIU Haidi, LI Fuzhi, ZHAO Xuan, et al. Preparation of hydroxyapatite with industrial gypsum and its adsorption upon Pb2+, Cu2+, Zn2+ and Ni2+ [J]. The Chinese Journal of Process Engineering, 2008, 8(1): 42-47. | |
| 36 | 王哲, 黄国和, 安春江, 等. Cu2+、Cd2+、Zn2+在高炉水淬渣上的竞争吸附特性[J]. 化工进展, 2015, 34(11): 4071-4078. |
| WANG Zhe, HUANG Guohe, AN Chunjiang, et al. Competitive adsorption characteristics of water-quenched blast furnace slag(WBFS) towards Cu2+, Cd2+ and Zn2+ [J]. Chemical Industry and Engineering Progress, 2015, 34(11): 4071-4078. | |
| 37 | 刘晶晶. Pb(Ⅱ)、Cu(Ⅱ)、Cd(Ⅱ)在黄土上竞争吸附及解吸特性研究[D]. 杭州: 浙江大学, 2014. |
| LIU Jingjing. Competitive adsorption and desorption behavior of loess soil towards Pb(Ⅱ), Cu(Ⅱ), Cd(Ⅱ)[D]. Hangzhou: Zhejiang University, 2014. | |
| 38 | 王国华, 崔雅茹, 施瑞盟, 等. Fe/SiO2比对渣结构及渣-锍分离特性的影响[J]. 硅酸盐学报, 2022, 50(10): 2676-2683. |
| WANG Guohua, CUI Yaru, SHI Ruimeng, et al. Effect of Fe/SiO2 ratio on slag structure and separation characteristic of slag and low nickel matte[J]. Journal of the Chinese Ceramic Society, 2022, 50(10): 2676-2683. | |
| 39 | PONTHIEU M, JUILLOT F, HIEMSTRA T, et al. Metal ion binding to iron oxides[J]. Geochimica et Cosmochimica Acta, 2006, 70(11): 2679-2698. |
| 40 | 赵越, 郑欣, 徐畅, 等. 改性硅酸钙(CSH)对重金属废水中Ni2+的吸附特性研究[J]. 安全与环境学报, 2017, 17(5): 1904-1908. |
| ZHAO Yue, ZHENG Xin, XU Chang, et al. On the adsorptive behaviors of calcium silicate hydrate(CSH) to Ni2+ [J]. Journal of Safety and Environment, 2017, 17(5): 1904-1908. | |
| 1 | FU Fenglian, WANG Qi. Removal of heavy metal ions from wastewaters: A review[J]. Journal of Environmental Management, 2011, 92(3): 407-418. |
| 2 | CHEN Quanyuan, YAO Yuan, LI Xinying, et al. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates[J]. Journal of Water Process Engineering, 2018, 26: 289-300. |
| 3 | MA Anthony, ABUSHAIKHA Ahmad, ALLEN Stephen J, et al. Ion exchange homogeneous surface diffusion modelling by binary site resin for the removal of nickel ions from wastewater in fixed beds[J]. Chemical Engineering Journal, 2019, 358: 1-10. |
| 4 | FOSTER Nancy S, NOBLE Richard D, KOVAL Carl A. Reversible photoreductive deposition and oxidative dissolution of copper ions in titanium dioxide aqueous suspensions[J]. Environmental Science and Technology, 1993, 27(2): 350-356. |
| 5 | WANG Chengyi, CHEN Lin, LIU Shanshan. Activated carbon fiber for adsorption/electrodeposition of Cu (Ⅱ) and the recovery of Cu(0) by controlling the applied voltage during membrane capacitive deionization[J]. Journal of Colloid and Interface Science, 2019, 548: 160-169. |
| 6 | HAN Huawen, RAFIQ Muhammad Khalid, ZHOU Tuoyu, et al. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants[J]. Journal of Hazardous Materials, 2019, 369: 780-796. |
| 7 | XU Lei, LIU Yani, WANG Jingang, et al. Selective adsorption of Pb2+ and Cu2+ on amino-modified attapulgite: Kinetic, thermal dynamic and DFT studies[J]. Journal of Hazardous Materials, 2021, 404: 124140. |
| 8 | MARIANA Mariana, ABDUL KHALIL H P S, MISTAR E M, et al. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption[J]. Journal of Water Process Engineering, 2021, 43: 102221. |
| 9 | FENG Xiaofang, LONG Runxuan, WANG Lingling, et al. A review on heavy metal ions adsorption from water by layered double hydroxide and its composites[J]. Separation and Purification Technology, 2022, 284: 120099. |
| 10 | 唐朝春, 王顺藤, 黄从新, 等. 介孔金属有机框架材料吸附水中重金属离子研究进展[J]. 化工进展, 2022, 41(6): 3263-3278. |
| TANG Chaochun, WANG Shunteng, HUANG Congxin, et al. Research progress on adsorption of heavy metal ions in water by mesoporous metal organic framework materials[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3263-3278. | |
| 11 | WU Hanrong, LIN Guo, LIU Chenchen, et al. Progress and challenges in molecularly imprinted polymers for adsorption of heavy metal ions from wastewater[J]. Trends in Environmental Analytical Chemistry, 2022, 36: e00178. |
| 12 | ZHAO Jing, ZHU Yingjie, WU Jin, et al. Chitosan-coated mesoporous microspheres of calcium silicate hydrate: Environmentally friendly synthesis and application as a highly efficient adsorbent for heavy metal ions[J]. Journal of Colloid and Interface Science, 2014, 418: 208-215. |
| 13 | BRISO Alejandro, QUINTANA Geraldine, Viviana IDE, et al. Integrated use of magnetic nanostructured calcium silicate hydrate and magnetic manganese dioxide adsorbents for remediation of an acidic mine water[J]. Journal of Water Process Engineering, 2018, 25: 247-257. |
| 14 | Renata ŻAK, DEJA Jan. Spectroscopy study of Zn, Cd, Pb and Cr ions immobilization on C—S—H phase[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015, 134: 614-620. |
| 15 | ZHAO Yue, CHEN Hui, YAN Qun. Enhanced phosphate removal during the simultaneous adsorption of phosphate and Ni2+ from electroless nickel wastewater by calcium silicate hydrate (CSH)[J]. Environmental Technology and Innovation, 2017, 8: 141-149. |
| 16 | 汤亚, 孙盛睿, 樊佳, 等. 粉煤灰衍生水合硅酸钙PEI改性及吸附去除Cu(Ⅱ)与催化降解有机污染物[J]. 无机材料学报, 2023, 38(11): 1281-1291. |
| TANG Ya, SUN Shengrui, FAN Jia, et al. PEI modified hydrated calcium silicate derived from fly ash and its adsorption for removal of Cu(Ⅱ) and catalytic degradation of organic pollutants[J]. Journal of Inorganic Materials, 2023, 38(11): 1281-1291. | |
| 17 | 佟钰, 何欣然, 罗超, 等. 硅藻基水化硅酸钙的表面改性与次甲基蓝吸附性能[J]. 沈阳建筑大学学报(自然科学版), 2019, 35(4): 755-761. |
| TONG Yu, HE Xinran, LUO Chao, et al. Surface modification of diatomite-based calcium silicate hydrate for adsorption of methylene blue[J]. Journal of Shenyang Jianzhu University (Natural Science), 2019, 35(4): 755-761. | |
| 18 | SHU Kaiqian, SASAKI Keiko. Occurrence of steel converter slag and its high value-added conversion for environmental restoration in China: A review[J]. Journal of Cleaner Production, 2022, 373: 133876. |
| 19 | 杨宇翔, 刘意成, 赵敏, 等. Co2+/Dy3+掺杂纳米立方Fe3O4的热还原制备及磁靶向滞留性能[J]. 高等学校化学学报, 2017, 38(10): 1709-1718. |
| YANG Yuxiang, LIU Yicheng, ZHAO Min, et al. Thermal reduation preparation of Co2+/Dy3+ doped cubic Fe3O4 and their magnetic targeting retention[J]. Chemical Journal of Chinese Universities, 2017, 38(10): 1709-1718. | |
| 20 | 高英力, 孟浩, 万红伟, 等. 电石渣碱激发矿渣/粉煤灰胶凝材料性能及微结构[J]. 中南大学学报(自然科学版), 2023, 54(5): 1739-1747. |
| GAO Yingli, MENG Hao, WAN Hongwei, et al. Properties and microstructure of alkali-activated cementitious materials prepared with carbide slag-slag-fly ash solid waste[J]. Journal of Central South University (Science and Technology), 2023, 54(5): 1739-1747. | |
| 21 | LI Yongkui, LI Suqin, PAN Xiaodong, et al. Eco-utilization of steel slag: Preparation of Fe-based calcium silicate hydrate and its application in As(Ⅴ) removal[J]. Applied Surface Science, 2022, 597: 153763. |
| 41 | HUANG Runlin, LIN Qintie, ZHONG Quanfa, et al. Removal of Cd(Ⅱ) and Pb(Ⅱ) from aqueous solution by modified attapulgite clay[J]. Arabian Journal of Chemistry, 2020, 13(4): 4994-5008. |
| 22 | 李秋芸, 陈雅鹏, 陈林楠, 等. 磁性硅酸钙重金属离子吸附剂用于光催化还原CO2 [J]. 硅酸盐学报, 2021, 49(10): 2045-2052. |
| LI Qiuyun, CHEN Yapeng, CHEN Linnan, et al. Magnetic calcium silicate heavy metal ion adsorbents for photocatalytic CO2 reduction[J]. Journal of the Chinese Ceramic Society, 2021, 49(10): 2045-2052. | |
| 23 | SHAO Ningning, TANG Siqi, LIU Ze, et al. Hierarchically structured calcium silicate hydrate-based nanocomposites derived from steel slag for highly efficient heavy metal removal from wastewater[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 14926-14935. |
| 24 | 戈明亮, 王雁武, 陈萌. 麦羟硅钠石对水中Zn2+的吸附性能研究[J]. 中国环境科学, 2015, 35(7): 2065-2071. |
| GE Mingliang, WANG Yanwu, CHEN Meng. Adsorption characteristics of Zn2+ onto magadiite[J]. China Environmental Science, 2015, 35(7): 2065-2071. | |
| 25 | 陶欢. 固废基水化硅酸钙的制备及其去除重金属性能研究[D]. 徐州:中国矿业大学, 2021. |
| TAO Huan. Preparation of solid-waste-based calcium silicate hydrate and its removal performance of heavy metals[D]. Xuzhou: China University of Mining and Technology, 2021. | |
| 26 | 王龙, 马杰, 邓迎璇, 等. 金属离子在铁(氢)氧化物与腐殖质微界面上的吸附机理和模型研究进展[J]. 农业资源与环境学报, 2017, 34(5): 405-413. |
| WANG Long, MA Jie, DENG Yingxuan, et al. Micro-interfacial mechanism and model of metal ions adsorption on the iron(hydr) oxides and humic substances: A review[J]. Journal of Agricultural Resources and Environment, 2017, 34(5): 405-413. | |
| 27 | 苗芳芳, 张一梅, 李鱼, 等. 废弃物填埋场土壤污染分析及重金属离子化学形态健康风险评估[J]. 环境工程, 2022, 40(7): 94-100. |
| [1] | 吴宇琦, 李江涛, 丁建智, 宋秀兰, 苏冰琴. 焙烧镁铝水滑石脱除厌氧消化沼气中CO2的效果及机制[J]. 化工进展, 2024, 43(9): 5250-5261. |
| [2] | 全翠, 高宁博, 张广涛, 索浩杰. 含油污泥热解残渣制备渗水砖的重金属和多环芳烃浸出特性[J]. 化工进展, 2024, 43(9): 5226-5233. |
| [3] | 孔祥蕊, 董玥岑, 张蒙雨, 王彪, 尹水娥, 陈冰, 陆家纬, 张媛, 冯乐乐, 王洪涛, 徐海云. 生活垃圾焚烧飞灰处理技术研究进展[J]. 化工进展, 2024, 43(7): 4102-4117. |
| [4] | 路广军, 韩晋钢, 陈英, 马志斌. 镁渣基多孔材料的制备及其对废水中Pb2+的吸附性能[J]. 化工进展, 2024, 43(4): 2126-2134. |
| [5] | 巩志强, 刘雷, 王少华, 韩悦, 郭俊山, 商攀峰, 祝令凯, 郑威. 矿物质化合物对含油污泥焚烧过程中重金属迁移转化的影响[J]. 化工进展, 2024, 43(3): 1614-1620. |
| [6] | 郑钰, 李靖杰, 张宇峰, 赵梦琦, 张娜, 周澳, 于伟, 谭厚章, 王学斌. 典型炉排炉和流化床垃圾焚烧飞灰及螯合产物的重金属浸出毒性[J]. 化工进展, 2024, 43(3): 1630-1636. |
| [7] | 彭程, 徐漪琳, 石钰婧, 张玟, 李宇涛, 王皓冉, 张卫, 占绣萍. 生物炭改性及其对除草剂污染水体和土壤修复的研究进展[J]. 化工进展, 2024, 43(2): 1069-1081. |
| [8] | 任鹏锟, 仲兆平, 杨宇轩, 张杉, 杜浩然, 李骞. 改性海泡石对污泥热解过程中重金属的控制[J]. 化工进展, 2024, 43(1): 541-550. |
| [9] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [10] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [11] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [12] | 李卫华, 于倩雯, 尹俊权, 吴寅凯, 孙英杰, 王琰, 王华伟, 杨玉飞, 龙於洋, 黄启飞, 葛燕辰, 何依洋, 赵灵燕. 酸雨环境下填埋飞灰吨袋破损后重金属的溶出行为[J]. 化工进展, 2023, 42(9): 4917-4928. |
| [13] | 张杉, 仲兆平, 杨宇轩, 杜浩然, 李骞. 磷酸盐改性高岭土对生活垃圾热解过程中重金属的富集[J]. 化工进展, 2023, 42(7): 3893-3903. |
| [14] | 李若琳, 何少林, 苑宏英, 刘伯约, 纪冬丽, 宋阳, 刘博, 余绩庆, 徐英俊. 原位热解对油页岩物性及地下水水质影响探索[J]. 化工进展, 2023, 42(6): 3309-3318. |
| [15] | 庄捷, 薛锦辉, 赵斌成, 张文艺. 猪粪厌氧消化进程中重金属与腐殖质的有机结合机制[J]. 化工进展, 2023, 42(6): 3281-3291. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||