化工进展 ›› 2023, Vol. 42 ›› Issue (4): 1934-1943.DOI: 10.16085/j.issn.1000-6613.2022-1111
镁锂分离复合纳滤膜研究进展
叶海星1( ), 陈宇昊1, 陈仪2, 孙海翔1(
), 陈宇昊1, 陈仪2, 孙海翔1( ), 牛青山2(
), 牛青山2( )
)
- 1.中国石油大学(华东)材料科学与工程学院,山东 青岛 266580
2.深圳大学高等研究院,广东 深圳 518061
-
收稿日期:2022-06-14修回日期:2022-07-22出版日期:2023-04-25发布日期:2023-05-08 -
通讯作者:孙海翔,牛青山 -
作者简介:叶海星(1998—),男,硕士研究生,研究方向为高分子膜材料。E-mail:z20140094@s.upc.edu.cn。 -
基金资助:国家自然科学基金联合基金(U2006230);国家自然科学基金(U1862120);山东省自然科学基金(ZR2019MB012);深圳市科创委基础研究面上项目(JCYJ20210324095202008)
Research progress of composite nanofiltration membrane for magnesium and lithium separation
YE Haixing1( ), CHEN Yuhao1, CHEN Yi2, SUN Haixiang1(
), CHEN Yuhao1, CHEN Yi2, SUN Haixiang1( ), NIU Qingshan2(
), NIU Qingshan2( )
)
- 1.School of Materials Science and Engineering, China University of Petroleum (East China), Qingdao 266580, Shandong, China
2.Institute for Advanced Study, Shenzhen University, Shenzhen 518061, Guangdong, China
-
Received:2022-06-14Revised:2022-07-22Online:2023-04-25Published:2023-05-08 -
Contact:SUN Haixiang, NIU Qingshan
摘要:
随着新能源等行业的快速发展,锂的需求量急剧增加。纳滤膜分离作为一种具有能耗低、成本低、绿色环保等优点的盐湖提锂方法,受到越来越广泛的关注。该方法能够减小卤水中的镁锂比,降低后续提锂难度,在盐湖提锂行业拥有巨大的潜力。但由于发展较晚,理论体系尚不完善,推动工业应用的进程缓慢。本文从分离机理入手,分析了近年来用于提升镁锂分离纳滤膜性能的改性方法,包括表面接枝改性、两相溶液添加剂、新单体设计、基膜改性和界面聚合(IP)工艺优化五种方法。同时,阐述了各种改性方法对纳滤膜结构和性质的影响。分析表明:表面改性的方法可以改变膜表面电荷但无法精确调控膜的孔径;添加剂可以减小水传输阻力但膜的耐受性有待考察;单体设计和基膜改性能够有效地提升选择性和渗透性;工艺优化较为复杂,进行工业放大有一定难度。总的来说,纳滤膜在盐湖提锂行业的发展十分具有前景,配合其他提锂工艺,以期实现绿色提锂、高效提锂。
中图分类号:
引用本文
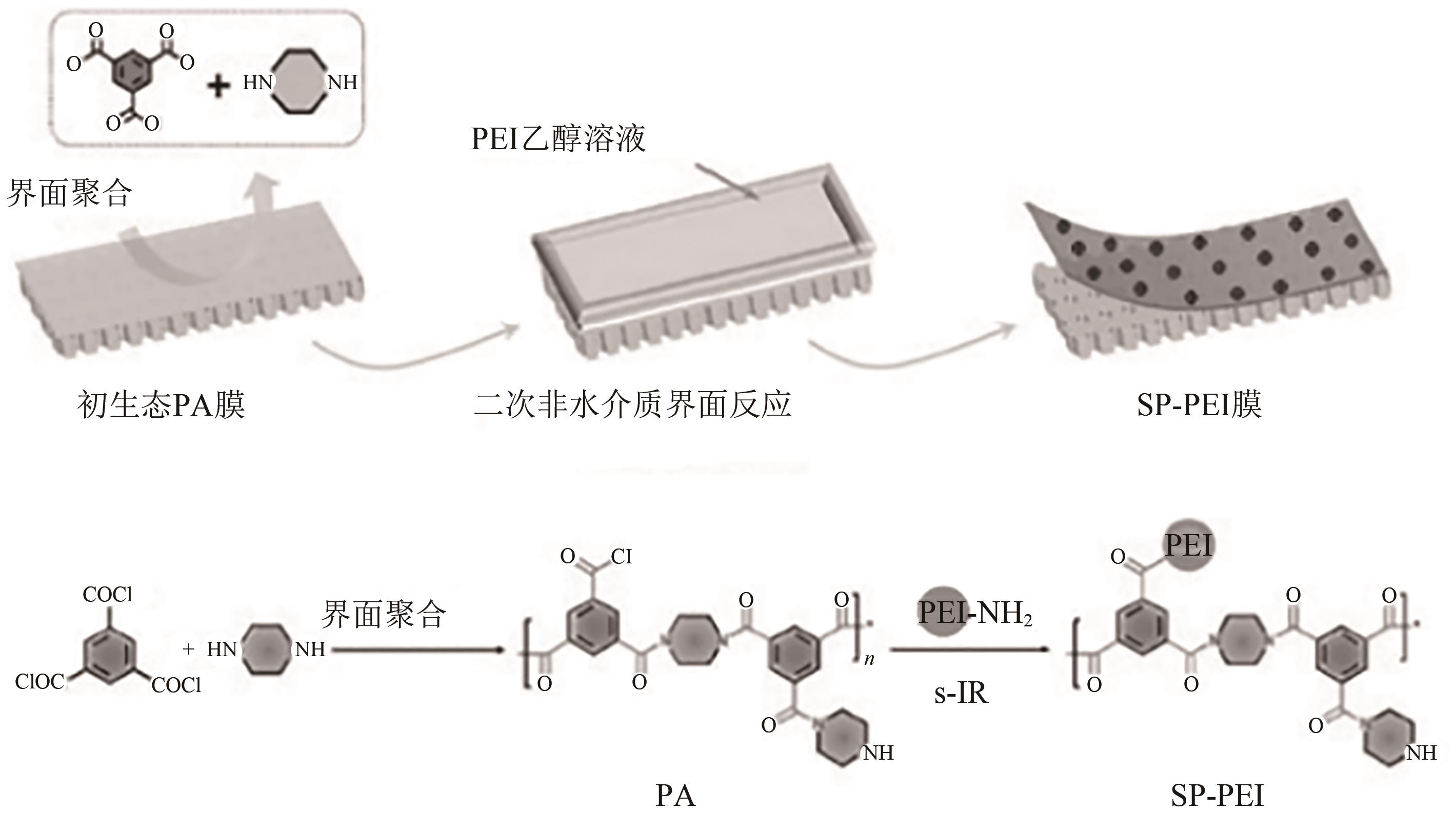
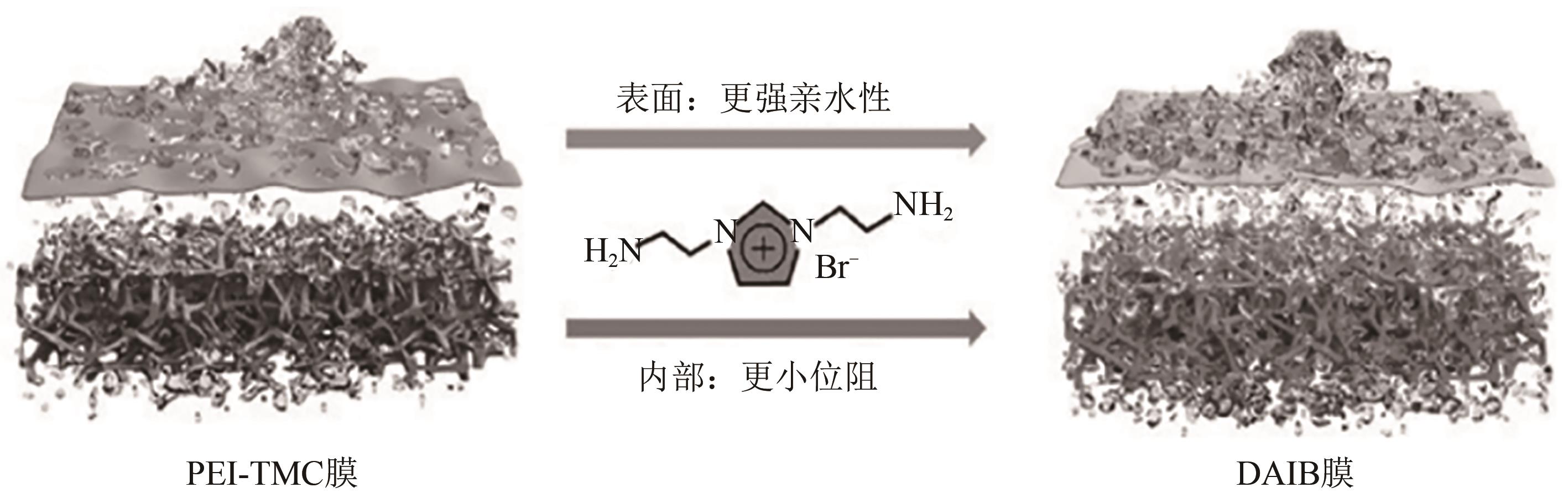
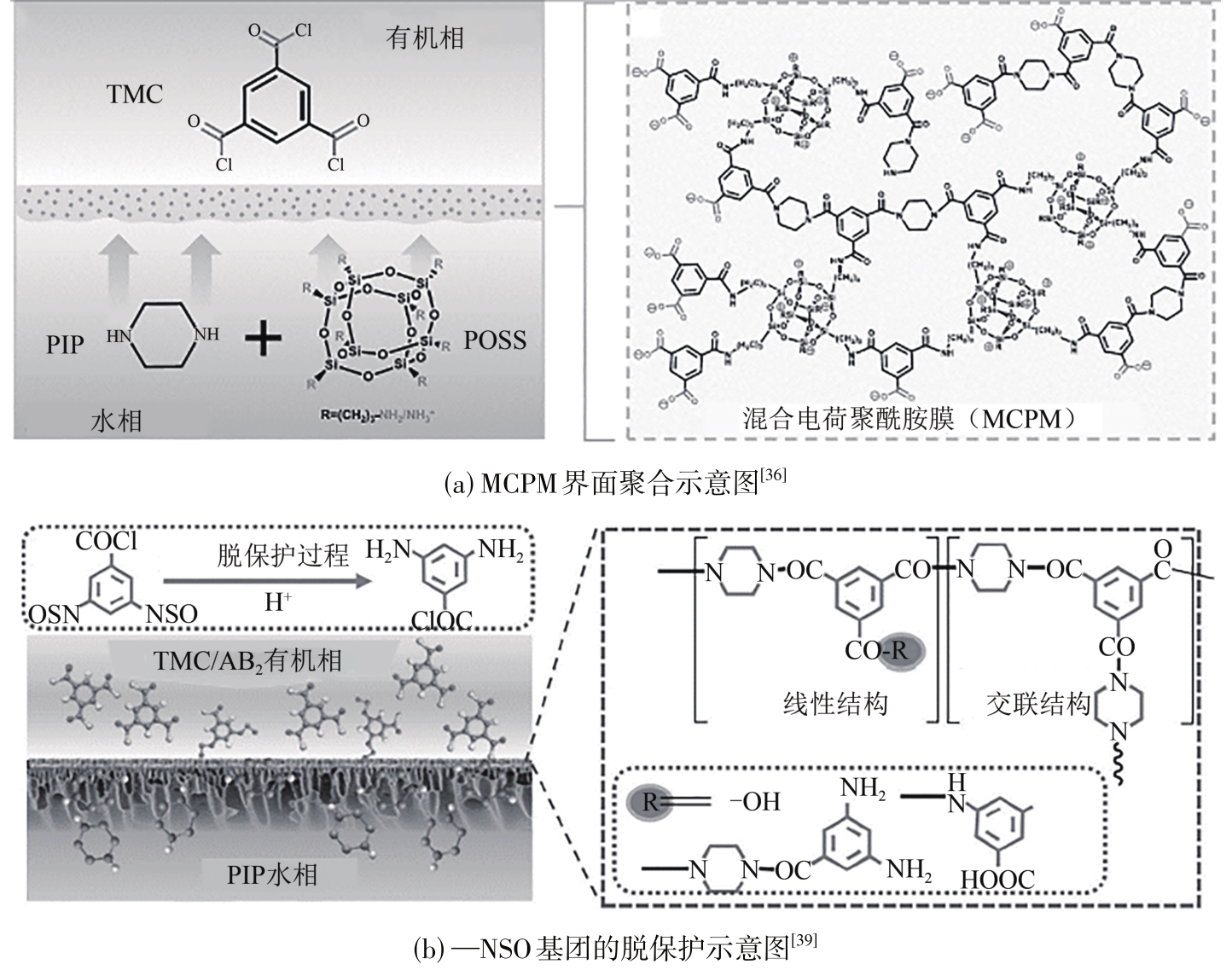
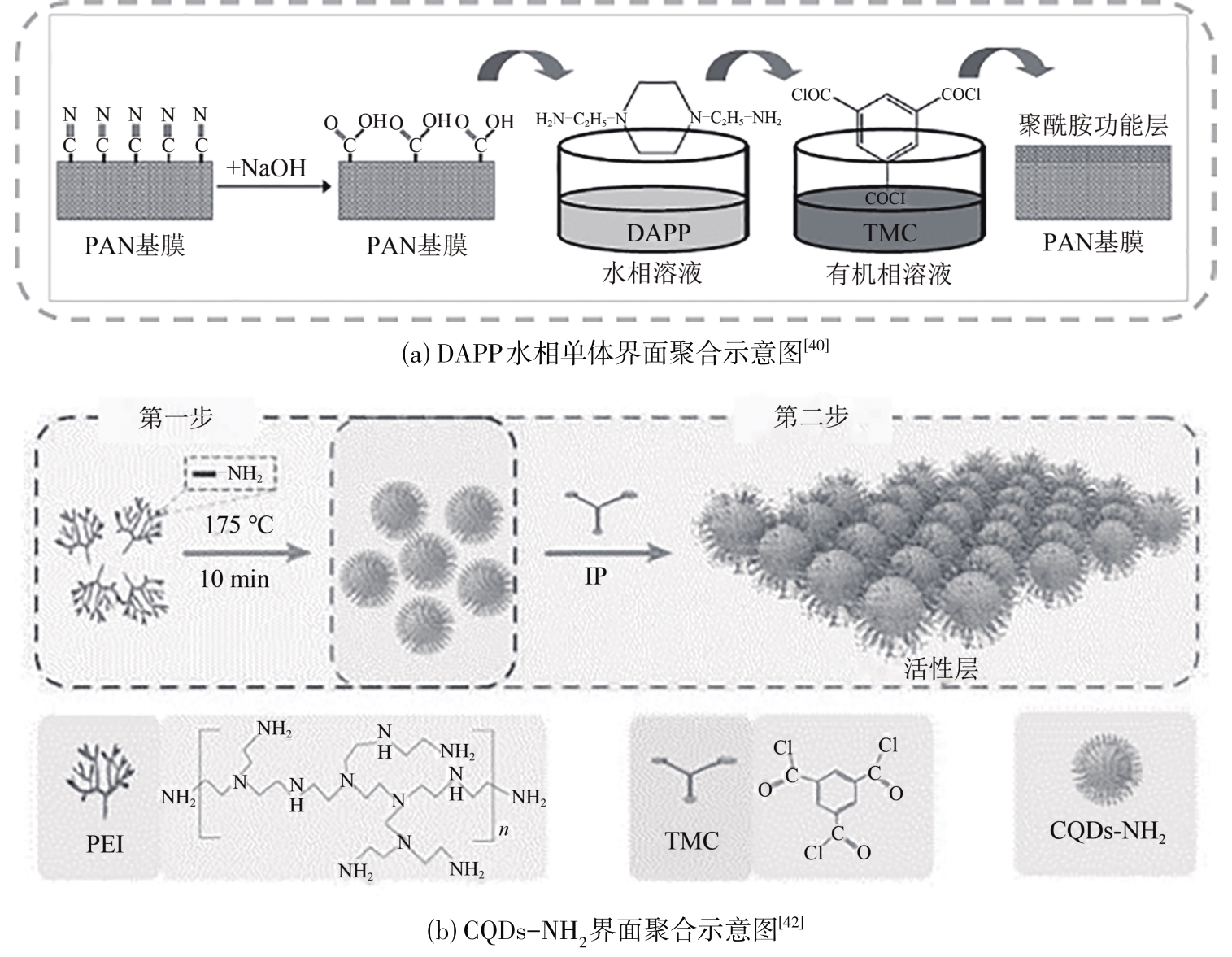
叶海星, 陈宇昊, 陈仪, 孙海翔, 牛青山. 镁锂分离复合纳滤膜研究进展[J]. 化工进展, 2023, 42(4): 1934-1943.
YE Haixing, CHEN Yuhao, CHEN Yi, SUN Haixiang, NIU Qingshan. Research progress of composite nanofiltration membrane for magnesium and lithium separation[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1934-1943.
| 改性方法 | 膜材料 | 盐截留/% | 渗透性 /L∙m-2∙h-1∙MPa-1 | 镁锂分离性能 | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|
| MgCl2 | LiCl | 溶质浓度 /mg∙L-1 | 原料液 (Mg2+/Li+) | 分离系数 | ||||
| 表面改性 | PSF/PIP-TMC-PEI | — | — | — | 2000 | 150 | 12.37 | [ |
PES/SWCNT/ PIP-TMC-PEI | 98.5 | 46.2 | 120.0 | 2000 | 21.3 | 33.4 | [ | |
| PES/PIP-TMC-DETA | 94.1 | 36.7 | 170.0 | 2000 | — | — | [ | |
| PSF/DDA&PIP -TMC-(CMPI)-PEI | 97.10 | 32.0 | — | 1000 | — | — | [ | |
| NF270/PDA-PEI | 86.7 | 5.3 | 66.3 | — | 30 | 7.15 | [ | |
| DL/PDA-PEI | 81.7 | 7.1 | 42.2 | — | 30 | 5.08 | [ | |
| DK/PDA-PEI | 98.6 | 16.0 | 33.7 | — | 30 | 59.54 | [ | |
| Ultem-TMC-BPEI-EDTA | 84.6 | 68.1 | 6.0 | 10000 | 24 | 9.2 | [ | |
| PAN/PIP-TMC-[MimAP][Tf2N] | 83.8 | 24.4 | 63.0 | 2100 | 20 | 8.12 | [ | |
| PSF/PEI-TMC-HMTAB | 93.3 | — | 163.2 | 2000 | 50 | 10.1 | [ | |
| PSF/PEI-TMC-DAIB | 95.8 | — | — | 10500 | 20 | 10 | [ | |
| PSF/PEI-TMC-QEDTP | 95.8 | 55.0 | 211.5 | 2000 | 120 | 15.6 | [ | |
| PSF/PEI-TMC-QPBD | 92.0 | — | 136.0 | 2000 | 50 | 5.88 | [ | |
| 添加剂 | PES/PIP@MWCNTs&PEI-TMC | 96.9 | 20.3 | 140.0 | 2000 | 21.4 | 16.5 | [ |
| PES/γ-CD&PEI-TMC | — | — | 48.6 | 2000 | 30 | 10.8 | [ | |
| PES/PHF&PIP-TMC | 89.9 | 16.3 | 67.0 | 2000 | 21.4 | 13.1 | [ | |
| PES/GQDs-NH3&PEI-TMC | — | — | 119.4 | 2000 | 20 | 21.9 | [ | |
| PAN/UiO-66-NH2&PEI-TMC | — | — | 306.0 | 2000 | 20 | 36.9 | [ | |
| PAN/POSS-NH2&PIP-TMC | 98.0 | — | — | 1000 | 1.56 | 43.9 | [ | |
| PES/PDA-C3N4&DAPP-TMC | 95.7 | 36.8 | — | 2000 | — | — | [ | |
| PES/BHC-CN&BAPP-TMC | 97.4 | — | — | 2000 | 73 | 23.9 | [ | |
| PES/PIP-TMC&AB2 | 99.1 | 35.2 | 127.3 | 2000 | 21.4 | 35.7 | [ | |
| 单体设计 | PAN/DAPP-TMC | 70.4 | 21.8 | — | 2000 | 20 | 2.6 | [ |
| PES/PEI-TMC | 94.8 | 30.6 | 50.2 | 2000 | 20 | 20 | [ | |
| PES/ GQDs-NH2-TMC | 94.7 | 22.9 | 119.8 | 2000 | 30 | 14.4 | [ | |
| 基膜改性 | PES&GO/PEI-TMC | — | — | 111.5 | 2000 | 20 | 16.13 | [ |
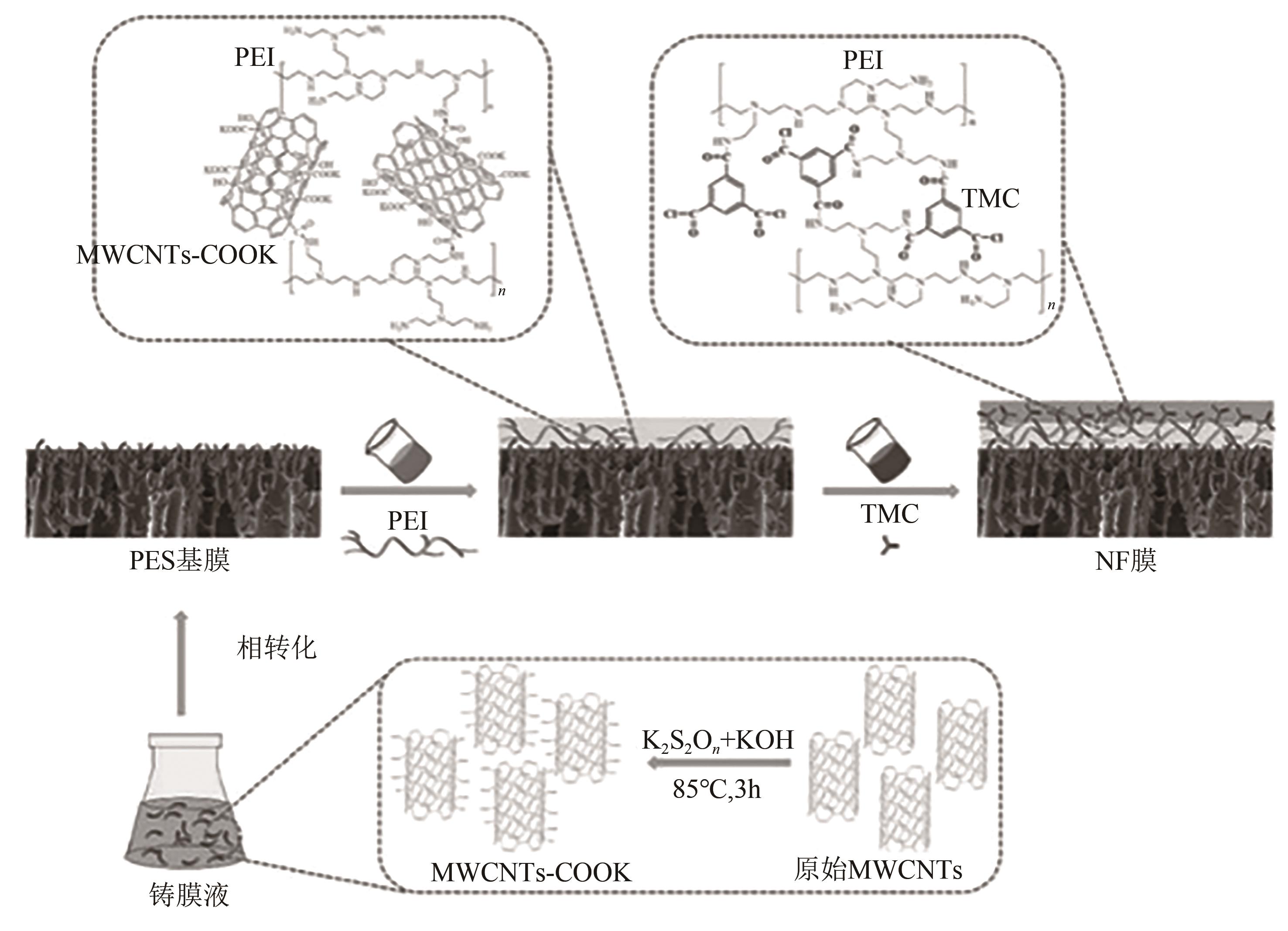
| PES&MWCNTs-COOK/PEI-TMC | — | — | 114.6 | 2000 | 20 | 58.66 | [ | |
| PES/CNC-COOH/PEI-TMC | — | — | 41.7 | 2000 | 30 | 12.15 | [ | |
| PES/CNC-COOH/PEI-TMC | — | — | 34.0 | 2000 | 60 | 5.84 | [ | |
| PSF/UIO-66-NH2/PIP-TMC | 97.9 | -66.7 | — | 10500 | 30.6 | 78.6 | [ | |
| 工艺优化 | PES/EDA-TMC(GLIP) | 98.3 | — | 43.0 | 2100 | 20 | 28 | [ |
| PES/Gu-MPD | 91.6 | 32.3 | 162.0 | 2000 | 23 | 8.0 | [ | |
表1 已报道镁锂分离复合纳滤膜性能对比
| 改性方法 | 膜材料 | 盐截留/% | 渗透性 /L∙m-2∙h-1∙MPa-1 | 镁锂分离性能 | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|
| MgCl2 | LiCl | 溶质浓度 /mg∙L-1 | 原料液 (Mg2+/Li+) | 分离系数 | ||||
| 表面改性 | PSF/PIP-TMC-PEI | — | — | — | 2000 | 150 | 12.37 | [ |
PES/SWCNT/ PIP-TMC-PEI | 98.5 | 46.2 | 120.0 | 2000 | 21.3 | 33.4 | [ | |
| PES/PIP-TMC-DETA | 94.1 | 36.7 | 170.0 | 2000 | — | — | [ | |
| PSF/DDA&PIP -TMC-(CMPI)-PEI | 97.10 | 32.0 | — | 1000 | — | — | [ | |
| NF270/PDA-PEI | 86.7 | 5.3 | 66.3 | — | 30 | 7.15 | [ | |
| DL/PDA-PEI | 81.7 | 7.1 | 42.2 | — | 30 | 5.08 | [ | |
| DK/PDA-PEI | 98.6 | 16.0 | 33.7 | — | 30 | 59.54 | [ | |
| Ultem-TMC-BPEI-EDTA | 84.6 | 68.1 | 6.0 | 10000 | 24 | 9.2 | [ | |
| PAN/PIP-TMC-[MimAP][Tf2N] | 83.8 | 24.4 | 63.0 | 2100 | 20 | 8.12 | [ | |
| PSF/PEI-TMC-HMTAB | 93.3 | — | 163.2 | 2000 | 50 | 10.1 | [ | |
| PSF/PEI-TMC-DAIB | 95.8 | — | — | 10500 | 20 | 10 | [ | |
| PSF/PEI-TMC-QEDTP | 95.8 | 55.0 | 211.5 | 2000 | 120 | 15.6 | [ | |
| PSF/PEI-TMC-QPBD | 92.0 | — | 136.0 | 2000 | 50 | 5.88 | [ | |
| 添加剂 | PES/PIP@MWCNTs&PEI-TMC | 96.9 | 20.3 | 140.0 | 2000 | 21.4 | 16.5 | [ |
| PES/γ-CD&PEI-TMC | — | — | 48.6 | 2000 | 30 | 10.8 | [ | |
| PES/PHF&PIP-TMC | 89.9 | 16.3 | 67.0 | 2000 | 21.4 | 13.1 | [ | |
| PES/GQDs-NH3&PEI-TMC | — | — | 119.4 | 2000 | 20 | 21.9 | [ | |
| PAN/UiO-66-NH2&PEI-TMC | — | — | 306.0 | 2000 | 20 | 36.9 | [ | |
| PAN/POSS-NH2&PIP-TMC | 98.0 | — | — | 1000 | 1.56 | 43.9 | [ | |
| PES/PDA-C3N4&DAPP-TMC | 95.7 | 36.8 | — | 2000 | — | — | [ | |
| PES/BHC-CN&BAPP-TMC | 97.4 | — | — | 2000 | 73 | 23.9 | [ | |
| PES/PIP-TMC&AB2 | 99.1 | 35.2 | 127.3 | 2000 | 21.4 | 35.7 | [ | |
| 单体设计 | PAN/DAPP-TMC | 70.4 | 21.8 | — | 2000 | 20 | 2.6 | [ |
| PES/PEI-TMC | 94.8 | 30.6 | 50.2 | 2000 | 20 | 20 | [ | |
| PES/ GQDs-NH2-TMC | 94.7 | 22.9 | 119.8 | 2000 | 30 | 14.4 | [ | |
| 基膜改性 | PES&GO/PEI-TMC | — | — | 111.5 | 2000 | 20 | 16.13 | [ |
| PES&MWCNTs-COOK/PEI-TMC | — | — | 114.6 | 2000 | 20 | 58.66 | [ | |
| PES/CNC-COOH/PEI-TMC | — | — | 41.7 | 2000 | 30 | 12.15 | [ | |
| PES/CNC-COOH/PEI-TMC | — | — | 34.0 | 2000 | 60 | 5.84 | [ | |
| PSF/UIO-66-NH2/PIP-TMC | 97.9 | -66.7 | — | 10500 | 30.6 | 78.6 | [ | |
| 工艺优化 | PES/EDA-TMC(GLIP) | 98.3 | — | 43.0 | 2100 | 20 | 28 | [ |
| PES/Gu-MPD | 91.6 | 32.3 | 162.0 | 2000 | 23 | 8.0 | [ | |
| 1 | SWAIN Basudev. Recovery and recycling of lithium: A review[J]. Separation and Purification Technology, 2017, 172: 388-403. |
| 2 | 王琪, 赵有璟, 刘洋, 等. 高镁锂比盐湖镁锂分离与锂提取技术研究进展[J]. 化工学报, 2021, 72(6): 2905-2921. |
| WANG Qi, ZHAO Youjing, LIU Yang, et al. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine with high magnesium/lithium ratio[J]. CIESC Journal, 2021, 72(6): 2905-2921. | |
| 3 | 蒋晨啸, 陈秉伦, 张东钰, 等. 我国盐湖锂资源分离提取进展[J]. 化工学报, 2022, 73(2): 481-503. |
| JIANG Chenxiao, CHEN Binglun, ZHANG Dongyu, et al. Progress in isolating lithium resources from China salt lake brine[J]. CIESC Journal, 2022, 73(2): 481-503. | |
| 4 | Geological Survey U.S.. Mineral commodity summaries 2022[R]. Virginia, U.S. Geological Survey, 2022. |
| 5 | 伍倩, 刘喜方, 郑绵平, 等. 我国盐湖锂资源开发现状、存在问题及对策[J]. 现代化工, 2017, 37(5): 1-5. |
| WU Qian, LIU Xifang, ZHENG Mianping, et al. Present situation, existing problems and countermeasures of development of salt lake lithium resources in China[J]. Modern Chemical Industry, 2017, 37(5): 1-5. | |
| 6 | PAUL M, JONS S D. Chemistry and fabrication of polymeric nanofiltration membranes: A review[J]. Polymer, 2016, 103: 417-456. |
| 7 | ZHOU Yong, YU Sanchuan, GAO Congjie, et al. Surface modification of thin film composite polyamide membranes by electrostatic self deposition of polycations for improved fouling resistance[J]. Separation and Purification Technology, 2009, 66(2): 287-294. |
| 8 | BOWEN W R, WELFOOT J S. Modelling of membrane nanofiltration—Pore size distribution effects[J]. Chemical Engineering Science, 2002, 57(8): 1393-1407. |
| 9 | XU Fang, DAI Liheng, WU Yulin, et al. Li+/Mg2+ separation by membrane separation: The role of the compensatory effect[J]. Journal of Membrane Science, 2021, 636: 119542. |
| 10 | 李志录, 王敏, 赵有璟, 等. 膜特征对锂资源提取过程的影响[J]. 化工进展, 2021, 40(9): 5061-5072. |
| LI Zhilu, WANG Min, ZHAO Youjing, et al. Effects of membrane characteristics for lithium extraction[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5061-5072. | |
| 11 | RICHARDS L A, SCHÄFER A I, RICHARDS B S, et al. The importance of dehydration in determining ion transport in narrow pores[J]. Small, 2012, 8(11): 1701-1709. |
| 12 | SAHU Subin, DI VENTRA Massimiliano, ZWOLAK Michael. Dehydration as a universal mechanism for ion selectivity in graphene and other atomically thin pores[J]. Nano Letters, 2017, 17(8): 4719-4724. |
| 13 | TANSEL Berrin. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects[J]. Separation and Purification Technology, 2012, 86: 119-126. |
| 14 | YAROSHCHUK A E. Rejection mechanisms of NF membranes[J]. Membrane Technology, 1998, 1998(100): 9-12. |
| 15 | WADEKAR S S, VIDIC R D. Influence of active layer on separation potentials of nanofiltration membranes for inorganic ions[J]. Environmental Science & Technology, 2017, 51(10): 5658-5665. |
| 16 | DONNAN F G. Theory of membrane equilibria and membrane potentials in the presence of non-dialysing electrolytes. A contribution to physical-chemical physiology[J]. Journal of Membrane Science, 1995, 100(1): 45-55. |
| 17 | GONG Lingyan, OUYANG Wei, LI Zirui, et al. Direct numerical simulation of continuous lithium extraction from high Mg2+/Li+ ratio brines using microfluidic channels with ion concentration polarization[J]. Journal of Membrane Science, 2018, 556: 34-41. |
| 18 | YAO Yuxing, CHEN Xiang, YAN Chong, et al. Regulating interfacial chemistry in lithium-ion batteries by a weakly solvating electrolyte[J]. Angewandte Chemie, 2021, 133: 4136-4143. |
| 19 | 杨哲, 戴若彬, 文越, 等. 新型纳滤膜在水处理与水回用中的研究进展[J]. 环境工程, 2021, 39(7): 1-12. |
| YANG Zhe, DAI Ruobin, WEN Yue, et al. Recent progress of nanofiltration membrane in water treatment and water reuse[J]. Environmental Engineering, 2021, 39(7): 1-12. | |
| 20 | LU Dan, MA Tao, LIN Saisai, et al. Constructing a selective blocked-nanolayer on nanofiltration membrane via surface-charge inversion for promoting Li+ permselectivity over Mg2+ [J]. Journal of Membrane Science, 2021, 635: 119504. |
| 21 | YANG Zhao, FANG Wangxi, WANG Zhenyi, et al. Dual-skin layer nanofiltration membranes for highly selective Li+/Mg2+ separation[J]. Journal of Membrane Science, 2021, 620: 118862. |
| 22 | HUANG Benqing, TANG Yongjian, GAO Anran, et al. Dually charged polyamide nanofiltration membranes fabricated by microwave-assisted grafting for heavy metals removal[J]. Journal of Membrane Science, 2021, 640: 119834. |
| 23 | QI Yawei, ZHU Lifang, SHEN Xin, et al. Polythyleneimine-modified original positive charged nanofiltration membrane: Removal of heavy metal ions and dyes[J]. Separation and Purification Technology, 2019, 222: 117-124. |
| 24 | ASHRAF M A, WANG J F, WU B H, et al. Enhancement in Li+/Mg2+ separation from salt lake brine with PDA-PEI composite nanofiltration membrane[J]. Journal of Applied Polymer Science, 2020, 137(47): 49549. |
| 25 | LI Wei, SHI Changkun, ZHOU Ayang, et al. A positively charged composite nanofiltration membrane modified by EDTA for LiCl/MgCl2 separation[J]. Separation and Purification Technology, 2017, 186: 233-242. |
| 26 | WU Huanhuan, LIN Yakai, FENG Wenyan, et al. A novel nanofiltration membrane with[MimAP][Tf2N]ionic liquid for utilization of lithium from brines with high Mg2+/Li+ ratio[J]. Journal of Membrane Science, 2020, 603: 117997. |
| 27 | LUO Hao, PENG Huawen, ZHAO Qiang. High flux Mg2+/Li+ nanofiltration membranes prepared by surface modification of polyethylenimine thin film composite membranes[J]. Applied Surface Science, 2022, 579: 152161. |
| 28 | PENG Huawen, ZHAO Qiang. A nano-heterogeneous membrane for efficient separation of lithium from high magnesium/lithium ratio brine[J]. Advanced Functional Materials, 2021, 31(14): 2009430. |
| 29 | XU Yang, PENG Huawen, LUO Hao, et al. High performance Mg2+/Li+ separation membranes modified by a bis-quaternary ammonium salt[J]. Desalination, 2022, 526: 115519. |
| 30 | FENG Yuxi, PENG Huawen, ZHAO Qiang. Fabrication of high performance Mg2+/Li+ nanofiltration membranes by surface grafting of quaternized bipyridine[J]. Separation and Purification Technology, 2022, 280: 119848. |
| 31 | ZHANG Haizhen, XU Zhenliang, DING Hao, et al. Positively charged capillary nanofiltration membrane with high rejection for Mg2+ and Ca2+ and good separation for Mg2+ and Li+ [J]. Desalination, 2017, 420: 158-166. |
| 32 | ZHAO Ying, LI Nan, SHI Jie, et al. Extra-thin composite nanofiltration membranes tuned by γ-cyclodextrins containing amphipathic cavities for efficient separation of magnesium/lithium ions[J]. Separation and Purification Technology, 2022, 286: 120419. |
| 33 | SHEN Qian, XU Sunjie, XU Zhenliang, et al. Novel thin-film nanocomposite membrane with water-soluble polyhydroxylated fullerene for the separation of Mg2+/Li+ aqueous solution[J]. Journal of Applied Polymer Science, 2019, 136(41): 48029. |
| 34 | XU Ping, HONG Jun, XU Zhenzhen, et al. Novel aminated graphene quantum dots (GQDs-NH2)-engineered nanofiltration membrane with high Mg2+/Li+ separation efficiency[J]. Separation and Purification Technology, 2021, 258: 118042. |
| 35 | AGHILI F, GHOREYSHI A A, BRUGGEN B, et al. A highly permeable UiO-66-NH2/polyethyleneimine thin-film nanocomposite membrane for recovery of valuable metal ions from brackish water[J]. Process Safety and Environmental Protection, 2021, 151: 244-256. |
| 36 | ZHAO Junhui, YOU Xinda, WANG Guangzhe, et al. Mix-charged polyamide membranes via molecular hybridization for selective ionic nanofiltration[J]. Journal of Membrane Science, 2022, 644: 120051. |
| 37 | BI Qiuyan, ZHANG Chao, LIU Jiandong, et al. A nanofiltration membrane prepared by PDA-C3N4 for removal of divalent ions[J]. Water Science and Technology, 2020, 81(2): 253-264. |
| 38 | BI Qiuyan, ZHANG Chao, LIU Jiandong, et al. Positively charged zwitterion-carbon nitride functionalized nanofiltration membranes with excellent separation performance of Mg2+/Li+ and good antifouling properties[J]. Separation and Purification Technology, 2021, 257: 117959. |
| 39 | HU P, YUAN B B, NIU Q J, et al. Modification of polyamide nanofiltration membrane with ultra-high multivalent cations rejections and mono-/divalent cation selectivity[J]. Desalination, 2022, 527: 115553. |
| 40 | LI Xianhui, ZHANG Chunjin, ZHANG Shuning, et al. Preparation and characterization of positively charged polyamide composite nanofiltration hollow fiber membrane for lithium and magnesium separation[J]. Desalination, 2015, 369: 26-36. |
| 41 | XU Ping, WANG Wei, QIAN Xiaoming, et al. Positive charged PEI-TMC composite nanofiltration membrane for separation of Li+ and Mg2+ from brine with high Mg2+/Li+ ratio[J]. Desalination, 2019, 449: 57-68. |
| 42 | GUO Changsheng, QIAN Xiaoming, TIAN Feng, et al. Amino-rich carbon quantum dots ultrathin nanofiltration membranes by double “one-step” methods: Breaking through trade-off among separation, permeation and stability[J]. Chemical Engineering Journal, 2021, 404: 127144. |
| 43 | XU Ping, HONG Jun, QIAN Xiaoming, et al. “Bridge” graphene oxide modified positive charged nanofiltration thin membrane with high efficiency for Mg2+/Li+ separation[J]. Desalination, 2020, 488: 114522. |
| 44 | XU Ping, HONG Jun, XU Zhenzhen, et al. Positively charged nanofiltration membrane based on (MWCNTs-COOK)-engineered substrate for fast and efficient lithium extraction[J]. Separation and Purification Technology, 2021, 270: 118796. |
| 45 | GUO Changsheng, LI Nan, QIAN Xiaoming, et al. Ultra-thin double Janus nanofiltration membrane for separation of Li+ and Mg2+: “Drag” effect from carboxyl-containing negative interlayer[J]. Separation and Purification Technology, 2020, 230: 115567. |
| 46 | YUAN Bingbing, WANG Ning, ZHAO Siheng, et al. Polyamide nanofiltration membrane fine-tuned via mixed matrix ultrafiltration support to maximize the sieving selectivity of Li+/Mg2+ and Cl–/SO4 2– [J]. Desalination, 2022, 538: 115929. |
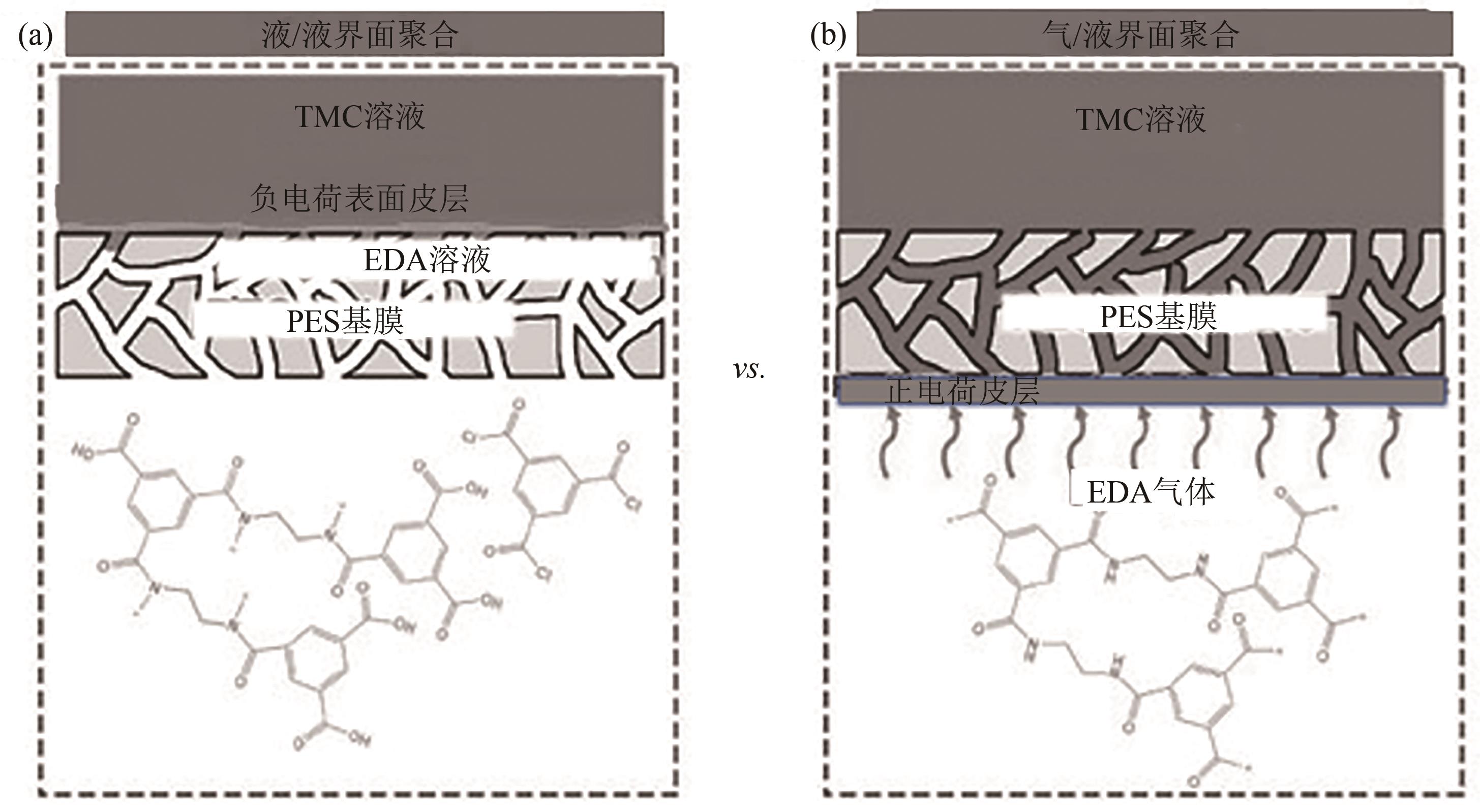
| 47 | WU Mingbang, YE Hao, ZHU Zhiyuan, et al. Positively-charged nanofiltration membranes constructed via gas/liquid interfacial polymerization for Mg2+/Li+ separation[J]. Journal of Membrane Science, 2022, 644: 119942. |
| 48 | XU Ping, HONG Jun, XU Zhenzhen, et al. Novel aminated graphene quantum dots (GQDs-NH2)-engineered nanofiltration membrane with high Mg2+/Li+ separation efficiency[J]. Separation and Purification Technology, 2021, 258: 118042. |
| 49 | 曹阳, 任玉灵, 郭世伟, 等. 聚酰胺薄层复合膜的界面聚合制备过程调控研究进展[J]. 化工进展, 2020, 39(6): 2125-2134. |
| CAO Yang, REN Yuling, GUO Shiwei, et al. Research progress on process optimization for preparation of polyamide thin-film composite membrane by interfacial polymerization[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2125-2134. | |
| 50 | WANG Li, REHMAN Danyal, SUN Pengfei, et al. Novel positively charged metal-coordinated nanofiltration membrane for lithium recovery[J]. ACS Applied Materials & Interfaces, 2021, 13(14): 16906-16915. |
| 51 | ZHANG Liufu, ZHANG Ruolin, JI Miaozhou, et al. Polyamide nanofiltration membrane with high mono/divalent salt selectivity via pre-diffusion interfacial polymerization[J]. Journal of Membrane Science, 2021, 636: 119478. |
| [1] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [2] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [3] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [4] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [5] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [6] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [7] | 王报英, 王皝莹, 闫军营, 汪耀明, 徐铜文. 聚合物包覆膜在金属分离回收中的研究进展[J]. 化工进展, 2023, 42(8): 3990-4004. |
| [8] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [9] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [10] | 王晓晗, 周亚松, 于志庆, 魏强, 孙劲晓, 姜鹏. 不同晶粒尺寸Y分子筛的合成及其加氢裂化反应性能[J]. 化工进展, 2023, 42(8): 4283-4295. |
| [11] | 李佳, 樊星, 陈莉, 李坚. 硝酸生产尾气中NO x 和N2O联合脱除技术研究进展[J]. 化工进展, 2023, 42(7): 3770-3779. |
| [12] | 汪嘉欣, 潘勇, 熊欣怡, 万晓月, 王建超. 甲苯一步催化硝化制备二硝基甲苯反应过程及危险性[J]. 化工进展, 2023, 42(7): 3420-3430. |
| [13] | 韩恒文, 韩伟, 李明丰. 烯烃水合反应工艺与催化剂研究进展[J]. 化工进展, 2023, 42(7): 3489-3500. |
| [14] | 殷鹏镇, 吴芹, 黎汉生. 甲基芳烃液相选择性催化氧化催化剂研究进展[J]. 化工进展, 2023, 42(6): 2916-2943. |
| [15] | 张巍, 秦川, 谢康, 周运河, 董梦瑶, 李婕, 汤云灏, 马英, 宋健. H2-SCR改性铂系催化剂低温脱硝的应用及性能强化挑战[J]. 化工进展, 2023, 42(6): 2954-2962. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||