化工进展 ›› 2025, Vol. 44 ›› Issue (10): 5703-5716.DOI: 10.16085/j.issn.1000-6613.2024-1455
• 能源加工与技术 • 上一篇
铁基钙钛矿载氧体化学链制合成气热力学性能分析与实验
张善超1( ), 张德亮2, 王露2, 梁豪1, 杨倩1, 杨冠杰1, 苏伟1, 马晓迅1, 朱燕燕1(
), 张德亮2, 王露2, 梁豪1, 杨倩1, 杨冠杰1, 苏伟1, 马晓迅1, 朱燕燕1( )
)
- 1.西北大学化工学院,国家碳氢资源清洁利用国际科技合作基地,陕北能源先进化工利用技术教育部工程研究中心,陕西省洁净煤转化工程技术研究中心,陕北能源化工产业发展协同创新中心,陕西 西安 710127
2.陕西煤业化工集团神木能源发展有限公司,陕西 榆林 719300
-
收稿日期:2024-09-05修回日期:2024-12-26出版日期:2025-10-25发布日期:2025-11-10 -
通讯作者:朱燕燕 -
作者简介:张善超(2001—),男,硕士研究生,研究方向为能源化工。E-mail:19861215961@163.com。 -
基金资助:国家自然科学基金(22378331);榆林学院-中国科学院洁净能源创新研究院联合基金(2021012)
Thermodynamic analysis and experimental study on the iron-based perovskite oxygen carrier for syngas production via chemical looping
ZHANG Shanchao1( ), ZHANG Deliang2, WANG Lu2, LIANG Hao1, YANG Qian1, YANG Guanjie1, SU Wei1, MA Xiaoxun1, ZHU Yanyan1(
), ZHANG Deliang2, WANG Lu2, LIANG Hao1, YANG Qian1, YANG Guanjie1, SU Wei1, MA Xiaoxun1, ZHU Yanyan1( )
)
- 1.School of Chemical Engineering, Northwest University, International Scientific and Technological Cooperation Base for Clean Utilization of Hydrocarbon Resources, Chemical Engineering Research Center of the Ministry of Education for Advanced Use Technology of Shanbei Energy, Shaanxi Research Center of Engineering Technology for Clean Coal Conversion, Collaborative Innovation Center for Development of Energy and Chemical industry in Northern Shaanxi, Xi’an 710127, Shaanxi, China
2.Shenmu Energy Developments Company Limited, Yulin 719300, Shaanxi, China
-
Received:2024-09-05Revised:2024-12-26Online:2025-10-25Published:2025-11-10 -
Contact:ZHU Yanyan
摘要:
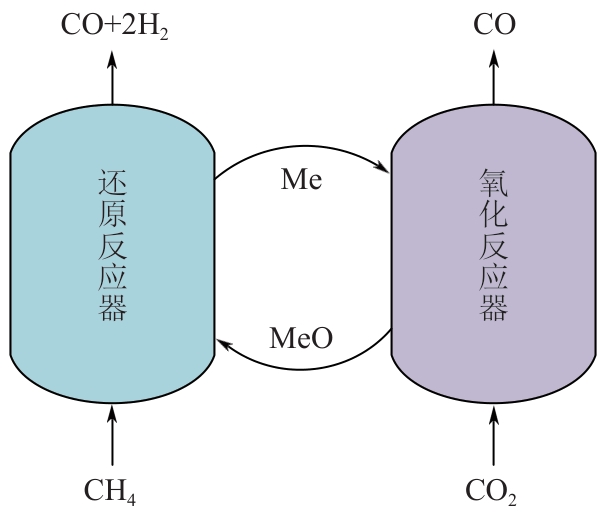
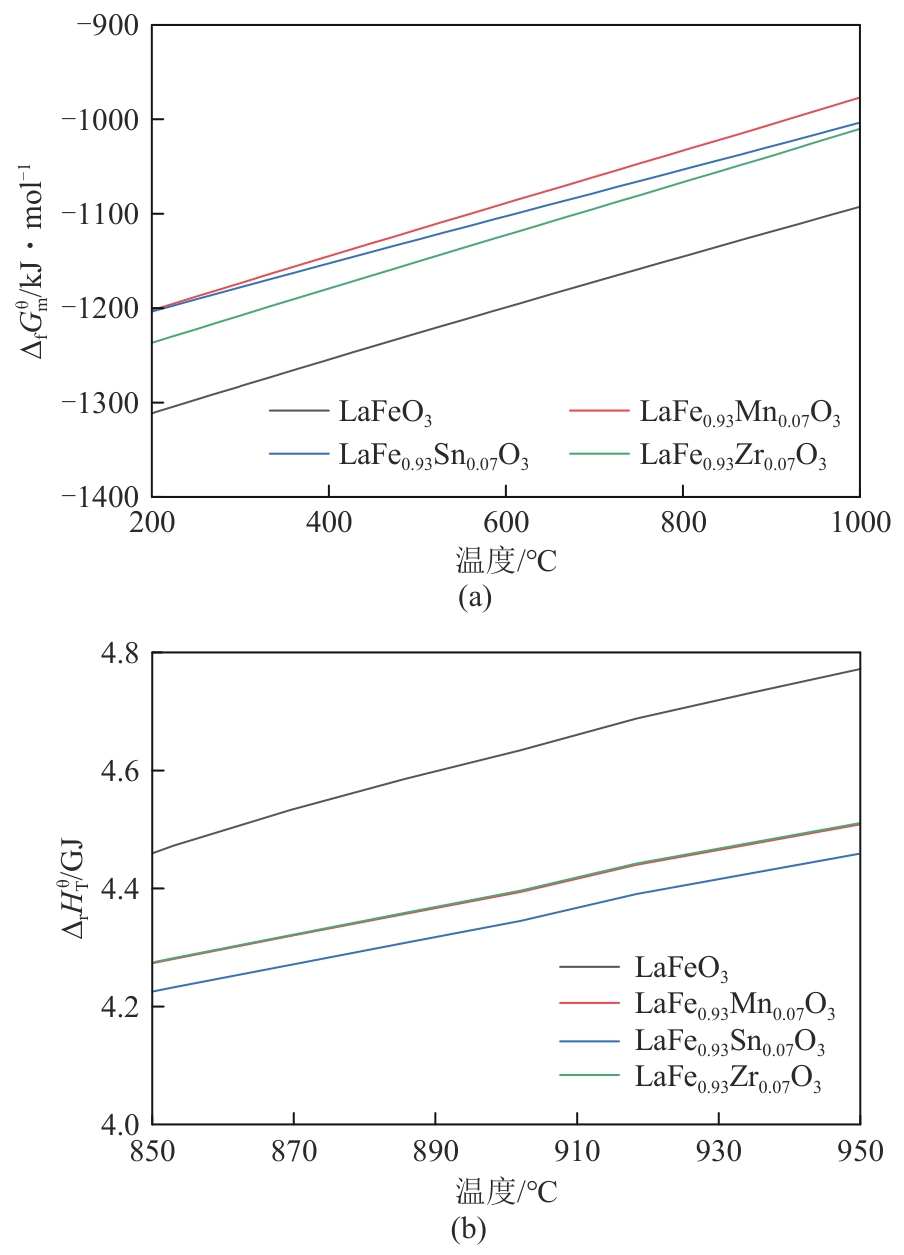
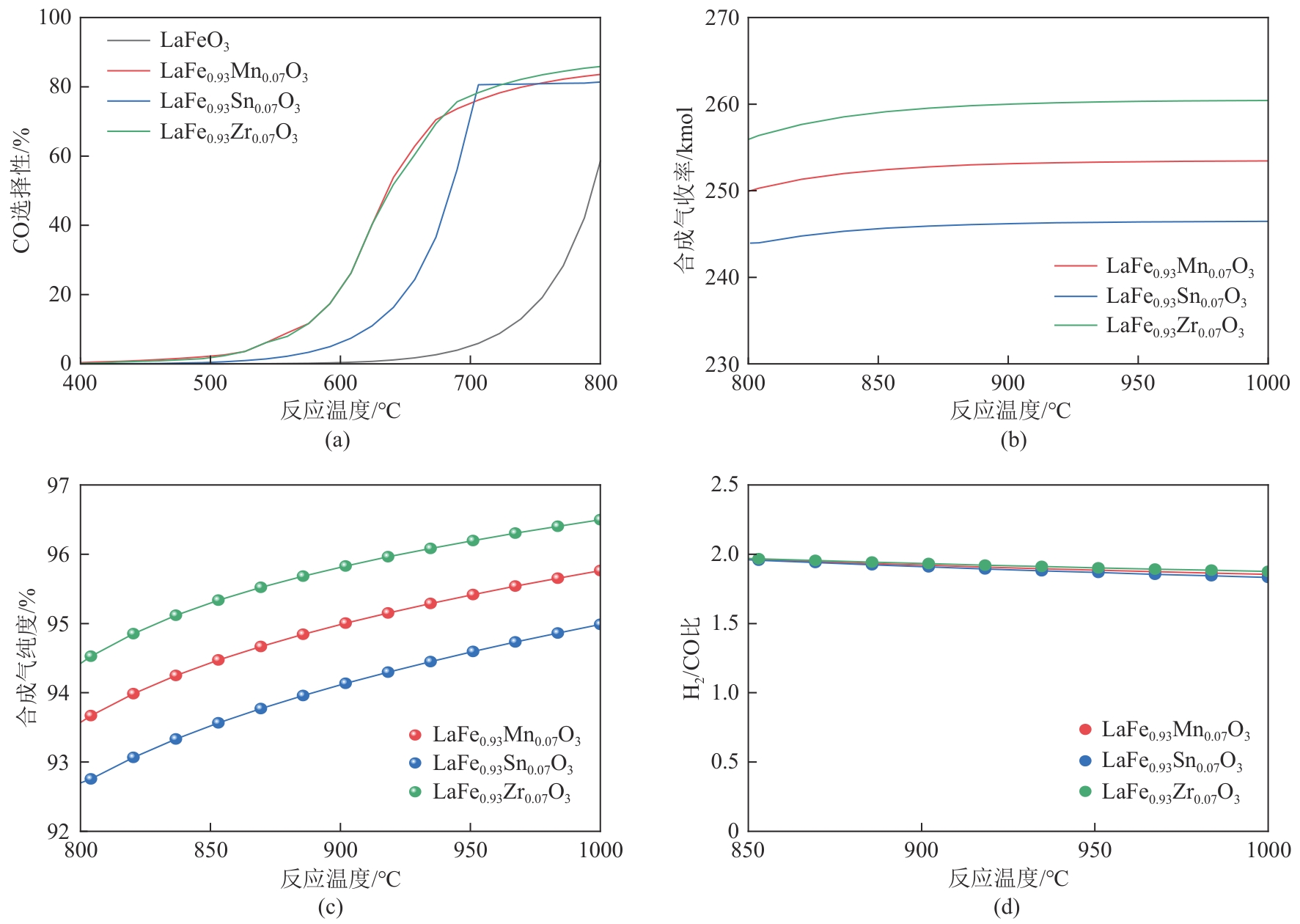
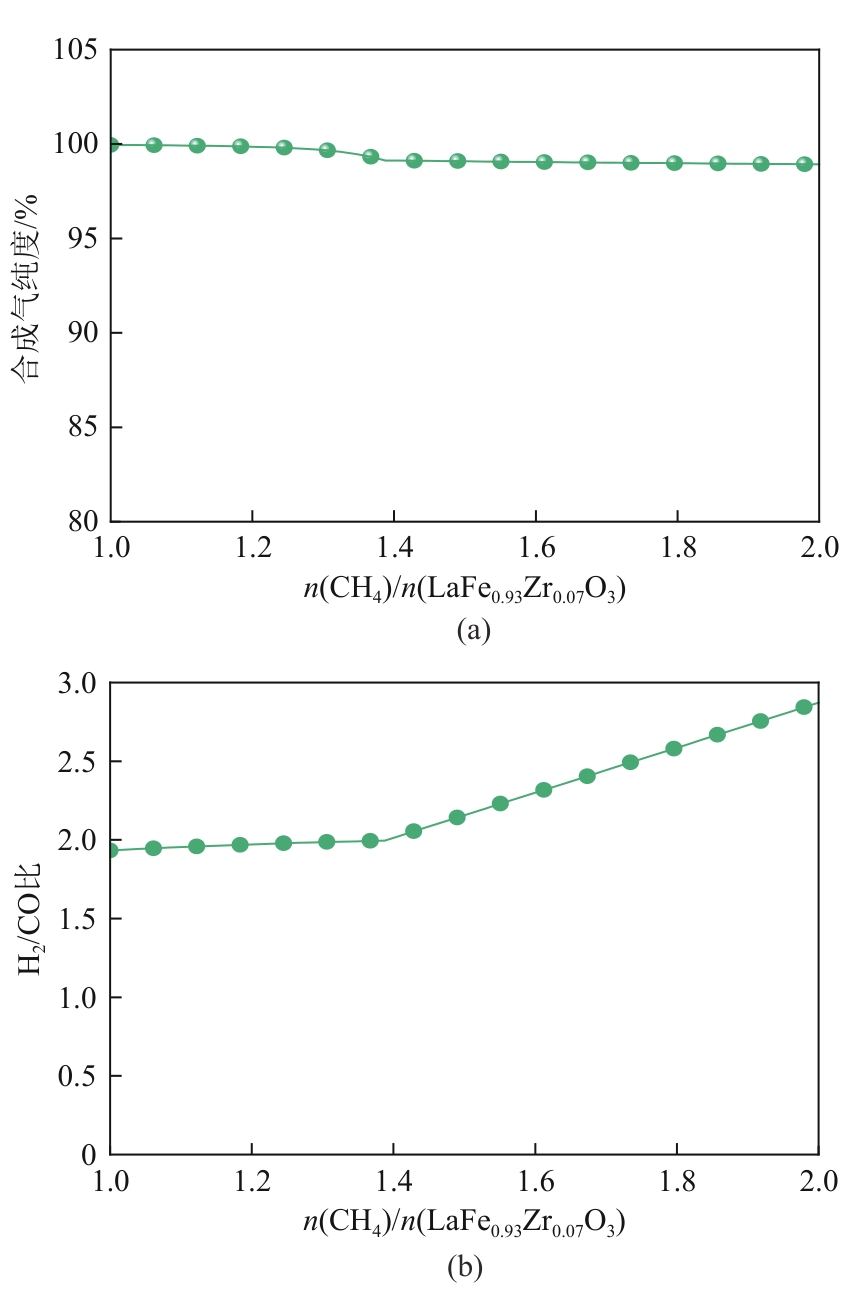
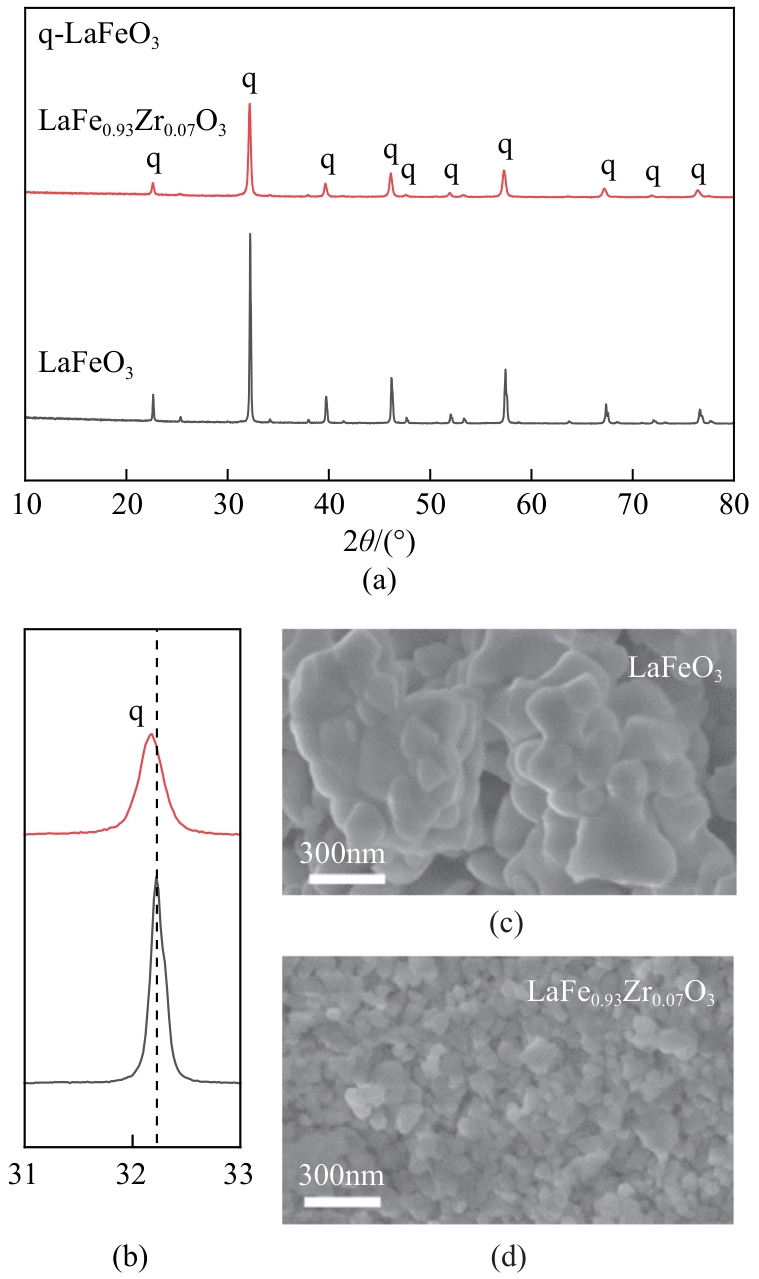
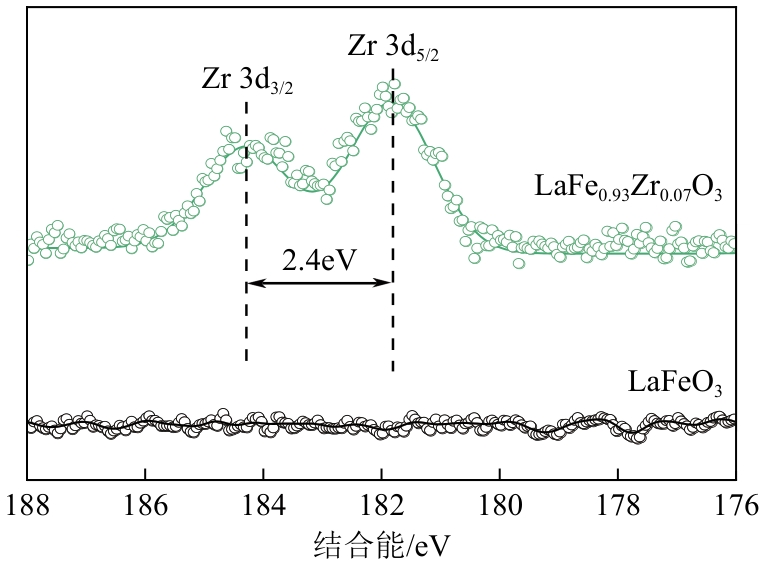
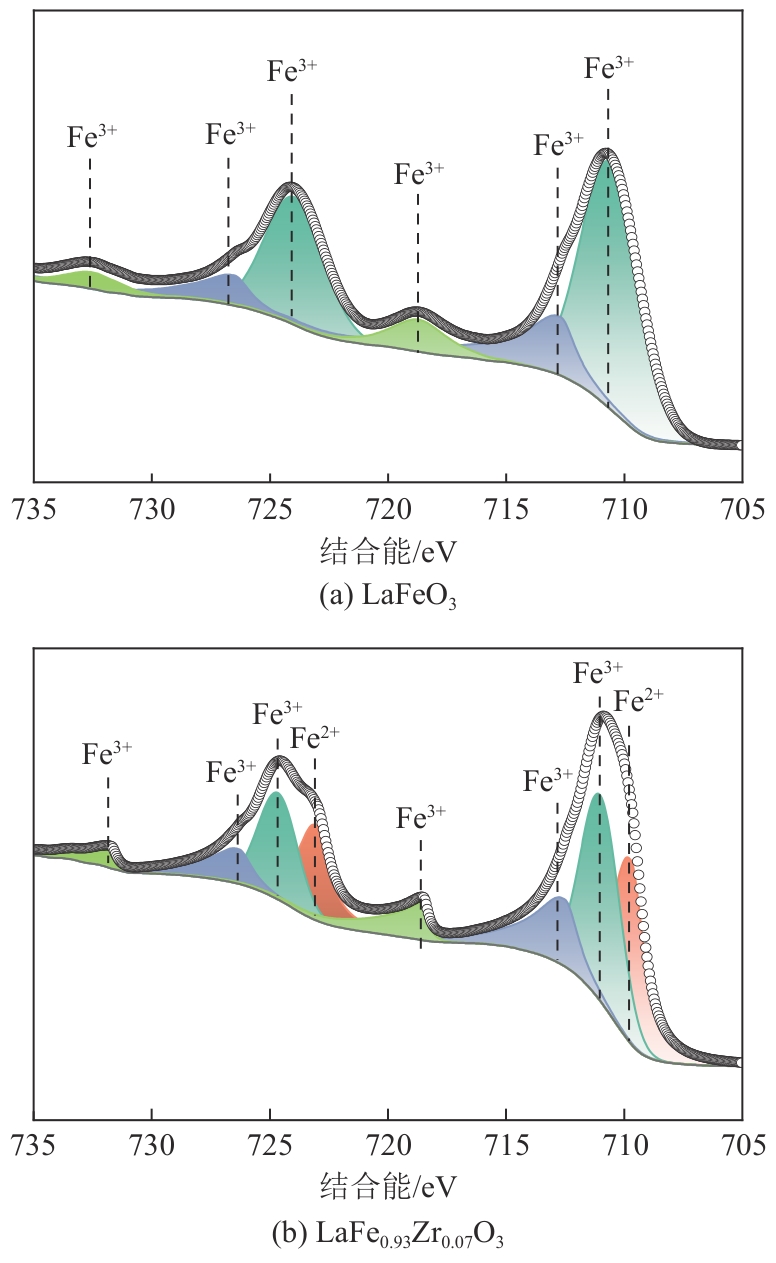
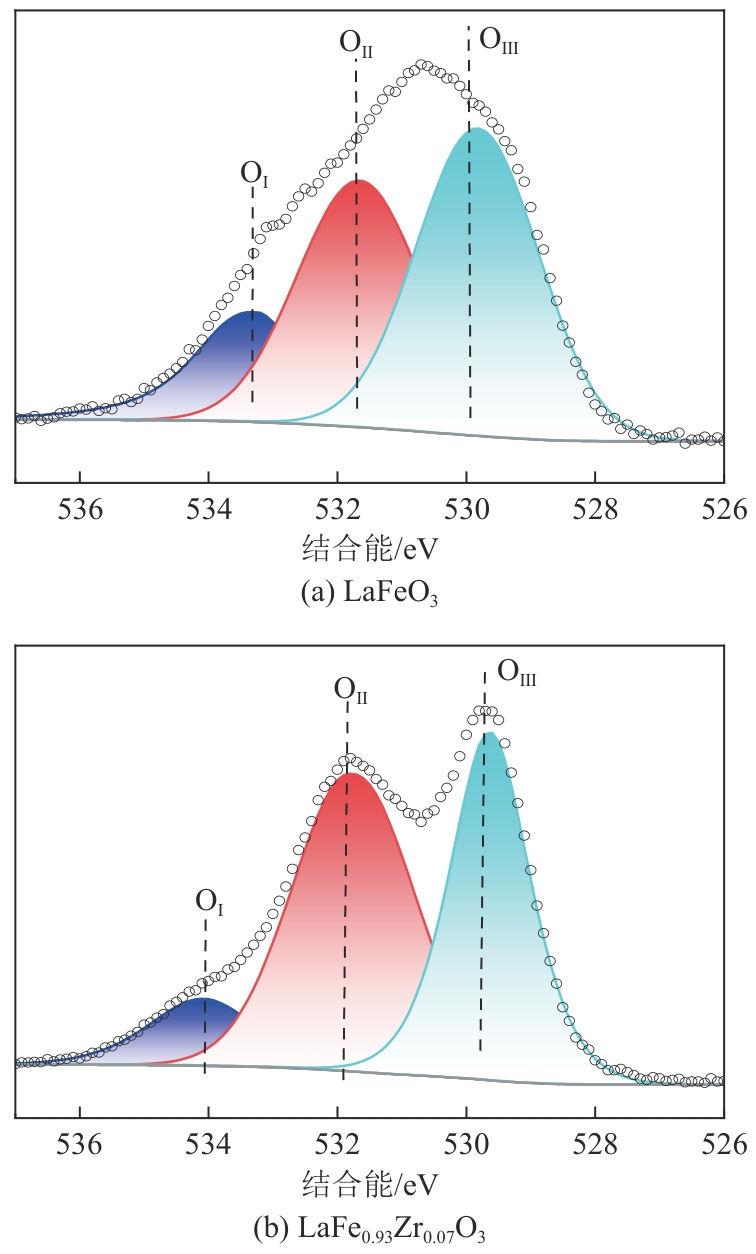
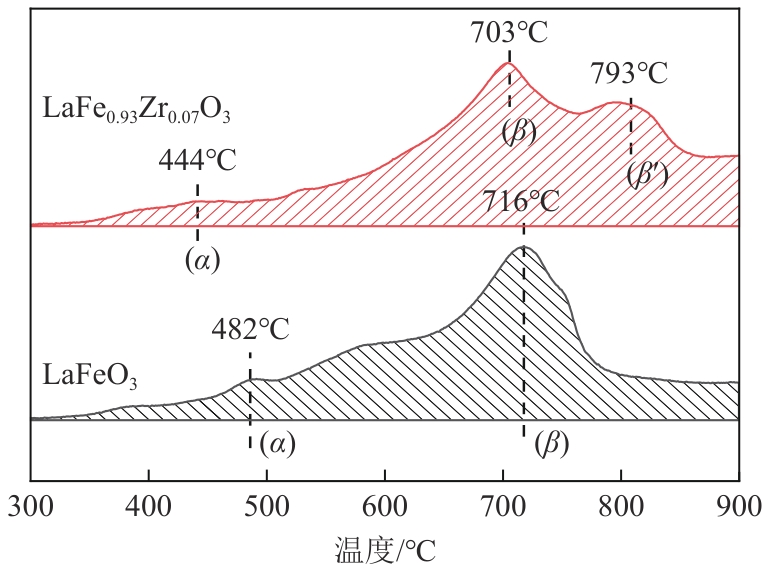
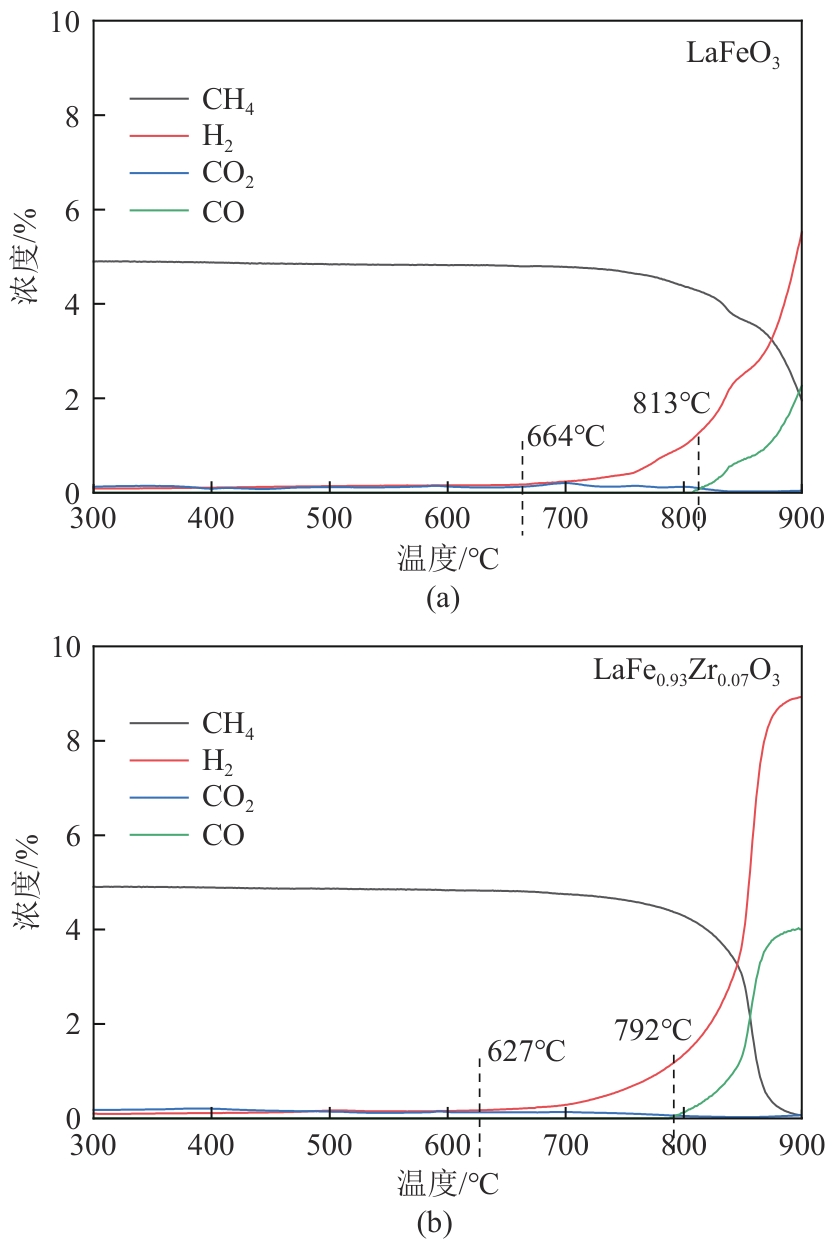
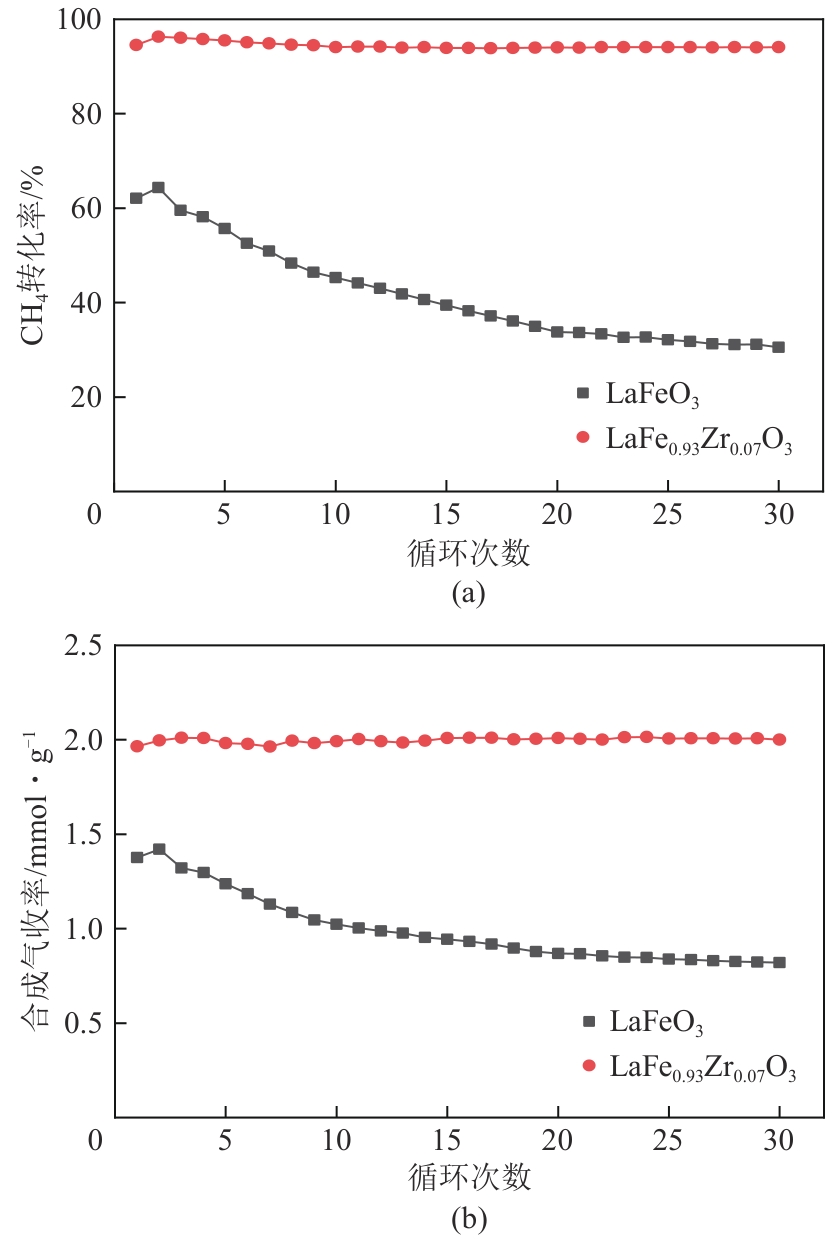
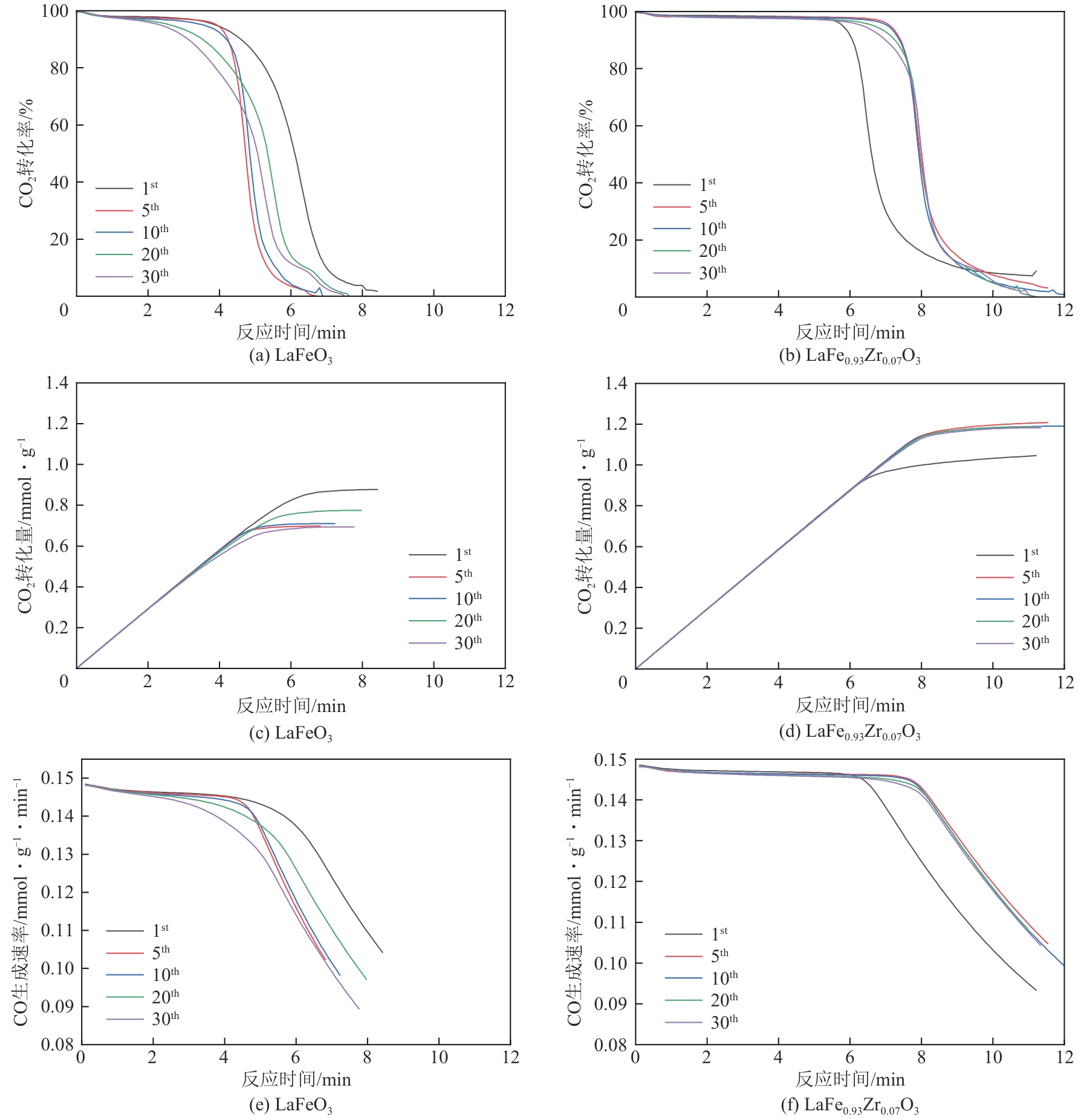
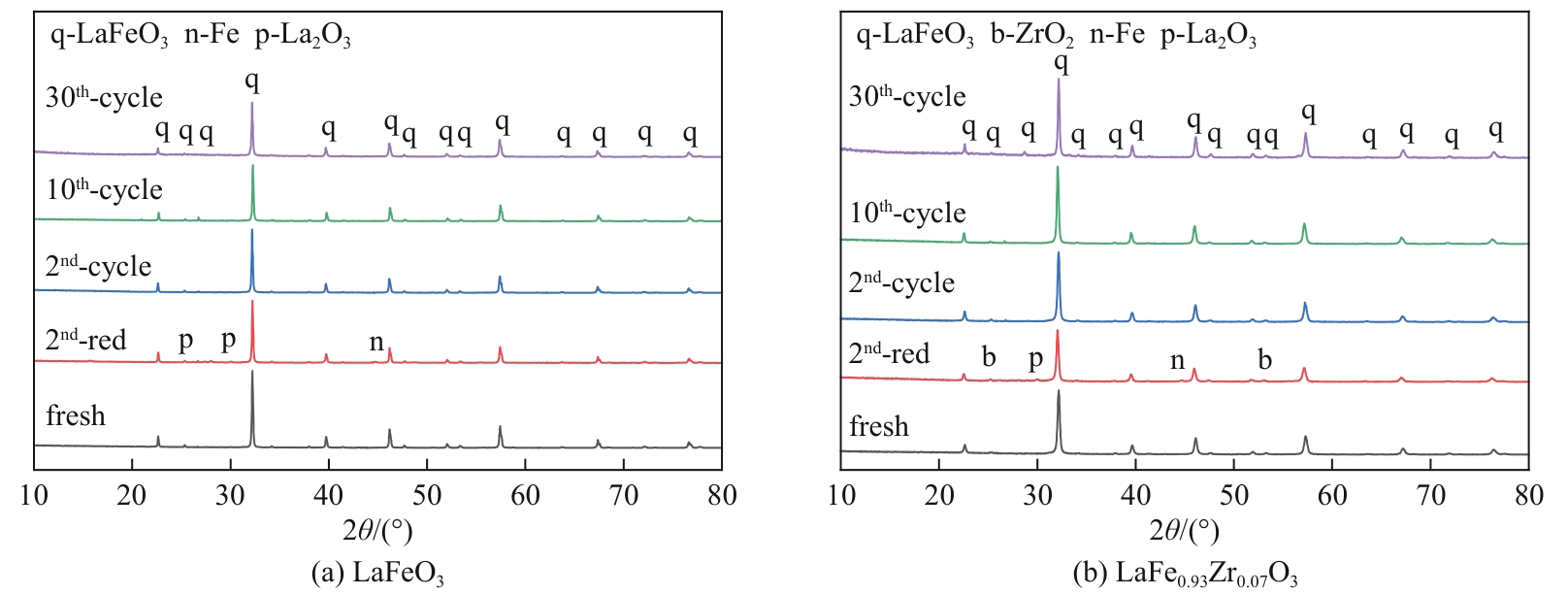
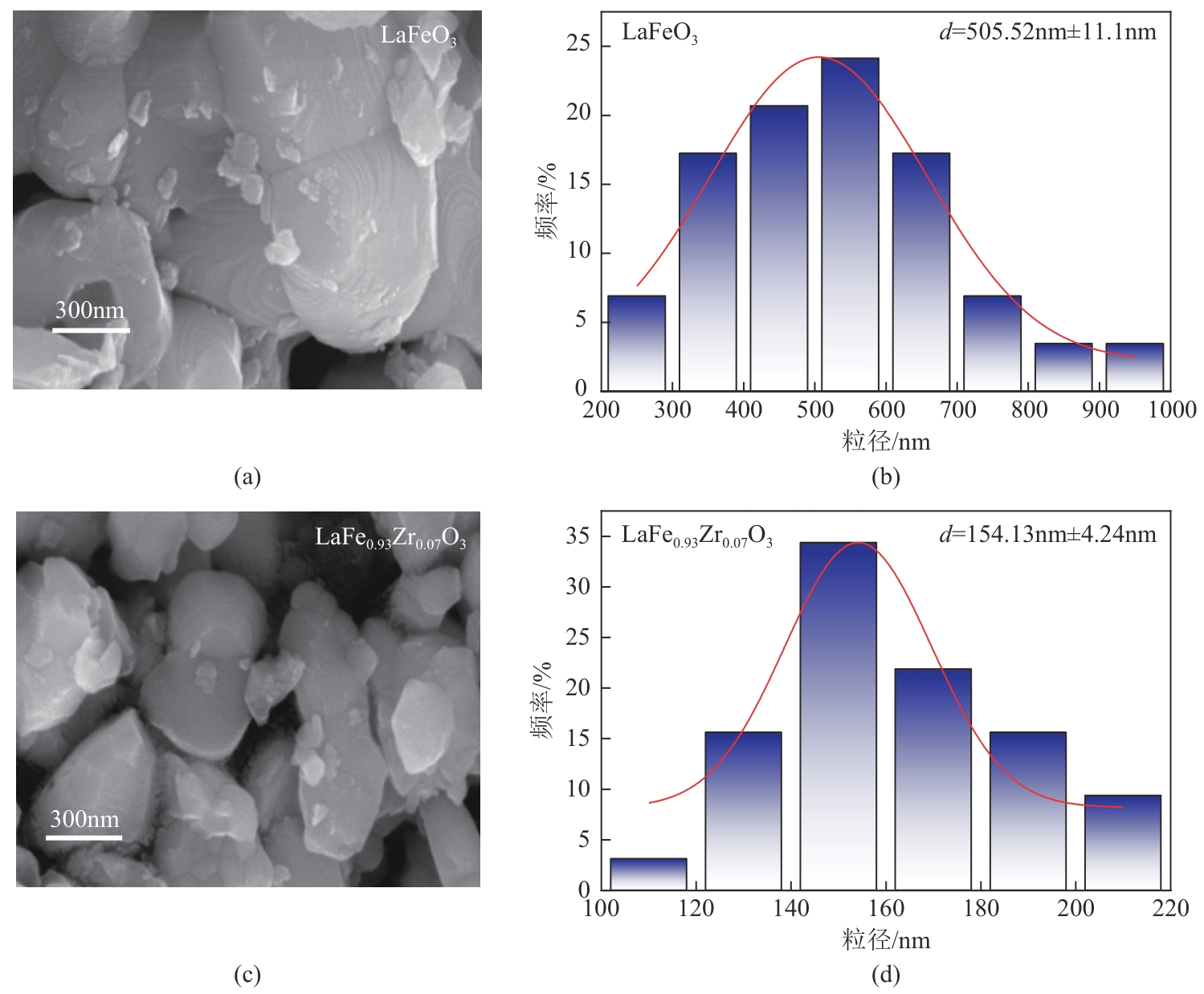
甲烷化学链干重整(CLDR)技术借助载氧体的循环将传统甲烷干重整反应进行时空解耦,可直接获得适合费托合成的合成气(H2/CO≈2),并实现温室气体CO2至CO的定向转化,其中,性能优异的载氧体(OC)是CLDR技术成功运行的关键。铁基钙钛矿(ABO3)具有优良的结构稳定性和合成气选择性,在化学链重整过程中备受关注,但它存在CH4活性低和循环稳定性差等不足,通过A和B位掺杂可调节载氧体的表面催化活性和晶格氧移动性,进而提高合成气产量和循环稳定性。本工作首先利用热力学软件研究了AFe1-x B x O3铁基钙钛矿载氧体A(Y、La、Pr、Sm)位和B位(Mn、Sn、Zr)金属取代对CLDR制合成气潜力的影响,发现A=La、B=Zr时载氧体性能最佳。之后,采用溶胶-凝胶法制备了LaFe0.93Zr0.07O3载氧体,发现Zr掺杂的新鲜LaFe0.93Zr0.07O3载氧体的甲烷转化率、合成气收率分别由LaFeO3的62.06%和1.38mmol/g提高至94.57%和1.97mmol/g,且在30次氧化还原循环反应过程中保持稳定(93.90%~96.29%和1.96~2.01mmol/g),在CO2氧化阶段也能够稳定高效地将更多CO2分子定向转化为CO(0.69mmol/g vs. 1.21mmol/g),无失活现象,这主要归因于Zr掺杂降低了载氧体的晶粒尺寸,造成了FeO6八面体畸变和更多的氧空位,增强了晶格氧活性和抗烧结性。本工作结合理论与实验的研究,能够为载氧体的优化设计提供理论和实验依据。
中图分类号:
引用本文
张善超, 张德亮, 王露, 梁豪, 杨倩, 杨冠杰, 苏伟, 马晓迅, 朱燕燕. 铁基钙钛矿载氧体化学链制合成气热力学性能分析与实验[J]. 化工进展, 2025, 44(10): 5703-5716.
ZHANG Shanchao, ZHANG Deliang, WANG Lu, LIANG Hao, YANG Qian, YANG Guanjie, SU Wei, MA Xiaoxun, ZHU Yanyan. Thermodynamic analysis and experimental study on the iron-based perovskite oxygen carrier for syngas production via chemical looping[J]. Chemical Industry and Engineering Progress, 2025, 44(10): 5703-5716.
| 项目 | 物相组成 |
|---|---|
| 气相 | CH4(g),CO(g),CO2(g),H2(g),H2O(g),O2(g),CH3(g) |
| 固相 | C,Fe,Fe0.947O,FeO,Fe2O3,Fe3O4,La,La2O3,LaFe12O19,La3Fe5O12,Mn,MnO,MnO2,Mn2O3,Mn3O4,Pr,PrO1.833,PrO2,Pr2O3,Pr6O11,Pr12O22,Pr3Fe5O12,PrFeO3,Sm,SmFeO3,Sm2O3,Sm3Fe5O12,Sn,SnO,SnO2,Y,YFeO3,Y2O3,Zr,ZrO2,LaFe0.93Mn0.07O3, LaFe0.93Sn0.07O3,LaFe0.93Zr0.07O3 |
表1 热力学计算中考虑的物相组分
| 项目 | 物相组成 |
|---|---|
| 气相 | CH4(g),CO(g),CO2(g),H2(g),H2O(g),O2(g),CH3(g) |
| 固相 | C,Fe,Fe0.947O,FeO,Fe2O3,Fe3O4,La,La2O3,LaFe12O19,La3Fe5O12,Mn,MnO,MnO2,Mn2O3,Mn3O4,Pr,PrO1.833,PrO2,Pr2O3,Pr6O11,Pr12O22,Pr3Fe5O12,PrFeO3,Sm,SmFeO3,Sm2O3,Sm3Fe5O12,Sn,SnO,SnO2,Y,YFeO3,Y2O3,Zr,ZrO2,LaFe0.93Mn0.07O3, LaFe0.93Sn0.07O3,LaFe0.93Zr0.07O3 |
| 样品 | 晶相组成 | 晶胞参数a/nm | 晶粒尺寸/nm |
|---|---|---|---|
| LaFeO3 | LaFeO3 | 3.9287 | 96 |
| LaFe0.93Zr0.07O3 | LaFeO3 | 3.9410 | 35 |
表2 LaFeO3和LaFe0.93Zr0.07O3载氧体的晶胞参数和晶粒尺寸
| 样品 | 晶相组成 | 晶胞参数a/nm | 晶粒尺寸/nm |
|---|---|---|---|
| LaFeO3 | LaFeO3 | 3.9287 | 96 |
| LaFe0.93Zr0.07O3 | LaFeO3 | 3.9410 | 35 |
| 载氧体 | 铁价态质量分数/% | Fe2+/Fe3+ | 氧物种质量分数/% | Oads/Olatt | |||
|---|---|---|---|---|---|---|---|
| Fe2+ | Fe3+ | OⅠ | OⅡ | OⅢ | |||
| LaFeO3 | 0 | 100 | 0 | 15.16 | 38.06 | 46.79 | 1.13 |
| LaFe0.93Zr0.07O3 | 36.69 | 63.31 | 0.560 | 10.28 | 49.56 | 40.16 | 1.49 |
表3 新鲜LaFe1-x Zr x O3(x=0、0.07)载氧体的XPS分析结果
| 载氧体 | 铁价态质量分数/% | Fe2+/Fe3+ | 氧物种质量分数/% | Oads/Olatt | |||
|---|---|---|---|---|---|---|---|
| Fe2+ | Fe3+ | OⅠ | OⅡ | OⅢ | |||
| LaFeO3 | 0 | 100 | 0 | 15.16 | 38.06 | 46.79 | 1.13 |
| LaFe0.93Zr0.07O3 | 36.69 | 63.31 | 0.560 | 10.28 | 49.56 | 40.16 | 1.49 |
| 样品 | 晶相组成 | 晶粒尺寸①/nm |
|---|---|---|
| LaFeO3 | LaFeO3 | 96 |
| LaFe0.93Zr0.07O3 | LaFeO3 | 35 |
| LaFeO3-10次循环 | LaFeO3 | 358 |
| LaFe0.93Zr0.07O3-10次循环 | LaFeO3 | 93 |
| LaFeO3-30次循环 | LaFeO3 | 502 |
| LaFe0.93Zr0.07O3-30次循环 | LaFeO3 | 135 |
表4 LaFeO3和LaFe0.93Zr0.07O3载氧体在循环前后的晶粒尺寸对比
| 样品 | 晶相组成 | 晶粒尺寸①/nm |
|---|---|---|
| LaFeO3 | LaFeO3 | 96 |
| LaFe0.93Zr0.07O3 | LaFeO3 | 35 |
| LaFeO3-10次循环 | LaFeO3 | 358 |
| LaFe0.93Zr0.07O3-10次循环 | LaFeO3 | 93 |
| LaFeO3-30次循环 | LaFeO3 | 502 |
| LaFe0.93Zr0.07O3-30次循环 | LaFeO3 | 135 |
| [1] | HUANG Chuande, WU Jian, CHEN Youtao, et al. In situ encapsulation of iron(0) for solar thermochemical syngas production over iron-based perovskite material[J]. Communications Chemistry, 2018, 1: 55. |
| [2] | LI Xinyu, LI Di, TIAN Hao, et al. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles[J]. Applied Catalysis B: Environmental, 2017, 202: 683-694. |
| [3] | JIAO Feng, LI Jinjing, PAN Xiulian, et al. Selective conversion of syngas to light olefins[J]. Science, 2016, 351(6277): 1065-1068. |
| [4] | YIN Xianglei, ZHANG Runsen, ZHANG Yulong, et al. Enhanced reactivity of methane partial oxidation of nickel doped LaMnO3+ δ perovskites for chemical looping process[J]. International Journal of Hydrogen Energy, 2024, 71: 481-492. |
| [5] | TSURU Toshinori, YAMAGUCHI Koji, YOSHIOKA Tomohisa, et al. Methane steam reforming by microporous catalytic membrane reactors[J]. AIChE Journal, 2004, 50(11): 2794-2805. |
| [6] | MURMURA M A, CERBELLI S, ANNESINI M C. Transport-reaction-permeation regimes in catalytic membrane reactors for hydrogen production. The steam reforming of methane as a case study[J]. Chemical Engineering Science, 2017, 162: 88-103. |
| [7] | JI Jinqing, SHEN Laihong. Enhanced co-production of high-quality syngas and highly-concentrated hydrogen via chemical looping steam methane reforming over Ni-substituted La0.6Ce0.4MnO3 oxygen carriers[J]. Fuel, 2024, 368: 131588. |
| [8] | DAI Xiaoping, CHENG Jie, LI Zhanzhao, et al. Reduction kinetics of lanthanum ferrite perovskite for the production of synthesis gas by chemical-looping methane reforming[J]. Chemical Engineering Science, 2016, 153: 236-245. |
| [9] | Axel LÖFBERG, KANE Tanushree, Jesús GUERRERO-CABALLERO, et al. Chemical looping dry reforming of methane: Toward shale-gas and biogas valorization[J]. Chemical Engineering and Processing: Process Intensification, 2017, 122: 523-529. |
| [10] | HUANG Linan, LI Danyang, TIAN Dong, et al. Optimization of Ni-based catalysts for dry reforming of methane via alloy design: A review[J]. Energy & Fuels, 2022, 36(10): 5102-5151. |
| [11] | Miryam GIL-CALVO, Cristina JIMÉNEZ-GONZÁLEZ, DE RIVAS Beatriz, et al. Novel nickel aluminate-derived catalysts supported on ceria and ceria-zirconia for partial oxidation of methane[J]. Industrial & Engineering Chemistry Research, 2017, 56(21): 6186-6197. |
| [12] | LI Yunhua, WANG Yaquan, HONG Xuebin, et al. Partial oxidation of methane to syngas over nickel monolithic catalysts[J]. AIChE Journal, 2006, 52(12): 4276-4279. |
| [13] | Axel LÖFBERG, Jesús GUERRERO-CABALLERO, KANE Tanushree, et al. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production[J]. Applied Catalysis B: Environmental, 2017, 212: 159-174. |
| [14] | HUANG Zhen, JIANG Huanqi, HE Fang, et al. Evaluation of multi-cycle performance of chemical looping dry reforming using CO2 as an oxidant with Fe-Ni bimetallic oxides[J]. Journal of Energy Chemistry, 2016, 25(1): 62-70. |
| [15] | MORE Amey, Götz VESER. Physical mixtures as simple and efficient alternative to alloy carriers in chemical looping processes[J]. AIChE Journal, 2017, 63(1): 51-59. |
| [16] | ZUO Huicong, LU Chunqiang, JIANG Lei, et al. Hydrogen production and CO2 capture from Linz-Donawitz converter gas via a chemical looping concept[J]. Chemical Engineering Journal, 2023, 477: 146870. |
| [17] | ZENG Dewang, QIU Yu, LI Min, et al. Spatially controlled oxygen storage materials improved the syngas selectivity on chemical looping methane conversion[J]. Applied Catalysis B: Environmental, 2021, 281: 119472. |
| [18] | LIU Fang, CHEN Liangyong, NEATHERY James K, et al. Cerium oxide promoted iron-based oxygen carrier for chemical looping combustion[J]. Industrial & Engineering Chemistry Research, 2014, 53(42): 16341-16348. |
| [19] | 王嘉锐, 刘大伟, 邓耀, 等. 载氧体在甲烷化学链重整反应中的研究进展[J]. 化工进展, 2024, 43(5): 2235-2253. |
| WANG Jiarui, LIU Dawei, DENG Yao, et al. Research progress of oxygen carriers in chemical looping reforming reaction of methane[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2235-2253. | |
| [20] | ADANEZ Juan, ABAD Alberto, Francisco GARCIA-LABIANO, et al. Progress in chemical-looping combustion and reforming technologies[J]. Progress in Energy and Combustion Science, 2012, 38(2): 215-282. |
| [21] | 向浩寅, 陈良勇. Ni、Ce、Zn和Cu修饰Fe2O3/Al2O3载氧体的甲烷化学链制氢特性[J]. 化工进展, 2024, 43(8): 4320-4332. |
| XIANG Haoyin, CHEN Liangyong. Evaluation of Ni, Ce, Zn and Cu modified Fe2O3/Al2O3 oxygen carriers for methane-fueled chemical looping hydrogen generation process[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4320-4332. | |
| [22] | GAO Yunfei, HAERI Farrah, HE Fang, et al. Alkali metal-promoted La x Sr2– x FeO4– δ redox catalysts for chemical looping oxidative dehydrogenation of ethane[J]. ACS Catalysis, 2018, 8(3): 1757-1766. |
| [23] | CHEN Chi, CIUCCI Francesco. Designing Fe-based oxygen catalysts by density functional theory calculations[J]. Chemistry of Materials, 2016, 28(19): 7058-7065. |
| [24] | FENG Yuan, JIN Hanyu, WANG Shuai. Oxygen migration performance of LaFeO3 perovskite-type oxygen carriers with Sr doping[J]. Physical Chemistry Chemical Physics, 2023, 25(13): 9216-9224. |
| [25] | ZHANG Li, HU Yue, XU Weibin, et al. Anti-coke BaFe1- x Sn x O3- δ oxygen carriers for enhanced syngas production via chemical looping partial oxidation of methane[J]. Energy & Fuels, 2020, 34(6): 6991-6998. |
| [26] | LOMBARDO Gabriele, EBIN Burçak, FOREMAN Mark R St J, et al. Chemical transformations in Li-ion battery electrode materials by carbothermic reduction[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(16): 13668-13679. |
| [27] | 韩天佑. 金属卤化物、氧化物与硫化物生成热的一些规则——金属卤化物、氧化物与硫化物生成热的近似计算方法[J]. 化学通报, 1966, 29(3): 60-64. |
| HAN Tianyou. Some rules of heat of formation of metal halides, oxides and sulfides — Approximate calculation method of heat of formation of metal halides, oxides and sulfides[J]. Chemistry, 1966, 29(3): 60-64. | |
| [28] | 王利. LiCoO2在酸性焙烧环境中反应的热力学及影响因素研究[D]. 兰州: 兰州理工大学, 2013. |
| WANG Li. Research on thermodynamics and influencing factors of the reaction of LiCoO2 in the acid roasting conditions[D]. Lanzhou: Lanzhou University of Technology, 2013. | |
| [29] | XIA Xue, CHANG Wenxi, CHENG Shuwen, et al. Oxygen activity tuning via FeO6 octahedral tilting in perovskite ferrites for chemical looping dry reforming of methane[J]. ACS Catalysis, 2022, 12(12): 7326-7335. |
| [30] | GOSAVI Priti V, BINIWALE Rajesh B. Pure phase LaFeO3 perovskite with improved surface area synthesized using different routes and its characterization[J]. Materials Chemistry and Physics, 2010, 119(1/2): 324-329. |
| [31] | KIM Jae-Nam, SHIN Kwang-Soo, KIM Dae-Hwan, et al. Changes in chemical behavior of thin film lead zirconate titanate during Ar+-ion bombardment using XPS[J]. Applied Surface Science, 2003, 206(1/2/3/4): 119-128. |
| [32] | THIRUMALAIRAJAN S, GIRIJA K, HEBALKAR Neha Y, et al. Shape evolution of perovskite LaFeO3 nanostructures: A systematic investigation of growth mechanism, properties and morphology dependent photocatalytic activities[J]. RSC Advances, 2013, 3(20): 7549-7561. |
| [33] | ROBBENNOLT Shauna, FORNELL Jordina, QUINTANA Alberto, et al. Structural and magnetic properties of Fe x Cu1– x sputtered thin films electrochemically treated to create nanoporosity for high-surface-area magnetic components[J]. ACS Applied Nano Materials, 2018, 1(4): 1675-1682. |
| [34] | HOLGADO J P, MUNUERA G, ESPINÓS J P, et al. XPS study of oxidation processes of CeO x defective layers[J]. Applied Surface Science, 2000, 158(1/2): 164-171. |
| [35] | ZHENG Yane, LI Kongzhai, WANG Hua, et al. Designed oxygen carriers from macroporous LaFeO3 supported CeO2 for chemical-looping reforming of methane[J]. Applied Catalysis B: Environmental, 2017, 202: 51-63. |
| [36] | LI Ranjia, YU Changchun, SHEN Shikong. Partial oxidation of methane to syngas using lattice oxygen of La1- x Sr x FeO3 perovskite oxide catalysts instead of molecular oxygen[J]. Journal of Natural Gas Chemistry, 2002, 11(3/4): 137-144. |
| [37] | XIAN Hui, ZHANG Xingwen, LI Xingang, et al. BaFeO3– x perovskite: An efficient NO x absorber with a high sulfur tolerance[J]. The Journal of Physical Chemistry C, 2010, 114(27): 11844-11852. |
| [1] | 陈子朝, 何方书, 胡强, 杨扬, 陈汉平, 杨海平. 甲烷干重整抗积炭Ni基催化剂研究进展[J]. 化工进展, 2025, 44(9): 4968-4978. |
| [2] | 王振, 张耀远, 吴芹, 史大昕, 陈康成, 黎汉生. 甲烷干重整用Ni/Al2O3基催化剂研究进展[J]. 化工进展, 2025, 44(9): 4979-4998. |
| [3] | 王晓光, 董青, 郎文丽, 洪翔鑫, 黄振祥, 谭凤玉, 雷以柱, 余子夷. 超低浓度甲烷减排与资源化利用研究进展[J]. 化工进展, 2025, 44(9): 5363-5376. |
| [4] | 杨嘉聪, 程光旭, 贾彤华, 姜召. 煤制甲醇与绿氢高效耦合新工艺模拟及技术经济分析[J]. 化工进展, 2025, 44(8): 4657-4668. |
| [5] | 高姣姣, 颜诗宇, 杨太顺, 谢尚志, 杨艳娟, 徐晶. 不同晶型Al2O3负载Ru催化剂对聚乙烯氢解的影响[J]. 化工进展, 2025, 44(7): 3917-3927. |
| [6] | 唐轩, 白晓炜, 张飞飞, 李晋平, 杨江峰. 沸石分子筛用于CO2-N2-CH4筛分分离的研究进展[J]. 化工进展, 2025, 44(7): 3938-3949. |
| [7] | 于宁, 王秋月, 王志才, 高子怡, 柴永明, 董斌. 双位点协同调控增强钙钛矿氧化物的水氧化活性[J]. 化工进展, 2025, 44(7): 3976-3984. |
| [8] | 谢武强, 张岭, 贺杠, 蒋里锋, 郑晰瑞, 张和鹏. CoTBrPP-PTAB-Cu电催化还原CO2制甲烷[J]. 化工进展, 2025, 44(6): 3093-3100. |
| [9] | 李白茹, 方志敏, 王爱丽, 罗龙, 张罗正, 李绿洲, 丁建宁. 钙钛矿太阳能电池研究进展[J]. 化工进展, 2025, 44(5): 2598-2624. |
| [10] | 付紫君, 宋学行, 沈群, 王晓波, 顾佳名, 汪丹峰, 魏伟, 孙楠楠. 基于LCA的CO2捕集-甲烷化一体化技术碳足迹分析[J]. 化工进展, 2025, 44(5): 2879-2887. |
| [11] | 张强, 孙楠, 郑俊杰, 吴强, 刘传海, 李元吉. 混合热力学促进剂对水合物法分离回收瓦斯的影响[J]. 化工进展, 2025, 44(1): 192-201. |
| [12] | 李依梦, 陈运全, 何畅, 张冰剑, 陈清林. 基于物理信息神经网络的甲烷无氧芳构化反应的正反问题[J]. 化工进展, 2024, 43(9): 4817-4823. |
| [13] | 张玉凤, 庞煜骞, 裴浩楠, 樊小青. 三种朝向链基有机骨架对天然气中C2~C3的分离[J]. 化工进展, 2024, 43(9): 5185-5192. |
| [14] | 郭长滨, 李蒙蒙, 冯梦晗, 原田, 张克强, 罗艳丽, 王风. 铈掺杂镧基钙钛矿制备及对水体磷酸盐和植酸的吸附性能[J]. 化工进展, 2024, 43(8): 4748-4756. |
| [15] | 梁国威, 金晶, 董波, 侯封校. 化学链燃烧中煤灰原位改性对钙基载氧体炭沉积的影响[J]. 化工进展, 2024, 43(8): 4253-4261. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||