化工进展 ›› 2021, Vol. 40 ›› Issue (3): 1178-1186.DOI: 10.16085/j.issn.1000-6613.2020-1806
嗜盐微生物底盘细胞:应用和前景
- 1.清华大学生命科学学院,北京 100084
2.清华大学化工系,北京 100084
-
收稿日期:2020-09-08出版日期:2021-03-05发布日期:2021-03-17 -
通讯作者:陈国强 -
作者简介:马悦原(1996—),女,博士研究生,研究方向为聚羟基脂肪酸酯的生物合成、合成生物学。E-mail:myy18@mails.tsinghua.edu.cn 。 -
基金资助:国家重点研发计划(2016YFB0302500);国家自然科学基金国际(地区)合作与交流项目NSFC-ANR项目(中法)(21761132013);国家自然科学基金国际(地区)合作与交流项目(31961133019)
Halophilic microorganisms as microbial chassis: applications and prospects
MA Yueyuan1( ), CHEN Jinchun1, CHEN Guoqiang1,2(
), CHEN Jinchun1, CHEN Guoqiang1,2( )
)
- 1.School of Life Sciences, Tsinghua University, Beijing 100084, China
2.Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
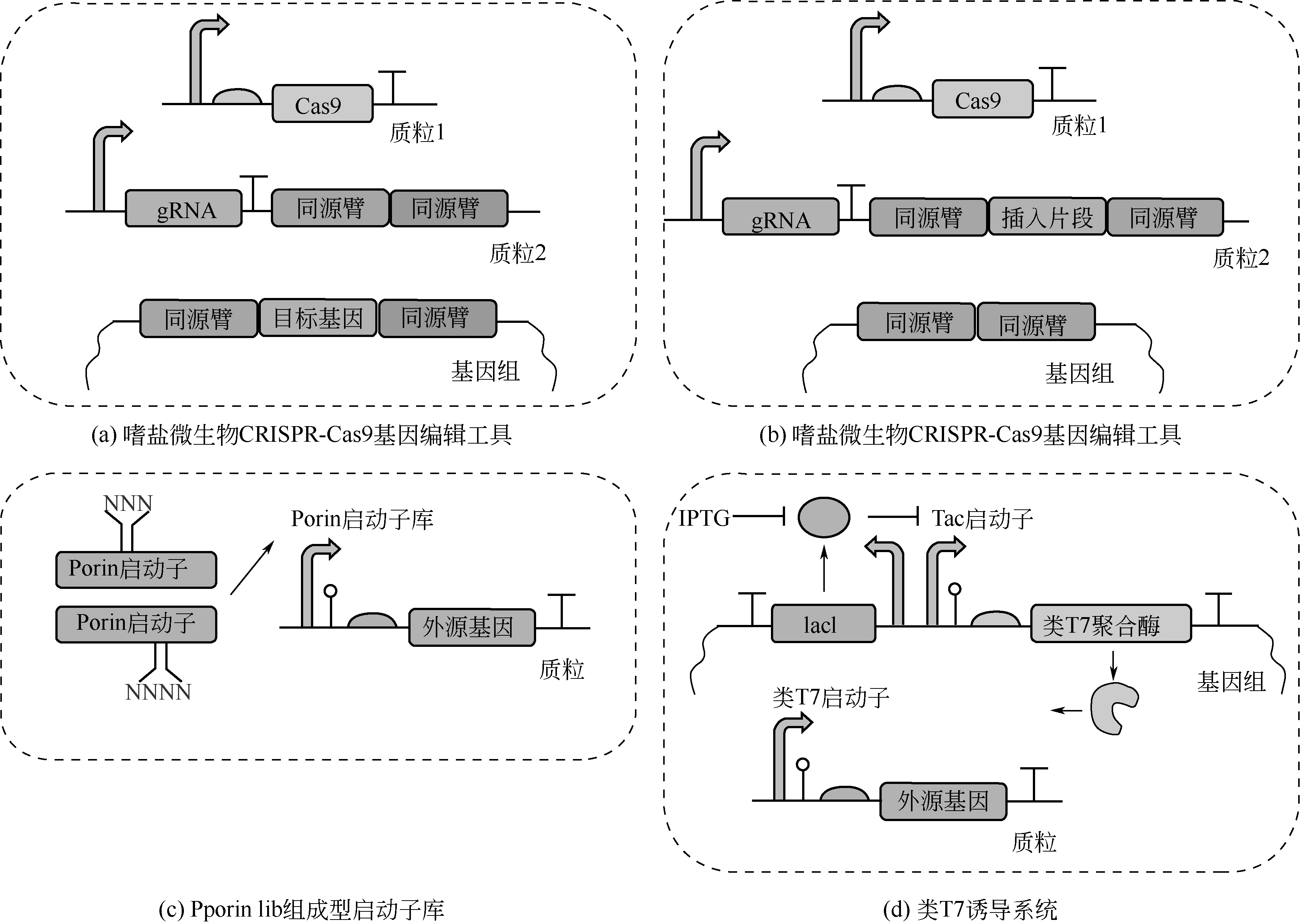
-
Received:2020-09-08Online:2021-03-05Published:2021-03-17 -
Contact:CHEN Guoqiang
摘要:
嗜盐微生物是一种耐高盐的微生物,有细菌、古菌、藻类等。其中的中度嗜盐菌耐受30~150g/L的氯化钠,有很高的研究价值和应用前景。嗜盐微生物可以在海水中生长,有的同时嗜碱,尤其不少嗜盐细菌在矿物培养基中生长迅速,具有作为底盘细胞的优势。随着近年来对嗜盐微生物分子操作工具的不断开发,嗜盐细菌的改造工作得到了迅猛发展。工程化的嗜盐微生物已经可以用于合成数种聚羟基脂肪酸酯(PHA),也用于大规模生产多种聚合物、蛋白质、小分子化合物、氨基酸和化妆品原料。合成生物学技术的使用也使嗜盐微生物实现了可控形变, 有利于更多的胞内产物的合成和下游的细胞分离。根据嗜盐微生物,特别是嗜盐细菌的特点和研究进展,本文提出了以嗜盐微生物作为底盘细胞的“下一代工业生物技术”,使发酵工业实现开放式的节能、节水、连续、全自动的过程,减少微生物大规模培养的复杂程度和对设备的高要求,同时能联产生产多种产品,大幅度降低工业生物技术的制造成本,提高产品的竞争性。
中图分类号:
引用本文
马悦原, 陈金春, 陈国强. 嗜盐微生物底盘细胞:应用和前景[J]. 化工进展, 2021, 40(3): 1178-1186.
MA Yueyuan, CHEN Jinchun, CHEN Guoqiang. Halophilic microorganisms as microbial chassis: applications and prospects[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1178-1186.
| 比较内容 | 嗜盐微生物底盘细胞 | 其他底盘细胞 |
|---|---|---|
| 用水 | 海水 | 淡水 |
| 碳源 | 常规碳源、厨余垃圾、秸秆等 | 各种糖类、脂肪酸等 |
| 灭菌/耗能 | 不需要灭菌/耗能小 | 需要灭菌/耗能大 |
| 是否易染菌 | 否 | 是 |
| 生长条件 | 灵活 | 苛刻 |
| 生长速度 | 快 | 快慢都有 |
| 发酵方式 | 可以连续发酵 | 很难连续发酵 |
| 产品数量 | 多种产物 | 单一产物 |
| 产品质量 | 纯度、分子量稳定 | 纯度、分子量不稳定 |
| 产品位置 | 胞内和胞外 | 胞内或胞外 |
| 发酵过程控制 | 容易自动控制 | 比较难自动控制 |
| 废水处理 | 废水可循环利用 | 废水不可循环利用 |
| 菌株遗传背景/代谢情况 | 不清晰 | 清晰 |
| 菌株基因编辑 | 困难 | 简便 |
表1 嗜盐微生物底盘细胞与其他底盘细胞的优缺点
| 比较内容 | 嗜盐微生物底盘细胞 | 其他底盘细胞 |
|---|---|---|
| 用水 | 海水 | 淡水 |
| 碳源 | 常规碳源、厨余垃圾、秸秆等 | 各种糖类、脂肪酸等 |
| 灭菌/耗能 | 不需要灭菌/耗能小 | 需要灭菌/耗能大 |
| 是否易染菌 | 否 | 是 |
| 生长条件 | 灵活 | 苛刻 |
| 生长速度 | 快 | 快慢都有 |
| 发酵方式 | 可以连续发酵 | 很难连续发酵 |
| 产品数量 | 多种产物 | 单一产物 |
| 产品质量 | 纯度、分子量稳定 | 纯度、分子量不稳定 |
| 产品位置 | 胞内和胞外 | 胞内或胞外 |
| 发酵过程控制 | 容易自动控制 | 比较难自动控制 |
| 废水处理 | 废水可循环利用 | 废水不可循环利用 |
| 菌株遗传背景/代谢情况 | 不清晰 | 清晰 |
| 菌株基因编辑 | 困难 | 简便 |
| 产物 | 菌株 | 产量 | 参考 文献 |
|---|---|---|---|
| 聚羟基脂肪酸酯(PHA) | |||
| PHB | Vibrio proteolyticu Haloarcula marismortui Halomonas boliviensis Halomonas bluephagenesis Halomonas campaniensis Halomonas sp. YLGW01 | 54.67% CDW 21% CDW 81% CDW 80% CDW 70% CDW 94.6% CDW | [ |
| P34HB | Halomonas bluephagenesis | 75% CDW | [ |
| PHBV | Natrinema altunense Halomonas campisalis Haloferax mediterranei Halogeometricum borinquense Halogranum amylolyticum | 61.02% CDW 81% CDW 24% CDW 73.51% CDW 26.6% CDW | [ |
| 蛋白质 | |||
| β-半乳糖苷酶 | Halorubrum lacusprofundi | 3.76U·mg-1 | [ |
| 乙醇脱氢酶 | Haloarcula marismortui | 0.021U·mg-1 | [ |
| 谷氨酸脱氢酶 | Halobacterium salinarum | [ | |
| 脂肪酶 | Halomonas sp. LM1C | 3000U·L-1 | [ |
| 淀粉酶 | Haloferax sp. HA10 | 4.2U·mg-1 | [ |
| PHA包涵体蛋白PhaP | Halomonas bluephagenesis | 1.86g·L-1 | [ |
| PHA调控蛋白PhaR | Halomonas bluephagenesis | [ | |
| 小分子化合物 | |||
| 四氢嘧啶 | Halomonas bluephagenesis Halomonas elongate Halomonas boliviensis | 28g·L-1 155mg·g CDW-1 11.1g·L-1·d-1 | [ |
| 甜菜碱 | Actinopolyspora halophila Ectothiorhodospirahalochloris | 0.7mg·mL-1 0.53mg·mL-1 | [ |
| β-胡萝卜素 | Halomonas elongate | 560mg·g CDW-1 | [ |
| 番茄红素 | Haloferax mediterranei | 119.25mg·g-1 | [ |
| 苏氨酸 | Halomonas bluephagenesis | 33g·L-1 | [ |
| 5-氨基乙酰丙酸(ALA) | Halomonas bluephagenesis | 0.7g·L-1 | [ |
表2 嗜盐微生物合成多种产品
| 产物 | 菌株 | 产量 | 参考 文献 |
|---|---|---|---|
| 聚羟基脂肪酸酯(PHA) | |||
| PHB | Vibrio proteolyticu Haloarcula marismortui Halomonas boliviensis Halomonas bluephagenesis Halomonas campaniensis Halomonas sp. YLGW01 | 54.67% CDW 21% CDW 81% CDW 80% CDW 70% CDW 94.6% CDW | [ |
| P34HB | Halomonas bluephagenesis | 75% CDW | [ |
| PHBV | Natrinema altunense Halomonas campisalis Haloferax mediterranei Halogeometricum borinquense Halogranum amylolyticum | 61.02% CDW 81% CDW 24% CDW 73.51% CDW 26.6% CDW | [ |
| 蛋白质 | |||
| β-半乳糖苷酶 | Halorubrum lacusprofundi | 3.76U·mg-1 | [ |
| 乙醇脱氢酶 | Haloarcula marismortui | 0.021U·mg-1 | [ |
| 谷氨酸脱氢酶 | Halobacterium salinarum | [ | |
| 脂肪酶 | Halomonas sp. LM1C | 3000U·L-1 | [ |
| 淀粉酶 | Haloferax sp. HA10 | 4.2U·mg-1 | [ |
| PHA包涵体蛋白PhaP | Halomonas bluephagenesis | 1.86g·L-1 | [ |
| PHA调控蛋白PhaR | Halomonas bluephagenesis | [ | |
| 小分子化合物 | |||
| 四氢嘧啶 | Halomonas bluephagenesis Halomonas elongate Halomonas boliviensis | 28g·L-1 155mg·g CDW-1 11.1g·L-1·d-1 | [ |
| 甜菜碱 | Actinopolyspora halophila Ectothiorhodospirahalochloris | 0.7mg·mL-1 0.53mg·mL-1 | [ |
| β-胡萝卜素 | Halomonas elongate | 560mg·g CDW-1 | [ |
| 番茄红素 | Haloferax mediterranei | 119.25mg·g-1 | [ |
| 苏氨酸 | Halomonas bluephagenesis | 33g·L-1 | [ |
| 5-氨基乙酰丙酸(ALA) | Halomonas bluephagenesis | 0.7g·L-1 | [ |
| 1 | NODA Shuhei, KONDO Akihiko. Recent advances in microbial production of aromatic chemicals and derivatives[J]. Trends in Biotechnology, 2017, 35(8): 785-796. |
| 2 | CHEN Guoqiang, JIANG Xiaoran. Next generation industrial biotechnology based on extremophilic bacteria[J].Current Opinion in Biotechnology, 2018, 50: 94-100. |
| 3 | CHEN Guoqiang. Next generation industrial biotechnology based on halophiles[J]. Abstracts of Papers of the American Chemical Society, 2019, 257: 610. |
| 4 | YU Linping, WU Fuqing, CHEN Guoqiang. Next-generation industrial biotechnology-transforming the current industrial biotechnology into competitive processes[J]. Biotechnology Journal, 2019, 14(9): 1800437. |
| 5 | LOURDES MORENO María, Dolores PÉREZ, María GARCÍA, et al. Halophilic bacteria as a source of novel hydrolytic enzymes[J]. Life, 2013, 3(1): 38-51. |
| 6 | OREN Aharon. Halophilic microbial communities and their environments[J]. Current Opinion in Biotechnology, 2015, 33: 119-124. |
| 7 | EDBEIB Mohamed Faraj, WAHAB Roswanira Abdul, HUYOP Fahrul. Halophiles: biology, adaptation, and their role in decontamination of hypersaline environments[J]. World Journal of Microbiology & Biotechnology, 2016, 32(8): 135. |
| 8 | 杜鹤童, 赵倚晴, 陈金春,等. 基于嗜盐微生物合成生物学的下一代工业生物技术[J]. 生命科学, 2019, 31(4): 67-72. |
| DU Hetong, ZHAO Yiqing, CHEN Jinchun, et al. Next generation industrial biotechnology based on synthetic biology of halophiles[J]. Chinese Bulletin of Life Sciences, 2019, 31(4): 67-72. | |
| 9 | CHEN Xiangbin, YU Linping, QIAO Guanqing, et al. Reprogramming Halomonas for industrial production of chemicals[J]. Journal of Industrial Microbiology and Biotechnology, 2018, 45:545-554. |
| 10 | ZHANG Xu, LIN Yina, CHEN Guoqiang. Halophiles as chassis for bioproduction[J]. Advanced Biosystems, 2018, 2(11): 1800088. |
| 11 | CONG Le, RAN F Ann, David COX, et al. Multiplex genome engineering using CRISPR/Cas systems[J]. Science, 2013, 339(6121): 819-823. |
| 12 | STOLL Britta, MAIER Lisa-Katharina, Sita LANGE J, et al. Requirements for a successful defence reaction by the CRISPR-Cas subtype Ⅰ-B system[J]. Biochemical Society Transactions, 2013, 41: 1444-1448. |
| 13 | MAIER Lisa-Katharina, Sita LANGE J, STOLL Britta, et al. Essential requirements for the detection and degradation of invaders by the Haloferax volcanii CRISPR/Cas system Ⅰ-B[J]. RNA Biology, 2013, 10(5): 865-874. |
| 14 | STACHLER Aris-Edda, MARCHFELDER Anita. Gene repression in haloarchaea using the CRISPR (clustered regularly interspaced short palindromic repeats)-Cas I-B system[J]. Journal of Biological Chemistry, 2016, 291(29): 15226-15242. |
| 15 | QIN Qin, LING Chen, ZHAO Yiqing, et al. CRISPR/Cas9 editing genome of extremophile Halomonas spp.[J]. Metabolic Engineering, 2018, 47: 219-229. |
| 16 | YIN Jin, FU Xiaozhi, WU Qiong, et al. Development of an enhanced chromosomal expression system based on porin synthesis operon for halophile Halomonas sp.[J]. Applied Microbiology and Biotechnology, 2014, 98(21): 8987-8997. |
| 17 | SHEN Rui, YIN Jin, YE Jianwen, et al. Promoter engineering for enhanced P(3HB-co-4HB) production by Halomonas bluephagenesis[J]. ACS Synthetic Biology, 2018, 7(8): 1897-1906. |
| 18 | YE Jianwen, HU Dingkai, YIN Jin, et al. Stimulus response-based fine-tuning of polyhydroxyalkanoate pathway in Halomonas[J]. Metabolic Engineering, 2020, 57: 85-95. |
| 19 | LARGE Andrew, STAMME Claudia, LANGE Christian, et al. Characterization of a tightly controlled promoter of the halophilic archaeon Haloferax volcanii and its use in the analysis of the essential cct1 gene[J]. Molecular Microbiology, 2007, 66(5): 1092-1106. |
| 20 | ALLERS Thorsten, Hien Ping NGO, MEVARECH Moshe, et al. Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based on the leuB and trpA genes.[J]. Applied and Environmental Microbiology, 2004, 70(2):943-953. |
| 21 | STRILLINGER Eva, GROETZINGER Stefan Wolfgang, ALLERS Thorsten, et al. Production of halophilic proteins using Haloferax volcanii H1895 in a stirred-tank bioreactor[J]. Applied Microbiology and Biotechnology, 2016, 100(3): 1183-1195. |
| 22 | ZHAO Han, ZHANG Haoqian, CHEN Xiangbin, et al. Novel T7-like expression systems used for Halomonas[J]. Metabolic Engineering, 2017, 39: 128-140. |
| 23 | SHEN Rui, NING Zhiyu, LAN Yuxuan, et al. Manipulation of polyhydroxyalkanoate granular sizes in Halomonas bluephagenesis[J]. Metabolic Engineering, 2019, 54: 117-126. |
| 24 | CHEN Yong, CHEN Xinyu, DU Hetong, et al. Chromosome engineering of the TCA cycle in Halomonas bluephagenesis for production of copolymers of 3-hydroxybutyrate and 3-hydroxyvalerate (PHBV)[J]. Metabolic Engineering, 2019, 54: 69-82. |
| 25 | YU Linping, YAN Xu, ZHANG Xu, et al. Biosynthesis of functional polyhydroxyalkanoates by engineered Halomonas bluephagenesis[J]. Metabolic Engineering, 2020, 59: 119-130. |
| 26 | HONG Ju Won, SONG Hun Suk, MOON Yu Mi, et al. Polyhydroxybutyrate production in halophilic marine bacteria Vibrio proteolyticus isolated from the Korean peninsula[J]. Bioprocess and Biosystems Engineering, 2019, 42(4): 603-610. |
| 27 | HAN Jing, LU Qiuhe, ZHOU Ligang, et al. Molecular characterization of the phaECHm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui[J]. Applied and Environmental Microbiology, 2007, 73(19): 6058-6065. |
| 28 | Jorge QUILLAGUAMÁN, DELGADO Osvaldo, MATTIASSON Bo, et al. Poly(β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1 using starch hydrolysate as substrate[J]. Journal of Applied Microbiology, 2005, 99(1): 151-157. |
| 29 | Jorge QUILLAGUAMÁN, Rajni HATTI-KAUL, MATTIASSON Bo, et al. Halomonas boliviensis sp. nov., an alkalitolerant, moderate halophile isolated from soil around a Bolivian hypersaline lake[J]. International Journal of Systematic and Evolutionary Microbiology, 2004, 54(3): 721-725. |
| 30 | VAN-THUOC D, QUILLAGUAMÁN J, MAMO G, et al. Utilization of agricultural residues for poly(3-hydroxybutyrate) production by Halomonas boliviensis LC1[J]. Journal of Applied Microbiology, 2007, 140: 420-428. |
| 31 | TAN Dan, XUE Yuansheng, AIBAIDULA Gulsimay, et al. Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01[J]. Bioresource Technology, 2011, 102(17): 8130-8136. |
| 32 | YUE Haitao, LING Chen, YANG Tao, et al. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates[J]. Biotechnology for Biofuels, 2014, 7(1): 108. |
| 33 | Jorge QUILLAGUAMÁN, Thuoc DOAN-VAN, Héctor GUZMÁN, et al. Poly(3-hydroxybutyrate) production by Halomonas boliviensis in fed-batch culture[J]. Applied Microbiology and Biotechnology, 2008, 78(2): 227-232. |
| 34 | PARK Ye-Lim, BHATIA Shashi Kant, GURAV Ranjit, et al. Fructose based hyper production of poly-3-hydroxybutyrate from Halomonas sp. YLGW01 and impact of carbon sources on bacteria morphologies[J]. International Journal of Biological Macromolecules, 2020, 154: 929-936. |
| 35 | YE Jianwen, HU Dingkai, CHE Xuemei, et al. Engineering of Halomonas bluephagenesis for low cost production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose[J]. Metabolic Engineering, 2018, 47: 143-152. |
| 36 | BHATTACHARYYA Anirban, PRAMANIK Arnab, MAJI Sudipta Kumar, et al. Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei[J]. AMB Express, 2012, 2(1):34. |
| 37 | MAHANSARIA Riddhi, DHARA Anusua, SAHA Amit, et al. Production enhancement and characterization of the polyhydroxyalkanoate produced by Natrinema ajinwuensis (as synonym) ≡ Natrinema altunense strain RM-G10[J]. International Journal of Biological Macromolecules, 2018, 107: 1480-1490. |
| 38 | KULKARNI S O, KANEKAR P P, NILEGAONKAR S S, et al. Production and characterization of a biodegradable poly (hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) copolymer by moderately haloalkalitolerant Halomonas campisalis MCM B-1027 isolated from Lonar Lake, India[J]. Bioresource Technology, 2010, 101(24): 9765-9771. |
| 39 | LU Qiuhe, HAN Jing, ZHOU Ligang, et al. Genetic and biochemical characterization of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthase in Haloferax mediterranei[J]. Journal of Bacteriology, 2008, 190(12): 4173-4180. |
| 40 | SALGAONKAR Bhakti B, BRAGANÇA Judith M. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Halogeometricum borinquense strain E3[J]. International Journal of Biological Macromolecules, 2015, 78: 339-346. |
| 41 | CHEN C Will, Trong Ming DON, Hsiao Feng YEN. Enzymatic extruded starch as a carbon source for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Haloferax mediterranei[J]. Process Biochemistry, 2006, 41(11): 2289-2296. |
| 42 | HAN Jing, WU Linping, HOU Jing, et al. Biosynthesis, characterization, and hemostasis potential of tailor-made poly(3-hydroxybutyrate-co-3-hydroxyvalerate) produced by Haloferax mediterranei[J]. Biomacromolecules, 2015, 16(2): 578-588. |
| 43 | ZHAO Youxi, RAO Zhiming, XUE Yanfen, et al. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by haloarchaeon Halogranum amylolyticum[J]. Applied Microbiology and Biotechnology, 2015, 99(18): 7639-7649. |
| 44 | KARAN Ram, Melinda CAPES D, DASSARMA Priya, et al. Cloning, overexpression, purification, and characterization of a polyextremophilic β-galactosidase from the antarctic haloarchaeon Halorubrum lacusprofundi[J]. BMC Biotechnology, 2013, 13(1): 3. |
| 45 | TIMPSON Leanne M, ALSAFADI Diya, DONNCHADHA Cillín MAC, et al. Characterization of alcohol dehydrogenase (ADH12) from Haloarcula marismortui, an extreme halophile from the Dead Sea[J]. Extremophiles, 2012, 16(1): 57-66. |
| 46 | MUNAWAR Nayla, ENGEL Paul C. Prospects for robust biocatalysis: engineering of novel specificity in a halophilic amino acid dehydrogenase[J]. Extremophiles, 2013, 17(1): 43-51. |
| 47 | Esther GUTIÉRREZ-ARNILLAS, ARELLANO María, DEIVE Francisco J, et al. Unravelling the suitability of biological induction for halophilic lipase production by Halomonas sp. LM1C cultures[J]. Bioresource Technology, 2017, 239: 368-377. |
| 48 | BAJPAI Bhakti, CHAUDHARY Monika, SAXENA Jyoti. Production and characterization of α-amylase from an extremely halophilic archaeon, Haloferax sp. HA10[J]. Food Technology and Biotechnology, 2015, 53(1): 11-17. |
| 49 | ZHAO Hongyu, YAO Zhenyu, CHEN Xiangbin, et al. Modelling of microbial polyhydroxyalkanoate surface binding protein PhaP for rational mutagenesis[J]. Microbial Biotechnology, 2017, 10(6): 1400-1411. |
| 50 | FU Xiaozhi, TAN Dan, AIBAIDULA Gulsimay, et al. Development of Halomonas TD01 as a host for open production of chemicals[J]. Metabolic Engineering, 2014, 23: 78-91. |
| 51 | MA Hong, ZHAO Yiqing, HUANG Wuzhe, et al. Rational flux-tuning of Halomonas bluephagenesis for co-production of bioplastic PHB and ectoine[J]. Nature Communications, 2020, 11(1): 3313. |
| 52 | GRAMMANN Katrin, VOLKE Angela, KUNTE Hans Jörg. New type of osmoregulated solute transporter identified in halophilic members of the bacteria domain: trap transporter TeaABC mediates uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581T[J]. Journal of Bacteriology, 2002, 184(11): 3078-3085. |
| 53 | SAUER Thomas, GALINSKI Erwin A. Bacterial milking: a novel bioprocess for production of compatible solutes[J]. Biotechnology and Bioengineering, 1998, 57(3): 8. |
| 54 | THUOC Doan VAN, Héctor GUZMÁN, Jorge QUILLAGUAMÁN, et al. High productivity of ectoines by Halomonas boliviensis using a combined two-step fed-batch culture and milking process[J]. Journal of Biotechnology, 2010, 147(1): 46-51. |
| 55 | Antti NYYSSÖLÄ, KEROVUO Janne, KAUKINEN Pasi, et al. Extreme halophiles synthesize betaine from glycine by methylation[J]. Journal of Biological Chemistry, American Society for Biochemistry and Molecular Biology, 2000, 275(29): 22196-22201. |
| 56 | Marta RODRÍGUEZ-SÁIZ, Cristina SÁNCHEZ-PORRO, LA FUENTE De Juan Luis, et al. Engineering the halophilic bacterium Halomonas elongata to produce β-carotene[J]. Applied Microbiology and Biotechnology, 2007, 77(3): 637-643. |
| 57 | ZUO Zhenqiang, XUE Qiong, ZHOU Jian, et al. Engineering Haloferax mediterranei as an efficient platform for high level production of lycopene[J]. Frontiers in Microbiology, 2018, 9: 2893. |
| 58 | DU Hetong, ZHAO Yiqing, WU Fuqing, et al. Engineering Halomonas bluephagenesis for L-threonine production[J]. Metabolic Engineering, 2020, 60: 119-127. |
| 59 | LI Tian, GUO Yingying, QIAO Guanqing, et al. Microbial synthesis of 5-aminolevulinic acid and its coproduction with polyhydroxybutyrate[J]. ACS Synthetic Biology, 2016, 5(11): 1264-1274. |
| 60 | CHEN Guoqiang. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry[J]. Chemical Society Reviews, 2009, 38(8): 2434. |
| 61 | ANJUM Anbreen, ZUBER Mohammad, Khalid Mahmood ZIA, et al. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements[J]. International Journal of Biological Macromolecules, 2016, 89: 161-174. |
| 62 | KESHAVARZ Tajalli, ROY Ipsita. Polyhydroxyalkanoates: bioplastics with a green agenda[J]. Current Opinion in Microbiology, 2010, 13(3): 321-326. |
| 63 | HAN Jing, WU Linping, LIU Xiaobin, et al. Biodegradation and biocompatibility of haloarchaea-produced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymers[J]. Biomaterials, 2017, 139: 172-186. |
| 64 | ZHAO Dahe, CAI Lei, WU Jinhua, et al. Improving polyhydroxyalkanoate production by knocking out the genes involved in exopolysaccharide biosynthesis in Haloferax mediterranei[J]. Applied Microbiology and Biotechnology, 2013, 97(7): 3027-3036. |
| 65 | CHEN Junyu, MITRA Ruchira, ZHANG Shengjie, et al. Unusual phosphoenolpyruvate (PEP) synthetase-like protein crucial to enhancement of polyhydroxyalkanoate accumulation in Haloferax mediterranei revealed by dissection of PEP-pyruvate interconversion mechanism[J]. Applied and Environmental Microbiology, 2019, 85(19): e00984. |
| 66 | LING Chen, QIAO Guanqing, SHUAI Bowen, et al. Engineering NADH/NAD+ ratio in Halomonas bluephagenesis for enhanced production of polyhydroxyalkanoates (PHA)[J]. Metabolic Engineering, 2018, 49: 275-286. |
| 67 | JIANG Xiaoran, YAO Zhihao, CHEN Guoqiang. Controlling cell volume for efficient PHB production by Halomonas[J]. Metabolic Engineering, 2017, 44: 30-37. |
| 68 | OREN Aharon. Industrial and environmental applications of halophilic microorganisms[J]. Environmental Technology, 2010, 31(8/9): 825-834. |
| 69 | YE Jianwen, HUANG Wuzhe, WANG Dongsheng, et al. Pilot scale-up of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) production by Halomonas bluephagenesis via cell growth adapted optimization process[J]. Biotechnology Journal, 2018, 13(5): 1800074. |
| 70 | FALB Michaela, Kerstin MÜLLER, Lisa KÖNIGSMAIER, et al. Metabolism of halophilic archaea[J]. Extremophiles, 2008, 12(2): 177-196. |
| 71 | Özlem ATES, ONER Ebru, ARGA Kazim Y. Genome-scale reconstruction of metabolic network for a halophilic extremophile, Chromohalobacter salexigens DSM 3043[J]. BMC Systems Biology, 2011, 5(1): 12. |
| 72 | OUYANG Pengfei, WANG Huan, HAJNAL Ivan, et al. Increasing oxygen availability for improving poly(3-hydroxybutyrate) production by Halomonas[J]. Metabolic Engineering, 2018, 45: 20-31. |
| 73 | QU Ge, LI Aitao, ACEVEDO‐ROCHA Carlos G, et al. The crucial role of methodology development in directed evolution of selective enzymes[J]. Angewandte Chemie: International Edition, 2020, 59(32): 13204-13231. |
| [1] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| [2] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [3] | 李伯耿, 罗英武, 刘平伟. 聚合物产品工程研究内容与方法的思考[J]. 化工进展, 2023, 42(8): 3905-3909. |
| [4] | 王报英, 王皝莹, 闫军营, 汪耀明, 徐铜文. 聚合物包覆膜在金属分离回收中的研究进展[J]. 化工进展, 2023, 42(8): 3990-4004. |
| [5] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [6] | 鲁少杰, 刘佳, 冀芊竹, 李萍, 韩月阳, 陶敏, 梁文俊. 硅藻土基复合填料制备及滴滤塔去除二甲苯的性能[J]. 化工进展, 2023, 42(7): 3884-3892. |
| [7] | 余希希, 张金帅, 雷文, 刘承果. 基于动态共价键自修复的光固化高分子材料研究进展[J]. 化工进展, 2023, 42(7): 3589-3599. |
| [8] | 于丁一, 李圆圆, 王晨钰, 纪永升. pH响应性木质素水凝胶的制备及药物控释[J]. 化工进展, 2023, 42(6): 3138-3146. |
| [9] | 杨发容, 顾丽莉, 刘洋, 李伟雪, 蔡洁云, 王惠平. 计算机模拟辅助特丁津分子印迹聚合物的制备及应用[J]. 化工进展, 2023, 42(6): 3157-3166. |
| [10] | 杨家添, 唐金铭, 梁恣荣, 黎胤宏, 胡华宇, 陈渊. 新型淀粉基高吸水树脂抑尘剂的制备及其应用[J]. 化工进展, 2023, 42(6): 3187-3196. |
| [11] | 何志勇, 郭天佛, 王金利, 吕锋. 二氧化碳/环氧化合物开环共聚催化剂进展[J]. 化工进展, 2023, 42(4): 1847-1859. |
| [12] | 谭德新, 曾佳欣, 梁莉敏, 申思慧, 曾子倩, 王艳丽. 取代烷基变化对芳炔单体及其聚合物性能影响[J]. 化工进展, 2023, 42(4): 2031-2037. |
| [13] | 张艺璇, 胡伟, 刘梦瑶, 鞠敬鸽, 赵义侠, 康卫民. 聚合物电解质在锌离子电池中的研究进展[J]. 化工进展, 2023, 42(3): 1397-1410. |
| [14] | 高江雨, 张耀君, 贺攀阳, 刘礼才, 张枫烨. 磷酸基地质聚合物的制备及其性能研究进展[J]. 化工进展, 2023, 42(3): 1411-1425. |
| [15] | 薛博, 杨婷婷, 王雪峰. 聚苯胺/碳纳米管气敏材料的研究进展[J]. 化工进展, 2023, 42(3): 1448-1456. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||