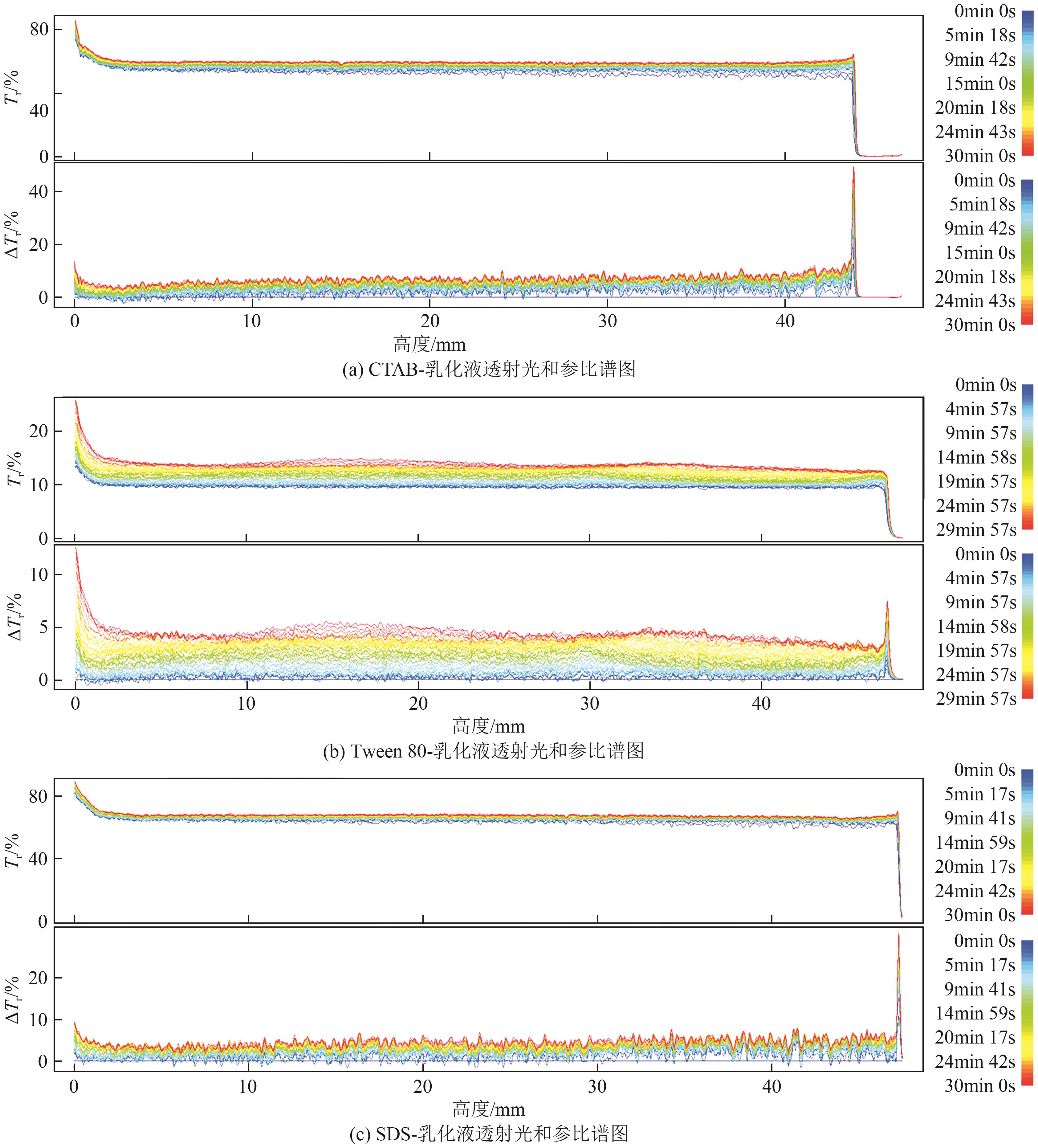
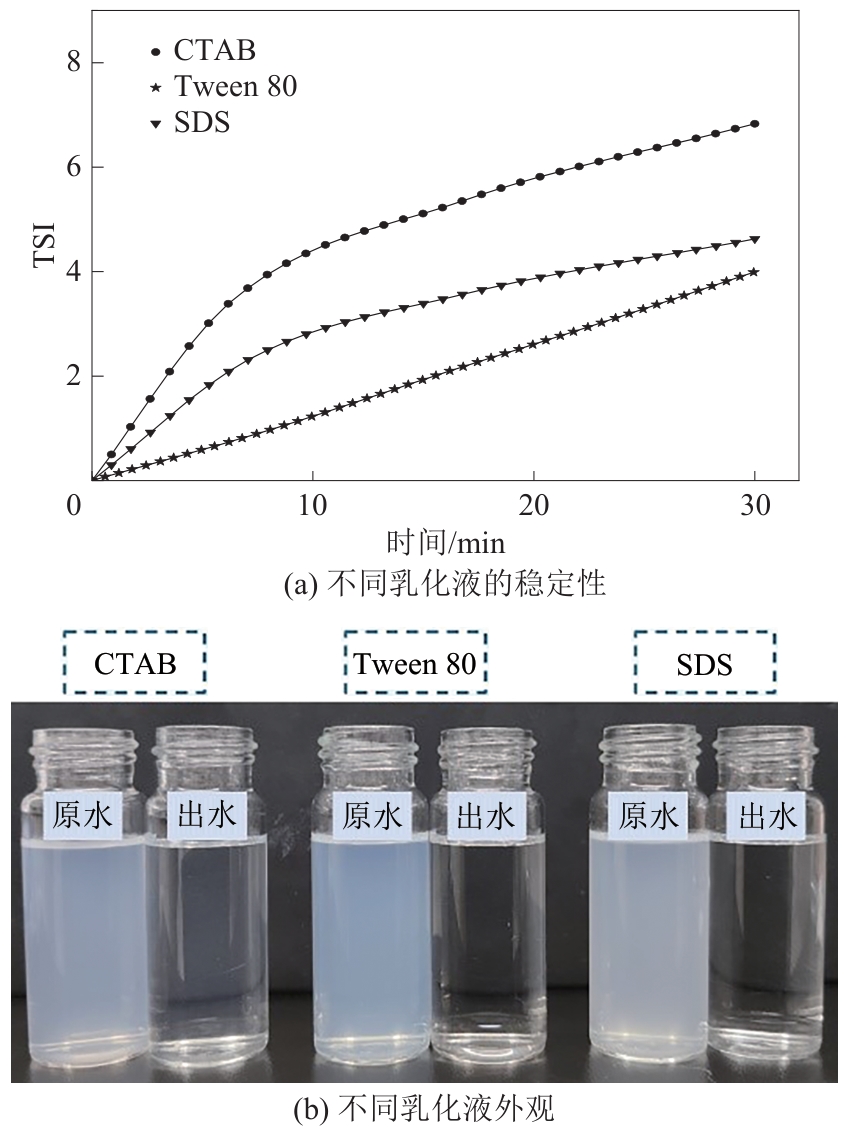
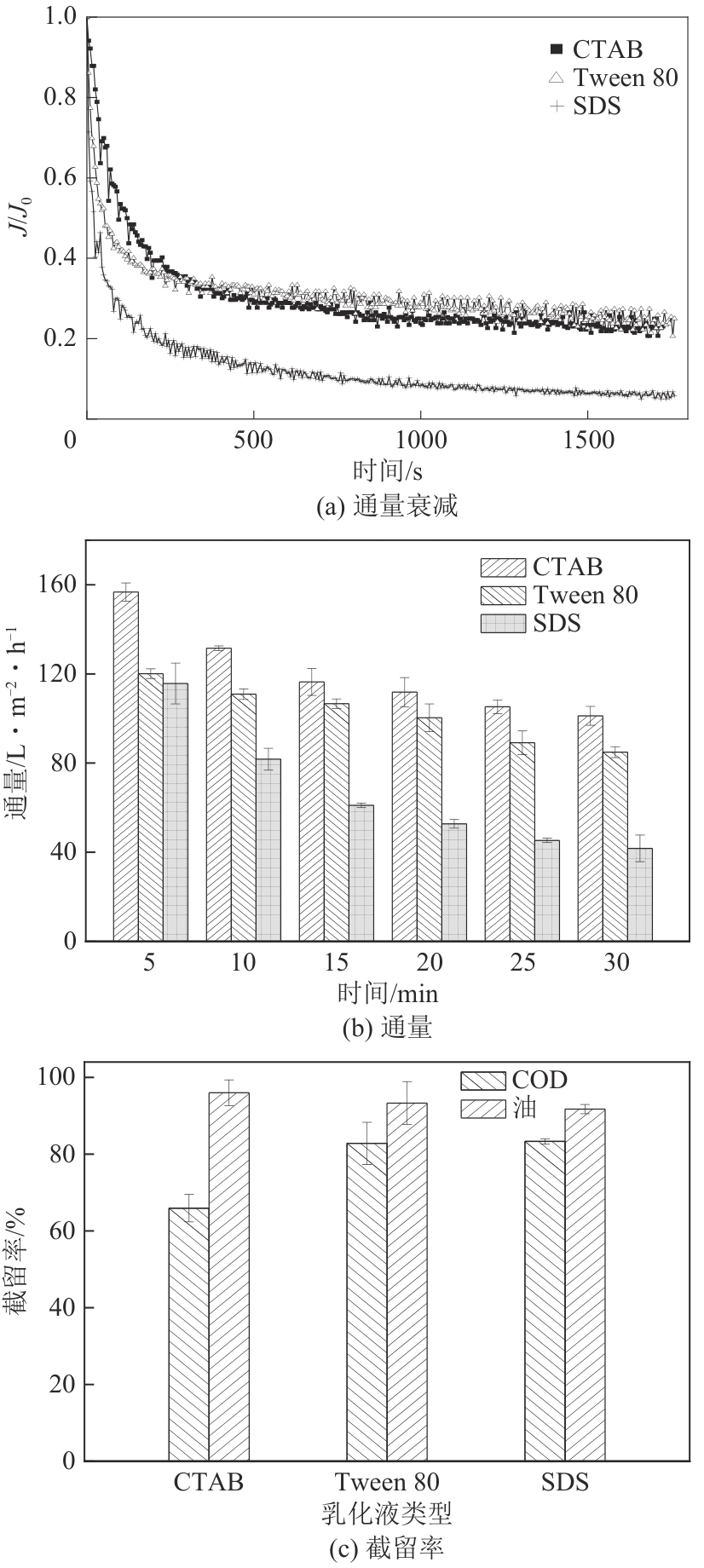
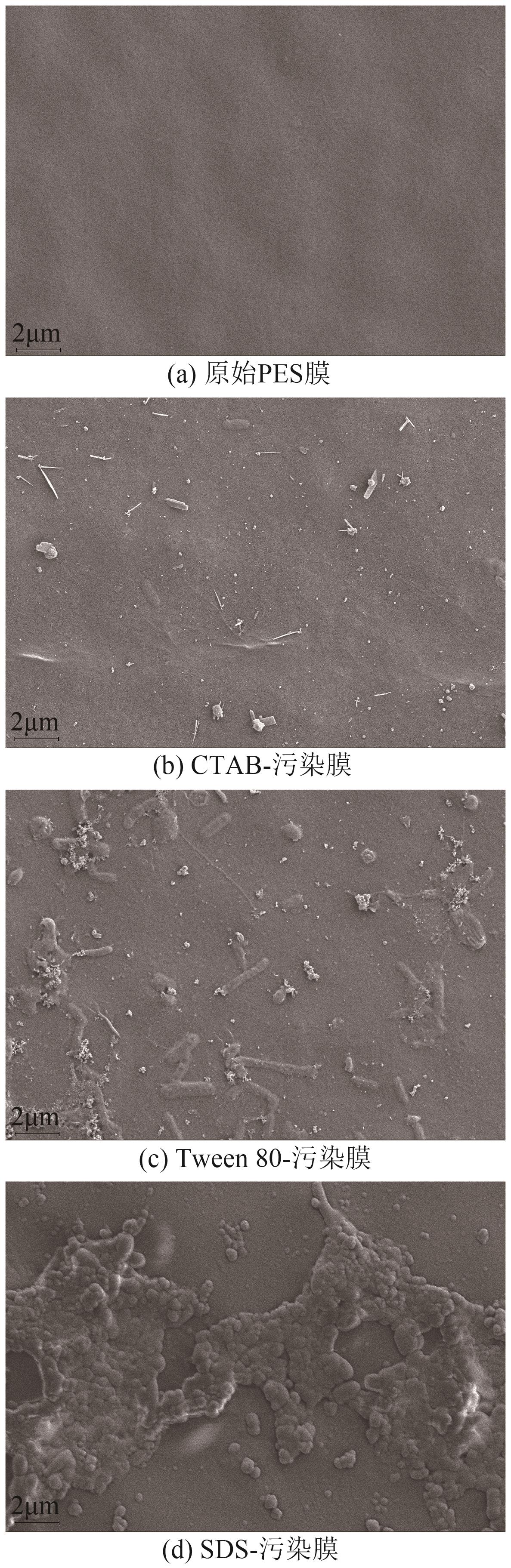
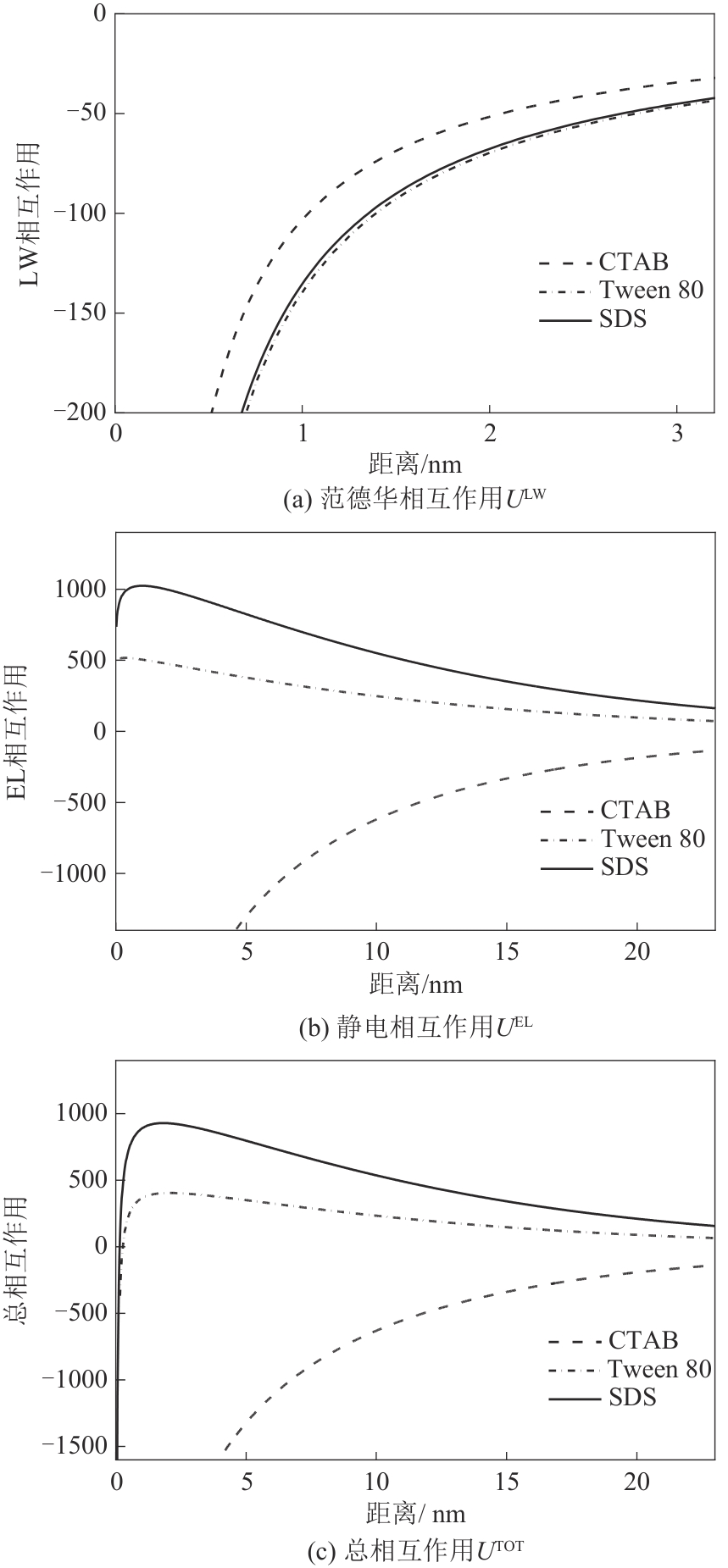
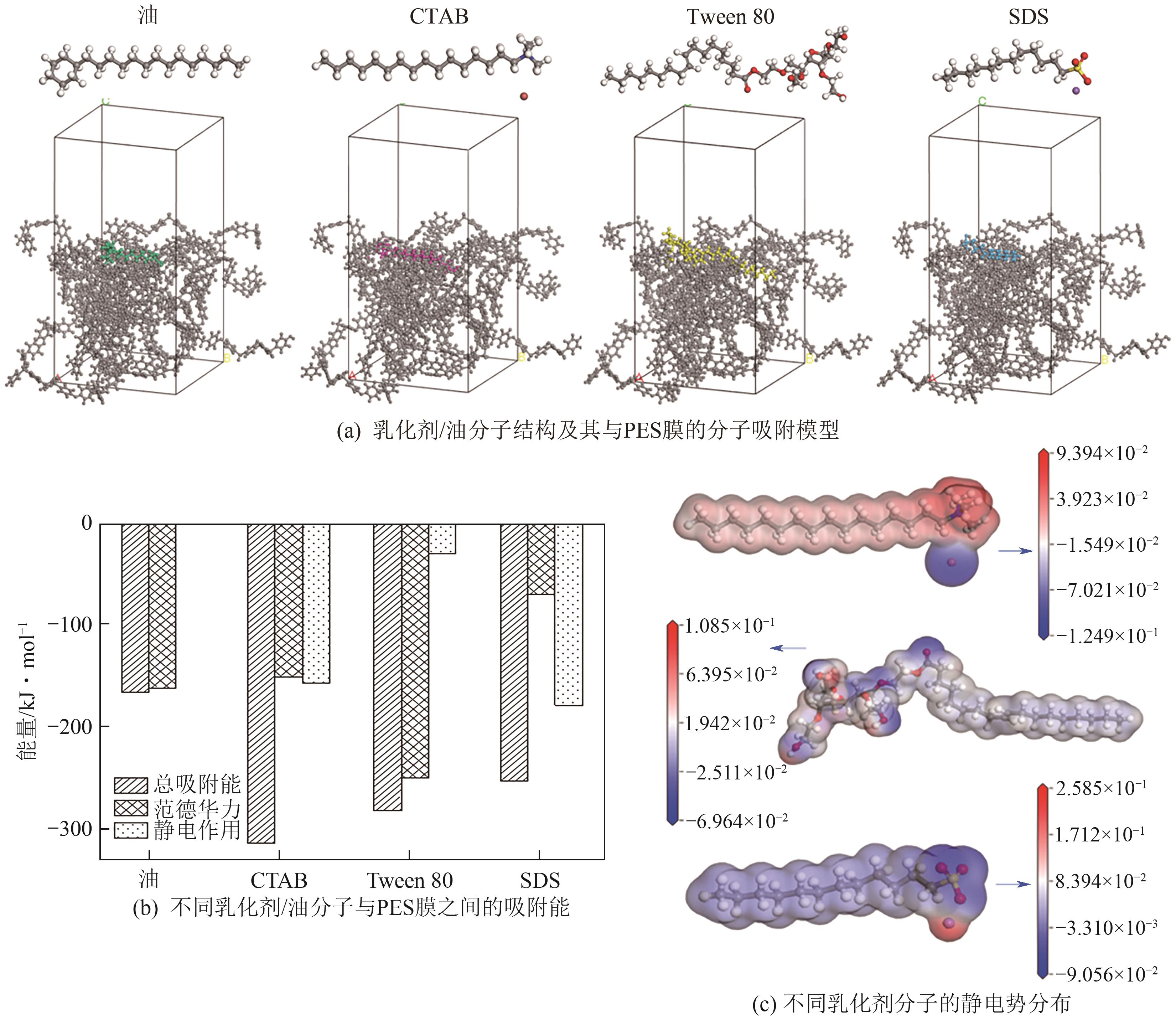
| [24] |
王可, 樊华, 侯得印, 等. XDLVO理论解析有机物在膜蒸馏过程中的膜污染行为机制[J]. 膜科学与技术, 2022 42(2): 25-33.
|
|
WANG Ke, FAN Hua, HOU Deyin, et al. Mechanism of membrane fouling in membrane distillation process by organic compounds: XDLVO theory[J]. Membrane Science and Technology, 2022 42(2): 25-33.
|
| [25] |
BRENELLI Lívia Beatriz, MARIUTTI Lilian Regina Barros, VILLARES PORTUGAL Rodrigo, et al. Modified lignin from sugarcane bagasse as an emulsifier in oil-in-water nanoemulsions[J]. Industrial Crops and Products, 2021, 167: 113532.
|
| [26] |
MATOS María, Gemma GUTIÉRREZ, LOBO Alberto, et al. Surfactant effect on the ultrafiltration of oil-in-water emulsions using ceramic membranes[J]. Journal of Membrane Science, 2016, 520: 749-759.
|
| [27] |
PARIA Santanu, KHILAR Kartic C. A review on experimental studies of surfactant adsorption at the hydrophilic solid-water interface[J]. Advances in Colloid and Interface Science, 2004, 110(3): 75-95.
|
| [1] |
ZHANG Ning, YANG Xianwen, WANG Yalun, et al. A review on oil/water emulsion separation membrane material[J]. Journal of Environmental Chemical Engineering, 2022, 10(2): 107257.
|
| [2] |
SUTRISNA Putu Doddy, KURNIA Kiki Adi, SIAGIAN Utjok W R, et al. Membrane fouling and fouling mitigation in oil-water separation: A review[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107532.
|
| [3] |
HUANG Shilin, RAS Robin H A, TIAN Xuelin. Antifouling membranes for oily wastewater treatment: Interplay between wetting and membrane fouling[J]. Current Opinion in Colloid & Interface Science, 2018, 36: 90-109.
|
| [4] |
GAO Shoujian, CHEN Jinhao, ZHENG Yanru, et al. Gradient adhesive hydrogel decorated superhydrophilic membranes for ultra-stable oil/water separation[J]. Advanced Functional Materials, 2022, 32(46): 2205990.
|
| [5] |
XIE Xiong, BAO Jianguo, HASSAN OMER Safaa, et al. Study for adsorption behaviors of emulsion oil on a novel ZrO2/PVDF modified membrane[J]. Desalination and Water Treatment, 2016, 57(25): 11736-11745.
|
| [6] |
ZHANG Bing, YU Shuili, ZHU Youbing, et al. Adsorption mechanisms of crude oil onto polytetrafluoroethylene membrane: Kinetics and isotherm, and strategies for adsorption fouling control[J]. Separation and Purification Technology, 2020, 235: 116212.
|
| [7] |
ZHAO Dongsheng, LIU Junxia, QIU Liping, et al. Roles of a mixed hydrophilic/hydrophobic interface in the regulation of nanofiltration membrane fouling in oily produced wastewater treatment: Performance and interfacial thermodynamic mechanisms[J]. Separation and Purification Technology, 2021, 257: 117970.
|
| [8] |
吕东伟. 陶瓷超滤膜分离乳化油过程中膜污染机制与抗污染改性研究[D]. 哈尔滨: 哈尔滨工业大学, 2016.
|
|
Dongwei LYU. Study on membrane fouling mechanism and anti-pollution modification in the process of separating emulsified oil by ceramic ultrafiltration membrane[D]. Harbin: Harbin Institute of Technology, 2016.
|
| [9] |
TANUDJAJA Henry J, CHEW Jia W. Assessment of oil fouling by oil-membrane interaction energy analysis[M]//Solid-liquid separation technologies. Boca Raton: CRC Press, 2022: 151-168.
|
| [10] |
HE Zhengwang, KASEMSET Sirirat, KIRSCHNER Alon Y, et al. The effects of salt concentration and foulant surface charge on hydrocarbon fouling of a poly(vinylidene fluoride) microfiltration membrane[J]. Water Research, 2017, 117: 230-241.
|
| [11] |
TIAN Ju, TRINH Thien An, KALYAN Muppalla Naga, et al. In-situ monitoring of oil emulsion fouling in ultrafiltration via electrical impedance spectroscopy (EIS): Influence of surfactant[J]. Journal of Membrane Science, 2020, 616: 118527.
|
| [12] |
TRINH Thien An, HAN Qi, MA Yunqiao, et al. Microfiltration of oil emulsions stabilized by different surfactants[J]. Journal of Membrane Science, 2019, 579: 199-209.
|
| [13] |
ZHANG Tong, WANG Qiaoying, YANG Yan, et al. Revealing the contradiction between DLVO/XDLVO theory and membrane fouling propensity for oil-in-water emulsion separation[J]. Journal of Hazardous Materials, 2024, 466: 133594.
|
| [14] |
LAY Huang Teik, Chi Siang ONG, WANG Rong, et al. Choice of DLVO approximation method for quantifying the affinity between latex particles and membranes[J]. Journal of Membrane Science, 2023, 666: 121121.
|
| [15] |
ESKHAN Asma, ALQASAS Neveen, JOHNSON Daniel. Interaction mechanisms and predictions of the biofouling of polymer films: A combined atomic force microscopy and quartz crystal microbalance with dissipation monitoring study[J]. Langmuir, 2023, 39(18): 6592-6612.
|
| [16] |
LIU Junxia, FAN Yaqian, SUN Yuhui, et al. Modelling the critical roles of zeta potential and contact angle on colloidal fouling with a coupled XDLVO-collision attachment approach[J]. Journal of Membrane Science, 2021, 623: 119048.
|
| [17] |
ZHANG Bing, TANG Heli, HUANG Dongmei, et al. Effect of pH on anionic polyacrylamide adhesion: New insights into membrane fouling based on XDLVO analysis[J]. Journal of Molecular Liquids, 2020, 320: 114463.
|
| [18] |
陆登荣, 刘红波, 钱大玮, 等. XDLVO理论解析中药共性高分子的超滤膜污染行为[J]. 中草药, 2022, 53(9): 2642-2649.
|
|
LU Dengrong, LIU Hongbo, QIAN Dawei, et al. Analysis of ultrafiltration membrane fouling behavior of common polymers in traditional Chinese medicine using XDLVO theory[J]. Chinese Traditional and Herbal Drugs, 2022, 53(9): 2642-2649.
|
| [19] |
ZHAO Fangchao, LI Zongxue, HAN Xixi, et al. The interaction between microalgae and membrane surface in filtration by uniform shearing vibration membrane[J]. Algal Research, 2020, 50: 102012.
|
| [20] |
BRANT Jonathan A, CHILDRESS Amy E. Assessing short-range membrane-colloid interactions using surface energetics[J]. Journal of Membrane Science, 2002, 203(1/2): 257-273.
|
| [21] |
李义雅, 龙军, 段庆华, 等. 分子动力学模拟研究矿物基础油润滑性能[J]. 石油学报(石油加工), 2017, 33(4): 619-625.
|
|
LI Yiya, LONG Jun, DUAN Qinghua, et al. Molecular dynamics simulation on the lubricating property of mineral base oil[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2017, 33(4): 619-625.
|
| [22] |
WANG Jianmei, DUAN Lintao, WANG Baoan. Interface microstructure and bonding energy of layered bimetallic ZCuSn6Pb6Zn3/steel coupling with temperature and pressure[J]. Tribology International, 2021, 155: 106754.
|
| [23] |
WANG Jianmei, XIA Quanzhi, MA Yang, et al. Interfacial bonding energy on the interface between ZChSnSb/Sn alloy layer and steel body at microscale[J]. Materials, 2017, 10(10): 1128.
|