化工进展 ›› 2022, Vol. 41 ›› Issue (3): 1409-1429.DOI: 10.16085/j.issn.1000-6613.2021-2203
硼基催化剂丙烷氧化脱氢制丙烯
高晓峰1,2( ), 黄永康2, 徐文豪2, 周娴2, 姚思宇2(
), 黄永康2, 徐文豪2, 周娴2, 姚思宇2( ), 马丁1(
), 马丁1( )
)
- 1.北京大学化学与分子工程学院,北京 100871
2.浙江大学化学工程与生物工程学院,浙江 杭州 310029
-
收稿日期:2021-10-28修回日期:2021-12-06出版日期:2022-03-23发布日期:2022-03-28 -
通讯作者:姚思宇,马丁 -
作者简介:高晓峰(1989—),男,博士后,研究方向为工业催化。E-mail:gaoxiaofeng2020@pku.edu.cn 。 -
基金资助:浙江省自然科学基金杰青项目(LR21B030001);国家重点研发计划(2021YFC2101800);北京分子科学国家研究中心开放课题(BNLMS202003)
Oxidative dehydrogenation of propane to propene over boron-based catalysts
GAO Xiaofeng1,2( ), HUANG Yongkang2, XU Wenhao2, ZHOU Xian2, YAO Siyu2(
), HUANG Yongkang2, XU Wenhao2, ZHOU Xian2, YAO Siyu2( ), MA Ding1(
), MA Ding1( )
)
- 1.College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
2.College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310029, Zhejiang, China
-
Received:2021-10-28Revised:2021-12-06Online:2022-03-23Published:2022-03-28 -
Contact:YAO Siyu,MA Ding
摘要:
自2016年Hermans课题组发现六方氮化硼(h-BN)在丙烷氧化脱氢制丙烯(ODHP)反应中优异的烯烃选择性,各类硼基材料引起了研究者强烈的研究兴趣并广泛地用于ODHP反应。与传统金属与金属氧化物基催化剂不同,非金属硼基催化体系能够有效抑制CO x 等过度氧化产物,提高烯烃产率,具有较广阔的工业应用前景。本综述对硼基丙烷氧化脱氢催化剂从催化剂的设计、合成策略和反应性能等方面进行了系统地讨论,阐明了催化剂的构效关系;总结了反应路线、关键中间体、决速步以及催化动力学行为,加深了硼基催化剂催化丙烷氧化脱氢活性位点和机理的理解。指出三配位B—O/B—OH活性位点的有效构建及实现表面与气相自由基反应的协同催化是提高硼基催化剂丙烷脱氢性能的关键。基于目前的研究现状和存在的问题,对硼基催化剂体系研发前景和未来工业化应用进行了展望。
中图分类号:
引用本文
高晓峰, 黄永康, 徐文豪, 周娴, 姚思宇, 马丁. 硼基催化剂丙烷氧化脱氢制丙烯[J]. 化工进展, 2022, 41(3): 1409-1429.
GAO Xiaofeng, HUANG Yongkang, XU Wenhao, ZHOU Xian, YAO Siyu, MA Ding. Oxidative dehydrogenation of propane to propene over boron-based catalysts[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1409-1429.
| 分类 | 催化剂 | 温度/℃ | 转化率/% | 选择性/% | 产率/golefin·gcat-1·h-1 | 参考 文献 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| C4= | C3= | C2= | CO | CO2 | ||||||
| BN基催化剂 | h-BN | 490 | 14 | — | 79 | 12 | 9 | — | 0.5 | [ |
| BNNTs | 490 | 16.5 | — | 72 | — | — | — | 3.7 | [ | |
| 高比表面积BN | 525 | 24 | — | 69 | — | — | — | 0.04 | [ | |
| 羟化BN | 530 | 20.6 | — | 80.6 | 10.7 | 7.9 | 0.5 | 7.74 | [ | |
| SS-BNNSs | 490 | 20 | — | 78 | 10 | — | — | — | [ | |
| 介孔B x CN | 350 | 6.7 | — | 89.4 | — | — | — | 0.05 | [ | |
| h-BN/堇青石 | 535 | 17 | — | 82.1 | 14.2 | 3.7 | — | 18.6 | [ | |
| B2O3基催化剂 | B2O3@BPO4-800 | 550 | 24.7 | — | 66.4 | 18.4 | 5.2 | 0.2 | 0.8 | [ |
| B2O3/SBA-15 | 450 | 14.8 | — | 73.3 | 14.1 | 10.8 | 1.8 | 1.0 | [ | |
| B2O3/Al2O3 | 550 | 24.1 | — | 42.6 | 12.5 | 22.1 | 22.5 | 0.4 | [ | |
| B2O3-O CNTs | 400 | 4.6 | — | 70 | — | — | — | 0.003 | [ | |
| B-CNTs | 400 | 0.6 | — | 83 | — | — | — | 0.16 | [ | |
| B-AnnealedND | 450 | 1.8 | — | 65 | 2 | 12 | 9 | 0.01 | [ | |
| B-mww | 530 | 15 | — | 80.4 | 11.2 | — | — | 0.52 | [ | |
| BS-1 | 560 | 41.4 | — | 54.9 | 26.3 | — | — | — | [ | |
| BPO4(OM) | 515 | 14.3 | — | 8.25 | 9 | 7.9 | 0.6 | 16 | [ | |
| 其他B基催化剂 | B4C | 500 | 7 | — | 84.2 | 9.3 | 3.4 | 0.7 | 0.6 | [ |
| SiB6 | 535 | 19.2 | — | 82.2 | 12.2 | 5.2 | — | 1.49 | [ | |
| B | 490 | 16.4 | — | 77.9 | 10 | 6.6 | 3.6 | 11 | [ | |
| Ti2B | 500 | 5.8 | — | 85.4 | 9.1 | 2.1 | 0.4 | 0.5 | [ | |
| NiB | 500 | 6.1 | — | 85.4 | 9.3 | 2.2 | 0.3 | 0.4 | [ | |
| CO2B/CO3B | 500 | 3.2 | — | 87.9 | 7.9 | 1.5 | 0.2 | 0.2 | [ | |
| HfB2 | 500 | 4.2 | — | 87.5 | 7.6 | 2 | 0.2 | 0.2 | [ | |
| WB | 500 | 2.5 | — | 87.9 | 7.3 | 1.7 | 0.5 | 0.1 | [ | |
表1 已报道的催化剂在ODH中的催化性能
| 分类 | 催化剂 | 温度/℃ | 转化率/% | 选择性/% | 产率/golefin·gcat-1·h-1 | 参考 文献 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| C4= | C3= | C2= | CO | CO2 | ||||||
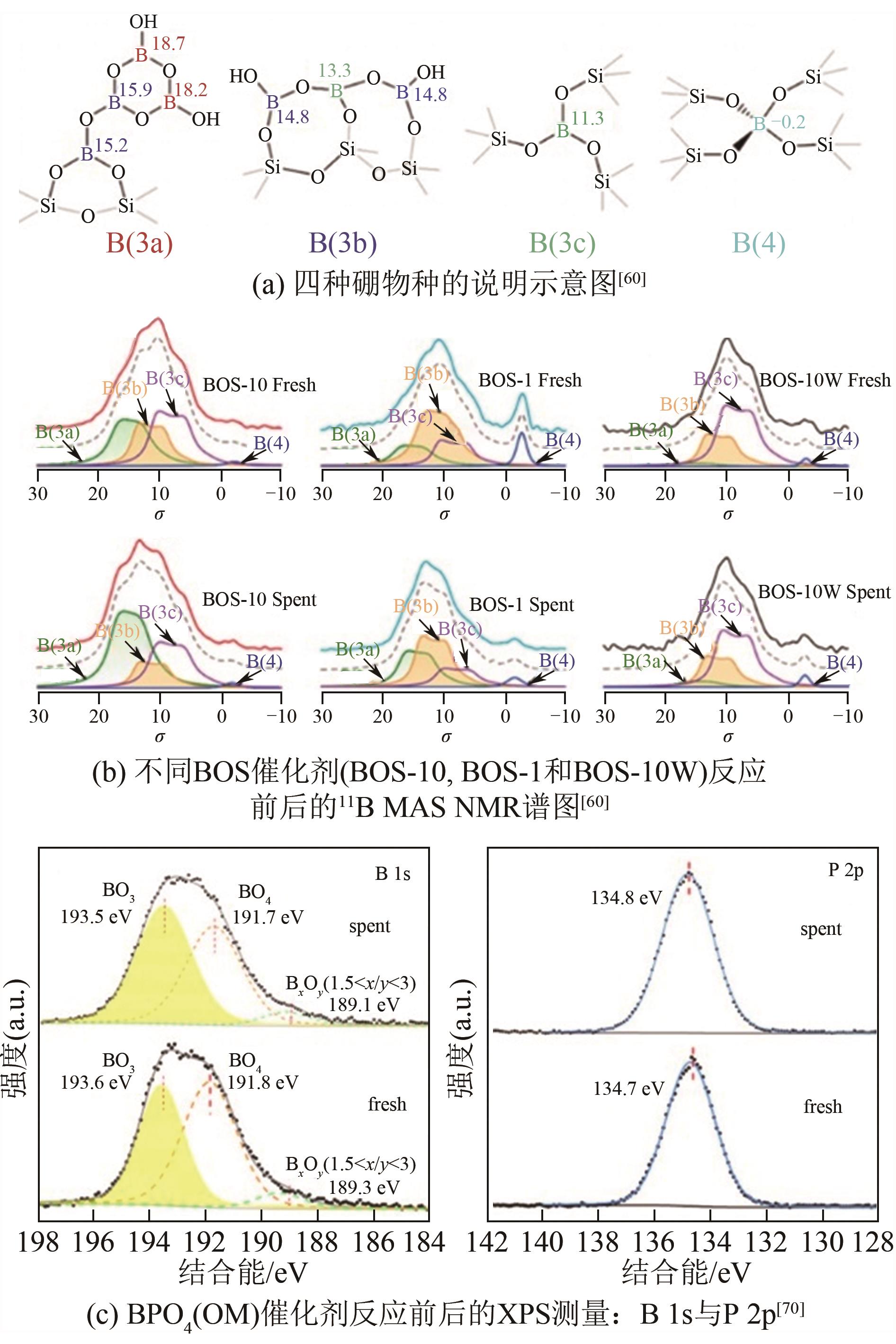
| BN基催化剂 | h-BN | 490 | 14 | — | 79 | 12 | 9 | — | 0.5 | [ |
| BNNTs | 490 | 16.5 | — | 72 | — | — | — | 3.7 | [ | |
| 高比表面积BN | 525 | 24 | — | 69 | — | — | — | 0.04 | [ | |
| 羟化BN | 530 | 20.6 | — | 80.6 | 10.7 | 7.9 | 0.5 | 7.74 | [ | |
| SS-BNNSs | 490 | 20 | — | 78 | 10 | — | — | — | [ | |
| 介孔B x CN | 350 | 6.7 | — | 89.4 | — | — | — | 0.05 | [ | |
| h-BN/堇青石 | 535 | 17 | — | 82.1 | 14.2 | 3.7 | — | 18.6 | [ | |
| B2O3基催化剂 | B2O3@BPO4-800 | 550 | 24.7 | — | 66.4 | 18.4 | 5.2 | 0.2 | 0.8 | [ |
| B2O3/SBA-15 | 450 | 14.8 | — | 73.3 | 14.1 | 10.8 | 1.8 | 1.0 | [ | |
| B2O3/Al2O3 | 550 | 24.1 | — | 42.6 | 12.5 | 22.1 | 22.5 | 0.4 | [ | |
| B2O3-O CNTs | 400 | 4.6 | — | 70 | — | — | — | 0.003 | [ | |
| B-CNTs | 400 | 0.6 | — | 83 | — | — | — | 0.16 | [ | |
| B-AnnealedND | 450 | 1.8 | — | 65 | 2 | 12 | 9 | 0.01 | [ | |
| B-mww | 530 | 15 | — | 80.4 | 11.2 | — | — | 0.52 | [ | |
| BS-1 | 560 | 41.4 | — | 54.9 | 26.3 | — | — | — | [ | |
| BPO4(OM) | 515 | 14.3 | — | 8.25 | 9 | 7.9 | 0.6 | 16 | [ | |
| 其他B基催化剂 | B4C | 500 | 7 | — | 84.2 | 9.3 | 3.4 | 0.7 | 0.6 | [ |
| SiB6 | 535 | 19.2 | — | 82.2 | 12.2 | 5.2 | — | 1.49 | [ | |
| B | 490 | 16.4 | — | 77.9 | 10 | 6.6 | 3.6 | 11 | [ | |
| Ti2B | 500 | 5.8 | — | 85.4 | 9.1 | 2.1 | 0.4 | 0.5 | [ | |
| NiB | 500 | 6.1 | — | 85.4 | 9.3 | 2.2 | 0.3 | 0.4 | [ | |
| CO2B/CO3B | 500 | 3.2 | — | 87.9 | 7.9 | 1.5 | 0.2 | 0.2 | [ | |
| HfB2 | 500 | 4.2 | — | 87.5 | 7.6 | 2 | 0.2 | 0.2 | [ | |
| WB | 500 | 2.5 | — | 87.9 | 7.3 | 1.7 | 0.5 | 0.1 | [ | |
| 1 | SATTLER J J H B, RUIZ-MARTINEZ J, SANTILLAN-JIMENEZ E, et al. Catalytic dehydrogenation of light alkanes on metals and metal oxides[J]. Chemical Reviews, 2014, 114(20): 10613-10653. |
| 2 | CAVANI F, BALLARINI N, CERICOLA A. Oxidative dehydrogenation of ethane and propane: how far from commercial implementation? [J]. Catalysis Today, 2007, 127(1/2/3/4): 113-131. |
| 3 | SANTHOSH KUMAR M, HAMMER N, RØNNING M, et al. The nature of active chromium species in Cr-catalysts for dehydrogenation of propane: new insights by a comprehensive spectroscopic study[J]. Journal of Catalysis, 2009, 261(1): 116-128. |
| 4 | Global propylene market in 2019[EB/OL]. . |
| 5 | Global propylene demand in 2025[EB/OL]. . |
| 6 | SHENG J, YAN B, LU W D, et al. Oxidative dehydrogenation of light alkanes to olefins on metal-free catalysts[J]. Chemical Society Reviews, 2021, 50(2): 1438-1468. |
| 7 | CORMA A, MELO F V, SAUVANAUD L, et al. Light cracked naphtha processing: controlling chemistry for maximum propylene production[J]. Catalysis Today, 2005, 107/108: 699-706. |
| 8 | JIAO F, LI J, PAN X, et al. Selective conversion of syngas to light olefins[J]. Science, 2016, 351(6277): 1065-1068. |
| 9 | LI J, WEI Y, CHEN J, et al. Observation of heptamethylbenzenium cation over SAPO-type molecular sieve DNL-6 under real MTO conversion conditions[J]. Journal of the American Chemical Society, 2012, 134(2): 836-839. |
| 10 | GÄRTNER C A, VAN VEEN A C, LERCHER J A. Oxidative dehydrogenation of ethane: common principles and mechanistic aspects[J]. ChemCatChem, 2013, 5(11): 3196-3217. |
| 11 | LI Q, SUI Z J, ZHOU X G, et al. Coke formation on Pt-Sn/Al2O3 catalyst in propane dehydrogenation: coke characterization and kinetic study[J]. Topics in Catalysis, 2011, 54(13/14/15): 888-896. |
| 12 | VU B K, SONG M B, AHN I Y, et al. Pt-Sn alloy phases and coke mobility over Pt-Sn/Al2O3 and Pt-Sn/ZnAl2O4 catalysts for propane dehydrogenation[J]. Applied Catalysis A: General, 2011, 400(1/2): 25-33. |
| 13 | XIAO L, MA F, ZHU Y A, et al. Improved selectivity and coke resistance of core-shell alloy catalysts for propane dehydrogenation from first principles and microkinetic analysis[J]. Chemical Engineering Journal, 2019, 377: 120049. |
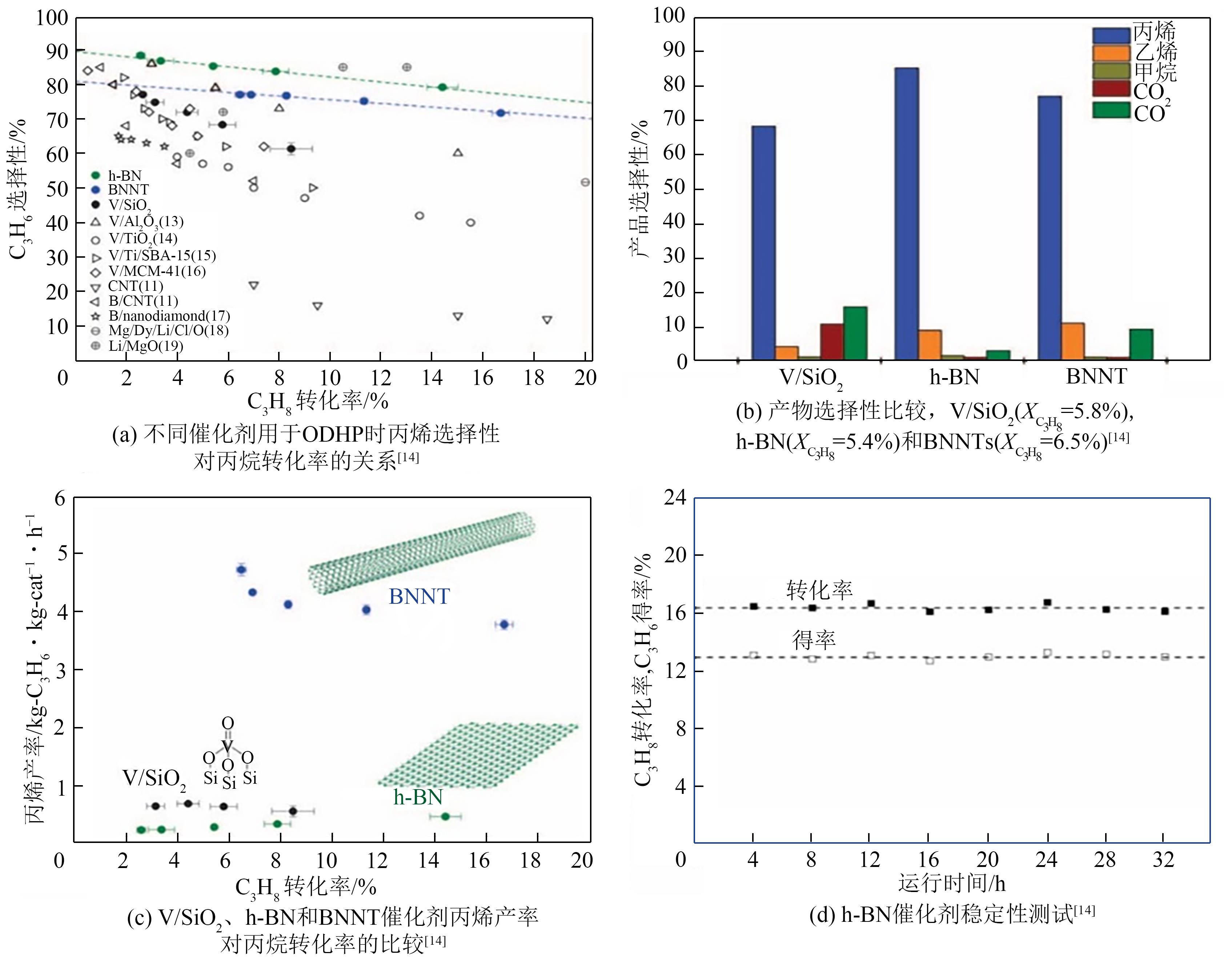
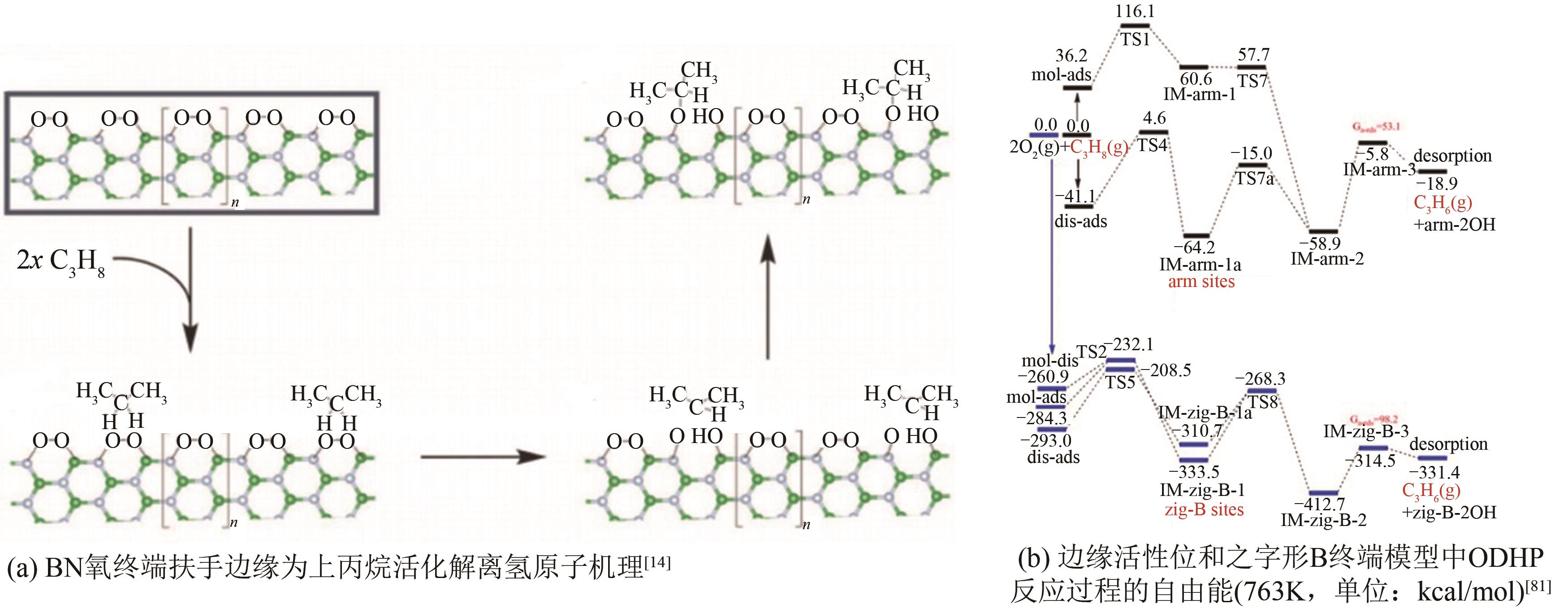
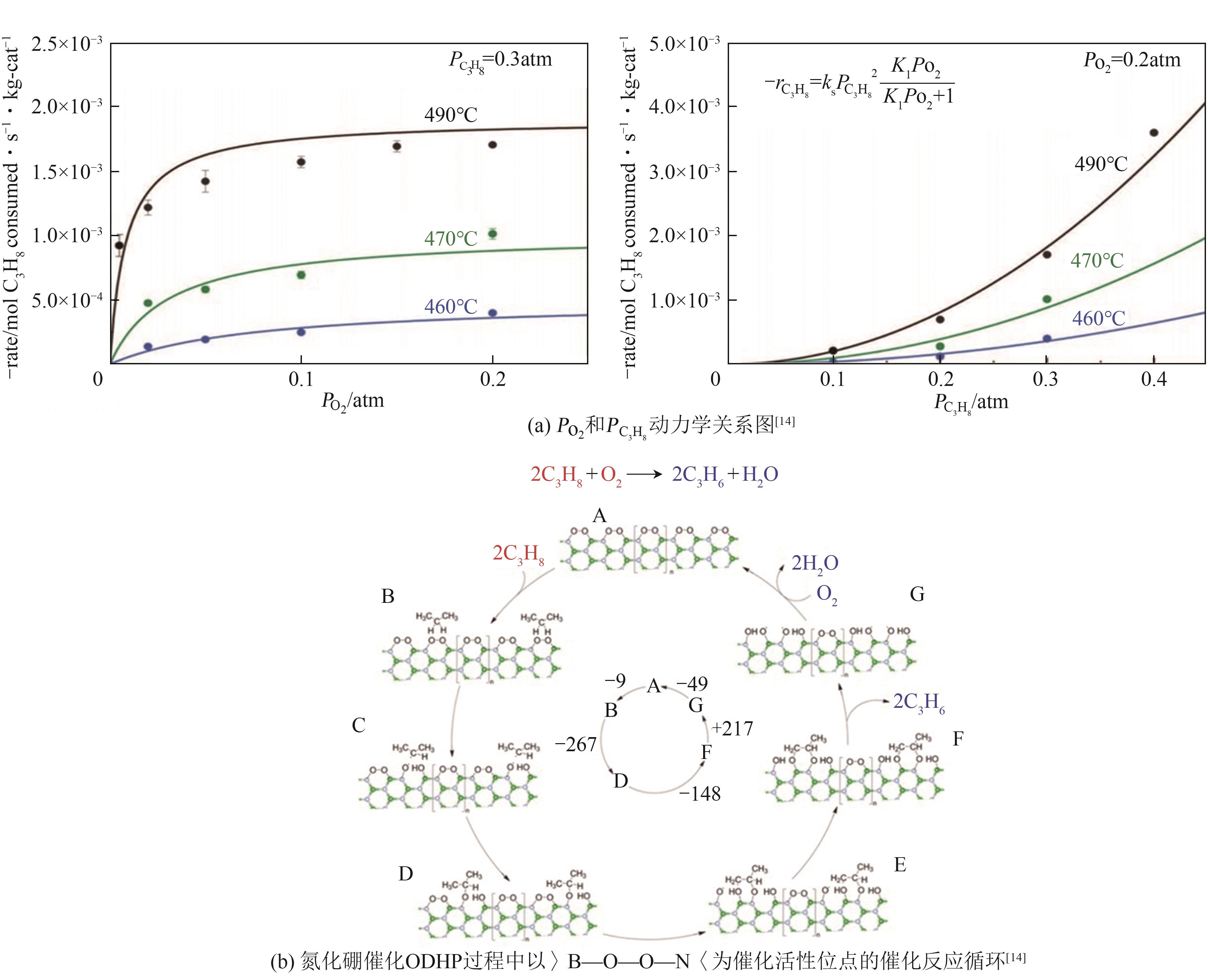
| 14 | GRANT J T, CARRERO C A, GOELTL F, et al. Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts[J]. Science, 2016, 354(6319): 1570-1573. |
| 15 | AYANDIRAN A A, BAKARE I A, BINOUS H, et al. Oxidative dehydrogenation of propane to propylene over VO x /CaO-γ-Al2O3 using lattice oxygen[J]. Catalysis Science & Technology, 2016, 6(13): 5154-5167. |
| 16 | DANIELL W, PONCHEL A, KUBA S, et al. Characterization and catalytic behavior of VO x -CeO2 catalysts for the oxidative dehydrogenation of propane[J]. Topics in Catalysis, 2002, 20(1/2/3/4): 65-74. |
| 17 | YANG S W, IGLESIA E, BELL A T. Oxidative dehydrogenation of propane over V2O5/MoO3/Al2O3 and V2O5/Cr2O3/Al2O3: structural characterization and catalytic function[J]. The Journal of Physical Chemistry B, 2005, 109(18): 8987-9000. |
| 18 | HOSSAIN M M. Kinetics of oxidative dehydrogenation of propane to propylene using lattice oxygen of VO x /CaO/γAl2O3 catalysts[J]. Industrial & Engineering Chemistry Research, 2017, 56(15): 4309-4318. |
| 19 | ZHANG S H, LIU H C. Insights into the structural requirements for oxidative dehydrogenation of propane on crystalline Mg-V-O catalysts[J]. Applied Catalysis A: General, 2018, 568: 1-10. |
| 20 | MISHAKOV I V, VEDYAGIN A A, BEDILO A F, et al. Aerogel VO x /MgO catalysts for oxidative dehydrogenation of propane[J]. Catalysis Today, 2009, 144(3/4): 278-284. |
| 21 | KLISIŃSKA A, SAMSON K, GRESSEL I, et al. Effect of additives on properties of V2O5/SiO2 and V2O5/MgO catalysts: I. Oxidative dehydrogenation of propane and ethane[J]. Applied Catalysis A: General, 2006, 309(1): 10-16. |
| 22 | PAK C, BELL A T, TILLEY T D. Oxidative dehydrogenation of propane over vanadia-magnesia catalysts prepared by thermolysis of OV(OtBu)3 in the presence of nanocrystalline MgO[J]. Journal of Catalysis, 2002, 206(1): 49-59. |
| 23 | ASCOOP I, GALVITA V V, ALEXOPOULOS K, et al. The role of CO2 in the dehydrogenation of propane over WO x -VO x /SiO2 [J]. Journal of Catalysis, 2016, 335: 1-10. |
| 24 | CHEN K D, BELL A T, IGLESIA E. Kinetics and mechanism of oxidative dehydrogenation of propane on vanadium, molybdenum, and tungsten oxides[J]. The Journal of Physical Chemistry B, 2000, 104(6): 1292-1299. |
| 25 | TEDEEVA M A, KUSTOV A L, PRIBYTKOV P V, et al. Dehydrogenation of propane with СО2 on supported CrO x /SiO2 catalysts[J]. Russian Journal of Physical Chemistry A, 2018, 92(12): 2403-2407. |
| 26 | BOUCETTA C, KACIMI M, ENSUQUE A, et al. Oxidative dehydrogenation of propane over chromium-loaded calcium-hydroxyapatite[J]. Applied Catalysis A: General, 2009, 356(2): 201-210. |
| 27 | YUN D, BAEK J, CHOI Y, et al. Promotional effect of Ni on a CrO x catalyst supported on silica in the oxidative dehydrogenation of propane with CO2 [J]. ChemCatChem, 2012, 4(12): 1952-1959. |
| 28 | VENEGAS J M, MCDERMOTT W P, HERMANS I. Serendipity in catalysis research: boron-based materials for alkane oxidative dehydrogenation[J]. Accounts of Chemical Research, 2018, 51(10): 2556-2564. |
| 29 | ZHANG X, YOU R, WEI Z Y, et al. Radical chemistry and reaction mechanisms of propane oxidative dehydrogenation over hexagonal boron nitride catalysts[J]. Angewandte Chemie, 2020, 132(21): 8119-8123. |
| 30 | MCDERMOTT W P, CENDEJAS M C, HERMANS I. Recent advances in the understanding of boron-containing catalysts for the selective oxidation of alkanes to olefins[J]. Topics in Catalysis, 2020, 63(19/20): 1700-1707. |
| 31 | SUN X, DING Y, ZHANG B, et al. New insights into the oxidative dehydrogenation of propane on borate-modified nanodiamond[J]. Chemical Communications, 2015, 51(44): 9145-9148. |
| 32 | CHATURBEDY P, AHAMED M, ESWARAMOORTHY M. Oxidative dehydrogenation of propane over a high surface area boron nitride catalyst: exceptional selectivity for olefins at high conversion[J]. ACS Omega, 2018, 3(1): 369-374. |
| 33 | ZHOU Y L, LIN J, LI L, et al. Enhanced performance of boron nitride catalysts with induction period for the oxidative dehydrogenation of ethane to ethylene[J]. Journal of Catalysis, 2018, 365: 14-23. |
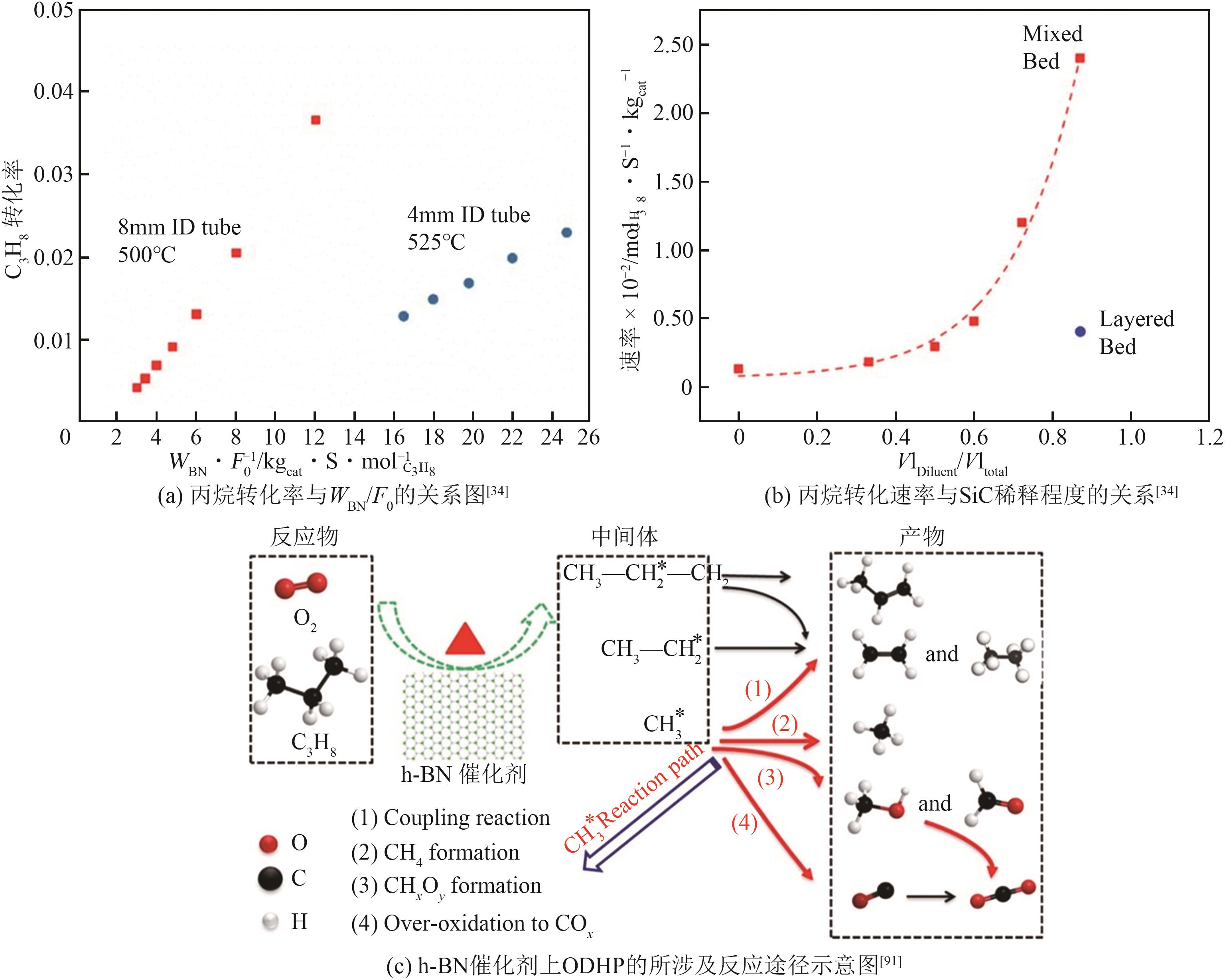
| 34 | VENEGAS J M, HERMANS I. The influence of reactor parameters on the boron nitride-catalyzed oxidative dehydrogenation of propane[J]. Organic Process Research & Development, 2018, 22(12): 1644-1652. |
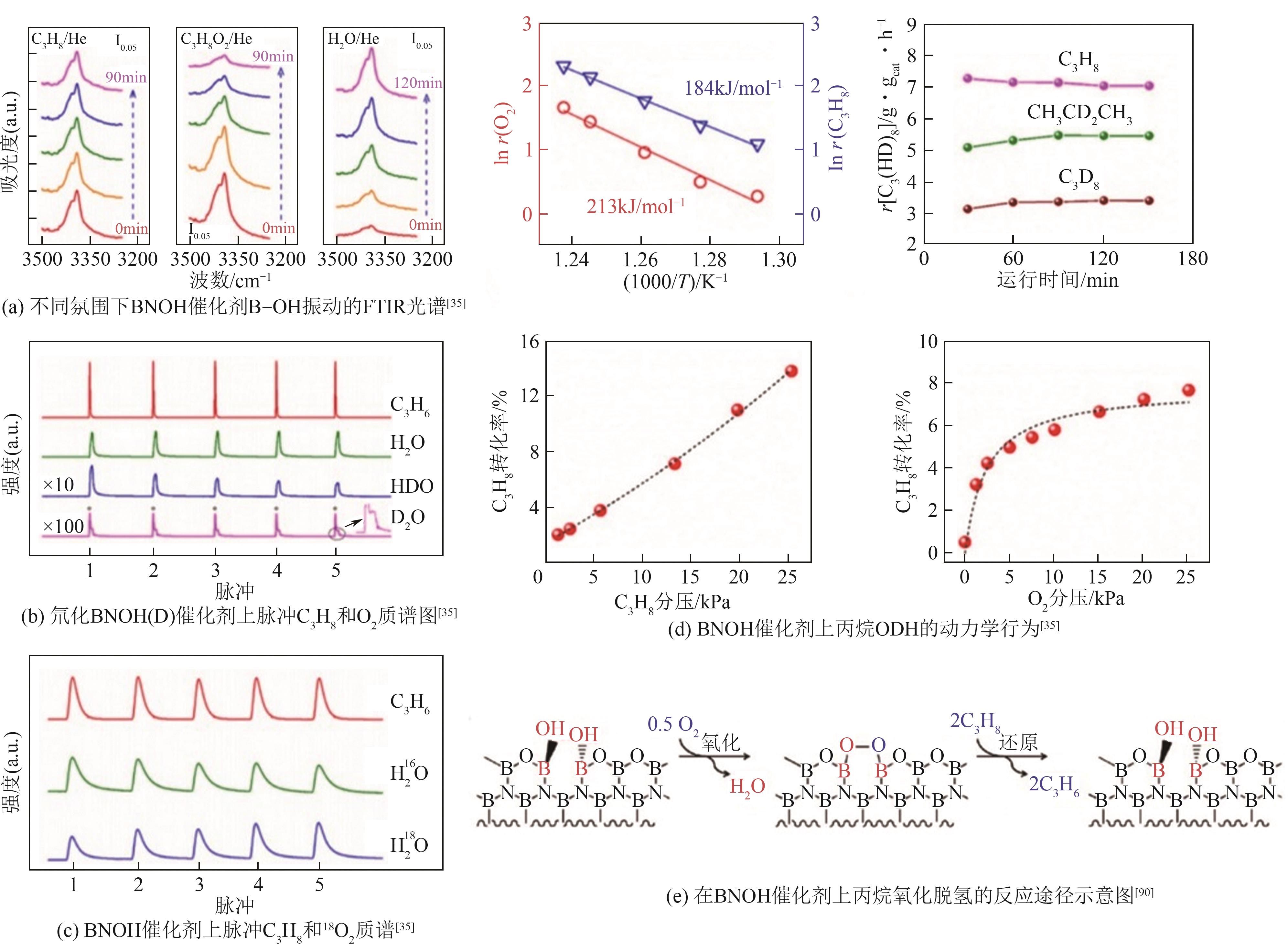
| 35 | SHI L, WANG D Q, SONG W, et al. Edge-hydroxylated boron nitride for oxidative dehydrogenation of propane to propylene[J]. ChemCatChem, 2017, 9(10): 1788-1793. |
| 36 | SHI L, YAN B, SHAO D, et al. Selective oxidative dehydrogenation of ethane to ethylene over a hydroxylated boron nitride catalyst[J]. Chinese Journal of Catalysis, 2017, 38(2): 389-395. |
| 37 | LINARES N, HARTMANN S, GALARNEAU A, et al. Continuous partial hydrogenation reactions by Pd@unconventional bimodal porous titania monolith catalysts[J]. ACS Catalysis, 2012, 2(10): 2194-2198. |
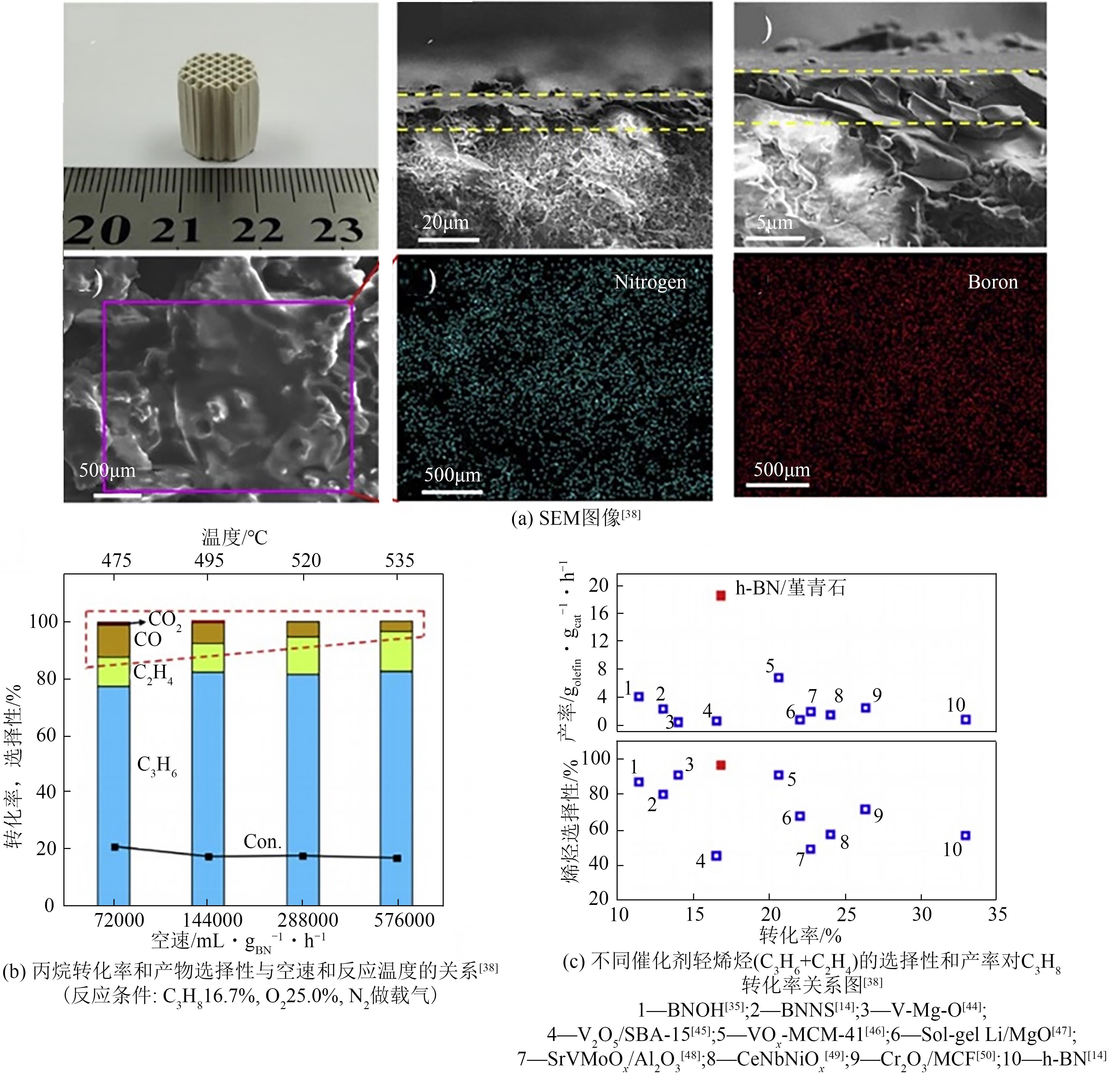
| 38 | WANG Y, LI W C, ZHOU Y X, et al. Boron nitride wash-coated cordierite monolithic catalyst showing high selectivity and productivity for oxidative dehydrogenation of propane[J]. Catalysis Today, 2020, 339: 62-66. |
| 39 | WU J C S, CHOU H C. Bimetallic Rh-Ni/BN catalyst for methane reforming with CO2 [J]. Chemical Engineering Journal, 2009, 148(2/3): 539-545. |
| 40 | HONDA Y, TAKAGAKI A, KIKUCHI R, et al. Oxidative dehydrogenation of ethane using ball-milled hexagonal boron nitride[J]. Chemistry Letters, 2018, 47(9): 1090-1093. |
| 41 | VENEGAS J M, GRANT J T, MCDERMOTT W P, et al. Selective oxidation of n-butane and isobutane catalyzed by boron nitride[J]. ChemCatChem, 2017, 9(12): 2118-2127. |
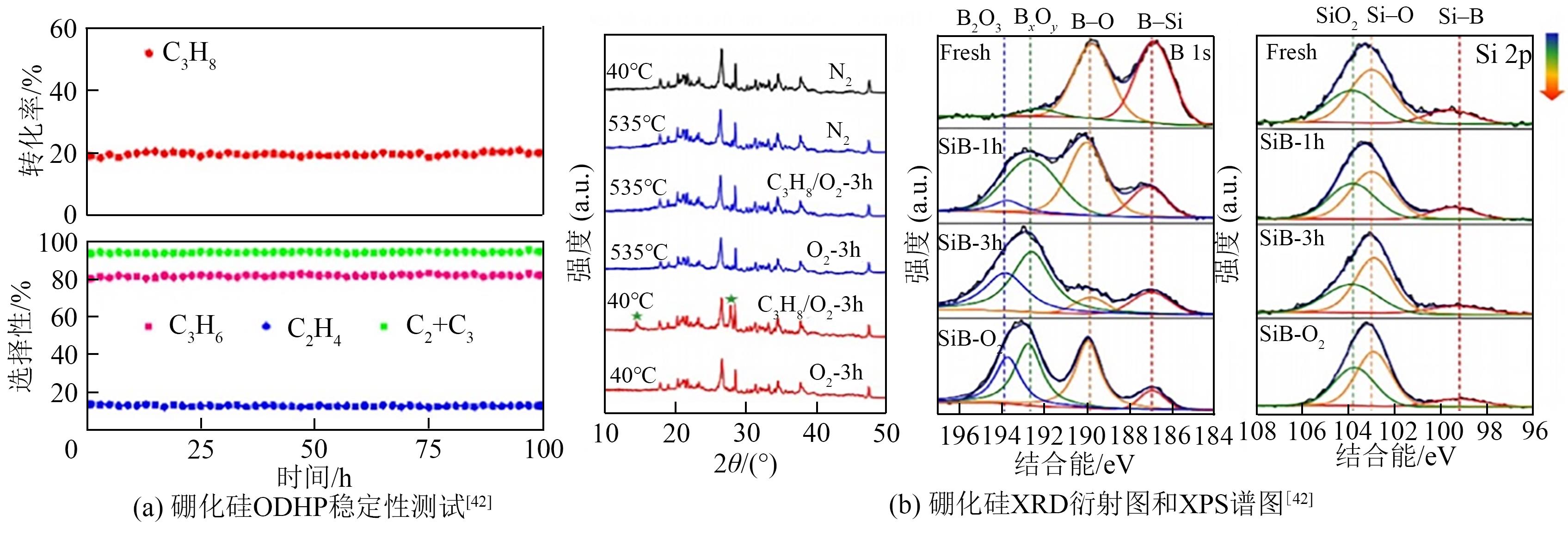
| 42 | YAN B, LI W C, LU A H. Metal-free silicon boride catalyst for oxidative dehydrogenation of light alkanes to olefins with high selectivity and stability[J]. Journal of Catalysis, 2019, 369: 296-301. |
| 43 | GUO F S, YANG P J, PAN Z M, et al. Carbon-doped BN nanosheets for the oxidative dehydrogenation of ethylbenzene[J]. Angewandte Chemie International Edition, 2017, 56(28): 8231-8235. |
| 44 | KONDRATENKO E V, BUYEVSKAYA O V, BAERNS M. Characterisation of vanadium-oxide-based catalysts for the oxidative dehydrogenation of propane to propene[J]. Topics in Catalysis, 2001, 15(2/3/4): 175-180. |
| 45 | LIU Y M, FENG W L, LI T C, et al. Structure and catalytic properties of vanadium oxide supported on mesocellulous silica foams(MCF)for the oxidative dehydrogenation of propane to propylene[J]. Journal of Catalysis, 2006, 239(1): 125-136. |
| 46 | SOLSONA B, BLASCO T, LÓPEZ NIETO J M, et al. Vanadium oxide supported on mesoporous MCM-41 as selective catalysts in the oxidative dehydrogenation of alkanes[J]. Journal of Catalysis, 2001, 203(2): 443-452. |
| 47 | TRIONFETTI C, BABICH I V, SESHAN K, et al. Formation of high surface area Li/MgO—Efficient catalyst for the oxidative dehydrogenation/cracking of propane[J]. Applied Catalysis A: General, 2006, 310: 105-113. |
| 48 | PUTRA M D, AL-ZAHRANI S M, ABASAEED A E. Oxidative dehydrogenation of propane to propylene over Al2O3-supported Sr-V-Mo catalysts[J]. Catalysis Communications, 2011, 14(1): 107-110. |
| 49 | LI J H, WANG C C, HUANG C J, et al. Low temperature catalytic performance of nanosized CeNbNiO mixed oxide for oxidative dehydrogenation of propane to propene[J]. Catalysis Letters, 2010, 137(1/2): 81-87. |
| 50 | LIU Y M, FENG W L, WANG L C, et al. Chromium supported on mesocellular silica foam(MCF)for oxidative dehydrogenation of propane[J]. Catalysis Letters, 2006, 106(3/4): 145-152. |
| 51 | CURTIN T, MCMONAGLE J B, HODNETT B K. Influence of boria loading on the acidity of B2O3/Al2O3 catalysts for the conversion of cyclohexanone oxime to caprolactam[J]. Applied Catalysis A: General, 1992, 93(1): 91-101. |
| 52 | XU B Q, CHENG S B, JIANG S, et al. Gas phase beckmann rearrangement of cyclohexanone oxime over zirconia-supported boria catalyst[J]. Applied Catalysis A: General, 1999, 188(1/2): 361-368. |
| 53 | XU B Q, CHENG S B, ZHANG X, et al. B2O3/ZrO2 for Beckmann rearrangement of cyclohexanone oxime: optimizing of the catalyst and reaction atmosphere[J]. Catalysis Today, 2000, 63(2/3/4): 275-282. |
| 54 | XU B Q, ZHANG X, YING S F, et al. High temperature calcination for a highly efficient and regenerable B2O3/ZrO2 catalyst for the synthesis of ɛ-caprolactam[J]. Chemical Communications, 2000(13): 1121-1122. |
| 55 | RAVINDRA D B, NIE Y T, JAENICKE S, et al. Isomerisation of α-pinene oxide over B2O3/SiO2 and Al-MSU catalysts[J]. Catalysis Today, 2004, 96(3): 147-153. |
| 56 | BUYEVSKAYA O V, BAERNS M. Catalytic selective oxidation of propane[J]. Catalysis Today, 1998, 42(3): 315-323. |
| 57 | BUYEVSKAYA O V, MÜLLER D, PITSCH I, et al. Selective oxidative conversion of propane to olefins and oxygenates on boria-containing catalysts[J]. Studies in Surface Science and Catalysis, 1998, 119: 671-676. |
| 58 | COLORIO G, VÉDRINE J C, AUROUX A, et al. Partial oxidation of ethane over alumina-boria catalysts[J]. Applied Catalysis A: General, 1996, 137(1): 55-68. |
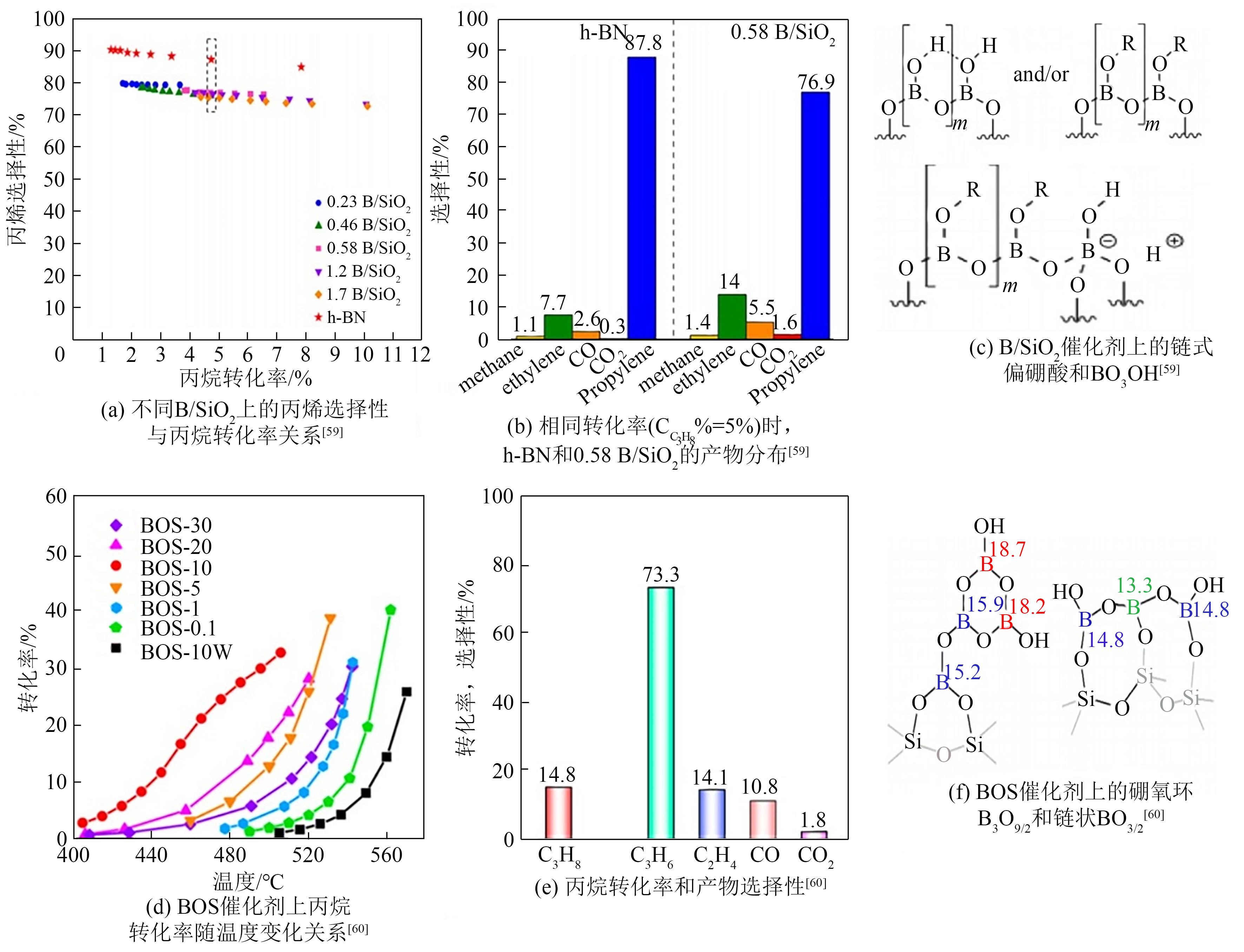
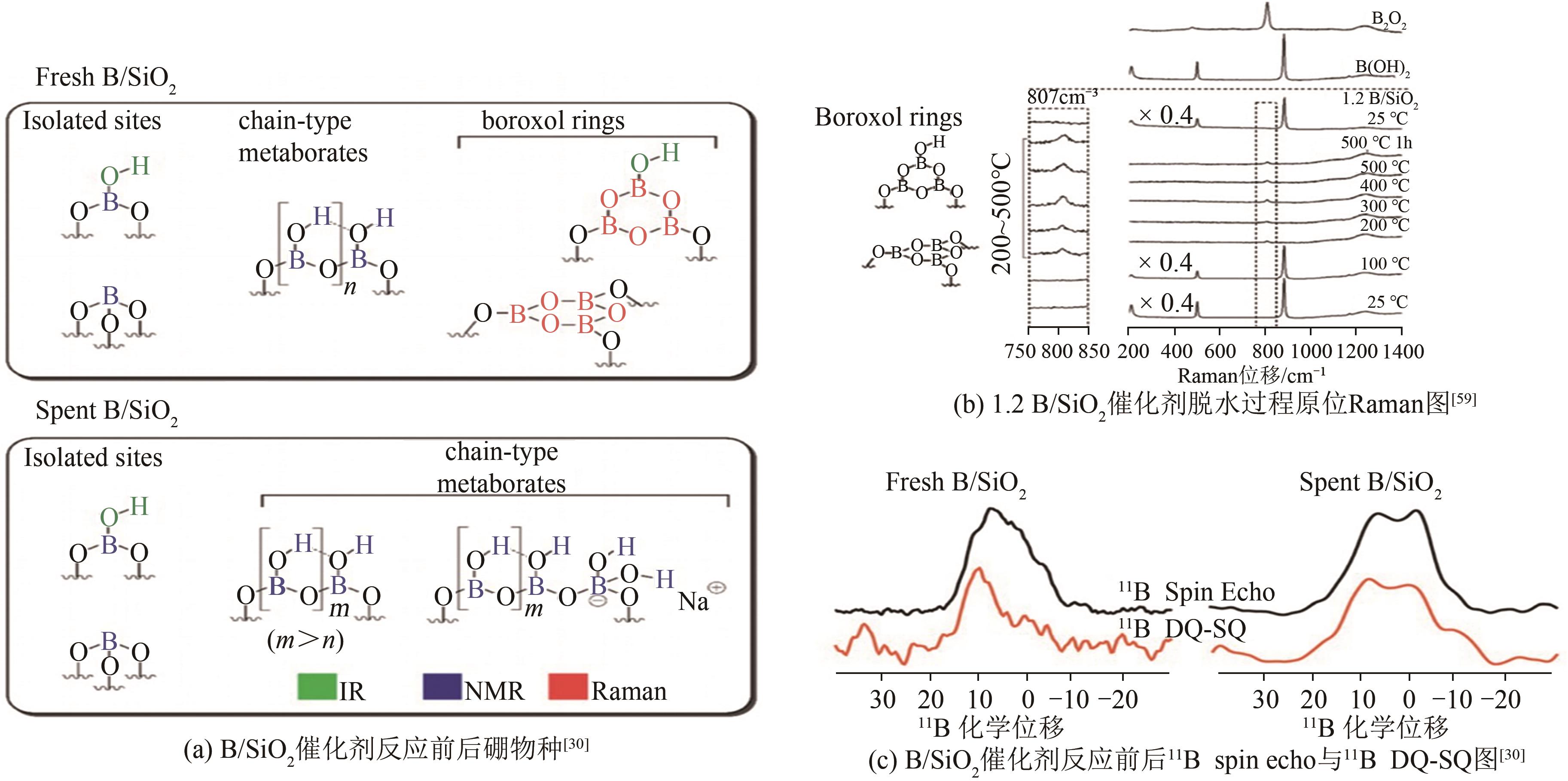
| 59 | LOVE A M, CENDEJAS M C, THOMAS B, et al. Synthesis and characterization of silica-supported boron oxide catalysts for the oxidative dehydrogenation of propane[J]. The Journal of Physical Chemistry C, 2019, 123(44): 27000-27011. |
| 60 | LU W D, WANG D Q, ZHAO Z C, et al. Supported boron oxide catalysts for selective and low-temperature oxidative dehydrogenation of propane[J]. ACS Catalysis, 2019, 9(9): 8263-8270. |
| 61 | ZHOU Y X, WANG Y, LU W D, et al. A high propylene productivity over B2O3/SiO2@honeycomb cordierite catalyst for oxidative dehydrogenation of propane[J]. Chinese Journal of Chemical Engineering, 2020, 28(11): 2778-2784. |
| 62 | RONY P R. Supported liquid-phase catalysts[J]. Chemical Engineering Science, 1968, 23(9): 1021-1034. |
| 63 | SELVAM T, MACHOKE A, SCHWIEGER W. Supported ionic liquids on non-porous and porous inorganic materials—A topical review[J]. Applied Catalysis A: General, 2012, 445/446: 92-101. |
| 64 | LIU Q W, WU Y W, XING F S, et al. B2O3@BPO4 sandwich-like hollow spheres as metal-free supported liquid-phase catalysts[J]. Journal of Catalysis, 2020, 381: 599-607. |
| 65 | YANG H G, ZENG H C. Preparation of hollow anatase TiO2 nanospheres via Ostwald ripening[J]. The Journal of Physical Chemistry B, 2004, 108(11): 3492-3495. |
| 66 | YUN J H, LOBO R F. Catalytic dehydrogenation of propane over iron-silicate zeolites[J]. Journal of Catalysis, 2014, 312: 263-270. |
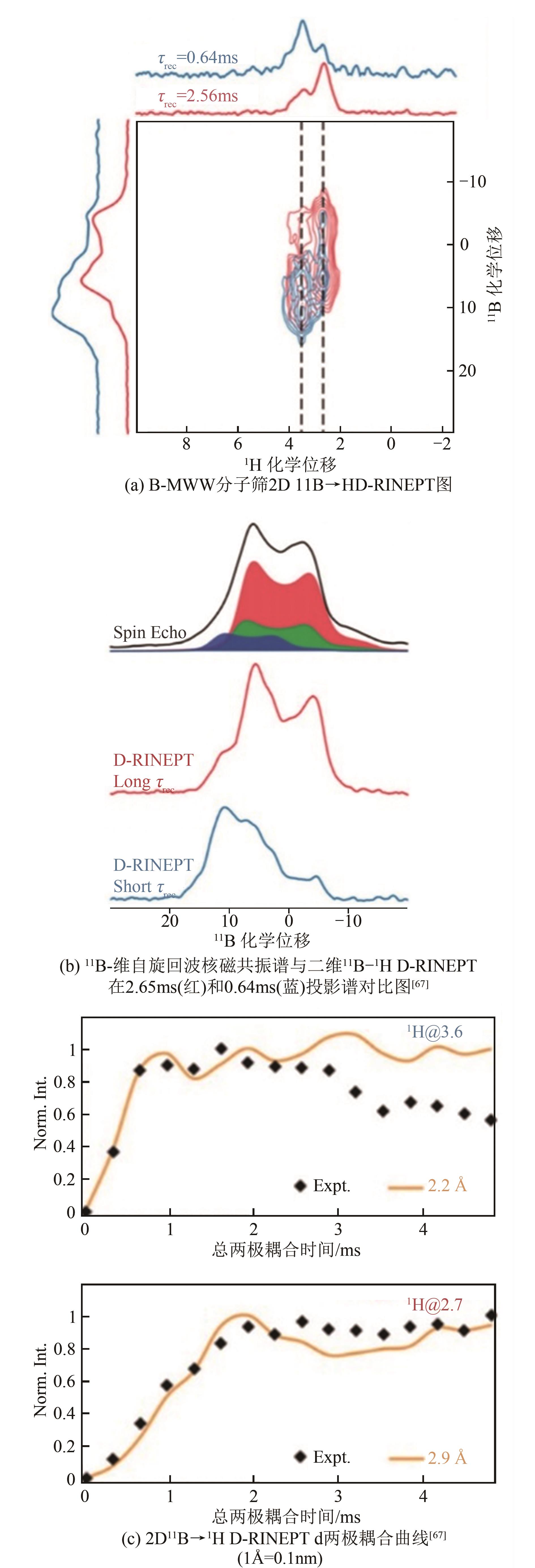
| 67 | ALTVATER N R, DORN R W, CENDEJAS M C, et al. B-MWW zeolite: the case against single-site catalysis[J]. Angewandte Chemie International Edition, 2020, 59(16): 6546-6550. |
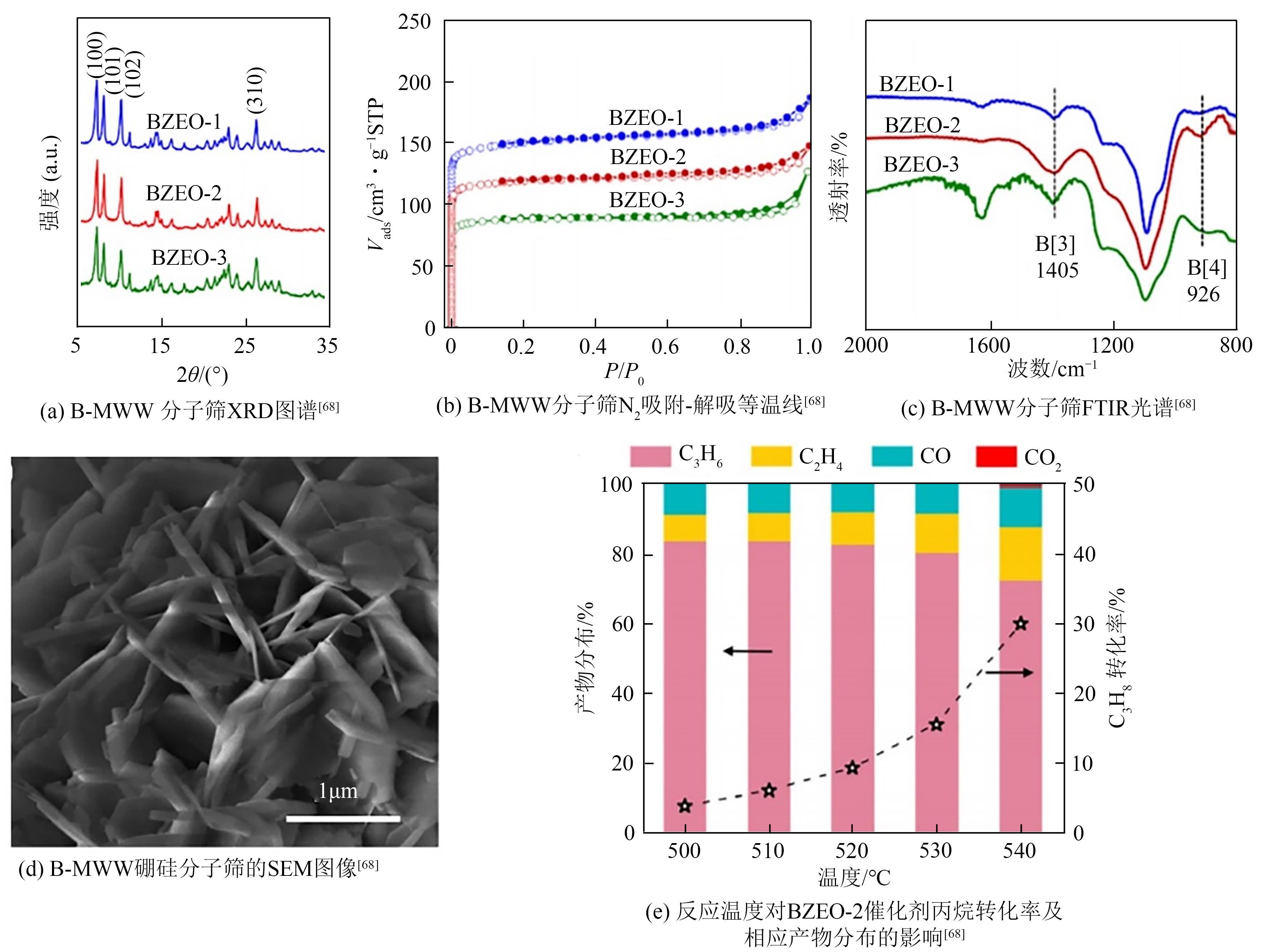
| 68 | QIU B, JIANG F, LU W D, et al. Oxidative dehydrogenation of propane using layered borosilicate zeolite as the active and selective catalyst[J]. Journal of Catalysis, 2020, 385: 176-182. |
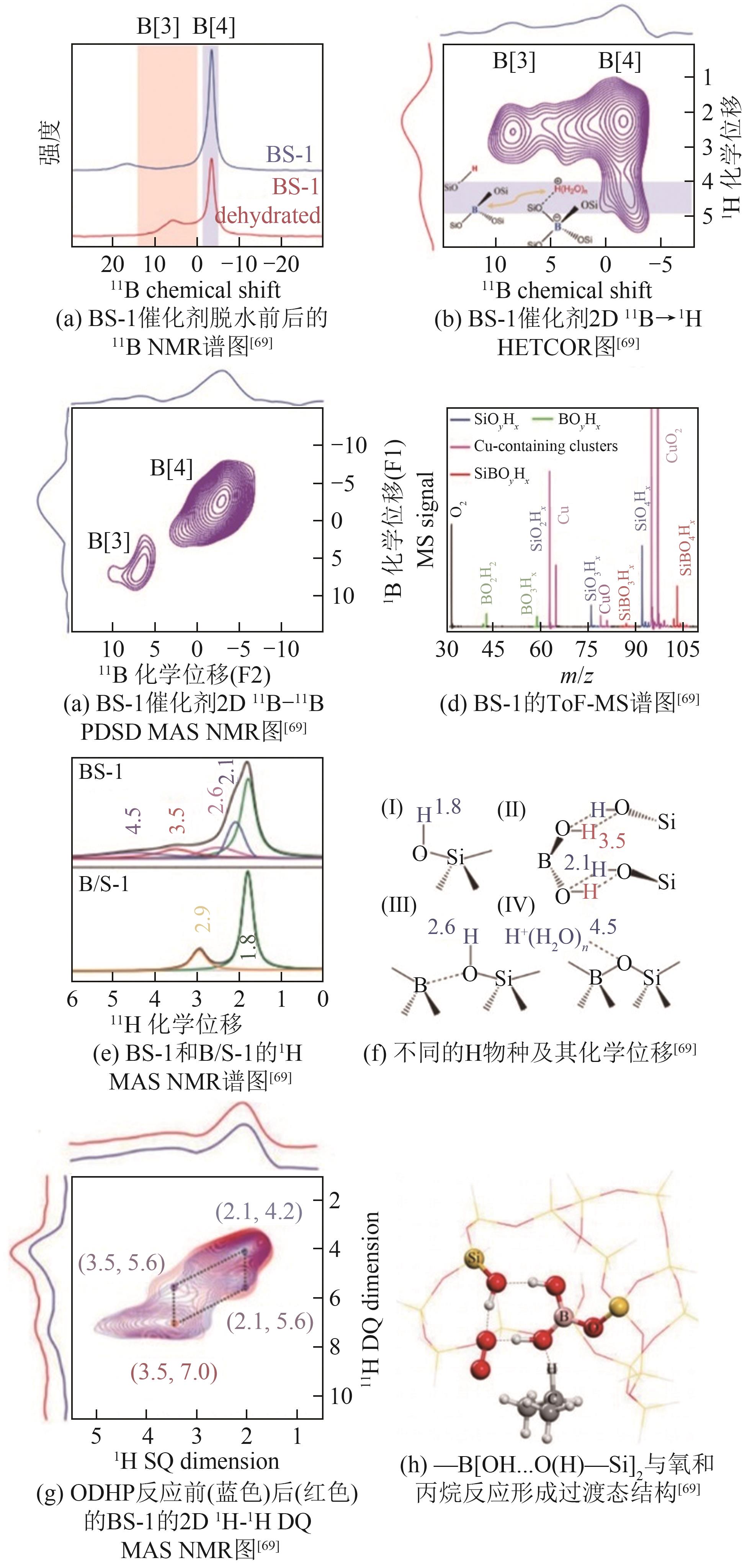
| 69 | ZHOU H, YI X, HUI Y, et al. Isolated boron in zeolite for oxidative dehydrogenation of propane[J]. Science, 2021, 372(6537): 76-80. |
| 70 | LU W D, GAO X Q, WANG Q G, et al. Ordered macroporous boron phosphate crystals as metal-free catalysts for the oxidative dehydrogenation of propane[J]. Chinese Journal of Catalysis, 2020, 41(12): 1837-1845. |
| 71 | CHEN Y F, CHUNG Y W, LI S Y. Boron carbide and boron carbonitride thin films as protective coatings in ultra-high density hard disk drives[J]. Surface and Coatings Technology, 2006, 200(12/13): 4072-4077. |
| 72 | LEE K E, LEE J Y, PARK M J, et al. Preparation of boron carbide thin films for HDD protecting layer[J]. Journal of Magnetism and Magnetic Materials, 2004, 272/273/274/275/276: 2197-2199. |
| 73 | LYU H, PENG T, WU P, et al. Nano-boron carbide supported platinum catalysts with much enhanced methanol oxidation activity and CO tolerance[J]. Journal of Materials Chemistry, 2012, 22(18): 9155. |
| 74 | MU S C, CHEN X, SUN R H, et al. Nano-size boron carbide intercalated graphene as high performance catalyst supports and electrodes for PEM fuel cells[J]. Carbon, 2016, 103: 449-456. |
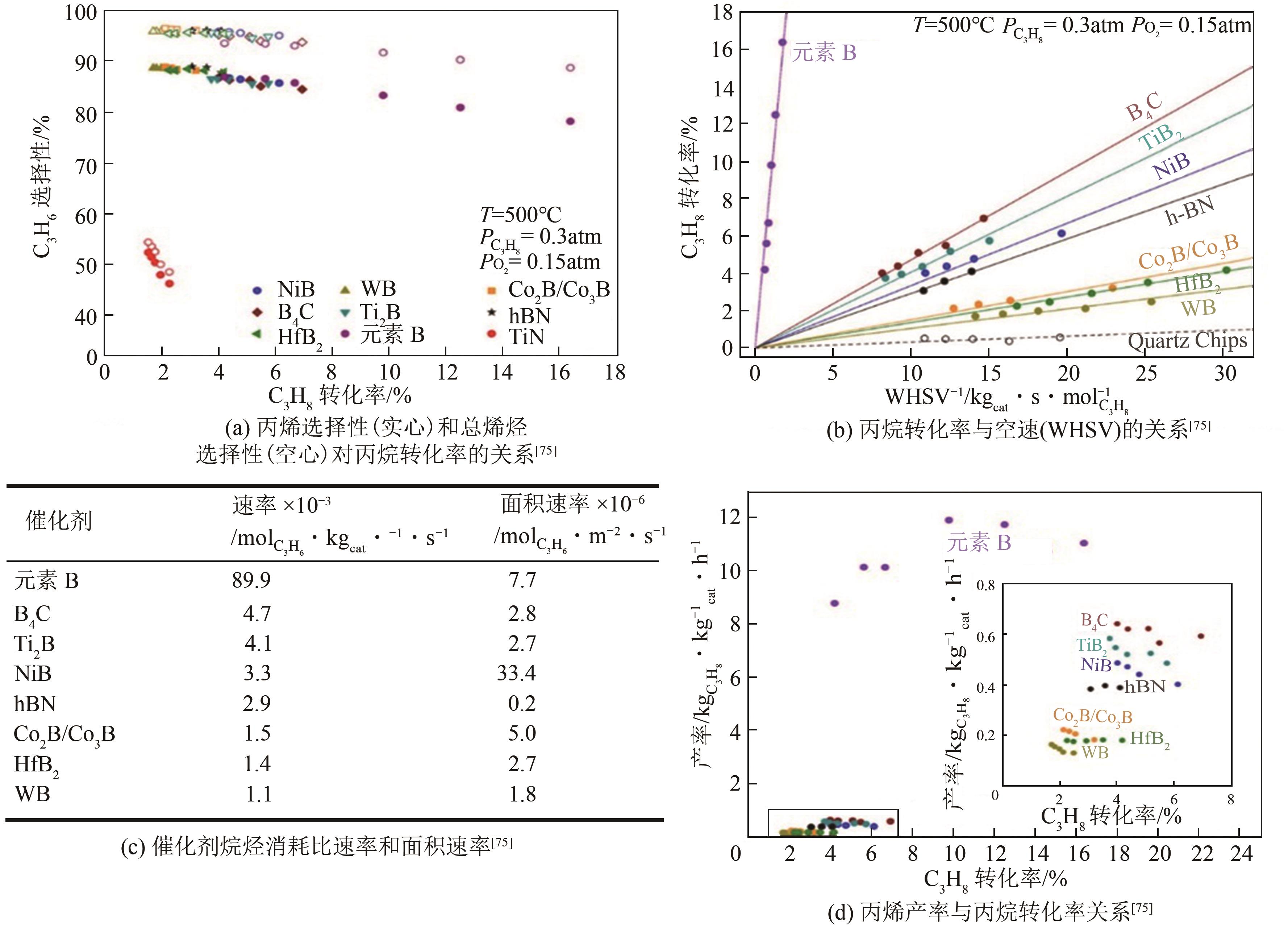
| 75 | GRANT J T, MCDERMOTT W P, VENEGAS J M, et al. Boron and boron-containing catalysts for the oxidative dehydrogenation of propane[J]. ChemCatChem, 2017, 9(19): 3623-3626. |
| 76 | LEVELES L, SESHAN K, LERCHER J A, et al. Oxidative conversion of propane over lithium-promoted magnesia catalyst: I. Kinetics and mechanism[J]. Journal of Catalysis, 2003, 218(2): 296-306. |
| 77 | SUI Z J, ZHOU J H, DAI Y C, et al. Oxidative dehydrogenation of propane over catalysts based on carbon nanofibers[J]. Catalysis Today, 2005, 106(1/2/3/4): 90-94. |
| 78 | FRANK B, ZHANG J, BLUME R, et al. Heteroatoms increase the selectivity in oxidative dehydrogenation reactions on nanocarbons[J]. Angewandte Chemie International Edition, 2009, 48(37): 6913-6917. |
| 79 | GOYAL R, SARKAR B, BAG A, et al. Single-step synthesis of hierarchical B x CN: a metal-free catalyst for low-temperature oxidative dehydrogenation of propane[J]. Journal of Materials Chemistry A, 2016, 4(47): 18559-18569. |
| 80 | CAO L, DAI P C, TANG J, et al. Spherical superstructure of boron nitride nanosheets derived from boron-containing metal-organic frameworks[J]. Journal of the American Chemical Society, 2020, 142(19): 8755-8762. |
| 81 | LI H P, ZHANG J R, WU P W, et al. O2 activation and oxidative dehydrogenation of propane on hexagonal boron nitride: mechanism revisited[J]. The Journal of Physical Chemistry C, 2019, 123(4): 2256-2266. |
| 82 | WU P W, YANG S Z, ZHU W S, et al. Tailoring N-terminated defective edges of porous boron nitride for enhanced aerobic catalysis[J]. Small, 2017, 13(44): 1701857. |
| 83 | LI L H, CERVENKA J, WATANABE K, et al. Strong oxidation resistance of atomically thin boron nitride nanosheets[J]. ACS Nano, 2014, 8(2): 1457-1462. |
| 84 | LEE K H, SHIN H J, KUMAR B, et al. Nanocrystalline-graphene-tailored hexagonal boron nitride thin films[J]. Angewandte Chemie International Edition, 2014, 53(43): 11493-11497. |
| 85 | DEAN C R, YOUNG A F, MERIC I, et al. Boron nitride substrates for high-quality graphene electronics[J]. Nature Nanotechnology, 2010, 5(10): 722-726. |
| 86 | HUANG R, ZHANG B S, WANG J, et al. Direct insight into ethane oxidative dehydrogenation over boron nitrides[J]. ChemCatChem, 2017, 9(17): 3293-3297. |
| 87 | LOVE A M, THOMAS B, SPECHT S E, et al. Probing the transformation of boron nitride catalysts under oxidative dehydrogenation conditions[J]. Journal of the American Chemical Society, 2019, 141(1): 182-190. |
| 88 | ZHANG Z, JIMENEZ-IZAL E, HERMANS I, et al. Dynamic phase diagram of catalytic surface of hexagonal boron nitride under conditions of oxidative dehydrogenation of propane[J]. The Journal of Physical Chemistry Letters, 2019, 10(1): 20-25. |
| 89 | FERLAT G, CHARPENTIER T, SEITSONEN A P, et al. Boroxol rings in liquid and vitreous B2O3 from first principles[J]. Physical Review Letters, 2008, 101(6): 065504. |
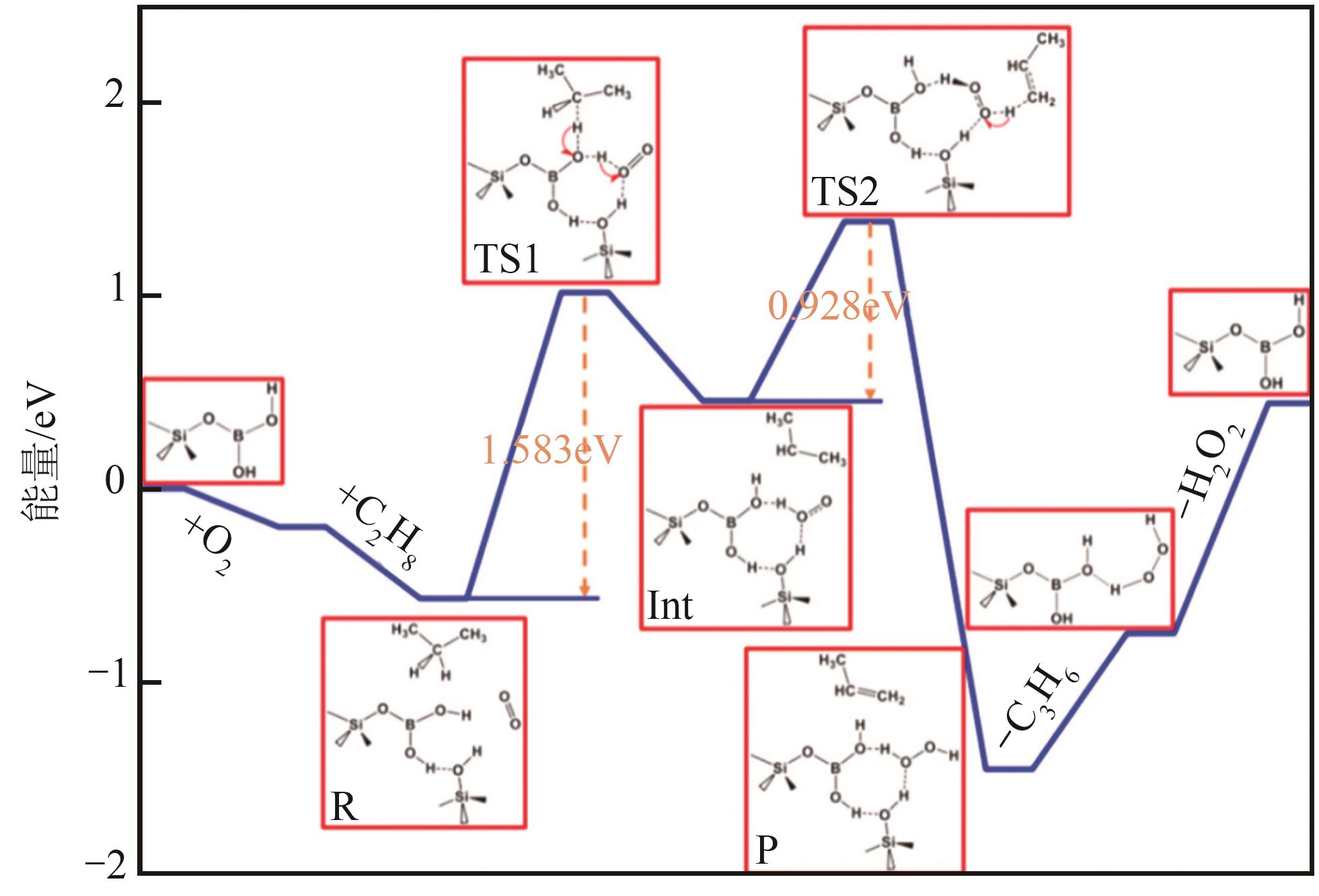
| 90 | SHI L, WANG D Q, LU A H. A viewpoint on catalytic origin of boron nitride in oxidative dehydrogenation of light alkanes[J]. Chinese Journal of Catalysis, 2018, 39(5): 908-913. |
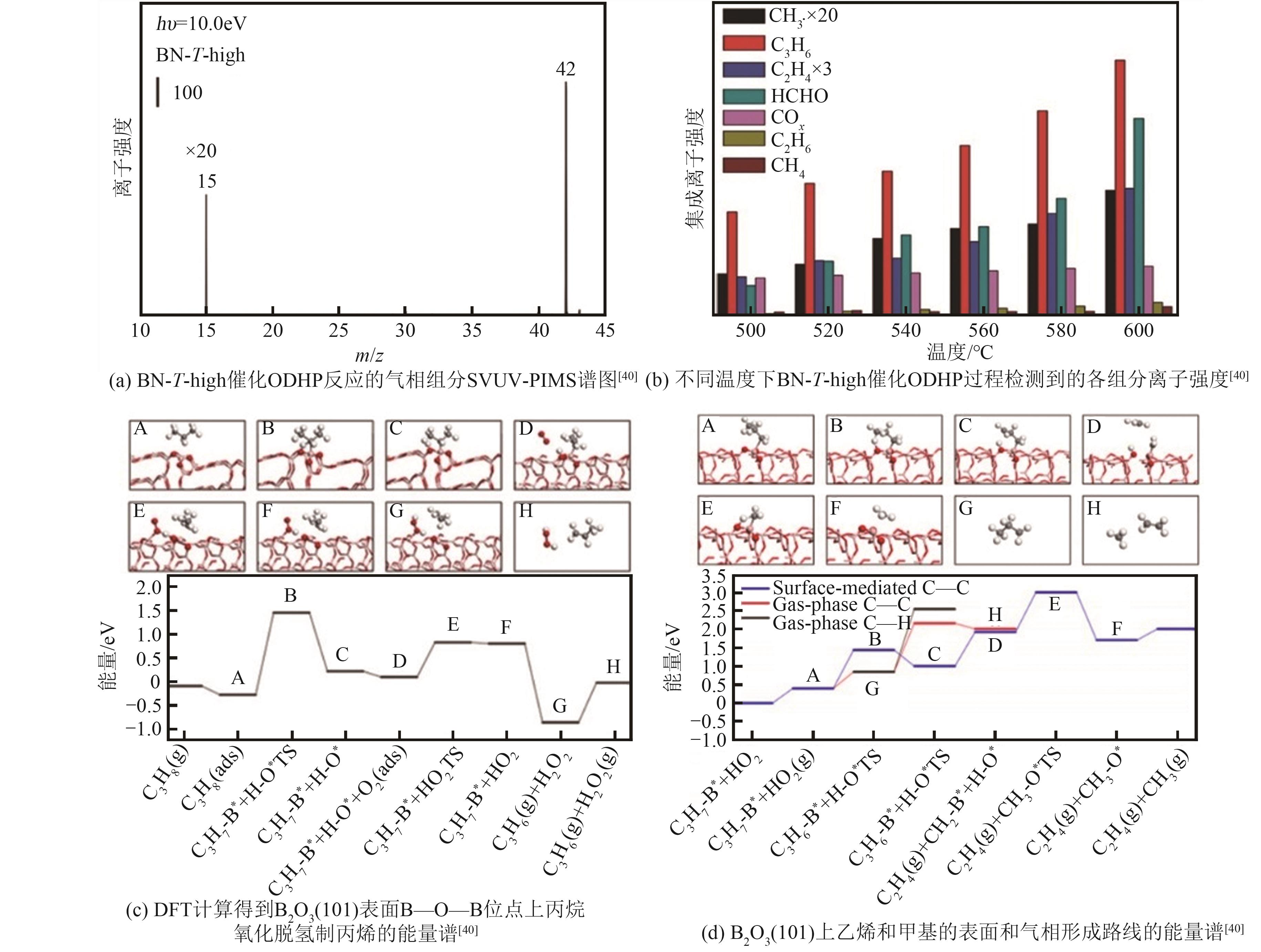
| 91 | TIAN J, TAN J, XU M, et al. Propane oxidative dehydrogenation over highly selective hexagonal boron nitride catalysts: the role of oxidative coupling of methyl[J]. Science Advances, 2019, 5(3): eaav8063. |
| [1] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [2] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [3] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [4] | 岳鑫, 李春迎, 孙道安, 李江伟, 杜咏梅, 马辉, 吕剑. 重氮化合物环丙烷化用多相催化剂研究进展[J]. 化工进展, 2023, 42(5): 2390-2401. |
| [5] | 王子宗, 刘罡, 王振维. 乙烯丙烯生产过程强化技术进展及思考[J]. 化工进展, 2023, 42(4): 1669-1676. |
| [6] | 常晓青, 彭东来, 李东洋, 张延武, 王景, 张亚涛. MOFs基丙烯/丙烷高效分离混合基质膜研究进展[J]. 化工进展, 2023, 42(4): 1961-1973. |
| [7] | 张孟旭, 王红琴, 李金, 安霓虹, 戴云生, 钱颖, 沈亚峰. PtSn/MgAl2O4-sheet催化剂的制备及其PDH反应性能[J]. 化工进展, 2023, 42(3): 1365-1372. |
| [8] | 周皞, 张恒, 温妮妮, 王旭瑞, 徐璐, 李玮, 苏亚欣. Cu-SAPO-44分子筛的制备及其C3H6-SCR脱硝性能[J]. 化工进展, 2023, 42(3): 1373-1382. |
| [9] | 胡兆岩, 张景新, 何义亮. Fe负载污泥生物炭催化热解聚丙烯及产物特性[J]. 化工进展, 2023, 42(2): 631-640. |
| [10] | 辛华, 彭琪, 李阳帆, 张岩, 陈悦, 李新琦. 含氟聚氨酯二甲基丙烯酸酯为芯材的微胶囊制备及自修复性能[J]. 化工进展, 2023, 42(10): 5406-5413. |
| [11] | 孙义明, 冉宝清, 卞武勋, 刘进超, 尹少鼎, 赵西坡. 聚丙烯蜡固-固相变材料的制备与工艺优化[J]. 化工进展, 2023, 42(1): 336-345. |
| [12] | 张广宇, 赵健, 孙峰, 姜杰, 孙冰, 徐伟. CO2催化转化制碳酸丙烯酯研究进展:催化剂设计、性能与反应机理[J]. 化工进展, 2022, 41(S1): 177-189. |
| [13] | 刘楠, 胡一铭, 杨颖, 李红晋, 高竹青, 郝秀丽. 废旧聚丙烯/活性炭微波共裂解制取可燃裂解气与轻质裂解油[J]. 化工进展, 2022, 41(S1): 150-159. |
| [14] | 张雨宸, 张耀远, 吴芹, 史大昕, 陈康成, 黎汉生. 丙烷脱氢用高稳定性Pt基催化剂研究进展[J]. 化工进展, 2022, 41(9): 4733-4753. |
| [15] | 常炜, 史秋兰, 赵正阳, 王瑞婷, 王志福, 赵俭波. 高内相乳液法制备聚丙烯酰胺多孔水凝胶及应用[J]. 化工进展, 2022, 41(7): 3832-3839. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||