化工进展 ›› 2020, Vol. 39 ›› Issue (2): 709-719.DOI: 10.16085/j.issn.1000-6613.2019-0909
铁对废水微生物脱氮的影响研究进展
- 广东省石油与精细化工研究院,广东省工业表面活性剂重点实验室,广东 广州 510665
-
收稿日期:2019-06-10出版日期:2020-02-05发布日期:2020-03-12 -
通讯作者:李彬 -
作者简介:吕冉(1992—),女,硕士,研究方向为环境微生物。E-mail:lvranchn@126.com 。 -
基金资助:广东省科学院建设国内一流研究机构行动专项(2019GDASYL-0301002);广东省科学院实施创新驱动发展能力建设专项(2017GDASCX-0856);广东省自然科学基金(2017A030310387)
Research progress on the effects of iron on microbiological nitrogen removal in wastewater
Ran LÜ( ),Bin LI(
),Bin LI( ),Ying XIAO,Jingwen ZHANG,Yuliang MAI
),Ying XIAO,Jingwen ZHANG,Yuliang MAI
- Guangdong Provincial Key Laboratory of Industrial Surfactant, Guangdong Research Institute of Petrochemical and Fine Chemical Engineering, Guangzhou 510665, Guangdong, China
-
Received:2019-06-10Online:2020-02-05Published:2020-03-12 -
Contact:Bin LI
摘要:
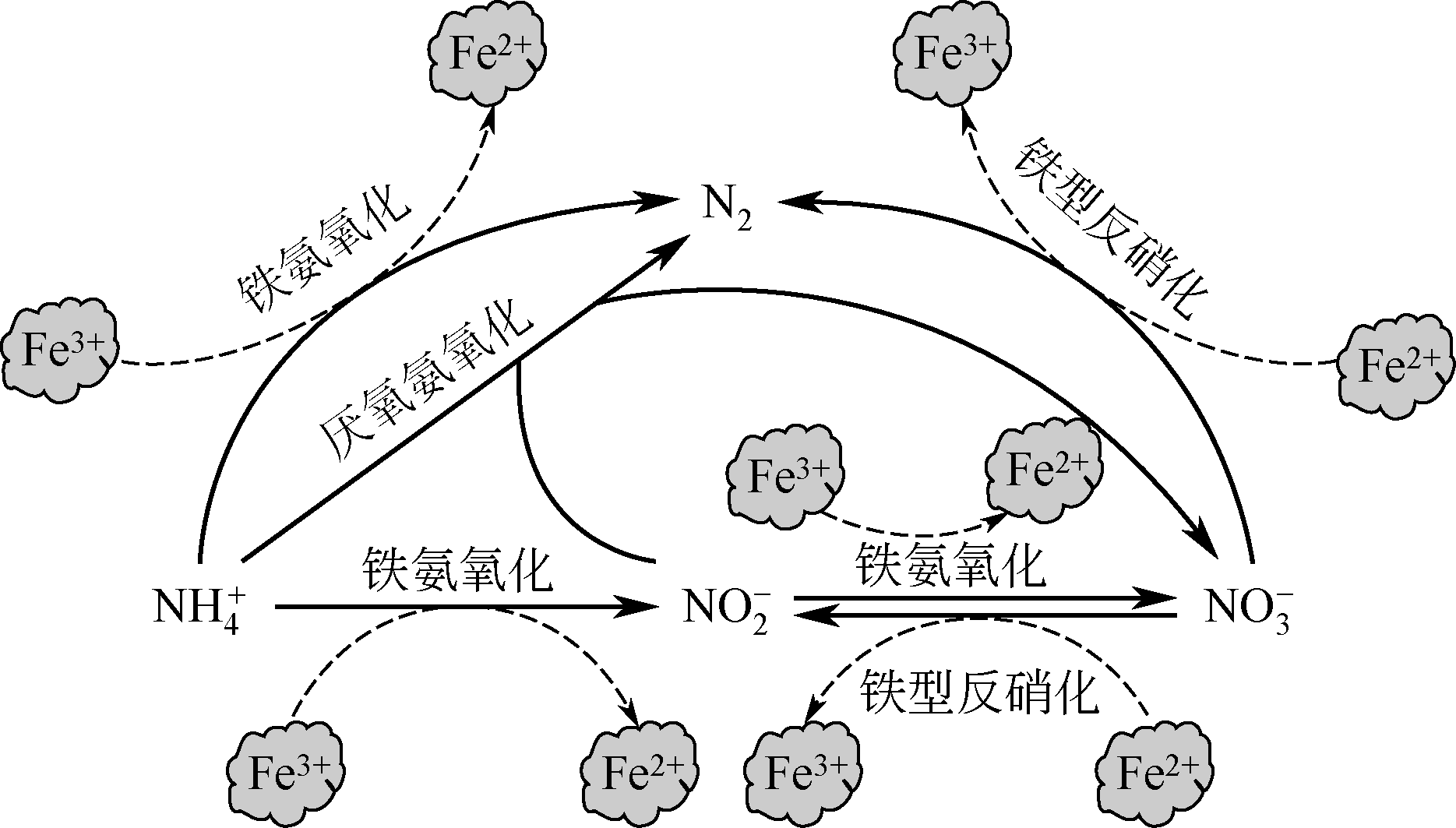
微生物脱氮是一种经济有效的治理水体氮污染的手段。目前微生物脱氮过程主要有厌氧氨氧化、硝化、反硝化及同时硝化反硝化等。铁是环境中普遍存在的金属元素,也是微生物所需的重要微量元素之一。在微生物脱氮系统中,铁盐或者含铁固体化合物等的投加会对微生物及脱氮工艺过程等产生一定的影响,且对于不同种类的微生物与不同的脱氮工艺,铁所产生的影响也将不同。本文全面综述了近些年的研究报道中铁对厌氧氨氧化、硝化、反硝化及同时硝化反硝化等不同脱氮过程中含氮污染物去除效果的影响,铁与脱氮微生物的酶活性、电子传递、增殖富集及脱氮反应器中生物膜、污泥絮体及颗粒形成等之间的作用关系,旨在全面理解铁对微生物脱氮系统的作用与内在机制,为实现利用铁强化微生物脱氮过程、提高微生物脱氮效率提供借鉴。
中图分类号:
引用本文
吕冉,李彬,肖盈,张靖雯,麦裕良. 铁对废水微生物脱氮的影响研究进展[J]. 化工进展, 2020, 39(2): 709-719.
Ran LÜ,Bin LI,Ying XIAO,Jingwen ZHANG,Yuliang MAI. Research progress on the effects of iron on microbiological nitrogen removal in wastewater[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 709-719.
| 投加形式 | 投加量 | 厌氧氨氧化菌/污泥 | 反应器(有效容积) | 对脱氮效果的影响 | 参考文献 |
|---|---|---|---|---|---|
| 毫米级零价铁mZVI;纳米级零价铁nZVI | 25g | 城市污水处理厂厌氧池收集的污泥 | 上流式厌氧污泥床反应器(4.75L) | 氮负荷率约630mg/(L·d)。稳定阶段,投加mZVI、nZVI和不投加铁,总氮去除速率分别为543mg/(L·d)、560mg/(L·d)和532mg/(L·d);氨氮去除率分别为92.6%、93.8%和91.8%;亚硝酸盐氮去除率分别为93.5%、96.6%和91.2% | [ |
| Fe(Ⅱ)(EDTA-FeNa2) | 0.06~0.18 mmol/L | 实验室规模厌氧氨氧化反应器污泥,70%~75%厌氧氨氧化菌KSU-1 | 上流式固定床反应器(0.3L) | 氮负荷率为5590g/(m3·d)时,0.06mmol/L及0.09mmol/L Fe(Ⅱ)对脱氮均有促进作用,氮去除速率由对照组[0.03mmol/L Fe(Ⅱ)]的3349g/(m3·d)提高至4429g/(m3·d)[0.09mmol/L Fe(Ⅱ)]。氮负荷率为12500g/(m3·d)时,氮去除速率由7033g/(m3·d)[0.03mmol/L Fe(Ⅱ)]提高至0.12 mmol/L Fe(Ⅱ)时的9135g/(m3·d),0.18mmol/L Fe(Ⅱ)时氮去除速率降至8198g/(m3·d) | [ |
| Fe2+ | 1mg/L | CANON反应器污泥与生物滤池生物膜的混合污泥 | 生物滤池(1L) | 进水总氮约105mg/L。在Fe2+的长期作用下,氮的去除速率由最初投加Fe2+时的约0.51kg/(m3·d)最终增至0.58kg/(m3·d) | [ |
| Fe2+ | 1~50mg/L | 厌氧氨氧化与硝化污泥污泥混合 | 生物滤池(1L) | 进水氨氮与亚硝酸盐氮各50mg/L。未投加Fe2+时,总氮去除速率为0.51kg/(m3·d);氨氮、亚硝酸盐氮与总氮去除率分别为58.9%、60.9%和58.2%。1~5mg/LFe2+能够促进脱氮,总氮去除速率增至0.57kg/(m3·d);氨氮、亚硝酸盐氮与总氮去除率分别增至94.4%、93.0%和80.8%。10~50mg/LFe2+则会不同程度地抑制脱氮 | [ |
| Fe3+ | 3.68mg/L | 稳定运行的实验室规模反应器中污泥 | 上流式厌氧污泥反应器(1L) | 进水氨氮125.9~168.3mg/L,亚硝酸盐氮149.0~182.5mg/L。添加Fe3+时的平均氮去除率和最大氮去除速率分别为67.4%和4.9kg/(m3·d)[对照组相应值分别为64.7%和4.1kg/(m3·d)] | [ |
| Fe3O4 | 2g | 实验室规模上流式厌氧污泥床反应器的厌氧氨氧化污泥 | 厌氧连续搅拌反应器(2L) | 进水氨氮和亚硝酸盐浓度分别为100.5mg/L和132.1mg/L。反应器稳定运行阶段,最大总氮去除率约98%,大于对照组约87% | [ |
Fe电极(阳极) (?60mm×90mm) | 上流式厌氧氨氧化反应器污泥 | 上流式厌氧反应器(0.7L) | 进水氨氮和亚硝酸盐氮浓度均为650mg/L。氨氮、亚硝酸盐氮和总氮去除速率分别为609.9mg/(L·d)、638.4mg/(L·d)和1209.6mg/(L·d);未设Fe-石墨电极的对照组中相应值分别仅为493.9mg/(L·d)、524.2mg/(L·d)和973.3mg/(L·d) | [ |
表1 铁对厌氧氨氧化的影响
| 投加形式 | 投加量 | 厌氧氨氧化菌/污泥 | 反应器(有效容积) | 对脱氮效果的影响 | 参考文献 |
|---|---|---|---|---|---|
| 毫米级零价铁mZVI;纳米级零价铁nZVI | 25g | 城市污水处理厂厌氧池收集的污泥 | 上流式厌氧污泥床反应器(4.75L) | 氮负荷率约630mg/(L·d)。稳定阶段,投加mZVI、nZVI和不投加铁,总氮去除速率分别为543mg/(L·d)、560mg/(L·d)和532mg/(L·d);氨氮去除率分别为92.6%、93.8%和91.8%;亚硝酸盐氮去除率分别为93.5%、96.6%和91.2% | [ |
| Fe(Ⅱ)(EDTA-FeNa2) | 0.06~0.18 mmol/L | 实验室规模厌氧氨氧化反应器污泥,70%~75%厌氧氨氧化菌KSU-1 | 上流式固定床反应器(0.3L) | 氮负荷率为5590g/(m3·d)时,0.06mmol/L及0.09mmol/L Fe(Ⅱ)对脱氮均有促进作用,氮去除速率由对照组[0.03mmol/L Fe(Ⅱ)]的3349g/(m3·d)提高至4429g/(m3·d)[0.09mmol/L Fe(Ⅱ)]。氮负荷率为12500g/(m3·d)时,氮去除速率由7033g/(m3·d)[0.03mmol/L Fe(Ⅱ)]提高至0.12 mmol/L Fe(Ⅱ)时的9135g/(m3·d),0.18mmol/L Fe(Ⅱ)时氮去除速率降至8198g/(m3·d) | [ |
| Fe2+ | 1mg/L | CANON反应器污泥与生物滤池生物膜的混合污泥 | 生物滤池(1L) | 进水总氮约105mg/L。在Fe2+的长期作用下,氮的去除速率由最初投加Fe2+时的约0.51kg/(m3·d)最终增至0.58kg/(m3·d) | [ |
| Fe2+ | 1~50mg/L | 厌氧氨氧化与硝化污泥污泥混合 | 生物滤池(1L) | 进水氨氮与亚硝酸盐氮各50mg/L。未投加Fe2+时,总氮去除速率为0.51kg/(m3·d);氨氮、亚硝酸盐氮与总氮去除率分别为58.9%、60.9%和58.2%。1~5mg/LFe2+能够促进脱氮,总氮去除速率增至0.57kg/(m3·d);氨氮、亚硝酸盐氮与总氮去除率分别增至94.4%、93.0%和80.8%。10~50mg/LFe2+则会不同程度地抑制脱氮 | [ |
| Fe3+ | 3.68mg/L | 稳定运行的实验室规模反应器中污泥 | 上流式厌氧污泥反应器(1L) | 进水氨氮125.9~168.3mg/L,亚硝酸盐氮149.0~182.5mg/L。添加Fe3+时的平均氮去除率和最大氮去除速率分别为67.4%和4.9kg/(m3·d)[对照组相应值分别为64.7%和4.1kg/(m3·d)] | [ |
| Fe3O4 | 2g | 实验室规模上流式厌氧污泥床反应器的厌氧氨氧化污泥 | 厌氧连续搅拌反应器(2L) | 进水氨氮和亚硝酸盐浓度分别为100.5mg/L和132.1mg/L。反应器稳定运行阶段,最大总氮去除率约98%,大于对照组约87% | [ |
Fe电极(阳极) (?60mm×90mm) | 上流式厌氧氨氧化反应器污泥 | 上流式厌氧反应器(0.7L) | 进水氨氮和亚硝酸盐氮浓度均为650mg/L。氨氮、亚硝酸盐氮和总氮去除速率分别为609.9mg/(L·d)、638.4mg/(L·d)和1209.6mg/(L·d);未设Fe-石墨电极的对照组中相应值分别仅为493.9mg/(L·d)、524.2mg/(L·d)和973.3mg/(L·d) | [ |
| 铁投加形式及投加量 | 反应器类型(有效容积) | 细菌或污泥 | 硝酸盐氮初始浓度 | 硝酸盐氮还原速率 | 参考文献 |
|---|---|---|---|---|---|
| ZVI,2g | 瓶子(0.25L) | 铁还原菌CC76 | 15mg/L | 0.28mg/(L·h) | [ |
| 还原铁粉(200目),每10天添加一次,投加量为10g | 升流式厌氧反应器(4L) | 实验室培养的自养型亚硝化污泥 | 平均 101.84mg/L | 29.3g/(m3·d) | [ |
| Fe2+,Fe/N摩尔比为2 | 血清瓶(0.1L) | 实验室规模高负荷脱氮反应器中反硝化颗粒污泥 | 98.89mg/L | 27.59mg/(g VSS·d) | [ |
| Fe2+,约30mg/L(与约70mg/L的Mn2+共存) | 血清瓶(0.25L) | 假单胞菌H117 | 约17mg/L | 0.2153mg/(L·h) | [ |
| Fe2+,800mg/L;或Fe(Ⅱ) [Fe(Ⅱ)EDTA),1500mg/L] | 上流式生物滤池反应器(4.71L) | 微杆菌属W5 | 约30mg/L | — | [ |
| Fe2+,约1250~1600mg/L | 上流式厌氧污泥床反应器(0.8L) | 用于处理造纸废水的大型上流式厌氧污泥床反应器中厌氧颗粒污泥 | 约106~ 112mg/L | 0.073kg/(m3·d) | [ |
| Fe(OH)2或FeS,电子供体/电子受体摩尔比为2 | 密闭玻璃瓶(0.3L) | 污水处理厂厌氧消化池污泥 | 50mg/L | 8.16mmol/(L·d) 或1.37mmol/(L·d) | [ |
| Fe(OH)2、FeS和S0共同作电子供体,总电子供体/受体物质的量之比1.15 | 上流式厌氧污泥床反应器(4L) | 污水处理厂厌氧消化池污泥 | 50mg/L | 7.01mmol/(L·d) | [ |
| 黄铁矿FeS2 | 聚丙烯瓶(0.05L) | 脱氮硫杆菌 | 2.46mmol/L | 0.54mmol/(kgpyrite·d) | [ |
| 磁黄铁矿Fe1-xS(x=0~0.125) | 上流式生物滤池反应器(1.8L) | 从厌氧污泥中富集的自养反硝化菌 | 21.11mg/L | 19.22mg/(L·d) | [ |
| 菱铁矿FeCO3 | 上流式生物滤池反应器(0.2417L) | 弗氏柠檬酸杆菌PXL1 | 150mg/L | 127.4~209.9mg/(kgsiderite·d) | [ |
| 零价铁屑(同碳合成IC-ME载体材料) | 玻璃瓶(与IC-ME耦合)(0.5L) | 煤化工废水处理厂氧化池污泥 | 90~98 mg/L | — | [ |
表2 不同形式含铁物质作电子供体的铁型反硝化过程
| 铁投加形式及投加量 | 反应器类型(有效容积) | 细菌或污泥 | 硝酸盐氮初始浓度 | 硝酸盐氮还原速率 | 参考文献 |
|---|---|---|---|---|---|
| ZVI,2g | 瓶子(0.25L) | 铁还原菌CC76 | 15mg/L | 0.28mg/(L·h) | [ |
| 还原铁粉(200目),每10天添加一次,投加量为10g | 升流式厌氧反应器(4L) | 实验室培养的自养型亚硝化污泥 | 平均 101.84mg/L | 29.3g/(m3·d) | [ |
| Fe2+,Fe/N摩尔比为2 | 血清瓶(0.1L) | 实验室规模高负荷脱氮反应器中反硝化颗粒污泥 | 98.89mg/L | 27.59mg/(g VSS·d) | [ |
| Fe2+,约30mg/L(与约70mg/L的Mn2+共存) | 血清瓶(0.25L) | 假单胞菌H117 | 约17mg/L | 0.2153mg/(L·h) | [ |
| Fe2+,800mg/L;或Fe(Ⅱ) [Fe(Ⅱ)EDTA),1500mg/L] | 上流式生物滤池反应器(4.71L) | 微杆菌属W5 | 约30mg/L | — | [ |
| Fe2+,约1250~1600mg/L | 上流式厌氧污泥床反应器(0.8L) | 用于处理造纸废水的大型上流式厌氧污泥床反应器中厌氧颗粒污泥 | 约106~ 112mg/L | 0.073kg/(m3·d) | [ |
| Fe(OH)2或FeS,电子供体/电子受体摩尔比为2 | 密闭玻璃瓶(0.3L) | 污水处理厂厌氧消化池污泥 | 50mg/L | 8.16mmol/(L·d) 或1.37mmol/(L·d) | [ |
| Fe(OH)2、FeS和S0共同作电子供体,总电子供体/受体物质的量之比1.15 | 上流式厌氧污泥床反应器(4L) | 污水处理厂厌氧消化池污泥 | 50mg/L | 7.01mmol/(L·d) | [ |
| 黄铁矿FeS2 | 聚丙烯瓶(0.05L) | 脱氮硫杆菌 | 2.46mmol/L | 0.54mmol/(kgpyrite·d) | [ |
| 磁黄铁矿Fe1-xS(x=0~0.125) | 上流式生物滤池反应器(1.8L) | 从厌氧污泥中富集的自养反硝化菌 | 21.11mg/L | 19.22mg/(L·d) | [ |
| 菱铁矿FeCO3 | 上流式生物滤池反应器(0.2417L) | 弗氏柠檬酸杆菌PXL1 | 150mg/L | 127.4~209.9mg/(kgsiderite·d) | [ |
| 零价铁屑(同碳合成IC-ME载体材料) | 玻璃瓶(与IC-ME耦合)(0.5L) | 煤化工废水处理厂氧化池污泥 | 90~98 mg/L | — | [ |
| 微生物脱氮过程 | 化学反应方程式 | 参考文献 |
|---|---|---|
| 厌氧氨氧化 | [ | |
| 氢自养反硝化 | [ | |
| 铁型反硝化 | [ | |
表3 铁与各微生物脱氮过程之间相互作用的化学式
| 微生物脱氮过程 | 化学反应方程式 | 参考文献 |
|---|---|---|
| 厌氧氨氧化 | [ | |
| 氢自养反硝化 | [ | |
| 铁型反硝化 | [ | |
| 1 | SUN Z Y, LV Y K, LIU Y X, et al. Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a novel metal resistant bacterium Cupriavidus sp. S1[J]. Bioresource Technology, 2016, 220: 142-150. |
| 2 | HUI C, GUO X X, SUN P F, et al. Removal of nitrite from aqueous solution by Bacillus amyloliquefaciens biofilm adsorption[J]. Bioresource Technology B, 2017, 248: 146-152. |
| 3 | AHN Y H. Sustainable nitrogen elimination biotechnologies: a review[J]. Process Biochemistry, 2006, 41(8): 1709-1721. |
| 4 | FEROUSI C, LINDHOUD S, BAYMANN F, et al. Iron assimilation and utilization in anaerobic ammonium oxidizing bacteria[J]. Current Opinion in Chemical Biology, 2017, 37: 129-136. |
| 5 | SIMON J, KLOTZ M G. Diversity and evolution of bioenergetic systems involved in microbial nitrogen compound transformations[J]. Biochimica et Biophysica Acta, 2013, 1827(2): 114-135. |
| 6 | JEFFERSON B, BURGESS J E, PICHON A, et al. Nutrient addition to enhance biological treatment of greywater[J]. Water Research, 2001, 35(11): 2702-2710. |
| 7 | XU P, ZENG G M, HUANG D L, et al. Use of iron oxide nanomaterials in wastewater treatment: a review[J]. Science of the Total Environment, 2012, 424(4): 1-10. |
| 8 | REN L F, NI S Q, LIU C, et al. Effect of zero-valent iron on the start-up performance of anaerobic ammonium oxidation (anammox) process[J]. Environmental Science and Pollution Research, 2015, 22(4): 2925-2934. |
| 9 | NI S Q, NI J Y, YANG N, et al. Effect of magnetic nanoparticles on the performance of activated sludge treatment system[J]. Bioresource Technology, 2013, 143(1): 555-561. |
| 10 | CRANE R A, SCOTT T B. Nanoscale zero-valent iron: future prospects for an emerging water treatment technology[J]. Journal of Hazardous Materials, 2012, 211/212: 112-125. |
| 11 | GLASS J B, ORPHAN V J. Trace metal requirements for microbial enzymes involved in the production and consumption of methane and nitrous oxide[J]. Frontiers in Microbiology, 2012, 3: 1-20. |
| 12 | KUENEN J G. Anammox bacteria: from discovery to application[J]. Nature Reviews Microbiology, 2008, 6(4): 320-326. |
| 13 | QIAO S, BI Z, ZHOU J T, et al. Long term effects of divalent ferrous ion on the activity of anammox biomass[J]. Bioresource Technology, 2013, 142(4): 490-497. |
| 14 | ZHANG X J, CHEN Z, ZHOU Y, et al. Impacts of the heavy metals Cu (Ⅱ), Zn (Ⅱ) and Fe (Ⅱ) on an Anammox system treating synthetic wastewater in low ammonia nitrogen and low temperature: Fe(Ⅱ) makes a difference[J]. Science of the Total Environment, 2019, 648: 798-804. |
| 15 | ZHANG X J, ZHOU Y, ZHAO S Y, et al. Effect of Fe (Ⅱ) in low-nitrogen sewage on the reactor performance and microbial community of an ANAMMOX biofilter[J]. Chemosphere, 2018, 200: 412-418. |
| 16 | CHEN H, YU J J, JIA X Y, et al. Enhancement of anammox performance by Cu(Ⅱ), Ni(Ⅱ) and Fe(Ⅲ) supplementation[J]. Chemosphere, 2014, 117(1): 610-616. |
| 17 | GAO F, ZHANG H M, YANG F L, et al. The effects of zero-valent iron (ZVI) and ferroferric oxide (Fe3O4) on anammox activity and granulation in anaerobic continuously stirred tank reactors (CSTR)[J]. Process Biochemistry, 2014, 49(11): 1970-1978. |
| 18 | ZHANG J X, ZHANG Y B, LI Y, et al. Enhancement of nitrogen removal in a novel anammox reactor packed with Fe electrode[J]. Bioresource Technology, 2012, 114(3): 102-108. |
| 19 | ZHANG H, JIN Z H, HAN L, et al. Synthesis of nanoscale zero-valent iron supported on exfoliated graphite for removal of nitrate[J]. Transactions of Nonferrous Metals Society of China, 2006, 16(s1): s345-s349. |
| 20 | BI Z, QIAO S, ZHOU J T, et al. Fast start-up of Anammox process with appropriate ferrous iron concentration[J]. Bioresource Technology, 2014, 170(5): 506-512. |
| 21 | LI X, YUAN Y, HUANG Y, et al. A novel method of simultaneous NH4+ and NO3- removal using Fe cycling as a catalyst: feammox coupled with NAFO[J]. Science of the Total Environment, 2018, 631/632: 153-157. |
| 22 | ZHENG Z J, LI W G, HUANG X F, et al. Effect of trace elements and optimization of their composition for the nitrification of a heterotrophic nitrifying bacterium, Acinetobacter harbinensis HITLi7T, at low temperature[J]. Annals of Microbiology, 2017, 67(11): 715-725. |
| 23 | 王秀蘅, 任南琪, 王爱杰, 等. 铁锰离子对硝化反应的影响效应研究[J]. 哈尔滨工业大学学报, 2003, 35(1): 122-125. |
| WANG X H, REN N Q, WANG A J, et al. Effect of ferrous and manganese ion on nitrification[J]. Journal of Harbin Institute of Technology, 2003, 35(1): 122-125. | |
| 24 | 王亚娥, 李杰, 翟思媛, 等. Fe0对SBBR工艺处理腈纶废水性能影响[J]. 化工学报, 2013, 64(8): 2996-3002. |
| WANG Y E, LI J, ZHAI S Y, et al. Effect of Fe0 on acrylic fiber wastewater treatment in SBBR[J]. CIESC Journal, 2013, 64(8): 2996-3002. | |
| 25 | MA B R, WANG S, LI Z W, et al. Magnetic Fe3O4 nanoparticles induced effects on performance and microbial community of activated sludge from a sequencing batch reactor under long-term exposure[J]. Bioresource Technology, 2017, 225: 377-385. |
| 26 | PINTATHONG P, RICHARDSON D J, SPIRO S, et al. Influence of metal ions and organic carbons on denitrification activity of the halotolerant bacterium, Paracoccus pantotrophus P16 a strain from shrimp pond[J]. Electronic Journal of Biotechnology, 2009, 12(2): 1-11. |
| 27 | JIA W L, WANG Q, ZHANG J, et al. Nutrients removal and nitrous oxide emission during simultaneous nitrification, denitrification, and phosphorus removal process: effect of iron[J]. Environmental Science and Pollution Research, 2016, 23(15): 15657-15664. |
| 28 | 王学, 李铁龙, 东美英, 等. 稳定纳米铁与反硝化菌耦合去除地下水中硝酸盐的研究[J]. 农业环境科学学报, 2011, 30(4): 739-745. |
| WANG X, LI T L, DONG M Y, et al. Reducing nitrate in groundwater by stabilized iron nanoparticles and denitrifying bacteria[J]. Journal of Agro-Environment Science, 2011, 30(4): 739-745. | |
| 29 | AN Y, LI T L, JIN Z H, et al. Nitrate degradation and kinetic analysis of the denitrification system composed of iron nanoparticles and hydrogenotrophic bacteria[J]. Desalination, 2010, 252(1): 71-74. |
| 30 | SUZUKI T, MORIBE M, OYAMA Y, et al. Mechanism of nitrate reduction by zero-valent iron: equilibrium and kinetics studies[J]. Chemical Engineering Journal, 2012, 183(3): 271-277. |
| 31 | ZHANG M, ZHENG P, WANG R, et al. Nitrate-dependent anaerobic ferrous oxidation (NAFO) by denitrifying bacteria: a perspective autotrophic nitrogen pollution control technology[J]. Chemosphere, 2014, 117(1): 604-609. |
| 32 | 王茹, 赵治国, 郑平, 等. 铁型反硝化:一种新型废水生物脱氮技术[J]. 化工进展, 2019, 38(4): 2003-2010. |
| WANG R, ZHAO Z G, ZHENG P, et al. Iron-dependent denitrification, a novel technology to remove nitrogen from wastewaters[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 2003-2010. | |
| 33 | LU J S, LIAN T T, SU J F. Effect of zero-valent iron on biological denitrification in the autotrophic denitrification system[J]. Research on Chemical Intermediates, 2018, 44: 6011-6022. |
| 34 | 张宁博,李祥,黄勇,等. 零价铁自养反硝化过程活性污泥矿化及解决措施[J]. 环境科学, 2017, 38(9): 3793-3800. |
| ZHANG N B, LI X, HUANG Y, et al. Activated sludge mineralization and solutions in the process of zero-valent iron autotrophic denitrification[J]. Environmental Science, 2017, 38(9): 3793-3800. | |
| 35 | LUO X X, SU J F, SHAO P H, et al. Efficient autotrophic denitrification performance through integrating thebio-oxidation of Fe(Ⅱ) and Mn(Ⅱ)[J]. Chemical Engineering Journal, 2018, 348: 669-677. |
| 36 | ZHOU J, WANG H Y, YANG K, et al. Autotrophic denitrification by nitrate-dependent Fe(Ⅱ) oxidation in a continuous up-flow biofilter[J]. Bioprocess and Biosystems Engineering, 2016, 39(2): 277-284. |
| 37 | ZHANG M, ZHENG P, LI W, et al. Performance of nitrate-dependent anaerobic ferrous oxidizing (NAFO) process: a novel prospective technology for autotrophic denitrification[J]. Bioresource Technology, 2015, 179: 543-548. |
| 38 | WEI Y Y, DAI J, MACKEY H R, et al. The feasibility study of autotrophic denitrification with iron sludge produced for sulfide control[J]. Water Research, 2017, 122: 226-233. |
| 39 | TORRENTÓ C, CAMA J, URMENETA J, et al. Denitrification of groundwater with pyrite and Thiobacillus denitrificans[J]. Chemical Geology, 2010, 278(1): 80-91. |
| 40 | LI R H, MORRISON L, COLLINS G, et al. Simultaneous nitrate and phosphate removal from wastewater lacking organic matter through microbial oxidation of pyrrhotite coupled to nitrate reduction[J]. Water Research, 2016, 96: 32-41. |
| 41 | YANG Y, CHEN T H, ZHANG X, et al. Simultaneous removal of nitrate and phosphate from wastewater by siderite based autotrophic denitrification[J]. Chemosphere, 2018, 199: 130-137. |
| 42 | ZHANG Z W, HAN Y X, XU C Y, et al. Microbial nitrate removal in biologically enhanced treated coal gasification wastewater of low COD to nitrate ratio by coupling biological denitrification with iron and carbon micro-electrolysis[J]. Bioresource Technology, 2018, 262: 65-73. |
| 43 | QIU X F, WANG T W, ZHONG X M, et al. Screening and characterization of an aerobic nitrifying-denitrifying bacterium from activated sludge[J]. Biotechnology and Bioprocess Engineering, 2012, 17(2): 353-360. |
| 44 | CHEN H, ZHAO X H, CHENG Y Y, et al. Iron robustly stimulates simultaneous nitrification and denitrification under aerobic conditions[J]. Environmental Science and Technology, 2018, 52(3): 1404-1412. |
| 45 | HUANG X L, GAO D W, PENG S, et al. Effects of ferrous and manganese ions on anammox process in sequencing batch biofilm reactors[J]. Journal of Environmental Sciences, 2014, 26(5): 1034-1039. |
| 46 | 于大禹, 张琳颖,高波. 异养硝化-好氧反硝化菌异养硝化性能的影响因素[J]. 化工进展, 2012, 31(12): 2797-2800. |
| YU D Y, ZHANG L Y, GAO B. Factors affecting the heterotrophic nitrification property of heterotrophic nitrification-aerobic denitrifier[J]. Chemical Industry and Engineering Progress, 2012, 31(12): 2797-2800. | |
| 47 | QIAN G S, HU X M, LI L, et al. Effect of iron ions and electric field on nitrification process in the periodic reversal bio-electrocoagulation system[J]. Bioresource Technology, 2017, 244: 382-390. |
| 48 | WASSER I M, De VRIES S, MOENNE-LOCCOZ P, et al. Nitric oxide in biological denitrification: Fe/Cu metalloenzyme and metal complex NO(x) redox chemistry[J]. Chemical Reviews, 2002, 102(4):1201-1234. |
| 49 | BOWMAN S E J, BREN K L. The chemistry and biochemistry of heme c: functional bases for covalent attachment[J]. Natural Product Reports, 2008, 25(6): 1118-1130. |
| 50 | OSHIKI M, ISHII S, YOSHIDA K, et al. Nitrate-dependent ferrous iron oxidation by anaerobic ammonium oxidation (anammox) bacteria[J]. Applied and Environmental Microbiology, 2013, 79(13): 4087-4093. |
| 51 | SU J F, CHENG C, HUANG T L, et al. Novel simultaneous Fe(Ⅲ) reduction and ammonium oxidation of Klebsiella sp. FC61 under the anaerobic conditions[J]. RSC Advances, 2016, 6(15): 12584-12591. |
| 52 | DING L J, AN X L, LI S, et al. Nitrogen loss through anaerobic ammonium oxidation coupled to iron reduction from paddy soils in a chronosequence[J]. Environmental Science and Technology, 2014, 48(18): 10641-10647. |
| 53 | OIKONOMIDIS I, BURROWS L J, CARLIELL-MARQUET C M. Mode of action of ferric and ferrous iron salts in activated sludge[J]. Journal of Chemical Technology and Biotechnology, 2010, 85(8): 1067-1076. |
| 54 | REN X M, CHEN Y, GUO L, et al. The influence of Fe2+, Fe3+ and magnet powder (Fe3O4) on aerobic granulation and their mechanisms[J]. Ecotoxicology and Environmental Safety, 2018, 164: 1-11. |
| 55 | KONCZAK B, KARCZ J, MIKSCH K. Influence of calcium, magnesium, and iron ions on aerobic granulation[J]. Applied Biochemistry and Biotechnology, 2014, 174(8): 2910-2918. |
| 56 | CHEN H, LIU Y F, XU X Q, et al. How does iron facilitate the aerated biofilter for tertiary simultaneous nutrient and refractory organics removal from real dyeing wastewater?[J]. Water Research, 2019(148): 344-358. |
| [1] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [2] | 汪鹏, 张洋, 范兵强, 何登波, 申长帅, 张贺东, 郑诗礼, 邹兴. 高碳铬铁盐酸浸出过程工艺及动力学[J]. 化工进展, 2023, 42(S1): 510-517. |
| [3] | 许中硕, 周盼盼, 王宇晖, 黄威, 宋新山. 硫铁矿介导的自养反硝化研究进展[J]. 化工进展, 2023, 42(9): 4863-4871. |
| [4] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [5] | 常印龙, 周启民, 王青月, 王文俊, 李伯耿, 刘平伟. 废弃聚烯烃的高值化学回收研究进展[J]. 化工进展, 2023, 42(8): 3965-3978. |
| [6] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [7] | 徐沛瑶, 陈标奇, KANKALA Ranjith Kumar, 王士斌, 陈爱政. 纳米材料用于铁死亡联合治疗的研究进展[J]. 化工进展, 2023, 42(7): 3684-3694. |
| [8] | 龚鹏程, 严群, 陈锦富, 温俊宇, 苏晓洁. 铁酸钴复合碳纳米管活化过硫酸盐降解铬黑T的性能及机理[J]. 化工进展, 2023, 42(7): 3572-3581. |
| [9] | 杨竞莹, 施万胜, 黄振兴, 谢利娟, 赵明星, 阮文权. 改性纳米零价铁材料制备的研究进展[J]. 化工进展, 2023, 42(6): 2975-2986. |
| [10] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| [11] | 杨红梅, 高涛, 鱼涛, 屈撑囤, 高家朋. 高铁酸盐处理难降解有机物磺化酚醛树脂[J]. 化工进展, 2023, 42(6): 3302-3308. |
| [12] | 王子宗, 刘罡, 王振维. 乙烯丙烯生产过程强化技术进展及思考[J]. 化工进展, 2023, 42(4): 1669-1676. |
| [13] | 贺山明, 潘界昌, 徐国钻, 李文君, 梁勇. 粗钨酸钠溶液亚铁盐沉淀法除铬、钒的热力学分析及实验验证[J]. 化工进展, 2023, 42(4): 2171-2179. |
| [14] | 赵重阳, 赵磊, 石详文, 黄俊, 李治尧, 沈凯, 张亚平. O2/H2O/SO2 对改性富铁凹凸棒石高温吸附PbCl2 的影响[J]. 化工进展, 2023, 42(4): 2190-2200. |
| [15] | 胡兆岩, 张景新, 何义亮. Fe负载污泥生物炭催化热解聚丙烯及产物特性[J]. 化工进展, 2023, 42(2): 631-640. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||