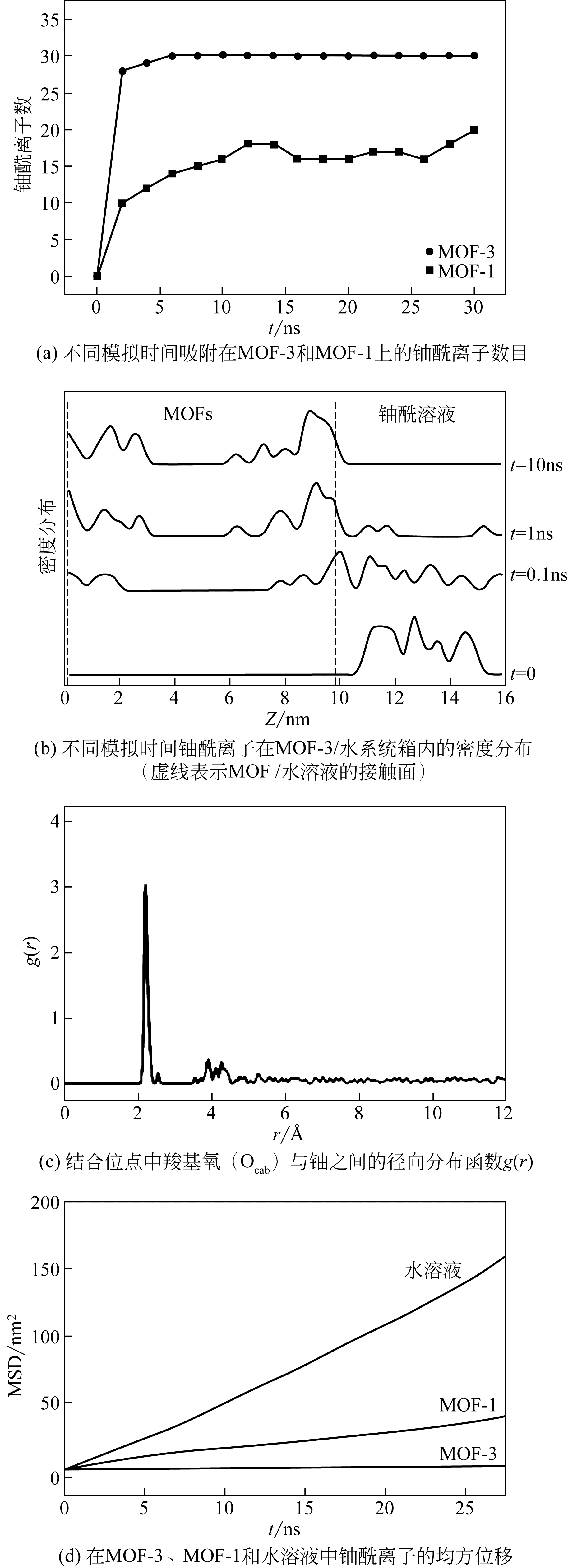
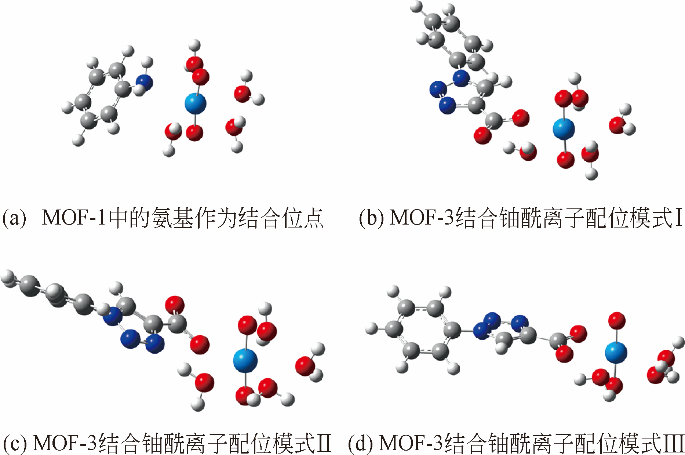
化工进展 ›› 2019, Vol. 38 ›› Issue (07): 3227-3242.DOI: 10.16085/j.issn.1000-6613.2018-1919
金属有机框架材料吸附分离水中铀的应用
- 南华大学资源环境与安全工程学院,湖南 衡阳 421001
-
收稿日期:2018-09-25出版日期:2019-07-05发布日期:2019-07-05 -
通讯作者:张晓文 -
作者简介:彭莹(1987—),女,博士研究生,研究方向为新型材料的合成及在环境领域的应用。E-mail:<email>pengying415@163.com</email>。 -
基金资助:国家自然科学基金(51874180);南华大学双一流建设项目(2017SYL05);湖南省铀尾矿库退役治理技术工程技术研究中心联合项目(2018YKZX2008)
Application of metal-organic frameworks in adsorption and separation of uranium from water
Ying PENG( ),Xiaowen ZHANG(
),Xiaowen ZHANG( ),Mi LI,Yu ZHANG,Xiaoyan WU
),Mi LI,Yu ZHANG,Xiaoyan WU
- College of Resources, Environment and Safety Engineering, University of South China, Hengyang 421001, Hunan, China
-
Received:2018-09-25Online:2019-07-05Published:2019-07-05 -
Contact:Xiaowen ZHANG
摘要:
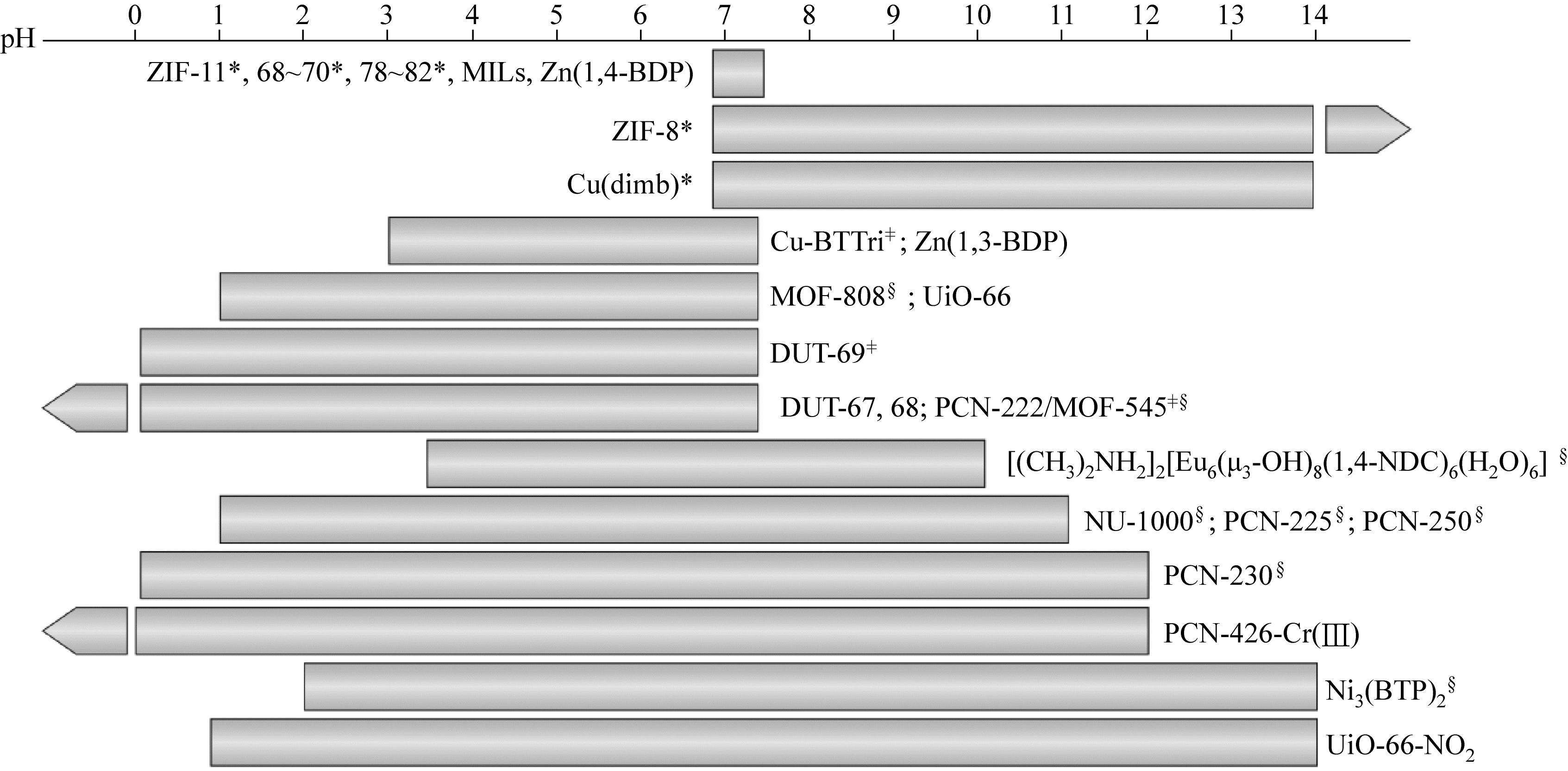
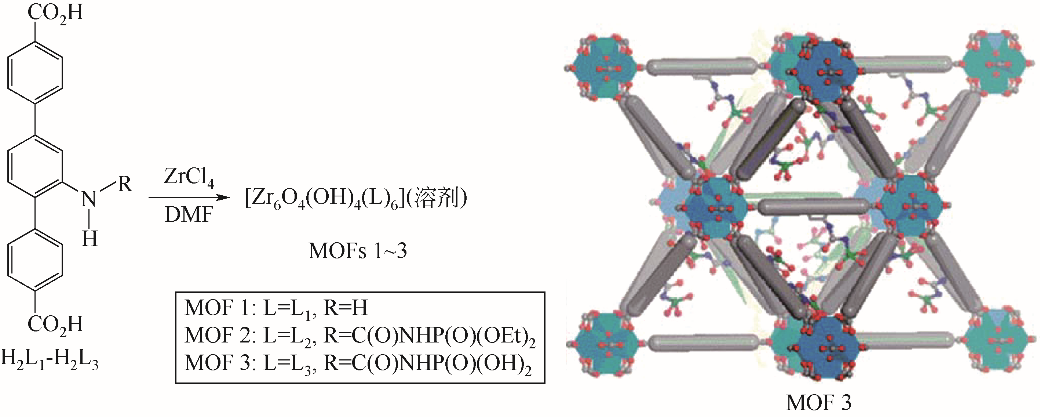
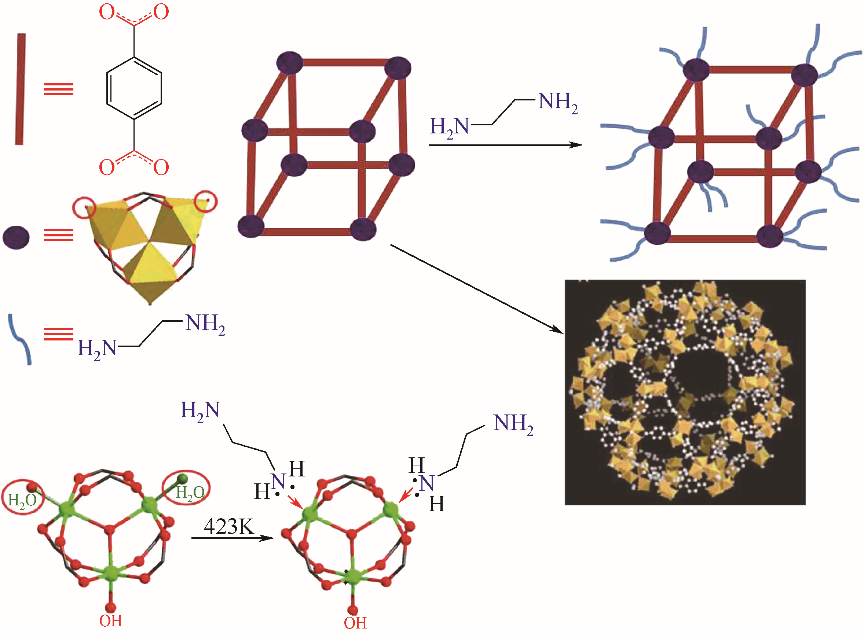
金属有机框架材料(metal-organic frameworks,MOFs)具有极高的比表面积和孔隙率,结构可设计调控,但在水相吸附分离方面存在水稳定和选择吸附性较差、分离困难、合成与再生成本偏高等问题。针对MOFs的缺陷,可以通过有目的的功能化改性从而提升其对目标污染物的吸附性能。本文介绍了MOFs的结构优势,分析了水稳定性的影响因素和判断手段,简述了具有代表性的高水稳定性MOFs材料的特性;根据MOFs改性方法的分类回顾了MOFs及改性MOFs在去除水相中放射性铀的应用;基于不同分析技术探讨了MOFs与铀酰离子的吸附机理;提出推动MOFs在吸附铀方面规模化应用发展的核心是合成高稳定性MOFs,通过改性提高MOFs的选择吸附性能和再生性以及深入研究吸附机理。
中图分类号:
引用本文
彭莹, 张晓文, 李密, 张宇, 吴晓燕. 金属有机框架材料吸附分离水中铀的应用[J]. 化工进展, 2019, 38(07): 3227-3242.
Ying PENG, Xiaowen ZHANG, Mi LI, Yu ZHANG, Xiaoyan WU. Application of metal-organic frameworks in adsorption and separation of uranium from water[J]. Chemical Industry and Engineering Progress, 2019, 38(07): 3227-3242.
| 吸附剂 | 比表面积 /m2·g-1 | 最佳pH | 固液比 /g·L-1 | q max /mg·g-1 | 符合的动力学模型 | 符合的热力学方程 | 吸附机理 | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| UiO-68-DPPU | 1350 | 2.5 | 1 | 217 | — | Langmuir | DFT计算得出DPPU与UO2 2+的吸附原理符合2:1的结合模式 | [ | |||
| MOF-76 | — | 3.0 | 0.4 | 298 | — | Langmuir | — | [ | |||
| HKUST-1 | 6 | 0.6 | 787.4 | 准二级 | Langmuir | 通过热力学参数ΔG、ΔH、ΔS推测吸附过程是自发吸热的过程 | [ | ||||
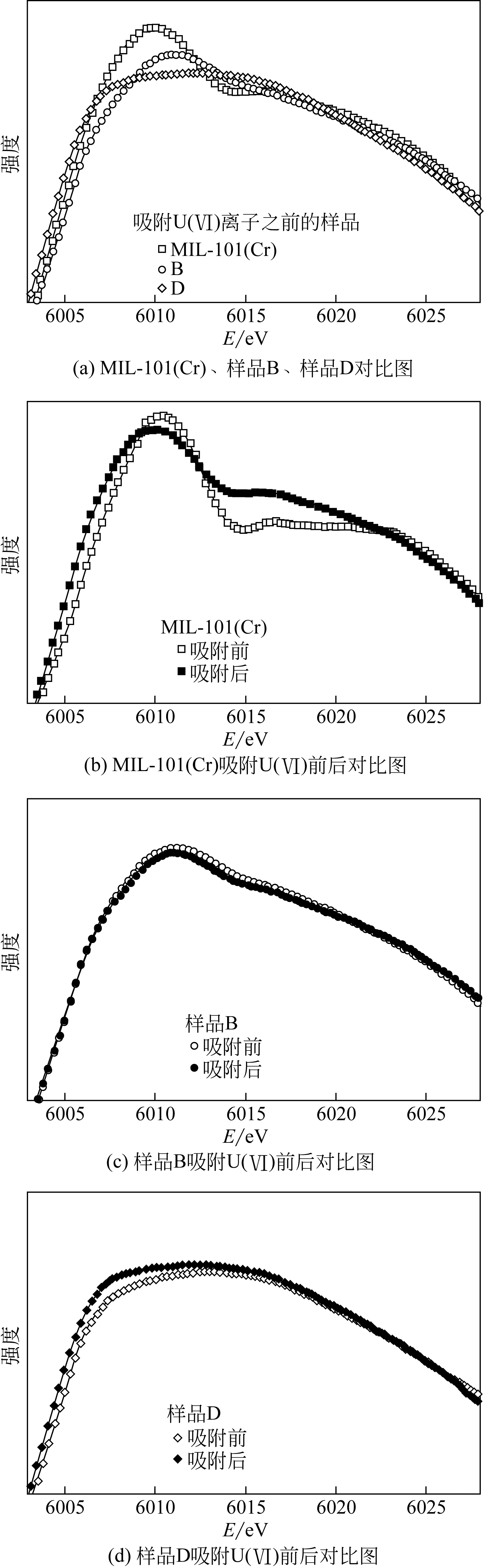
| MIL-101(Cr)-ED | 517 | 4.5 | 0.5 | 200(exp, c 0=100mg·L-1) | — | — | X射线吸收光谱(XANES+EXAFS)研究了铀与氨基的结合模式和ED嫁接量的影响 | [ | |||
| MIL-101 | 3065 | 5 | 0.4 | 20(exp) | 准二级 | Langmuir | FTIR和EXAFS联合分析得出MIL-101-NH2上的NH2由于苯环的空间位阻作用而影响了U-N的结合 | [ | |||
| MIL-101-NH2 | 1645 | 90(exp) | |||||||||
| MIL-101-DETA | 1074 | 350(exp) | |||||||||
| MIL-101-ED | 753 | 200(exp) | |||||||||
| Zn(HBTC)(L)·(H2O)2 | — | 2 | — | 0.53① | 准二级 | Langmuir | IR光谱分析推测吸附作用主要是由于MOF材料1D通道上独立的羧基与UO2 2+的U-O键合作用 | [ | |||
| MIL-101-COOH | 890.5 | 7 | — | 314 | 准二级 | Langmuir | MDS(分子动力学模拟)和DFT(密度泛函理论计算)联合从分子水平分析了MOFs与U的结合 | [ | |||
| Coumarin-modified Zn-MOF-74 | — | 4 | 1 | 360(exp, c 0=400mg·L-1) | 准二级 | — | — | [ | |||
| UiO-66 | 1382 | 109.9 | 准二级 | Langmuir | 简单推测NH2并没有提高UiO-66吸附能力的原因是苯环上氨基的低活性、比表面积的降低和NH2之间氢键的形成 | [ | |||||
| UiO-66-NH2 | 1050 | 5.5 | 0.4 | 114.9 | |||||||
| HKUST-1@H3PW12O40 | 467 | 6 | 0.2 | 14.58 | 准二级 | Langmuir | 通过热力学参数ΔG,ΔH,ΔS推测吸附过程是自发吸热的过程 | [ | |||
| UiO-66-AO | 711 | — | — | 2.68② | 准二级 | — | EXAFS分析得出偕胺肟能够与U(Ⅵ)进行螯合,形成六角形双锥体配位几何体 | [ | |||
| MIL-53(Al)-AO | — | 5.5 | 0.4 | 454.54 | 准二级 | Freundlich | — | [ | |||
| CMPO@MIL-101(Cr) | 1014 | — | — | — | — | — | 该篇文献主要是研究材料很好的选择性和可重复利用性,批实验和机理分析少 | [ | |||
| MIL-101-Ship | 2365 | 4 | 1 | 27.99 | 准二级 | Langmuir | — | [ | |||
| Fe3O4@ZIF-8 | 1137 | 3 | — | 523.5(exp) | 准二级 | Langmuir | — | [ | |||
| Fe3O4@AMCA-MIL53(Al) | 197.8 | 5.5 | 0.8 | 227.3 | 准二级 | Langmuir | 通过热力学参数ΔG、ΔH、ΔS推测吸附过程是自发吸热的过程 | [ | |||
| GO–COOH/UiO-66 | 767.12 | 8 | 0.5 | 998(exp, c 0=502.2mg·L-1) | 准二级 | Langmuir | FTIR光谱法、X射线光电子能谱(XPS)和XRD联合分析得出COOH和铀主要是螯合作用,还有一点离子交换作用 | [ | |||
| Co-SLUG-35 | — | 9 | — | 1.03③ | 准二级 | Langmuir | FTIR、SEM、XPS分析得出MOFs对铀的吸附机理是阴离子交换 | [ | |||
| MIL-100(Al) | — | 5 | — | 110(exp, c e=10000mg·L-1) | 准二级 | Freundlich | — | [ | |||
| SZ-1 | 10.2 | — | — | — | — | — | XAS(XANES+EXAFS)、XPS光谱和MDS模拟表明,UO2 2 +和SZ-2之间的强静电相互作用有效地将UO2 2+驱动到SZ-2的骨架中,而更紧密的氢键网络的形成导致UO2 2+被充分捕获 | [ | |||
| SZ-2 | 225 | 4.5 | — | 58.18 | — | Langmuir | |||||
| SZ-3 | 594 | 4.5 | 58.18 | — | Langmuir | ||||||
表1 原始MOFs及改性MOFs材料对铀的去除效果以及机理
| 吸附剂 | 比表面积 /m2·g-1 | 最佳pH | 固液比 /g·L-1 | q max /mg·g-1 | 符合的动力学模型 | 符合的热力学方程 | 吸附机理 | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| UiO-68-DPPU | 1350 | 2.5 | 1 | 217 | — | Langmuir | DFT计算得出DPPU与UO2 2+的吸附原理符合2:1的结合模式 | [ | |||
| MOF-76 | — | 3.0 | 0.4 | 298 | — | Langmuir | — | [ | |||
| HKUST-1 | 6 | 0.6 | 787.4 | 准二级 | Langmuir | 通过热力学参数ΔG、ΔH、ΔS推测吸附过程是自发吸热的过程 | [ | ||||
| MIL-101(Cr)-ED | 517 | 4.5 | 0.5 | 200(exp, c 0=100mg·L-1) | — | — | X射线吸收光谱(XANES+EXAFS)研究了铀与氨基的结合模式和ED嫁接量的影响 | [ | |||
| MIL-101 | 3065 | 5 | 0.4 | 20(exp) | 准二级 | Langmuir | FTIR和EXAFS联合分析得出MIL-101-NH2上的NH2由于苯环的空间位阻作用而影响了U-N的结合 | [ | |||
| MIL-101-NH2 | 1645 | 90(exp) | |||||||||
| MIL-101-DETA | 1074 | 350(exp) | |||||||||
| MIL-101-ED | 753 | 200(exp) | |||||||||
| Zn(HBTC)(L)·(H2O)2 | — | 2 | — | 0.53① | 准二级 | Langmuir | IR光谱分析推测吸附作用主要是由于MOF材料1D通道上独立的羧基与UO2 2+的U-O键合作用 | [ | |||
| MIL-101-COOH | 890.5 | 7 | — | 314 | 准二级 | Langmuir | MDS(分子动力学模拟)和DFT(密度泛函理论计算)联合从分子水平分析了MOFs与U的结合 | [ | |||
| Coumarin-modified Zn-MOF-74 | — | 4 | 1 | 360(exp, c 0=400mg·L-1) | 准二级 | — | — | [ | |||
| UiO-66 | 1382 | 109.9 | 准二级 | Langmuir | 简单推测NH2并没有提高UiO-66吸附能力的原因是苯环上氨基的低活性、比表面积的降低和NH2之间氢键的形成 | [ | |||||
| UiO-66-NH2 | 1050 | 5.5 | 0.4 | 114.9 | |||||||
| HKUST-1@H3PW12O40 | 467 | 6 | 0.2 | 14.58 | 准二级 | Langmuir | 通过热力学参数ΔG,ΔH,ΔS推测吸附过程是自发吸热的过程 | [ | |||
| UiO-66-AO | 711 | — | — | 2.68② | 准二级 | — | EXAFS分析得出偕胺肟能够与U(Ⅵ)进行螯合,形成六角形双锥体配位几何体 | [ | |||
| MIL-53(Al)-AO | — | 5.5 | 0.4 | 454.54 | 准二级 | Freundlich | — | [ | |||
| CMPO@MIL-101(Cr) | 1014 | — | — | — | — | — | 该篇文献主要是研究材料很好的选择性和可重复利用性,批实验和机理分析少 | [ | |||
| MIL-101-Ship | 2365 | 4 | 1 | 27.99 | 准二级 | Langmuir | — | [ | |||
| Fe3O4@ZIF-8 | 1137 | 3 | — | 523.5(exp) | 准二级 | Langmuir | — | [ | |||
| Fe3O4@AMCA-MIL53(Al) | 197.8 | 5.5 | 0.8 | 227.3 | 准二级 | Langmuir | 通过热力学参数ΔG、ΔH、ΔS推测吸附过程是自发吸热的过程 | [ | |||
| GO–COOH/UiO-66 | 767.12 | 8 | 0.5 | 998(exp, c 0=502.2mg·L-1) | 准二级 | Langmuir | FTIR光谱法、X射线光电子能谱(XPS)和XRD联合分析得出COOH和铀主要是螯合作用,还有一点离子交换作用 | [ | |||
| Co-SLUG-35 | — | 9 | — | 1.03③ | 准二级 | Langmuir | FTIR、SEM、XPS分析得出MOFs对铀的吸附机理是阴离子交换 | [ | |||
| MIL-100(Al) | — | 5 | — | 110(exp, c e=10000mg·L-1) | 准二级 | Freundlich | — | [ | |||
| SZ-1 | 10.2 | — | — | — | — | — | XAS(XANES+EXAFS)、XPS光谱和MDS模拟表明,UO2 2 +和SZ-2之间的强静电相互作用有效地将UO2 2+驱动到SZ-2的骨架中,而更紧密的氢键网络的形成导致UO2 2+被充分捕获 | [ | |||
| SZ-2 | 225 | 4.5 | — | 58.18 | — | Langmuir | |||||
| SZ-3 | 594 | 4.5 | 58.18 | — | Langmuir | ||||||
| 1 | 王建龙, 刘海洋 . 放射性废水的膜处理技术研究进展[J]. 环境科学学报, 2013, 33(10): 2639-2656. |
| WANG J L , LIU H Y . Research advances in radioactive wastewater treatment using membrane processes[J]. Acta Scientiae Circumstantiae, 2013, 33(10): 2639-2656. | |
| 2 | 陈海军, 黄舒怡, 张志宾, 等 . 功能性纳米零价铁的构筑及其对环境放射性核素铀的富集应用研究进展[J]. 化学学报, 2017, 75(6): 560-574. |
| CHEN H J , HUANG S Y , ZHANG Z B , et al . Synthesis of functional nanoscale zero-valent iron composites for the application of radioactive uranium enrichment from environment: a review[J]. Acta Chimica Sinica, 2017, 75(6): 560-574. | |
| 3 | YAN S , HUA B , BAO Z Y , et al . Uranium(Ⅵ) removal by nanoscale zerovalent iron in anoxic batch systems[J]. Environmental Science & Technology, 2010, 44(20): 7783-778. |
| 4 | VELLINGIRI K , K-H KIM , POURNARA A , et al . Towards high-efficiency sorptive capture of radionuclides in solution and gas[J]. Progress in Materials Science, 2018, 94: 1-67. |
| 5 | ABNEY C W , MAYES R T , SAITO T , et al . Materials for the recovery of uranium from seawater[J]. Chemical Reviews, 2017, 117(23): 13935-14013. |
| 6 | 魏金枝, 张少平, 王雪亮, 等 . 巯基修饰Cu-MOFs材料的制备及其汞吸附性能[J]. 化工学报, 2017, 68(5): 2186-2194. |
| WEI J Z , ZHANG S P , WANG X L , et al . Preparation of ethanedithiol modified Cu-MOFs and its adsorption performance for mercury[J]. Journal of Chemical Industry and Engineering, 2017, 68(5): 2186-2194. | |
| 7 | 赵田, 董茗, 赵熠, 等 . 纳米金属-有机框架材料的制备及应用[J]. 化学进展, 2017, 29(10): 1252-1259. |
| ZHAO T , DONG M , ZHAO Y , et al . Preparation and application of nano-sized metal-organic frameworks[J]. Progress in Chemistry, 2017, 29(10): 1252-1259. | |
| 8 | WEN J , FANG Y , ZENG G M . Progress and prospect of adsorptive removal of heavy metal ions from aqueous solution using metal organic frameworks: a review of studies from the last decade[J]. Chemosphere, 2018, 201: 627-643. |
| 9 | LUSTIG W P , MUKHERJEE S , DUDD N D , et al . Metal-organic frameworks: functional luminescent and photonic materials for sensing applications[J]. Chemical Society Reviews, 2017, 46: 3242-3285. |
| 10 | 冯丹, 隗翠香, 夏炎 . 功能化金属有机骨架材料去除饮用水污染物的研究进展[J]. 色谱, 2017, 35(3): 237-244. |
| FENG D , WEI C X , XIA Y . Progress in the applications of funcionalized metal-organic frameworks for adsorption and removal of pollutants in drinking water[J]. Chinese Journal of Chromatograghy, 2017, 35(3): 237-244. | |
| 11 | 张贺, 李国良, 张可刚, 等 . 金属有机骨架材料在吸附分离研究中的应用进展[J]. 化学学报, 2017, 75: 841-859. |
| ZHANG H , LI G L , ZHANG K G , et al . Advances of metal-organic frameworks in adsorption and separation applications[J]. Acta Chimica Sinica, 2017, 75: 841-859. | |
| 12 | KOBIELSKA P A , HOWARTH A J , FARHA O K , et al . Metal-organic frameworks for heavy metal removal from water[J]. Coordination Chemistry Reviews, 2018, 358: 92-107. |
| 13 | MON M, BRUNO R , FERRANDO-SORIA J , et al . Metal-organic framework technologies for water remediation: towards a sustainable ecosystem[J]. Journal of Materials Chemistry A, 2018, 6: 4912-4947. |
| 14 | 李小娟, 何长发, 黄斌, 等 . 金属有机骨架材料吸附去除环境污染物的进展[J]. 化工进展, 2016, 35(2): 586-594. |
| LI X J , HE C F , HUANG B , et al . Progress in the applications of metal-organic frameworks in adsorption removal of hazardous materials[J].Chemical Industry and Engineering Progress, 2016, 35(2): 586-594. | |
| 15 | CARBONI M , ABNEY C W , LIU S B , et al . Highly porous and stable metal-organic frameworks for uranium extraction[J]. Chemical Science, 2013, 4: 2396-2402. |
| 16 | 姜宁, 邓志勇, 王公应, 等 . 金属有机框架材料的制备及在吸附分离CO2中的应用[J]. 化学进展, 2014, 26(10): 1645-1654. |
| JIANG N , DENG Z Y , WANG G Y , et al . Preparation of metal-organic frameworks and application for CO2 adsorption and separation[J]. Progress in Chemistry, 2014, 26(10): 1645-1654. | |
| 17 | 翟睿, 焦丰龙, 林虹君, 等 . 金属有机框架材料的研究进展[J]. 色谱, 2014, 32(2): 107-116. |
| ZHAI R , JIAO F L , LIN H J , et al . Progress in metal-organic frameworks[J]. Chinese Journal of Chromatography, 2014, 32(2): 107-116. | |
| 18 | CAVKA J H , JAKOBSEN S , OLSBYE U , et al . A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability[J]. Journal of the American Chemical Society, 2008, 130: 13850-13851. |
| 19 | LI H , EDDAOUDI M , O’KEEFFE M , et al . Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J]. Nature, 1999, 402(6759): 276-279. |
| 20 | FURUKAWA H , KO N, GO Y B, et al . Ultrahigh porosity in metal-organic frameworks[J]. Science, 2010, 329(5990): 424-428. |
| 21 | FARHA O K , ERYAZICI I , JEONG N C , et al . Metal-organic framework materials with ultrahigh surface areas: is the sky the limit? [J]. Journal of the American Chemical Society, 2012, 134(36): 15016-15021. |
| 22 | 刘兵, 介素云, 李伯耿 . 金属有机框架化合物在非均相催化反应中的应用[J]. 化学进展, 2013, 25(1): 36-45. |
| LIU B , JIE S Y, Li B G . Metal-organic frameworks for heterogeneous catalysis[J]. Progress in Chemistry, 2013, 25(1): 36-45. | |
| 23 | SCHAATE A , ROY P, GODT A , et al . Modulated synthesis of Zr-based metal-organic frameworks: from nano to single crystals[J]. European Journal of Chemistry, 2011, 17: 6643-6651. |
| 24 | KATZ M J , BROWN Z J , COLÓN Y J , et al . A facile synthesis of UiO-66, UiO-67 and their derivatives[J]. Chemical Communications, 2013, 49: 9449-9451. |
| 25 | CHEN L , BAI Z L , ZHU L , et al . Ultrafast and efficient extraction of uranium from seawater using an amidoxime appended metal-organic framework[J]. ACS Applied Materials & Interfaces, 2017, 9: 32446-32451. |
| 26 | LI F B , LI X Y , CUI P , et al . Plasma-grafting amidoxime/metal-organic framework composites for the selective sequestration of U(Ⅵ)[J]. Environmental Science-Nano, 2018, 5(8): 2000-2008. |
| 27 | LIU J M , LIU T , WANG C C , et al . Introduction of amidoxime groups into metal-organic frameworks to synthesize MIL-53(Al)-AO for enhanced U(Ⅵ) sorption[J]. Journal of Molecular Liquids, 2017, 242: 531-536. |
| 28 | BAI Y , DOU Y B , XIE L H , et al . Zr-based metal-organic frameworks: design, synthesis, structure, and applications[J]. Chemical Society Reviews, 2016, 45: 2327-2367. |
| 29 | CANIVET J , EATEEVA A , GUO Y , et al . Water adsorption in MOFs: fundamentals and applications[J]. Chemical Society Reviews, 2014, 43: 5594-5617. |
| 30 | BURTCH N C , JASUJA H , WALTON K S , et al . Water stability and adsorption in metal organic frameworks[J]. Chemical Reviews, 2014, 114:10575-10612. |
| 31 | HOWARTH A J , LIU Y Y , LI P , et al . Chemical, thermal and mechanical stabilities of metal-organic frameworks[J]. Nature Reviews Materials, 2016, 1: 1-15. |
| 32 | WANG C H , LIU X L , DEMIR N K , et al . Applications of water stable metal-organic frameworks[J]. Chemical Society Reviews, 2016, 45(18): 5107-5134. |
| 33 | FENG M B , ZHANG P , ZHOU H C , et al . Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: a review[J]. Chemosphere, 2018, 209: 783-800. |
| 34 | PARK K S , NI Z , CÔTÉ A P , et al . Exceptional chemical and thermal stability of zeolitic imidazolate frameworks[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(27): 10187-10191. |
| 35 | LIU X L , LI Y S , ZHU G Q , et al . An organophilic pervaporation membrane derived from metal-organic framework nanoparticles for efficient recovery of bio-alcohols[J]. Angewandte Chemie:International Edition, 2011, 50(45): 10636-10639. |
| 36 | BARTHELET K , MARROT J , RIOU D , et al . A breathing hybrid organic-inorganic solid with very large pores and high magnetic characteristics[J]. Angewandte Chemie: International Edition, 2002, 41(2): 281-284. |
| 37 | SERRE C , MILLANGE F , THOUVENOT C , et al . Very large breathing effect in the first nanoporous chromium(Ⅲ)-based solids: MIL-53 or CrIII(OH)•{O2C-C6H4-CO2}•{HO2C-C6H4-CO2H} x •H2O y [J]. Journal of the American Chemical Society, 2002, 124: 13519-13526. |
| 38 | LOISEAU T , SERRE C , HUGUENARD C , et al . A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration[J]. European Journal of Chemistry, 2004, 10(6): 1373-1382. |
| 39 | KHAN N A , HASA Z , JHUNG S H . Adsorptive removal of hazardous materials using metal-organic frameworks(MOFs): a review[J]. Journal of Hazardous Materials, 2013, 244/245: 444-456. |
| 40 | FÉREY G , SERRE C , M-D Caroline , et al . A hybrid solid with giant pores prepared by a combination of targeted chemistry, simulation, and powder diffraction[J]. Angewandte Chemie: International Edition, 2004, 43, 6296-6301. |
| 41 | FÉREY G , MELLOT-DRAZNIEKS C , SERRE C , et al . A chromium terephthalate-based solid with unusually large pore volumes and surface area[J]. Science, 2005, 309: 2040-2042. |
| 42 | 李正杰 . 金属-有机骨架材料气相分离性能的分子模拟研究[D]. 北京: 北京化工大学, 2013. |
| LI Z J . Molecular simulation study on the gas separation performance of metal-organic frameworks[D]. Beijing: Beijing University of Chemical Technology, 2013. | |
| 43 | KANDIAH M , NILSEN M H , USSEGLIO S , et al . Synthesis and stability of tagged UiO-66 Zr-MOFs[J]. Chemistry of Materials, 2010, 22: 6632-6640. |
| 44 | WU H , CHUA Y S , KRUNGLEVICIUTE V , et al . Unusual and highly tunable missing-linker defects in zirconium metal-organic framework UiO-66 and their important effects on gas adsorption[J]. Journal of the American Chemical Society, 2013, 135: 10525-10532. |
| 45 | FENG Y F , JIANG H , LI S N , et al . Metal-organic frameworks HKUST-1 for liquid-phase adsorption of uranium[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2013, 431: 87-92. |
| 46 | FALAIS C , VOLKRINGER C , GIOVINE R , et al . Capture of actinides (Th4+, [UO2]2+) and surrogating lanthanide (Nd3+) in porous metal-organic framework MIL-100(Al) from water: selectivity and imaging of embedded nanoparticles[J]. Dalton Transactions, 2017, 46(36): 12010-12014. |
| 47 | WU Y H , PANG H W , YAO W , et al . Synthesis of rod-like metal-organic framework(MOF-5) nanomaterial for efficient removal of U(Ⅵ): batch experiments and spectroscopy study[J]. Science Bulletin, 2018, 63: 831-839. |
| 48 | SU S Z , CHE R , LIU Q , et al . Zeolitic imidazolate framework-67: a promising candidate for recovery of uranium (Ⅵ) from seawater[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 547: 73-80. |
| 49 | YUAN L Y , TIAN M , LAN J H , et al . Defect engineering in metal-organic frameworks: a new strategy to develop applicable actinide sorbents[J]. Chemical Communications, 2018, 54: 370-373. |
| 50 | XIAO C L , SILVER M A , WANG S A . Metal-organic frameworks for radionuclide sequestration from aqueous solution: a brief overview and outlook[J]. Dalton Transactions, 2017, 46: 16381-16386. |
| 51 | ZHENG T , YANG Z X , GUI D X , et al . Overcoming the crystallization and designability issues in the ultrastable zirconium phosphonate framework system[J]. Nature Communications, 2014, 8: 15369. |
| 52 | LI L N , MA W, SHEN S S , et al . A combined experimental and theoretical study on the extraction of uranium by amino-derived metal organic frameworks through post-synthetic strategy[J]. ACS Applied Materials & Interfaces, 2016, 8: 31032-31041. |
| 53 | DECKER J D , CLERCQ J D , VERMEIR P , et al . Functionalized metal-organic-framework CMPO@MIL-101(Cr) as a stable and selective rare earth adsorbent[J]. Journal of Materials Science, 2016, 51: 5019-5026. |
| 54 | ZHAO Y G , WANG X , LI J X ,et al . Amidoxime functionalization of mesoporous silica and its high removal of U(Ⅵ)[J]. Polymer Chemistry, 2015, 6: 5376-5384. |
| 55 | WANG Y , GU Z X , YANG J J , et al . Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium(Ⅵ)[J]. Applied Surface Science, 2014, 320: 10-20. |
| 56 | CHEN H , SHAO D D , LI J X , et al . The uptake of radionuclides from aqueous solution by poly(amidoxime) modified reduced graphene oxide[J]. Chemical Engineering Journal, 2014, 254: 623-634. |
| 57 | SONG L L , CHEN C , LUO F , et al . Isoreticular MOFs functionalized in the pore wall by different organic groups for high-performance removal of uranyl ions[J]. Journal of Radioanalytical and Nuclear Chemistry , 2016, 310: 317-327. |
| 58 | LUO B C , YUAN L Y , CHAI Z F , et al . U(Ⅵ) capture from aqueous solution by highly porous and stable MOFs: UiO-66 and its amine derivative[J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 307: 269-276. |
| 59 | LIU S J , LUO M B , LI J Q , et al . Adsorption equilibrium and kinetics of uranium onto porous azo-metal-organic frameworks[J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 310: 353-36. |
| 60 | TAO Y , YANG L X , LI J H , et al . A new azo metal-organic framework showing polycatenated 3D array and ultrahigh U(Ⅵ) removal[J]. Journal of Solid State Chemistry, 2018, 266: 244-249. |
| 61 | YANG L X , FENG X F , YIN W H , et al . Metal-organic framework containing both azo and amide groups for effective U(Ⅵ) removal[J]. Journal of Solid State Chemistry, 2018, 265: 148-154. |
| 62 | ZHANG J Y , ZHANG N , ZHANG L J , et al . Adsorption of uranyl ions on amine-functionalization of MIL-101(Cr) nanoparticles by a facile coordination-based post-synthetic strategy and X-ray absorption spectroscopy studies[J]. Scientific Reports, 2015, 5(13514): 1-10. |
| 63 | ZHANG L , WANG L L , GONG L L , et al . Coumarin-modified microporous-mesoporous Zn-MOF-74 showing ultra-high uptake capacity and photo-switched storage/release of UⅥ ions[J]. Journal of Hazardous Materials, 2016, 311: 30-36. |
| 64 | LIU F T , SONG S S , CHENG G , et al . MIL-101(Cr) metal-organic framework functionalized with tetraethylenepentamine for potential removal of uranium(Ⅵ) from waste water[J]. Adsorption Science & Technology, 2018, 36(7/8): 1550-1567. |
| 65 | BAI Z Q , YUAN L Y , ZHU L , et al . Introduction of amino groups into acid-resistant MOFs for enhanced U(Ⅵ) sorption[J]. Journal of Materials Chemistry A, 2015, 3: 525-534. |
| 66 | WANG L L , LUO F , DANG L L , et al . Ultrafast high-performance extraction of uranium from seawater without pretreatment using an acylamide- and carboxyl-functionalized metal-organic framework[J]. Journal of Materials Chemistry A, 2015, 3: 13724-13730. |
| 67 | DECKER J D , Kl FOLENS , CLERCQ J D , et al . Ship-in-a-bottle CMPO in MIL-101(Cr) for selective uranium recovery from aqueous streams through adsorption[J]. Journal of Hazardous Materials, 2017, 335: 1-9. |
| 68 | MIN X , YANG W T , HUI Y F , et al . Fe3O4@ZIF-8: a magnetic nanocomposite for highly efficient UO2 2+ adsorption and selective UO2 2+/Ln3+ separation[J]. Chemical Communications., 2017, 53: 4199-4202. |
| 69 | ALQADAMI A A , NAUSHAD M , ALOTHMAN Z A , et al . Novel metal-organic framework(MOF) based composite material for the sequestration of U(Ⅵ) and Th(Ⅳ) metal ions from aqueous environment[J]. ACS Applied Materials & Interfaces, 2017, 9(41): 36026-36037. |
| 70 | ZHANG X M , LIU Y , JIAO Y , et al . Facile construction of Fe@zeolite imidazolate framework-67 to selectively remove uranyl ions from aqueous solution[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 91: 309-315. |
| 71 | YANG P P , LIU Q , LIU J Y , et al . Interfacial growth of a metal-organic framework (UiO-66) on functionalized graphene oxide (GO) as a suitable seawater adsorbent for extraction of uranium(Ⅵ) [J]. Journal of Materials Chemistry A, 2017, 5(34): 17933-17942. |
| 72 | WANG C H , ZHENG T , LUO R , et al . In situ growth of ZIF-8 on PAN fibrous filters for highly efficient U(Ⅵ) removal[J]. ACS Applied Materials & Interfaces, 2018, 10: 24164-24171. |
| 73 | LI J , WU Z , DUAN Q Y , et al . Decoration of ZIF-8 on polypyrrole nanotubes for highly efficient and selective capture of U(Ⅵ)[J]. Journal of Cleaner Production, 2018, 204: 896-905. |
| 74 | FOTOVAT H , KHAJEH M , OVEISI A R , et al . A hybrid material composed of an amino-functionalized zirconium-based metal-organic framework and a urea-based porous organic polymer as an efficient sorbent for extraction of uranium(Ⅵ)[J]. Microchimica Acta, 2018, 185: 469-476. |
| 75 | LUSTIG W P , MUKHERJEE S , RUDD N D , et al . Metal-organic frameworks: functional luminescent and photonic materials for sensing applications[J]. Chemical Society Review, 2017, 46: 3242-3285. |
| 76 | ZHANG Y M , YUAN S , DAY G, et al . Luminescent sensors based on metal-organic frameworks[J]. Coordination Chemistry Reviews, 2018, 35: 28-45. |
| 77 | YANG W T , BAI Z Q , SHI W Q , et al . MOF-76: from a luminescent probe to highly efficient UVI sorption material[J]. Chemical Communications, 2013, 49: 10415-10417. |
| 78 | LIU W , DAI X , BAI Z L , et al . Highly sensitive and selective uranium detection in natural water systems using a luminescent mesoporous metal-organic framework equipped with abundant Lewis basic sites: a combined batch, X-ray absorption spectroscopy, and first principles simulation investigation[J]. Environmental Science & Technology, 2017, 51: 3911-3921. |
| 79 | CHEN W M , MENG X L , ZHANG G L , et al . A superior fluorescent sensor for Al3+ and UO2 2+ based on a Co(II) metal-organic framework with exposed pyrimidyl Lewis base site[J]. Journal of Materials Chemistry A, 2017, 5 (25): 13079-13085. |
| 80 | YE J W , BOGALE R F , SHI Y W , et al . A water-stable dual-channel luminescence sensor for UO2 2+ ions based on an anionic terbium(Ⅲ) metal-organic framework[J]. Chemistry:A European Journal , 2017, 23(32): 7657-7662. |
| 81 | ABNEY C W , TAYLOR-PASHOW K M L , RUSSELL S R , et al . Topotactic transformations of metal-organic frameworks to highly porous and stable inorganic sorbents for efficient radionuclide sequestration[J]. Chemistry of Materials, 2014, 26(18): 5231-5243. |
| 82 | HUANG S Y , PANG H W , LI L , et al . Unexpected ultrafast and high adsorption of U(Ⅵ) and Eu(Ⅲ) from solution using porous Al2O3 microspheres derived from MIL-53[J]. Chemical Engineering Journal, 2018, 353: 157-166. |
| 83 | LIU F T , XIONG W J , LIU J Y , et al . Novel amino-functionalized carbon material derived from metal organic framework: a characteristic adsorbent for U(Ⅵ) removal from aqueous environment[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 556: 72-80. |
| 84 | XIONG Y Y , LI J Q , YAN C S , et al . MOF catalysis of FeII-to-FeIII reaction for an ultrafast and one-step generation of the Fe2O3@MOF composite and uranium(Ⅵ) reduction by iron(Ⅱ) under ambient conditions[J]. Chemical Communications, 2016, 52(61): 9538-9541. |
| 85 | LI J Q , GONG L L , FENG X F , et al . Direct extraction of U(Ⅵ) from alkaline solution and seawater via anion exchange by metal-organic framework[J]. Chemical Engineering Journal, 2017, 316: 154-159. |
| 86 | LI J , WANG X X , ZHAO G X , et al . Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions[J]. Chemical Society Reviews, 2018, 47: 2322-2356. |
| 87 | HU R , WANG X K , DAI S Y , et al . Application of graphitic carbon nitride for the removal of Pb(Ⅱ) and aniline from aqueous solutions[J]. Chemical Engineering Journal, 2015, 260: 469-477. |
| 88 | ZHANG H , XUE J H , HU N , et al . Selective removal of U(Ⅵ) from low concentration wastewater by functionalized HKUST-1@H3PW12O40 [J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 308(3): 865-875. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [3] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [4] | 徐若思, 谭蔚. C形管池沸腾两相流流场模拟与流固耦合分析[J]. 化工进展, 2023, 42(S1): 47-55. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 张凤岐, 崔成东, 鲍学伟, 朱炜玄, 董宏光. 胺液吸收-分步解吸脱硫工艺的设计与评价[J]. 化工进展, 2023, 42(S1): 518-528. |
| [7] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [8] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [9] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [10] | 张瑞杰, 刘志林, 王俊文, 张玮, 韩德求, 李婷, 邹雄. 水冷式复叠制冷系统的在线动态模拟与优化[J]. 化工进展, 2023, 42(S1): 124-132. |
| [11] | 王太, 苏硕, 李晟瑞, 马小龙, 刘春涛. 交流电场中贴壁气泡的动力学行为[J]. 化工进展, 2023, 42(S1): 133-141. |
| [12] | 赵晨, 苗天泽, 张朝阳, 洪芳军, 汪大海. 负压状态窄缝通道乙二醇水溶液传热特性[J]. 化工进展, 2023, 42(S1): 148-157. |
| [13] | 孙继鹏, 韩靖, 唐杨超, 闫汉博, 张杰瑶, 肖苹, 吴峰. 硫黄湿法成型过程数值模拟与操作参数优化[J]. 化工进展, 2023, 42(S1): 189-196. |
| [14] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [15] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||