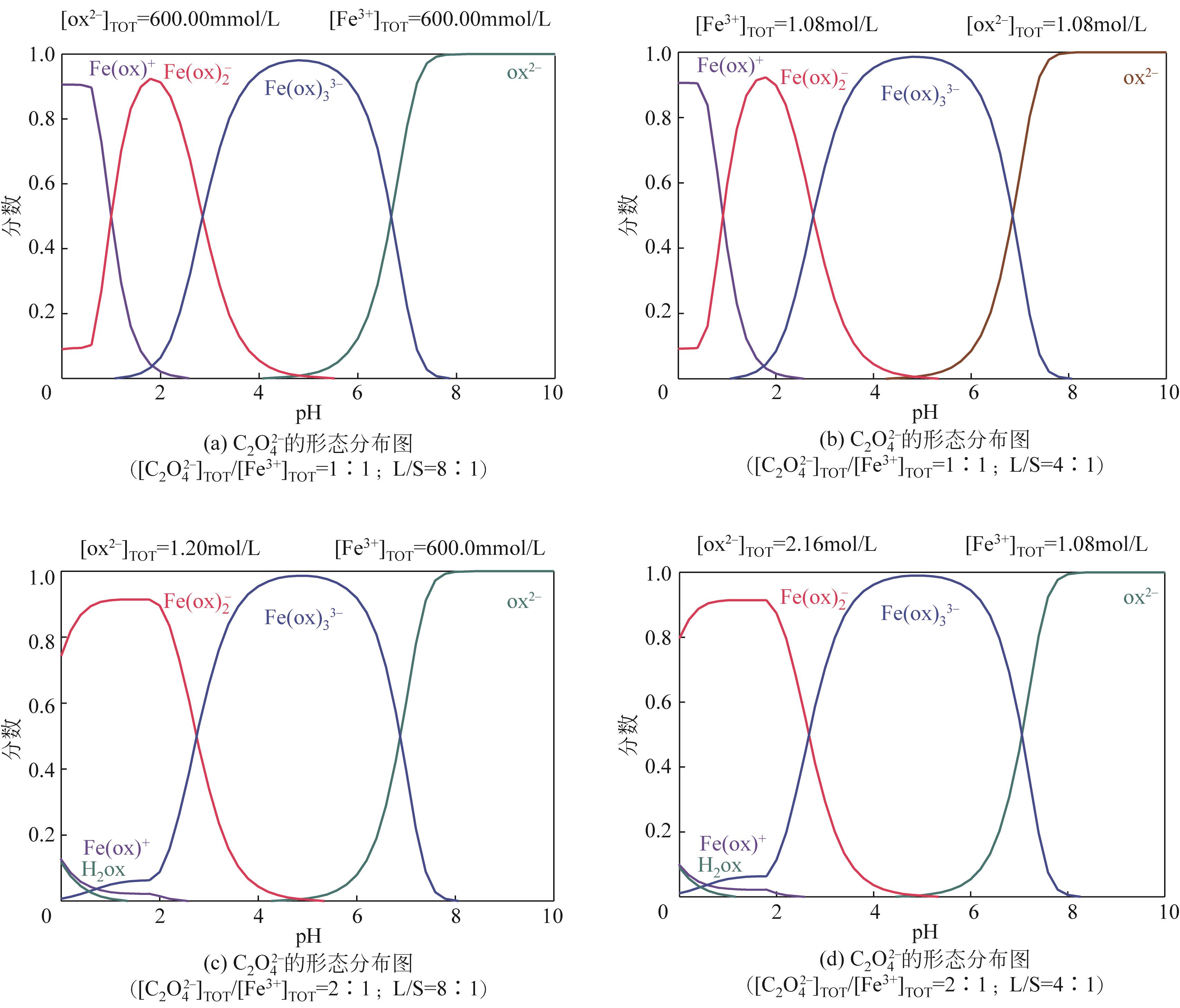
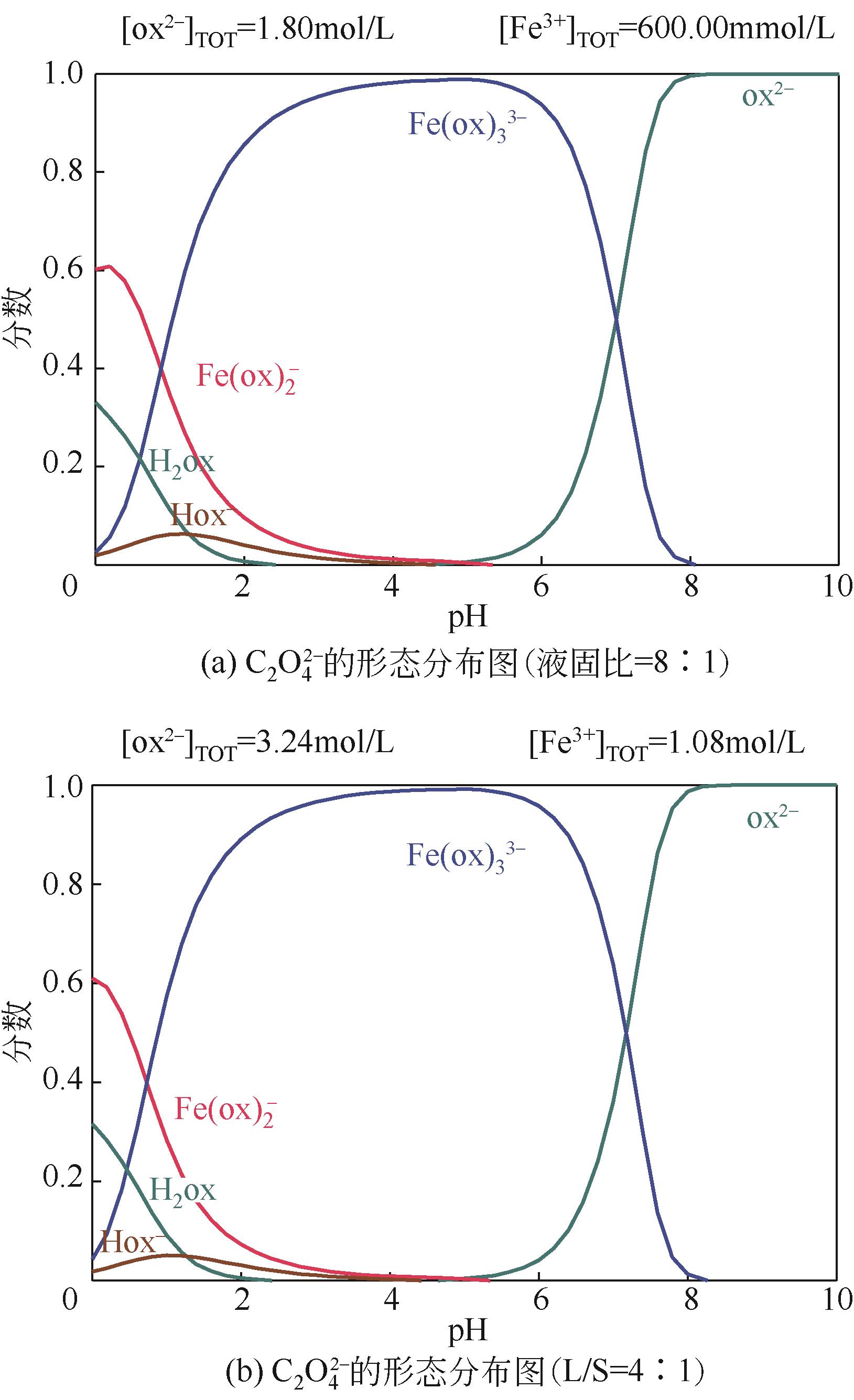
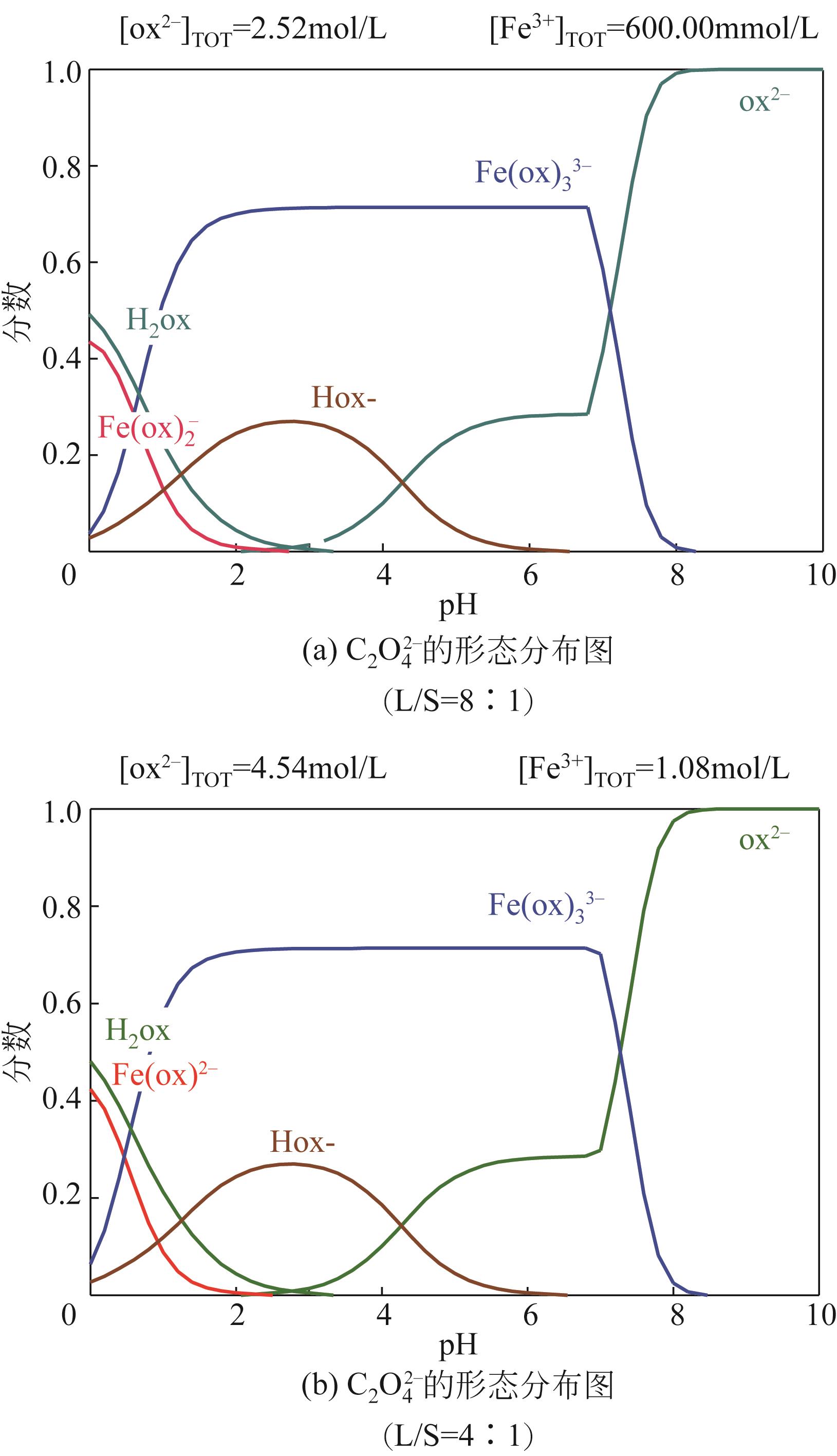
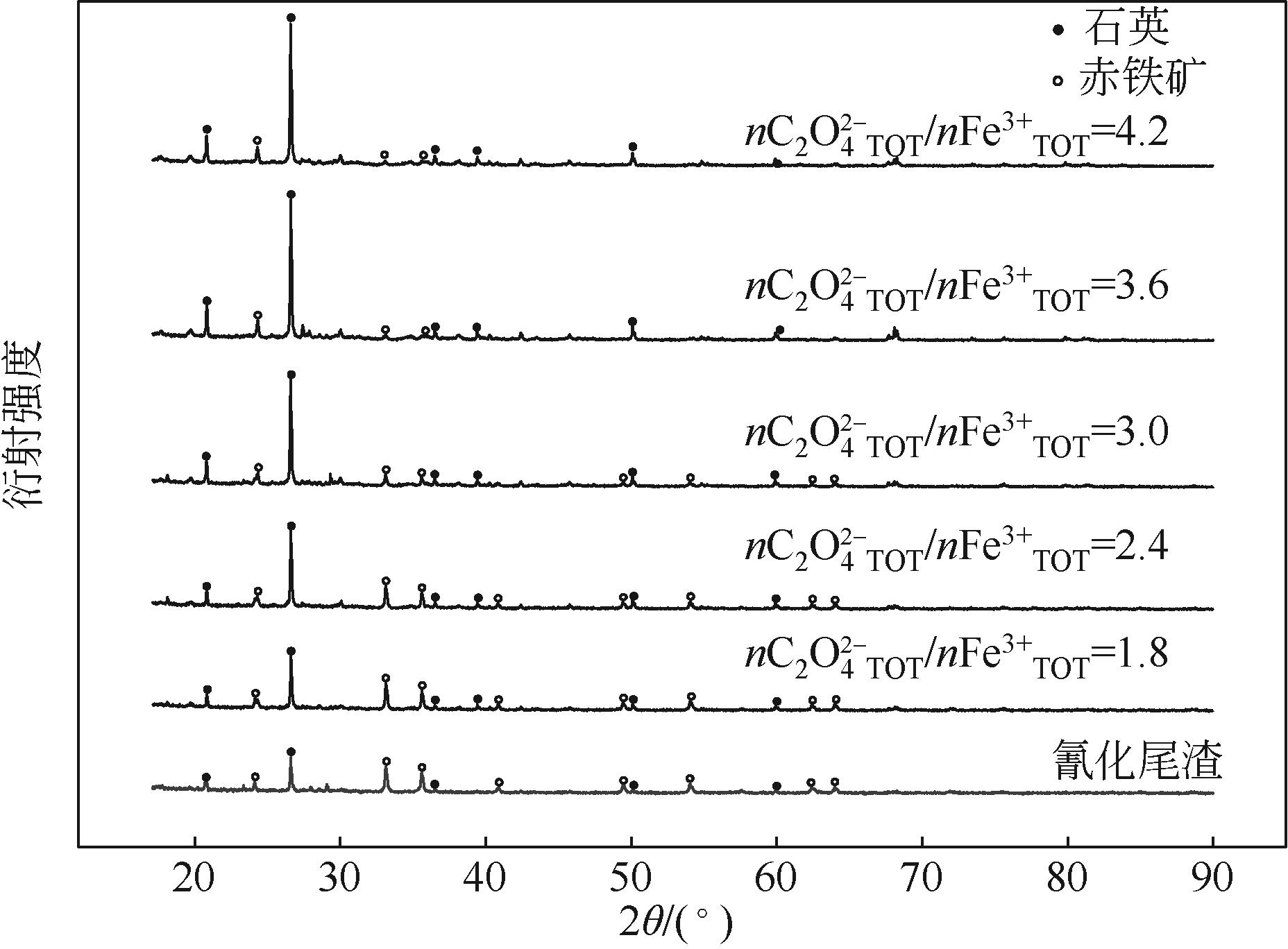
| 1 |
袁嘉声, 畅永锋, 郑春龙, 等. 氰化尾渣脱氰综述[J]. 中国有色金属学报, 2021, 31(6): 1568-1581.
|
|
YUAN Jiasheng, CHANG Yongfeng, ZHENG Chunlong, et al. Review on treatment technologies of cyanide tailing[J]. Journal of Chinese Nonferrous Metals, 2021, 31(6): 1568-1581.
|
| 2 |
阮书锋, 桑胜华, 李云, 等. 含金银硫酸烧渣综合利用研究-选择性脱铜[J]. 矿冶, 2017, 26(3): 46-49.
|
|
RUAN Shufeng, SANG Shenghua, LI Yun, et al. Study on selective copper removal for comprehensiveutilization of gold and silver bearing pyrite cinders[J]. Mining & Metallurgy, 2017, 26(3): 46-49.
|
| 3 |
Alp I, Deveci H, Yazıcı E Y, et al. Potential use of pyrite cinders as raw material in cement production: Results of industrial scale trial operations[J]. Journal of Hazardous Materials, 2009, 166(1): 144-149.
|
| 4 |
郑哗. 难处理金矿石预处理技术及应用现状[J]. 黄金, 2009, 30(1): 36-41.
|
|
ZHENG Hua. Pretreatment technique and its application of refractory gold ore[J]. Gold, 2009, 30(1): 36-41.
|
| 5 |
HANG Hanquan, CHEN Guanhua, CAI Xiang, et al. The leaching behavior of copper and iron recovery from reduction roasting pyrite cinder[J]. Journal of Hazardous Materials, 2021, 420, 126561.
|
| 6 |
LI Haoyu, LONG Hailin, ZHANG Libo, et al. Effectiveness of microwave-assisted thermal treatment in the extraction of gold in cyanide tailings[J]. Journal of Hazardous Materials, 2020, 384: 121456.
|
| 7 |
LONG Hailin, LI Haoyu, PEI Jiannan, et al. Cleaner recovery of multiple valuable metals from cyanide tailings via chlorination roasting[J]. Separation Science and Technology, 2021, 56(12): 2113-2123.
|
| 8 |
WANG Weiwei, LI Zhengyao. Recovery and kinetics of gold and iron from cyanide tailings by one-step chlorination-reduction roasting. Minerals Engineering, 2020, 155(15): 1-4.
|
| 9 |
CHEN Dong, GUO Hongwei, XU Jifang, et al. Recovery of Iron from cyrite cinder containing non-ferrous metals using high-temperature chloridizing-reduction-magnetic separation[J]. Metallurgical and materials transactions b-process metallurgy and materials processing science, 2017, 48(2): 933-942.
|
| 10 |
常耀超, 王云, 刘大学, 等. 氰化尾渣熔融氯化提金扩大试验[J]. 有色金属(冶炼部分), 2016(4): 43-45.
|
|
CHANG Yaochao, WANG Yun, LIU Daxue, et al. Expanding test on gold recovery from cyanide residue by molten chlorination[J]. Nonferrous Metals(Extractive Metallurgy), 2016(4): 43-45.
|
| 11 |
杨永斌, 曾冠武, 李骞, 等. 高硫砷金矿焙砂的硫酸熟化法预处理[J]. 中国有色金属学报, 2014, 24(9): 2380-2386.
|
|
YANG Yongbin, ZENG Guanwu, LI qian, et al. Pretreatment by sulfuric acid-curing of calcine roasting for gold ores with high sulfur and arsenic contents[J]. Journal of Chinese Nonferrous Metals, 2014, 24(9): 2380-2385.
|
| 12 |
郑雅杰,陈白珍. 硫铁矿烧渣的熟化及机理[J]. 中国有色金属学报, 2001,11(1) :144-147.
|
|
ZHENG Yajie, CHEN Baizhen. Maturation of pytite cinders and its mechanism[J]. Journal of Chinese Nonferrous Metals, 2001,11(1): 144-147.
|
| 13 |
马红周, 王丁丁, 王耀宁, 等 .硫酸熟化法浸出焙烧氰化尾渣中的铁[J]. 有色金属(冶炼部分), 2020, (10): 19-22.
|
|
MA Hongzhou, WANG Dingding, WANG Yaoning, et al. Leaching of iron from roasting cyanide tailings by sulfuric acid curing method[J].Nonferrous Metals(Extractive Metallurgy), 2020, (10): 19-22.
|
| 14 |
郑雅杰, 龚昶, 孙召明. 氰化尾渣还原焙烧酸浸提铁及氰化浸金新工艺[J]. 中国有色金属学报, 2014, 24(9): 2426-2433
|
|
ZHENG Yajie, GONG Chang, SUN Zhaoming. New technology of iron extraction and gold recovery from cyanide tailings by cyanide tailings by cyanide process after reduction roasting and acid leaching[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2426-2433.
|
| 15 |
刘志建, 金哲男, 王保仁,等. 黄金冶炼氰渣火法处理研究现状及展望[J]. 有色金属(冶炼部分), 2021(1): 84-88.
|
|
LIU Zhijian, JIN Zhenan, WANG Baoren, et al. Research status prospect of pyrometallurgical treatment of cyanide residue residue from gold smelting[J]. Nonferrous Metals(Extractive Metallurgy), 2021(1): 84-88.
|
| 16 |
张永峰, 武鑫. 两段焙烧工艺在黄金生产中的应用[J]. 中国有色冶金, 2010(5): 37-40.
|
|
ZHANG Yongfeng, WU Xin. Application of two-stage roasting process in gold production[J]. China Nonferrous Metallurgy, 2010(5): 37-40.
|
| 17 |
金程, 李登新. 硫酸烧渣还原浸取铁[J]. 有色金属(冶炼部分), 2012(1): 9-12.
|
|
JIN Cheng, LI Dengxin. Reductive leaching of iron from sulfate slag[J]. Nonferrous Metals(Extractive Metallurgy), 2012(1): 9-12.
|
| 18 |
王威, 高照国, 曹耀华, 等. 提高含砷金精矿二段焙砂金浸出率的研究[J]. 中国矿业, 2015, 24(7): 104-107.
|
|
WANG Wei, GAO Zhaoguo, CAO Yaohua, et al. The research on enhancing gold leaching rate from a arsenic-containing gold concentrate with two-stage roasting calcine[J]. China Mining Magazine, 2015, 24(7): 104-107.
|
| 19 |
杨保俊, 方晓宇, 王百年, 等. 草酸助剂强化酸浸法提取硫酸烧渣中的铁[J]. 化工进展, 2019, 38(3):1552-1560.
|
|
YANG Baojun, FANG Xiaoyu, WANG Bainian, et al. Extraction of iron from pyrite cinder via oxalic acid-intensified leaching method[J]. Chemical Industry and Engineering Progress, 2019, 38(3):1552-1560.
|