化工进展 ›› 2021, Vol. 40 ›› Issue (4): 2016-2033.DOI: 10.16085/j.issn.1000-6613.2020-2036
丙烯酸催化合成新进展
- 中国科学院大连化学物理研究所,辽宁 大连 116023
-
收稿日期:2020-10-10出版日期:2021-04-05发布日期:2021-04-14 -
通讯作者:王峰 -
作者简介:张志鑫(1988—),男,博士,工程师,研究方向为多相催化。E-mail:zhangzhixin@dicp.ac.cn 。 -
基金资助:国家自然科学基金(21706250);辽宁省博士启动基金(2019-BS-244);大连市科技创新基金(2019J11CY009);中国科学院大连化学物理研究所创新基金(DICP I201945)
New advances in catalytic synthesis of acrylic acid
ZHANG Zhixin( ), WANG Yehong, ZHANG Chaofeng, WANG Feng(
), WANG Yehong, ZHANG Chaofeng, WANG Feng( )
)
- Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
-
Received:2020-10-10Online:2021-04-05Published:2021-04-14 -
Contact:WANG Feng
摘要:
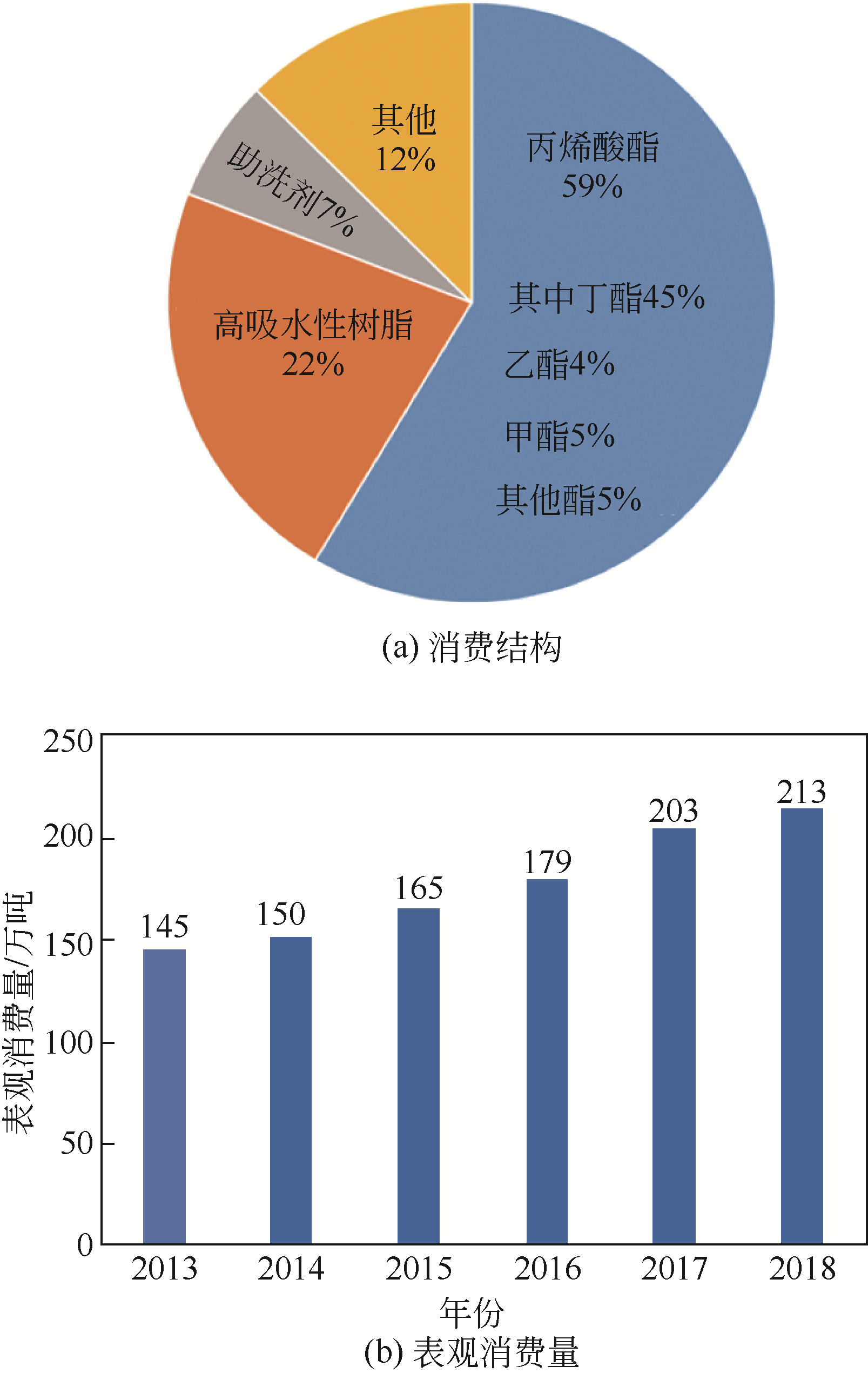
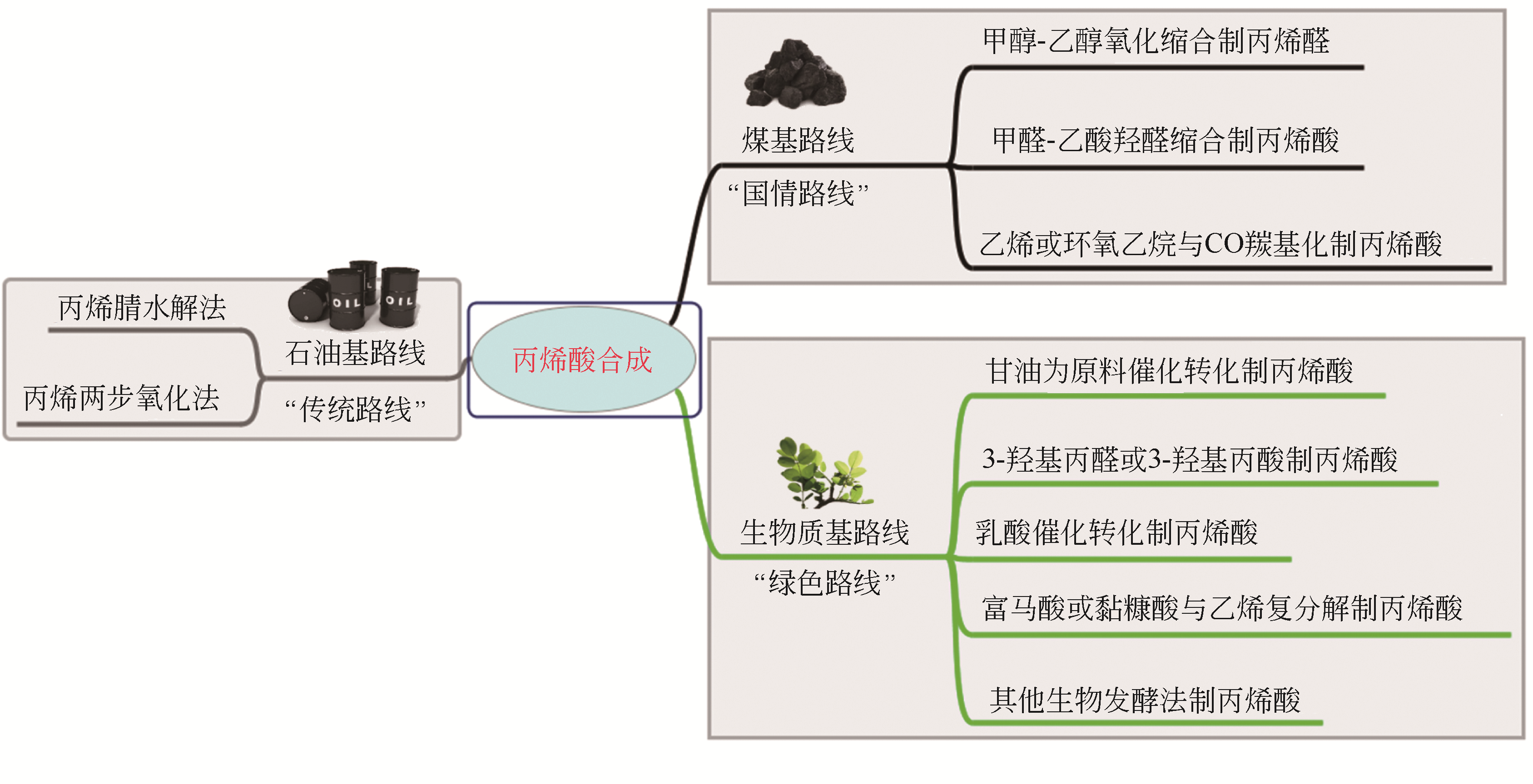
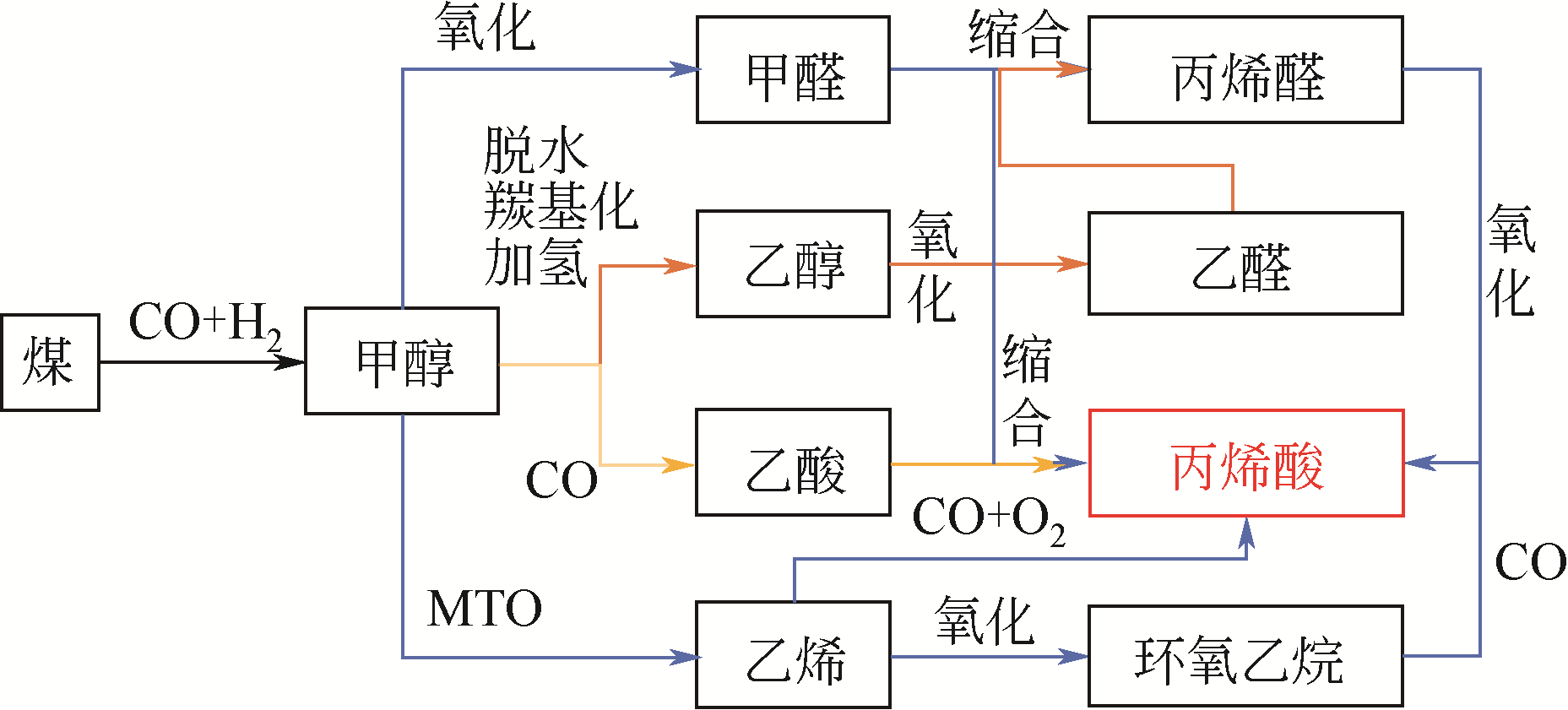
丙烯酸是一种重要的化工中间体和聚合物单体,需求量巨大。我国丰富的煤炭资源和可再生生物质资源为煤基和生物质基丙烯酸合成路线提供坚实的物质保障。本文将综述这两条主要路线,具体包括以煤基化工原料CO、低碳醇(甲醇和乙醇)、甲醛、乙酸、乙烯等为原料的丙烯酸合成路线;以生物质基平台化合物甘油、3-羟基丙酸、乳酸、富马酸、黏糠酸等为原料的丙烯酸合成路线;并对这些路线进行了比较,为路线的选择提供参考。重点关注了这些过程中的催化问题:反应所需的活性位、副反应分析、催化剂的类型与特点以及催化性能与失活机理,为未来实用煤基和生物质基丙烯酸生产用高效稳定廉价催化剂的设计开发提供理论参考。
中图分类号:
引用本文
张志鑫, 王业红, 张超锋, 王峰. 丙烯酸催化合成新进展[J]. 化工进展, 2021, 40(4): 2016-2033.
ZHANG Zhixin, WANG Yehong, ZHANG Chaofeng, WANG Feng. New advances in catalytic synthesis of acrylic acid[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2016-2033.
| 合成路线 | 反应步数 (反应类型) | 原子 经济性 | 研究阶段 |
|---|---|---|---|
| 甲醇-乙醇氧化缩合制丙烯醛,丙烯醛氧化制丙烯酸 | 3(氧化-缩合-氧化) | 57% | 基础研究初期、文献较少 |
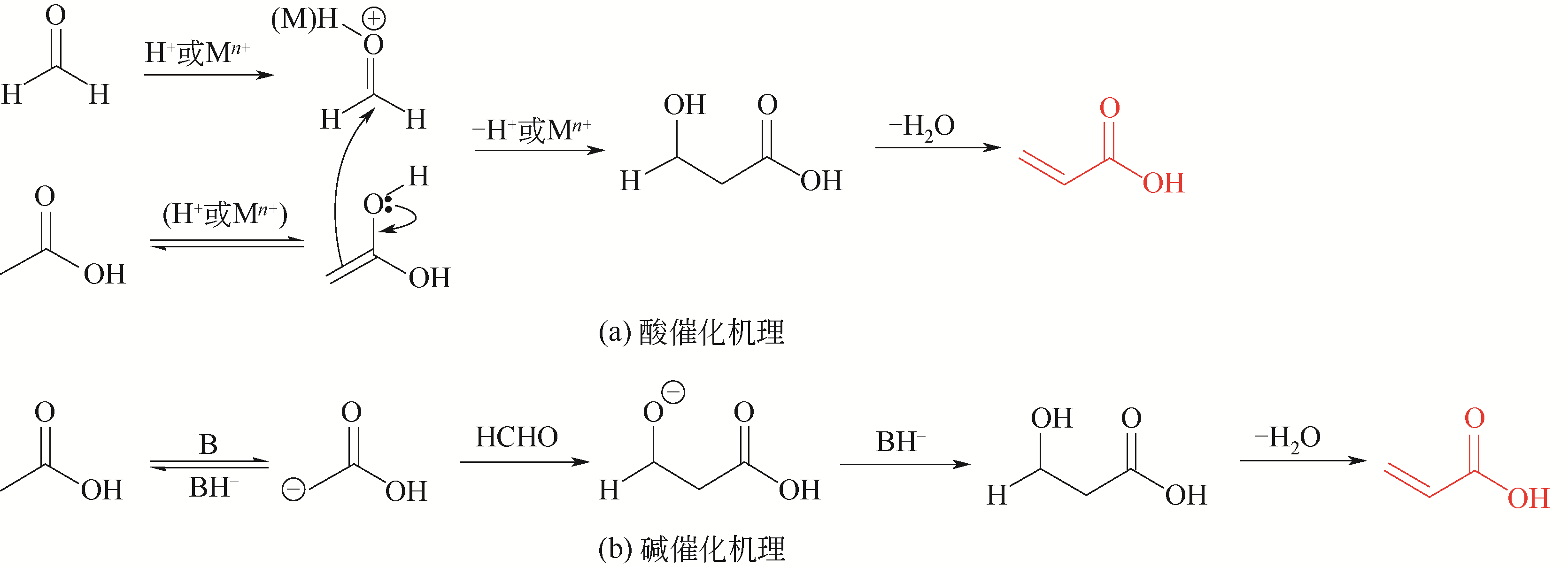
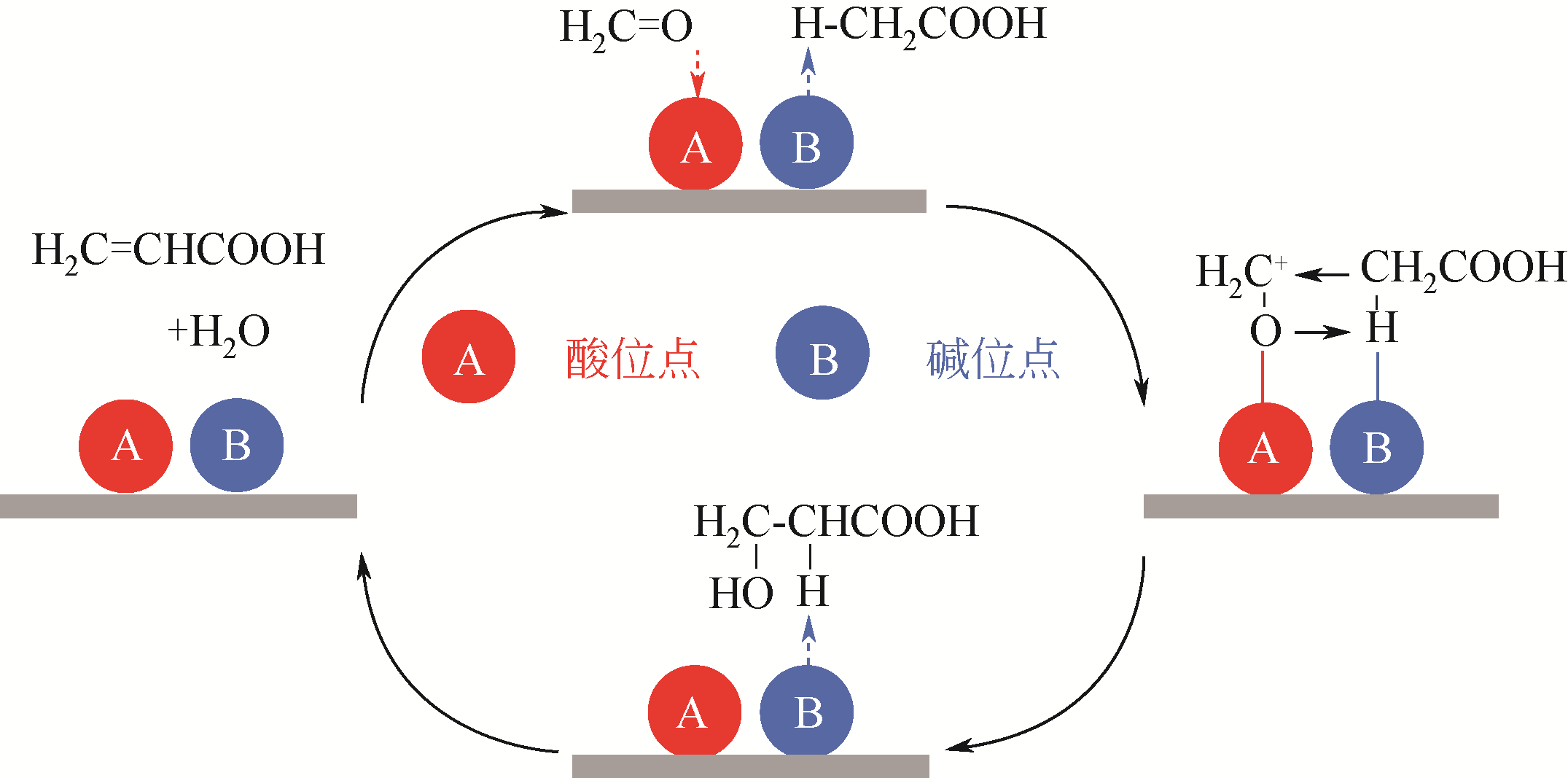
| 甲醛-乙酸羟醛缩合制丙烯酸 | 1(缩合) | 80% | 基础研究较为充分、文献较多 |
| 乙烯或环氧乙烷与CO羰基化制丙烯酸 | 1(氧化羰基化/羰基化) | 100% | 仅有少量专利报道、文献较少 |
表1 煤基丙烯酸合成路线对比
| 合成路线 | 反应步数 (反应类型) | 原子 经济性 | 研究阶段 |
|---|---|---|---|
| 甲醇-乙醇氧化缩合制丙烯醛,丙烯醛氧化制丙烯酸 | 3(氧化-缩合-氧化) | 57% | 基础研究初期、文献较少 |
| 甲醛-乙酸羟醛缩合制丙烯酸 | 1(缩合) | 80% | 基础研究较为充分、文献较多 |
| 乙烯或环氧乙烷与CO羰基化制丙烯酸 | 1(氧化羰基化/羰基化) | 100% | 仅有少量专利报道、文献较少 |
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| FeVO(FeOx/FeVO4) | O2,300℃,10h | C=100%,S=14% | [ |
| WNbVO | O2,285℃ | C=100%,S=46% | [ |
| H3PO4/WNbVO | O2,285℃,1~2h | C=100%,S=59% | [ |
| WVO | O2,318℃,2h | C=100%,S=26% | [ |
| WNbVO | O2,265℃,37h | C=100%,S=51% | [ |
| WMoVO | O2,290℃,69h | C=100%,S=42% | [ |
| H0.1Cs(VO)0.2(PMo12O40)0.5(PW12O40)0.5 | O2,340℃,1h | C=100%,S=60% | [ |
| VSiO | O2,320℃,1h | C=94%,S=85% | [ |
| H-Fe-MCM-22 | O2,320℃,10h | Y=53% | [ |
| Cs2.5H0.5PW12O40/Nb2O5+MoVO-SiC | O2,300℃,70h | C=100%,S=75% | [ |
| V-H3SiW12O40/HZSM-5 | H2O2,90℃,6h | Y=36% | [ |
| Cu/SiO2-MnO2 | H2O2,70℃,30h | C=77%,S=75% | [ |
表2 甘油一步氧化脱水制丙烯酸催化剂及其催化性能
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| FeVO(FeOx/FeVO4) | O2,300℃,10h | C=100%,S=14% | [ |
| WNbVO | O2,285℃ | C=100%,S=46% | [ |
| H3PO4/WNbVO | O2,285℃,1~2h | C=100%,S=59% | [ |
| WVO | O2,318℃,2h | C=100%,S=26% | [ |
| WNbVO | O2,265℃,37h | C=100%,S=51% | [ |
| WMoVO | O2,290℃,69h | C=100%,S=42% | [ |
| H0.1Cs(VO)0.2(PMo12O40)0.5(PW12O40)0.5 | O2,340℃,1h | C=100%,S=60% | [ |
| VSiO | O2,320℃,1h | C=94%,S=85% | [ |
| H-Fe-MCM-22 | O2,320℃,10h | Y=53% | [ |
| Cs2.5H0.5PW12O40/Nb2O5+MoVO-SiC | O2,300℃,70h | C=100%,S=75% | [ |
| V-H3SiW12O40/HZSM-5 | H2O2,90℃,6h | Y=36% | [ |
| Cu/SiO2-MnO2 | H2O2,70℃,30h | C=77%,S=75% | [ |
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| WO3/ZrO2 | 280℃,10h | C=100%,S=65% | [ |
| WO3/TiO2 | 280℃,14h | C=100%,S=73% | [ |
| Nb2O5 | 315℃,10h | C=88%,S=51% | [ |
| Nb2O5/SiO2-ZrO2 | 325℃,8h | C=77%,S=45% | [ |
| HY | 250℃,10h | C=89%,S=100% | [ |
| 丝光沸石 | 250℃,10h | C=92%,S=100% | [ |
| FePO4 | 280℃,5h | C=100%,S=92% | [ |
| VPO | 320℃,2h | C=100%,S=70% | [ |
| Nd4(P2O7)3 | 320℃,7~8h | C=96%,S=83% | [ |
| H3PW12O40/ZrO2 | 315℃,10h | C=76%,S=71% | [ |
| H4SiW12O40/SiO2 | 275℃,5h | C=98%,S=86% | [ |
| Cs2.5H0.5PW12O40 | 275℃,1h | C=100%,S=98% | [ |
| CsSiW12O40/Al2O3 | 250℃,3h | Y=96%,S=96% | [ |
表3 甘油催化脱水制丙烯醛催化剂及其催化性能
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| WO3/ZrO2 | 280℃,10h | C=100%,S=65% | [ |
| WO3/TiO2 | 280℃,14h | C=100%,S=73% | [ |
| Nb2O5 | 315℃,10h | C=88%,S=51% | [ |
| Nb2O5/SiO2-ZrO2 | 325℃,8h | C=77%,S=45% | [ |
| HY | 250℃,10h | C=89%,S=100% | [ |
| 丝光沸石 | 250℃,10h | C=92%,S=100% | [ |
| FePO4 | 280℃,5h | C=100%,S=92% | [ |
| VPO | 320℃,2h | C=100%,S=70% | [ |
| Nd4(P2O7)3 | 320℃,7~8h | C=96%,S=83% | [ |
| H3PW12O40/ZrO2 | 315℃,10h | C=76%,S=71% | [ |
| H4SiW12O40/SiO2 | 275℃,5h | C=98%,S=86% | [ |
| Cs2.5H0.5PW12O40 | 275℃,1h | C=100%,S=98% | [ |
| CsSiW12O40/Al2O3 | 250℃,3h | Y=96%,S=96% | [ |
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| 硅胶 | 15% 3-HPA水溶液,300℃,WHSV=1h-1 | C=100%,Y>99% | [ |
| TiO2 | — | Y=95% | [ |
| SiO2 | 20% 3-HPA水溶液,250℃ | C=100%,Y=97% | [ |
| Al2O3 | 60%~80% 3-HPA水溶液,250℃ | C=100%,Y=97% | [ |
表4 3-羟基丙酸(3-HPA)催化脱水制丙烯酸催化剂及其催化性能
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| 硅胶 | 15% 3-HPA水溶液,300℃,WHSV=1h-1 | C=100%,Y>99% | [ |
| TiO2 | — | Y=95% | [ |
| SiO2 | 20% 3-HPA水溶液,250℃ | C=100%,Y=97% | [ |
| Al2O3 | 60%~80% 3-HPA水溶液,250℃ | C=100%,Y=97% | [ |
| 催化剂 | 反应条件 | 催化性能或效果 | 参考文献 |
|---|---|---|---|
| HAP | 360℃,乳酸WHSV=1.4~2.1h-1, 8h | S=71%~74%,Y=50%~62% | [ |
| Rb0.95Na0.05β | 360℃,乳酸WHSV=2.1h-1, 10h | S=70%,Y=60%~65% | [ |
| K0.97Na0.03ZSM-5 | 360℃,乳酸WHSV=2.1h-1, 10h | S=80%,Y=74%~78% | [ |
| Na2HPO4/NaY | 340℃ | Y=58%~74% | [ |
| Ca2P2O7 | 25%(质量分数),乳酸,375℃,WHSV=3 | C=100%,Y=78% | [ |
| BaSO4 | 400℃ | Y=74% | [ |
表5 乳酸催化脱水制丙烯酸催化剂及其催化性能
| 催化剂 | 反应条件 | 催化性能或效果 | 参考文献 |
|---|---|---|---|
| HAP | 360℃,乳酸WHSV=1.4~2.1h-1, 8h | S=71%~74%,Y=50%~62% | [ |
| Rb0.95Na0.05β | 360℃,乳酸WHSV=2.1h-1, 10h | S=70%,Y=60%~65% | [ |
| K0.97Na0.03ZSM-5 | 360℃,乳酸WHSV=2.1h-1, 10h | S=80%,Y=74%~78% | [ |
| Na2HPO4/NaY | 340℃ | Y=58%~74% | [ |
| Ca2P2O7 | 25%(质量分数),乳酸,375℃,WHSV=3 | C=100%,Y=78% | [ |
| BaSO4 | 400℃ | Y=74% | [ |
| 合成路线 | 反应步数(反应类型) | 原子经济性,原料成本(以丙烯酸为基准) | 研究阶段 |
|---|---|---|---|
| 甘油为原料催化转化制丙烯酸 | 1~2(脱水、氧化/氨氧化等) | <67%,6327CNY·t-1 | 基础研究充分,大量文献报道 |
| 3-羟基丙醛或3-羟基丙酸催化脱水制丙烯酸 | 1(脱水) | 80%,— | 基础研究较为充分,大量文献报道 |
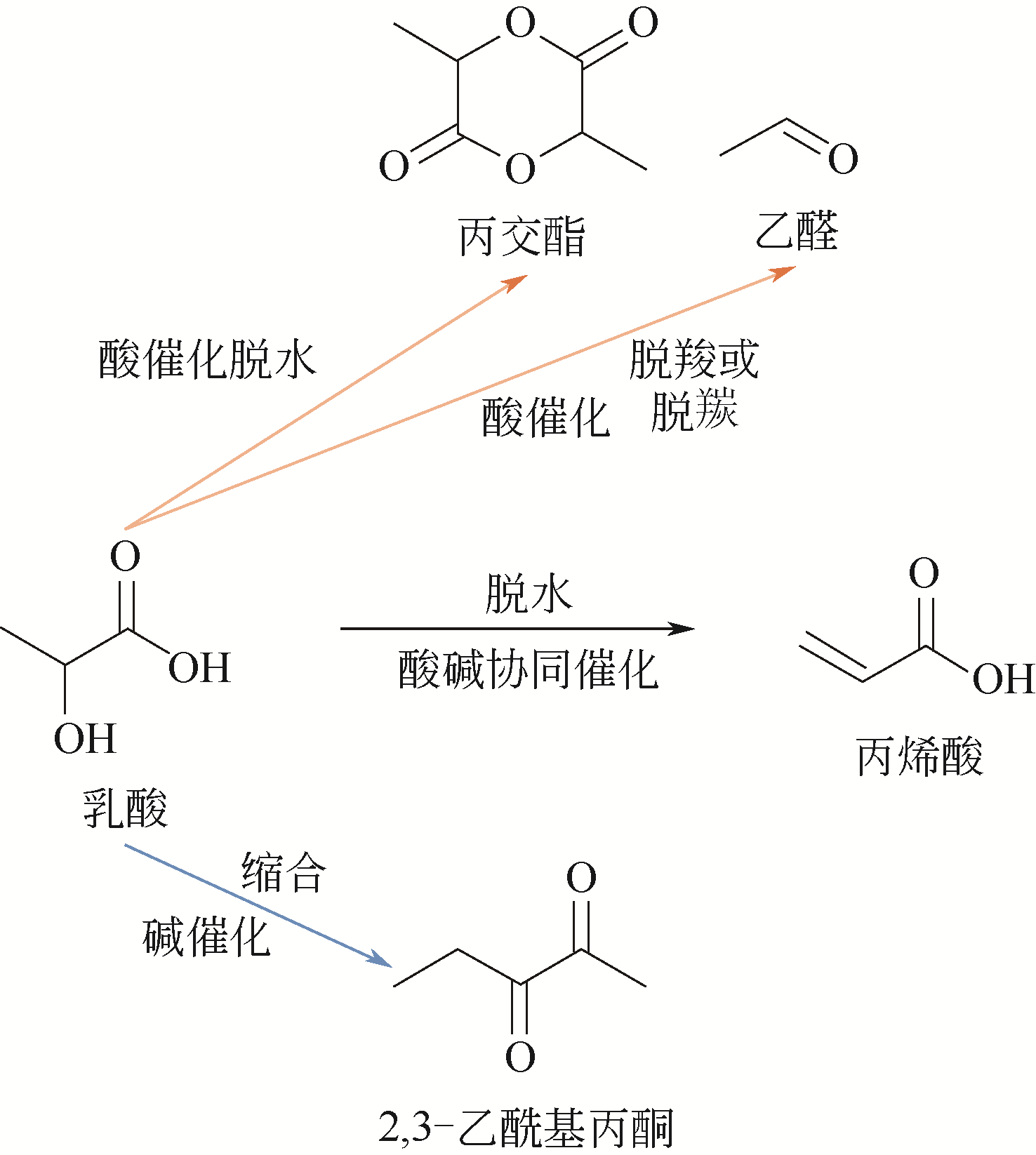
| 乳酸催化脱水制丙烯酸 | 1(脱水) | 80%,10750CNY·t-1 | 基础研究较为充分,大量文献报道 |
| 富马酸与乙烯复分解制丙烯酸 | 1(烯烃复分解) | 100%,8396CNY·t-1 | 仅有少量专利报道、文献较少 |
| 黏糠酸与乙烯复分解制丙烯酸 | 1(烯烃复分解) | 100%,23620CNY·t-1 | 仅有少量专利报道、文献较少 |
表6 生物质基丙烯酸合成路线对比
| 合成路线 | 反应步数(反应类型) | 原子经济性,原料成本(以丙烯酸为基准) | 研究阶段 |
|---|---|---|---|
| 甘油为原料催化转化制丙烯酸 | 1~2(脱水、氧化/氨氧化等) | <67%,6327CNY·t-1 | 基础研究充分,大量文献报道 |
| 3-羟基丙醛或3-羟基丙酸催化脱水制丙烯酸 | 1(脱水) | 80%,— | 基础研究较为充分,大量文献报道 |
| 乳酸催化脱水制丙烯酸 | 1(脱水) | 80%,10750CNY·t-1 | 基础研究较为充分,大量文献报道 |
| 富马酸与乙烯复分解制丙烯酸 | 1(烯烃复分解) | 100%,8396CNY·t-1 | 仅有少量专利报道、文献较少 |
| 黏糠酸与乙烯复分解制丙烯酸 | 1(烯烃复分解) | 100%,23620CNY·t-1 | 仅有少量专利报道、文献较少 |
| 1 | BEERTHUIS R, ROTHENBERG G, SHIJU N R. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables[J]. Green Chemistry, 2015, 17(3): 1341-1361. |
| 2 | GAUTAM P, NEHA, UPADHYAY S N, et al. Bio-methanol as a renewable fuel from waste biomass: current trends and future perspective[J]. Fuel, 2020, 273: 117783. |
| 3 | HAHN-HAGERDAL B, GALBE M, GORWA-GRAUSLUND M F, et al. Bio-ethanol - the fuel of tomorrow from the residues of today[J]. Trends in Biotechnology, 2006, 24(12): 549-556. |
| 4 | BOROWIEC A, DEVAUX J F, DUBOIS J L, et al. An acrolein production route from ethanol and methanol mixtures over FeMo-based catalysts[J]. Green Chemistry, 2017, 19(11): 2666-2674. |
| 5 | BOROWIEC A, LILIĆ A, MORIN J C, et al. Acrolein production from methanol and ethanol mixtures over La- and Ce-doped FeMo catalysts[J]. Applied Catalysis B: Environmental, 2018, 237: 149-157. |
| 6 | LILIĆ A, BENNICI S, DEVAUX J F, et al. Influence of catalyst acid/base properties in acrolein production by oxidative coupling of ethanol and methanol[J]. ChemSusChem, 2017, 10(9): 1916-1930. |
| 7 | LILIĆ A, WEI T T, BENNICI S, et al. A comparative study of basic, amphoteric, and acidic catalysts in the oxidative coupling of methanol and ethanol for acrolein production[J]. ChemSusChem, 2017, 10(17): 3459-3472. |
| 8 | STOŠIĆ D, HOSOGLU F, BENNICI S, et al. Methanol and ethanol reactivity in the presence of hydrotalcites with Mg/Al ratios varying from 2 to 7[J]. Catalysis Communications, 2017, 89: 14-18. |
| 9 | VITCHA J F, SIMS V A. Vapor phase aldol reaction. Acrylic acid by reaction of acetic acid and formaldehyde[J]. I&EC Product Research and Development, 1966, 5(1): 50-53. |
| 10 | GUO Xinpeng, YANG Dan, ZUO Cuncun, et al. Catalysts, process optimization, and kinetics for the production of methyl acrylate over vanadium phosphorus oxide catalysts[J]. Industrial & Engineering Chemistry Research, 2017, 56(20): 5860-5871. |
| 63 | LI Xiukai, ZHANG Yugen. Highly efficient process for the conversion of glycerol to acrylic acid via gas phase catalytic oxidation of an allyl alcohol intermediate[J]. ACS Catalysis, 2016, 6(1): 143-150. |
| 64 | SHIRAMIZU M, TOSTE F D. Deoxygenation of biomass-derived feedstocks: oxorhenium-catalyzed deoxydehydration of sugars and sugar alcohols[J]. Angewandte Chemie International Edition, 2012, 51(32): 8082-8086. |
| 11 | ZUO Cuncun, LI Chunshan, GE Tingting, et al. Spherical P-modified catalysts for heterogeneous cross-aldol condensation of formaldehyde with methyl acetate for methyl acrylate production[J]. Canadian Journal of Chemical Engineering, 2017, 95(11): 2104-2111. |
| 12 | MA Zhanling, MA Xiangang, LIU Hongchao, et al. A green route to methyl acrylate and acrylic acid by an aldol condensation reaction over H-ZSM-35 zeolite catalysts[J]. Chemical Communications, 2017, 53(65): 9071-9074. |
| 13 | YAN Jianbiao, ZHANG Chunlei, NING Chunli, et al. Vapor phase condensation of methyl acetate with formaldehyde to preparing methyl acrylate over cesium supported SBA-15 catalyst[J]. Journal of Industrial and Engineering Chemistry, 2015, 25: 344-351. |
| 14 | AI M. Vapor-phase aldol condensation of formaldehyde with acetic acid on V2O5-P2O5 catalysts[J]. Journal of Catalysis, 1987, 107(1): 201-208. |
| 15 | AI M. Effects of organic-compounds used in preparing V/Ti binary phosphate catalysts[J]. Journal of Catalysis, 1988, 113(2): 562-566. |
| 16 | FENG Xinzhen, SUN Bo, YAO Yao, et al. Renewable production of acrylic acid and its derivative: new insights into the aldol condensation route over the vanadium phosphorus oxides[J]. Journal of Catalysis, 2014, 314: 132-141. |
| 17 | LIU Jun, WANG Pengcheng, FENG Yina, et al. Precisely phase-modulated VPO catalysts with enhanced inter-phase conjunction for acrylic acid production through the condensation of acetic acid and formaldehyde[J]. Journal of Catalysis, 2019, 374: 171–182. |
| 18 | YANG Dan, LI Dan, YAO Haoyu, et al. Reaction of formalin with acetic acid over vanadium-phosphorus oxide bifunctional catalyst[J]. Industrial & Engineering Chemistry Research, 2015, 54(27): 6865-6873. |
| 19 | HU Jing, LU Zhipeng, YIN Hengbo, et al. Aldol condensation of acetic acid with formaldehyde to acrylic acid over SiO2-, SBA-15-, and HZSM-5-supported V-P-O catalysts[J]. Journal of Industrial and Engineering Chemistry, 2016, 40: 145-151. |
| 20 | ZHAO Hui, ZUO Cuncun, YANG Dan, et al. Effects of support for vanadium phosphorus oxide catalysts on vapor-phase aldol condensation of methyl acetate with formaldehyde[J]. Industrial & Engineering Chemistry Research, 2016, 55(50): 12693-12702. |
| 21 | WANG Yumeng, WANG Zhenlu, HAO Xue, et al. Nb-doped vanadium phosphorus oxide catalyst for the aldol condensation of acetic acid with formaldehyde to acrylic acid[J]. Industrial & Engineering Chemistry Research, 2018, 57(36): 12055-12060. |
| 22 | WANG Gang, SARARUK C, LI Zengxi, et al. Studies on mild catalytic synthesis of methyl acrylate via one-step aldol reaction[J]. AIChE Journal, 2018, 64(4): 1359-1372. |
| 23 | WANG Gang, LI Zengxi, LI Chunshan, et al. Preparation of methyl acrylate from methyl acetate and methanol with mild catalysis of cobalt complex[J]. Chemical Engineering Journal, 2019, 359: 863-873. |
| 24 | WANG Xiao, WANG Hui, SUN Yuhan. Synthesis of acrylic acid derivatives from CO2 and ethylene[J]. Chem, 2017, 3(2): 211-228. |
| 25 | SUN Daolai, YAMADA Y, SATO S, et al. Glycerol as a potential renewable raw material for acrylic acid production[J]. Green Chemistry, 2017, 19(14): 3186-3213. |
| 26 | GRASSELLI R K, TRIFIRO F. Acrolein and acrylic acid from biomass[J]. Rendiconti Lincei-Scienze Fisiche E: Naturali, 2017, 28: 59-67. |
| 27 | 花东龙, 庄晓煜, 童东绅, 等.催化甘油胶水氧化连串反应制丙烯酸[J]. 化学进展, 2016, 28(S2): 375-390. |
| HUA Donglong, ZHUANG Xiaoyu, TONG Dongshen, et al. Catalytic oxidehydration of glycerol to acrylic acid[J]. Progress in Chemistry, 2016, 28(S2): 375-390. | |
| 28 | WANG Feng, XU Jie, DUBOIS J L, et al. Catalytic oxidative dehydration of glycerol over a catalyst with iron oxide domains embedded in an iron orthovanadate phase[J]. ChemSusChem, 2010, 3(12): 1383-1389. |
| 29 | OMATA K, MATSUMOTO K, MURAYAMA T, et al. Direct oxidative transformation of glycerol into acrylic acid over phosphoric acid-added W-V-Nb complex metal oxide catalysts[J]. Chemistry Letters, 2014, 43(4): 435-437. |
| 30 | OMATA K, MATSUMOTO K, MURAYAMA T, et al. Direct oxidative transformation of glycerol to acrylic acid over Nb-based complex metal oxide catalysts[J]. Catalysis Today, 2016, 259: 205-212. |
| 31 | CHIEREGATO A, SORIANO M D, BASILE F, et al. One-pot glycerol oxidehydration to acrylic acid on multifunctional catalysts: Focus on the influence of the reaction parameters in respect to the catalytic performance[J]. Applied Catalysis B: Environmental, 2014, 150: 37-46. |
| 32 | CHIERGATO A, SORIANO M D, GARÍA-GONZÁLEZ E, et al. Multielement crystalline and pseudocrystalline oxides as efficient catalysts for the direct transformation of glycerol into acrylic acid[J]. ChemSusChem, 2015, 8(2): 398-406. |
| 33 | YUN Yang Sik, Kyung Rok LEE, PARK Hongseok, et al. Rational design of a bifunctional catalyst for the oxydehydration of glycerol: a combined theoretical and experimental study[J]. ACS Catalysis, 2015, 5(1): 82-94. |
| 34 | LI Xiukai, ZHANG Yugen. Oxidative dehydration of glycerol to acrylic acid over vanadium-substituted cesium salts of Keggin-type heteropolyacids[J]. ACS Catalysis, 2016, 6(5): 2785-2791. |
| 35 | PAULA A S, POSSATO L G, RATERO D R, et al. One-step oxidehydration of glycerol to acrylic acid using ETS-10-like vanadosilicates[J]. Microporous and Mesoporous Materials, 2016, 232: 151-160. |
| 36 | SANTOS M B DOS, ANDRADE H M C, MASCARENHAS A J S. Oxidative dehydration of glycerol over alternative H,Fe-MCM-22 catalysts: sustainable production of acrylic acid[J]. Microporous and Mesoporous Materials, 2019, 278: 366-377. |
| 37 | LIU Rong, WANG Tiefeng, LIU Chang, et al. Highly selective and stable CsPW/Nb2O5 catalysts for dehydration of glycerol to acrolein[J]. Chinese Journal of Catalysis, 2013, 34(12): 2174–2182. |
| 38 | THANASILP S, SCHWANK J W, MEEYOO V, et al. One-pot oxydehydration of glycerol to value-added compounds over metal-doped SiW/HZSM-5 catalysts: effect of metal type and loading[J]. Chemical Engineering Journal, 2015, 275: 113-124. |
| 39 | SARKAR B, PENDEM C, SIVAKUMAR KONATHALA L N, et al. Cu nanoclusters supported on nanocrystalline SiO2-MnO2: a bifunctional catalyst for the one-step conversion of glycerol to acrylic acid[J]. Chemical Communications, 2014, 50(68): 9707-9710. |
| 40 | SORIANO M D, CONCEPCIÓN P, NIETO J M L, et al. Tungsten-vanadium mixed oxides for the oxidehydration of glycerol into acrylic acid[J]. Green Chemistry, 2011, 13(10): 2954-2962. |
| 41 | KATRYNIOK B, PAUL S, CAPRON M, et al. Towards the sustainable production of acrolein by glycerol dehydration[J]. ChemSusChem, 2009, 2(8): 719-730. |
| 42 | KATRYNIOK B, PAUL S, DUMEIGNIL F. Recent developments in the field of catalytic dehydration of glycerol to acrolein[J]. ACS Catalysis, 2013, 3(8): 1819-1834. |
| 43 | WANG Zichun, WANG Leizhi, JIANG Yijiao, et al. Cooperativity of Brønsted and Lewis acid sites on zeolite for glycerol dehydration[J]. ACS Catalysis, 2014, 4(4): 1144-1147. |
| 44 | YUN Danim, YUN Yang Sik, KIM Tae Yong, et al. Mechanistic study of glycerol dehydration on Brønsted acidic amorphous aluminosilicate[J]. Journal of Catalysis, 2016, 341: 33-43. |
| 45 | CHAI Songhai, WANG Haopeng, LIANG Yu, et al. Sustainable production of acrolein: investigation of solid acid-base catalysts for gas-phase dehydration of glycerol[J]. Green Chemistry, 2007, 9(10): 1130-1136. |
| 46 | DALIL M, DE CARNEVALI D, EDAKE M, et al. Gas phase dehydration of glycerol to acrolein: coke on WO3/TiO2 reduces by-products[J]. Journal of Molecular Catalysis A: Chemical, 2016, 421: 146-155. |
| 47 | CHAI Songhai, WANG Haopeng, LIANG Yu, et al. Sustainable production of acrolein: gas-phase dehydration of glycerol over Nb2O5 catalyst[J]. Journal of Catalysis, 2007, 250(2): 342-349. |
| 48 | GARCÍA-SANCHO C, CECILIA J A, MORENO-RUIZ A, et al. Influence of the niobium supported species on the catalytic dehydration of glycerol to acrolein[J]. Applied Catalysis B: Environmental, 2015, 179: 139-149. |
| 49 | DE OLIVEIRA A S, VASCONCELOS S J S, DE SOUSA J R, et al. Catalytic conversion of glycerol to acrolein over modified molecular sieves: activity and deactivation studies[J]. Chemical Engineering Journal, 2011, 168(2): 765-774. |
| 50 | DECOLATTI H P, COSTA B O D, QUERINI C A. Dehydration of glycerol to acrolein using H-ZSM5 zeolite modified by alkali treatment with NaOH[J]. Microporous and Mesoporous Materials, 2015, 204: 180-189. |
| 51 | DELEPLANQUE J, DUBOIS J L, DEVAUX J F, et al. Production of acrolein and acrylic acid through dehydration and oxydehydration of glycerol with mixed oxide catalysts[J]. Catalysis Today, 2010, 157(1/2/3/4): 351-358. |
| 52 | FENG Xinzhen, YAO Yao, SU Qin, et al. Vanadium pyrophosphate oxides: the role of preparation chemistry in determining renewable acrolein production from glycerol dehydration[J]. Applied Catalysis B: Environmental, 2015, 164: 31-39. |
| 53 | LIU Qingbo, ZHANG Zhen, DU Ying, et al. Rare earth pyrophosphates: effective catalysts for the production of acrolein from vapor-phase dehydration of glycerol[J]. Catalysis Letters, 2009, 127(3/4): 419-428. |
| 54 | CHAI Songhai, WANG Haopeng, LIANG Yu, et al. Sustainable production of acrolein: preparation and characterization of zirconia-supported 12-tungstophosphoric acid catalyst for gas-phase dehydration of glycerol[J]. Applied Catalysis A: General, 2009, 353(2): 213-222. |
| 55 | TSUKUDA E, SATO S, TAKAHASHI R, et al. Production of acrolein from glycerol over silica-supported heteropoly acids[J]. Catalysis Communications, 2007, 8(9): 1349-1353. |
| 56 | ALHANASH A, KOZHEVNIKOVA E F, KOZHEVNIKOV I V. Gas-phase dehydration of glycerol to acrolein catalysed by caesium heteropoly salt[J]. Applied Catalysis A: General, 2010, 378(1): 11-18. |
| 57 | HAIDER M H, DUMMER N F, ZHANG Dazhi, et al. Rubidium- and caesium-doped silicotungstic acid catalysts supported on alumina for the catalytic dehydration of glycerol to acrolein[J]. Journal of Catalysis, 2012, 286: 206-213. |
| 58 | CHAI Songhai, TAO Lizhi, YAN Bo, et al. Sustainable production of acrolein: effects of reaction variables, modifiers doping and ZrO2 origin on the performance of WO3/ZrO2 catalyst for the gas-phase dehydration of glycerol[J]. RSC Advances, 2014, 4(9): 4619-4630. |
| 59 | SUN Daolai, YAMADA Y, SATO S, et al. Glycerol hydrogenolysis into useful C3 chemicals[J]. Applied Catalysis B: Environmental, 2016, 193: 75-92. |
| 60 | ARCEO E, MARSDEN P, BERGMAN R G, et al. An efficient didehydroxylation method for the biomass-derived polyols glycerol and erythritol. Mechanistic studies of a formic acid-mediated deoxygenation[J]. Chemical Communications, 2009(23): 3357-3359. |
| 61 | ARCEO E, ELLMAN J A, BERGMAN R G. A direct, biomass-based synthesis of benzoic acid: formic acid-mediated deoxygenation of the glucose-derived materials quinic acid and shikimic acid[J]. ChemSusChem, 2010, 3(7): 811-813. |
| 62 | KAMM O, MARVEL C S. Allyl alcohol[J]. Organic Syntheses, 1921, 1: 15-19. |
| 65 | YI Jing, LIU Shuo, ABU-OMAR M M. Rhenium-catalyzed transfer hydrogenation and deoxygenation of biomass-derived polyols to small and useful organics[J]. ChemSusChem, 2012, 5(8): 1401-1404. |
| 66 | CANALE V, TONUCCI L, BRESSAN M, et al. Deoxydehydration of glycerol to allyl alcohol catalyzed by rhenium derivatives[J]. Catalysis Science & Technology, 2014, 4(10): 3697-3704. |
| 67 | LIU Yong, TÜYSÜZ H, JIA Chunjiang, et al. From glycerol to allyl alcohol: iron oxide catalyzed dehydration and consecutive hydrogen transfer[J]. Chemical Communications, 2010, 46(8): 1238-1240. |
| 68 | KONAKA A, TAGO T, YOSHIKAWA T, et al. Conversion of biodiesel-derived crude glycerol into useful chemicals over a zirconia-iron oxide catalyst[J]. Industrial & Engineering Chemistry Research, 2013, 52(44): 15509-15515. |
| 69 | KONAKA A, TAGO T, YOSHIKAWA T, et al. Conversion of glycerol into allyl alcohol over potassium-supported zirconia-iron oxide catalyst[J]. Applied Catalysis B: Environmental, 2014, 146: 267-273. |
| 70 | SANTOS R C R, BRAGA D M V, PINHEIRO A N, et al. Role of Cu, Ni and Co metals in the acidic and redox properties of Mo catalysts supported on Al2O3 spheres for glycerol conversion[J]. Catalysis Science & Technology, 2016, 6(13): 4986-5002. |
| 71 | TAZAWA S, OTA N, TAMURA M, et al. Deoxydehydration with molecular hydrogen over ceria-supported rhenium catalyst with gold promoter[J]. ACS Catalysis, 2016, 6(10): 6393-6397. |
| 72 | YANG Sungpil, KIM Minsu, YANG Sungeun, et al. Production of acrylic acid from biomass-derived allyl alcohol by selective oxidation using Au/ceria catalysts[J]. Catalysis Science & Technology, 2016, 6(10): 3616-3622. |
| 73 | KIM Minsu, Hyunjoo LEE. Highly selective production of acrylic acid from glycerol via two steps using Au/CeO2 catalysts[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(12): 11371-11376. |
| 74 | SERESHKI B R, BALAN S J, PATIENCE G S, et al. Reactive vaporization of crude glycerol in a fluidized bed reactor[J]. Industrial & Engineering Chemistry Research, 2010, 49(3): 1050-1056. |
| 75 | LIU Rong, Shuting LYU, WANG Tiefeng. Sustainable production of acrolein from biodiesel-derived crude glycerol over H3PW12O40 supported on Cs-modified SBA-15[J]. Journal of Industrial and Engineering Chemistry, 2016, 37: 354-360. |
| 76 | VALDEHUESA K N G, LIU Huaiwei, NISOLA G M, et al. Recent advances in the metabolic engineering of microorganisms for the production of 3-hydroxypropionic acid as C3 platform chemical[J]. Applied Microbiology and Biotechnology, 2013, 97(8): 3309-3321. |
| 77 | CHEN Yun, NIELSEN J. Advances in metabolic pathway and strain engineering paving the way for sustainable production of chemical building blocks[J]. Current Opinion in Biotechnology, 2013, 24(6): 965-972. |
| 78 | CHEN Zhen, LIU Dehua. Toward glycerol biorefinery: metabolic engineering for the production of biofuels and chemicals from glycerol[J]. Biotechnology for Biofuels, 2016, 9: 205. |
| 79 | LI Chao, ZHU Qiangqiang, CUI Ziheng, et al. Highly efficient and selective production of acrylic acid from 3-hydroxypropionic acid over acidic heterogeneous catalysts[J]. Chemical Engineering Science, 2018, 183: 288-294. |
| 80 | TAM M S, CRACIUN R, MILLER D J, et al. Reaction and kinetic studies of lactic acid conversion over alkali-metal salts[J]. Industrial & Engineering Chemistry Research, 1998, 37(6): 2360-2366. |
| 81 | DUSSELIER M, WOUWE P VAN, DEWAELE A, et al. Shape-selective zeolite catalysis for bioplastics production[J]. Science, 2015, 349(6243): 78-80. |
| 82 | KATRYNIOK B, PAUL S, DUMEIGNIL F. Highly efficient catalyst for the decarbonylation of lactic acid to acetaldehyde[J]. Green Chemistry, 2010, 12(11): 1910-1913. |
| 83 | MÄKI-ARVELA P, SIMAKOVA I L, SALMI T, et al. Production of lactic acid/lactates from biomass and their catalytic transformations to commodities[J]. Chemical Reviews, 2014, 114(3): 1909-1971. |
| 84 | MATSUURA Y, ONDA A, OGO S, et al. Acrylic acid synthesis from lactic acid over hydroxyapatite catalysts with various cations and anions[J]. Catalysis Today, 2014, 226: 192-197. |
| 85 | MATSUURA Y, ONDA A, YANAGISAWA K. Selective conversion of lactic acid into acrylic acid over hydroxyapatite catalysts[J]. Catalysis Communications, 2014, 48: 5-10. |
| 86 | BLANCO E, DELICHERE P, MILLET J M M, et al. Gas phase dehydration of lactic acid to acrylic acid over alkaline-earth phosphates catalysts[J]. Catalysis Today, 2014, 226: 185-191. |
| 87 | YAN Bo, TAO Lizhi, LIANG Yu, et al. Sustainable production of acrylic acid: catalytic performance of hydroxyapatites for gas-phase dehydration of lactic acid[J]. ACS Catalysis, 2014, 4(6): 1931-1943. |
| 88 | YAN Bo, TAO Lizhi, LIANG Yu, et al. Sustainable production of acrylic acid: alkali-ion exchanged beta zeolite for gas-phase dehydration of lactic acid[J]. Chemsuschem, 2014, 7(6): 1568-1578. |
| 89 | YAN Bo, MAHMOOD A, LIANG Yu, et al. Sustainable production of acrylic acid: Rb+- and Cs+-exchanged beta zeolite catalysts for catalytic gas-phase dehydration of lactic acid[J]. Catalysis Today, 2016, 269: 65-73. |
| 90 | NÄFE G, L􀆕PEZ-MARTÍNEZ M A, DYBALLA M,et al. Deactivation behavior of alkali-metal zeolites in the dehydration of lactic acid to acrylic acid[J]. Journal of Catalysis, 2015, 329: 413-424. |
| 91 | ZHANG Xianghui, LIN Lu, ZHANG Tong, et al. Catalytic dehydration of lactic acid to acrylic acid over modified ZSM-5 catalysts[J]. Chemical Engineering Journal, 2016, 284: 934-941. |
| 92 | GUO Zhen, THENG De Sheng, TANG K Yuanting, et al. Dehydration of lactic acid to acrylic acid over lanthanum phosphate catalysts: the role of Lewis acid sites[J]. Physical Chemistry Chemical Physics, 2016, 18(34): 23746-23754. |
| 93 | Shuting LÜ, WANG Tiefeng. Efficient production of acrylic acid by dehydration of lactic acid over BaSO4 with crystal defects[J]. RSC Advances, 2017, 7(17): 10278-10286. |
| 94 | YAN Bo, TAO Lizhi, MAHMOOD A, et al. Potassium-ion-exchanged zeolites for sustainable production of acrylic acid by gas-phase dehydration of lactic acid[J]. ACS Catalysis, 2017, 7(1): 538-550. |
| 95 | ZHANG Junfeng, ZHAO Yuling, FENG Xinzhen, et al. Na2HPO4-modified NaY nanocrystallites: efficient catalyst for acrylic acid production through lactic acid dehydration[J]. Catalysis Science & Technology, 2014, 4(5): 1376-1385. |
| 96 | ZHANG Junfeng, ZHAO Yuling, PAN Min, et al. Efficient acrylic acid production through bio lactic acid dehydration over NaY zeolite modified by alkali phosphates[J]. ACS Catalysis, 2011, 1(1): 32-41. |
| 97 | SUN Junming, WANG Yong. Recent advances in catalytic conversion of ethanol to chemicals[J]. ACS Catalysis, 2014, 4(4): 1078-1090. |
| 98 | 孟青青, 杨建国, 王凤寰. 生物法合成丙烯酸的研究进展[J]. 中国生物工程杂志, 2012, 32(10): 119-127. |
| MENG Qingqing, YANG Jianguo, WANG Fenghuan. Advances in the research of biological production of acrylic acid[J]. China Biotechnology, 2012, 32(10): 119-127. | |
| 99 | O’BRIEN D J, PANZER C C, EISELE W P. Biological production of acrylic-acid from cheese whey by resting cells of clostridium-propionicum[J]. Biotechnology Progress, 1990, 6(4): 237-242. |
| 100 | DANNER H, ÜRMÖS M, GARTNER M, et al. Biotechnological production of acrylic acid from biomass[J]. Applied Biochemistry and Biotechnology, 1998, 70/71/72(1): 887-894. |
| 101 | CHU Hun Su, Jin-Ho AHN, YUN Jiae, et al. Direct fermentation route for the production of acrylic acid[J]. Metabolic Engineering, 2015, 32: 23-29. |
| 102 | LIU Zhijie, LIU Tiangang. Production of acrylic acid and propionic acid by constructing a portion of the 3-hydroxypropionate/ 4-hydroxybutyrate cycle from Metallosphaera sedula in Escherichia coli[J]. Journal of Industrial Microbiology & Biotechnology, 2016, 43(12): 1659-1670. |
| 103 | Yoo-Sung KO, KIM Je Woong, CHAE Tong Un, et al. A novel biosynthetic pathway for the production of acrylic acid through beta-alanine route in Escherichia coli[J]. ACS Synthetic Biology, 2020, 9(5): 1150-1159. |
| 104 | KAMAL A, KUMAR M S, KUMAR C G, et al. Bioconversion of acrylonitrile to acrylic acid by rhodococcus ruber strain AKSH-84[J]. Journal of Microbiology and Biotechnology, 2011, 21(1): 37-42. |
| 105 | ZHU Linqi, CHEN Hao, HUANG Lei, et al. Electrochemical analysis of clostridium propionicum and its acrylic acid production in microbial fuel cells[J]. Engineering in Life Sciences, 2011, 11(3): 238-244. |
| 106 | STRAATHOF A J J, SIE S, FRANCO T T, et al. Feasibility of acrylic acid production by fermentation[J]. Applied Microbiology and Biotechnology, 2005, 67(6): 727-734. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 赵巍, 赵德银, 李世瀚, 刘洪达, 孙进, 郭艳秋. 三嗪型天然气管道缓蚀型减阻剂合成与应用[J]. 化工进展, 2023, 42(S1): 391-399. |
| [7] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [8] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [9] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [10] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [11] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [12] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [13] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [14] | 罗成, 范晓勇, 朱永红, 田丰, 崔楼伟, 杜崇鹏, 王飞利, 李冬, 郑化安. 中低温煤焦油加氢反应器不同分配器中液体分布的CFD模拟[J]. 化工进展, 2023, 42(9): 4538-4549. |
| [15] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||