化工进展 ›› 2025, Vol. 44 ›› Issue (4): 2008-2019.DOI: 10.16085/j.issn.1000-6613.2024-0566
光热驱动褐煤固定床气化过程热质传递规律
- 太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
-
收稿日期:2024-04-07修回日期:2024-09-12出版日期:2025-04-25发布日期:2025-05-07 -
通讯作者:宋云彩 -
作者简介:袁梦丽(1998—),女,硕士研究生,研究方向为光热驱动煤气化中热质传递规律。E-mail:yml_150370@163.com。 -
基金资助:国家重点研发计划(2022YFE0208400);山西省重点研发计划(202202090301002);山西省自然科学基金(20210302124096);中央高校基本科研业务费专项资金(2022ZFJH04)
Heat and mass transfer law of photothermal-driven lignite fixed-bed gasification process
YUAN Mengli( ), SONG Yuncai(
), SONG Yuncai( ), LI Wenying, FENG Jie
), LI Wenying, FENG Jie
- State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2024-04-07Revised:2024-09-12Online:2025-04-25Published:2025-05-07 -
Contact:SONG Yuncai
摘要:
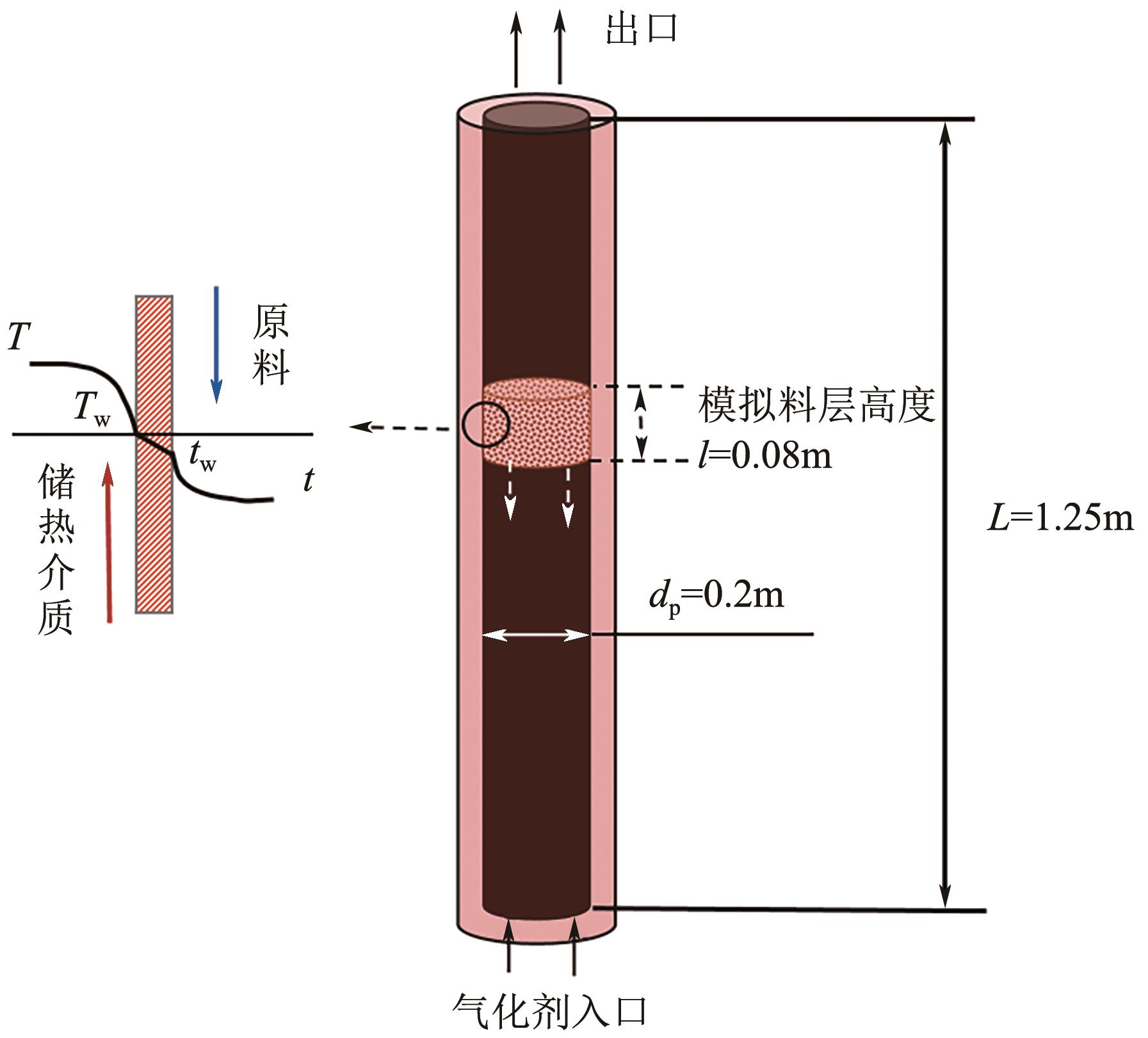
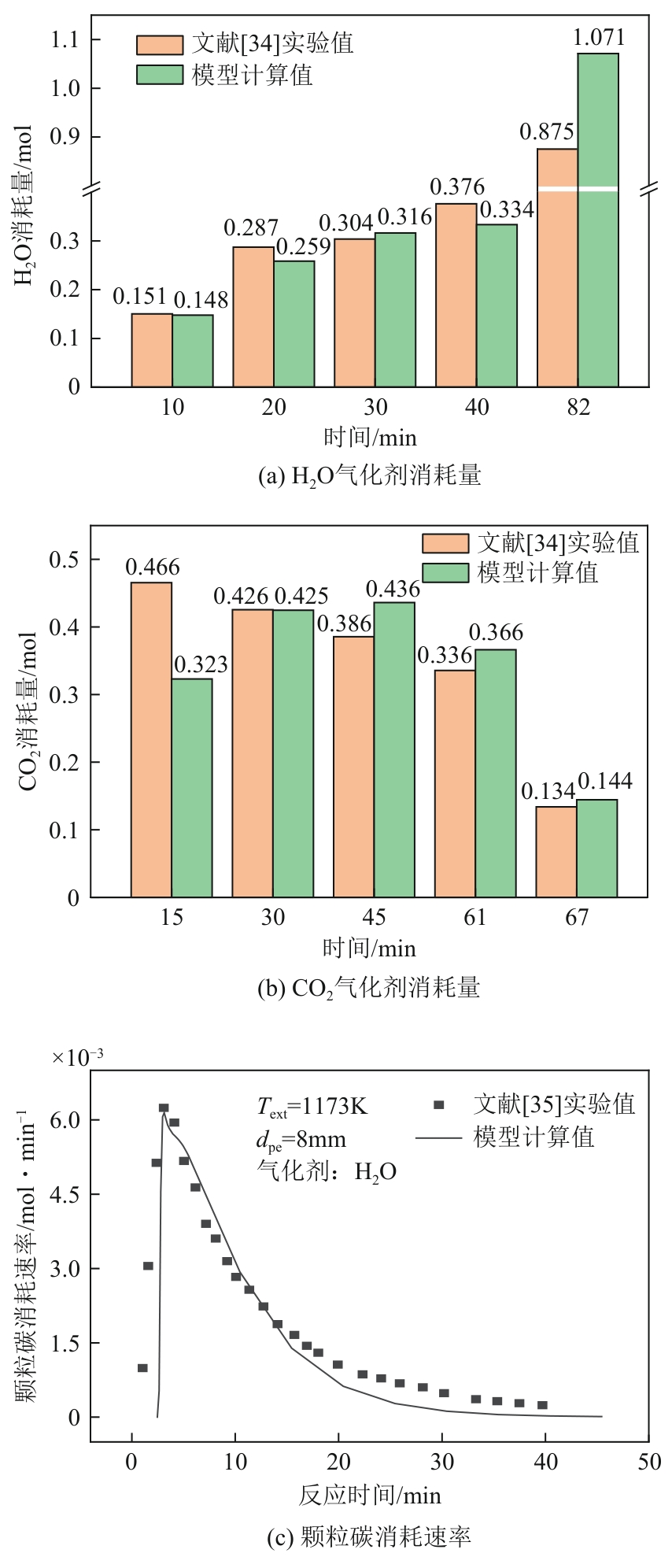
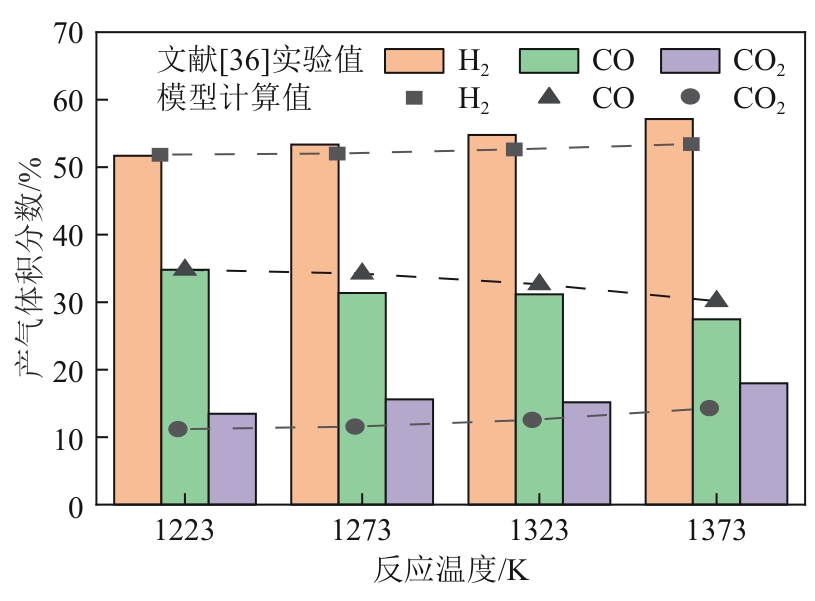
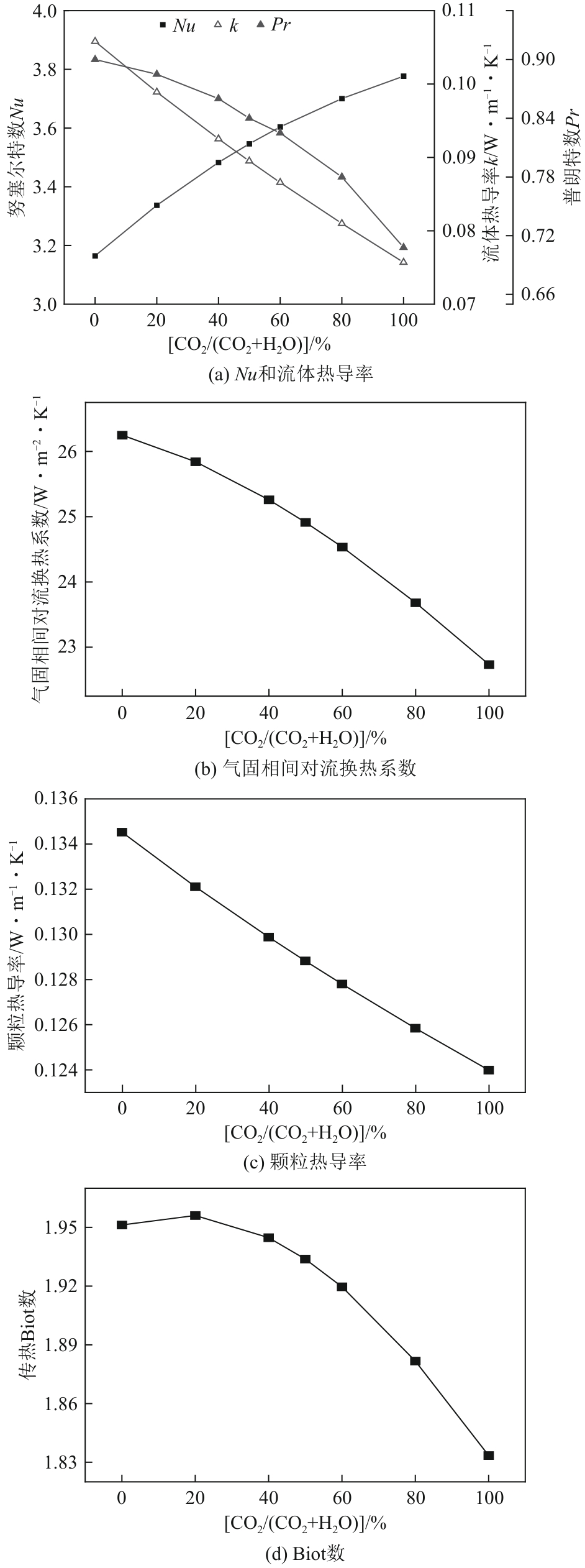
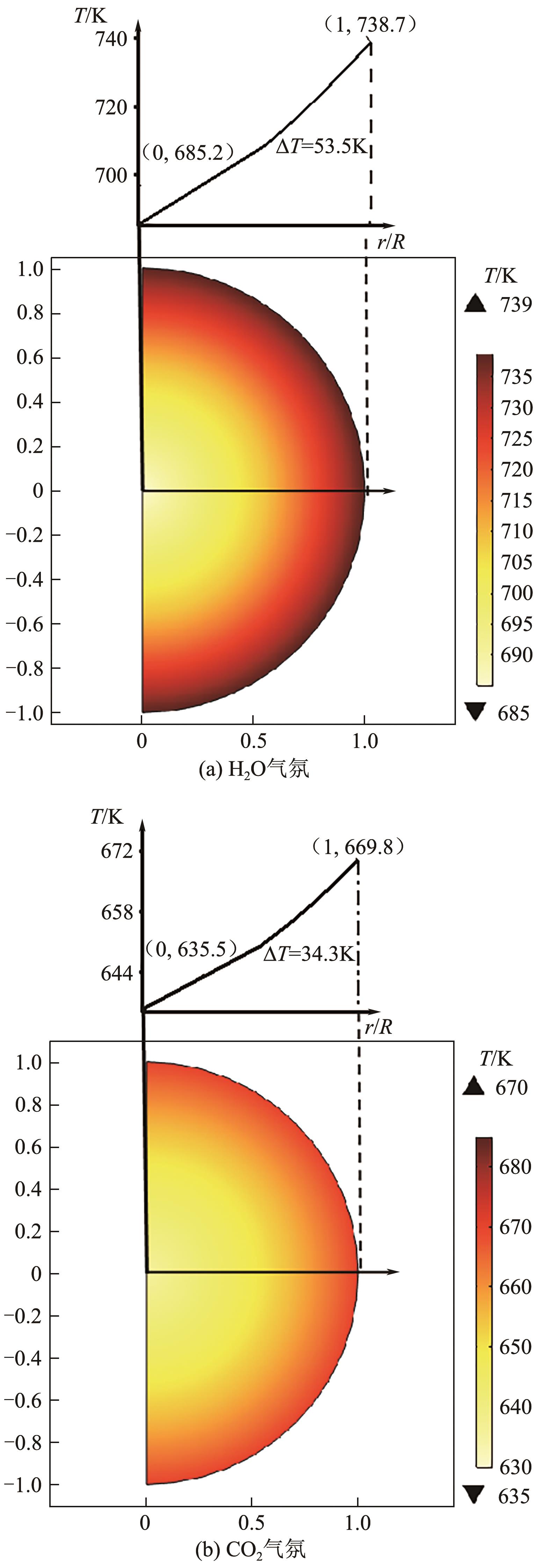
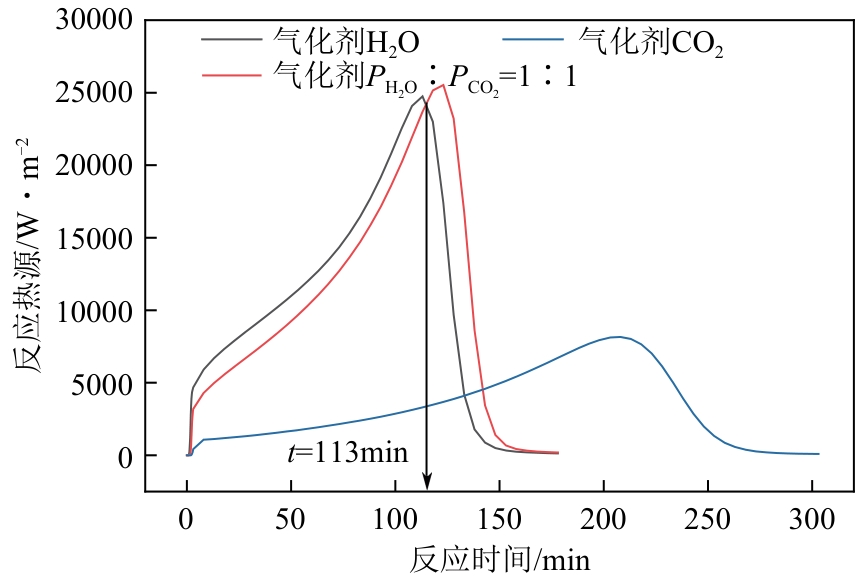
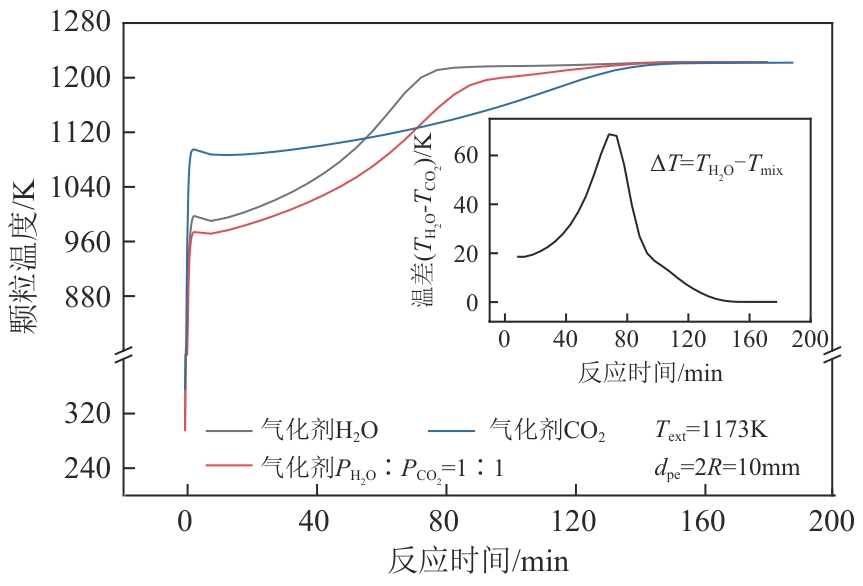
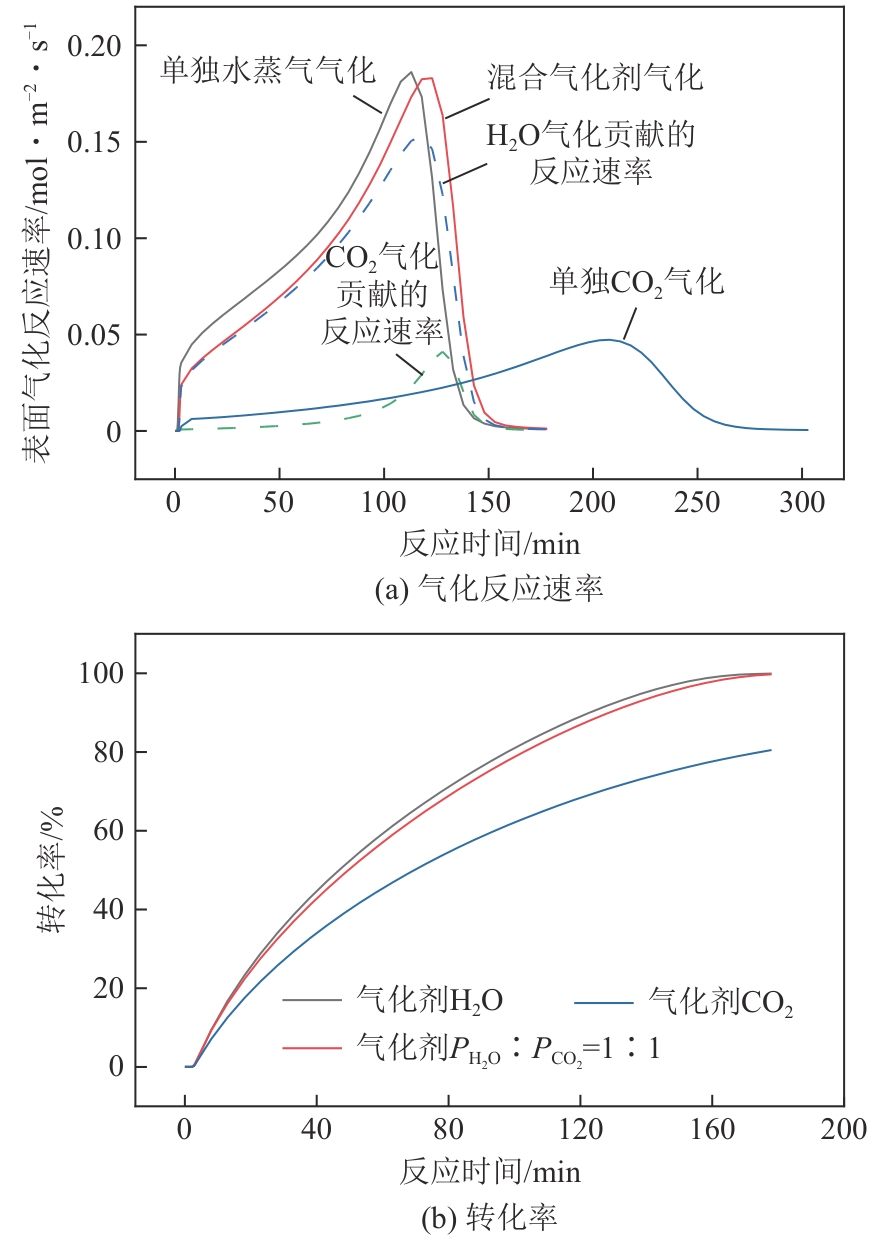
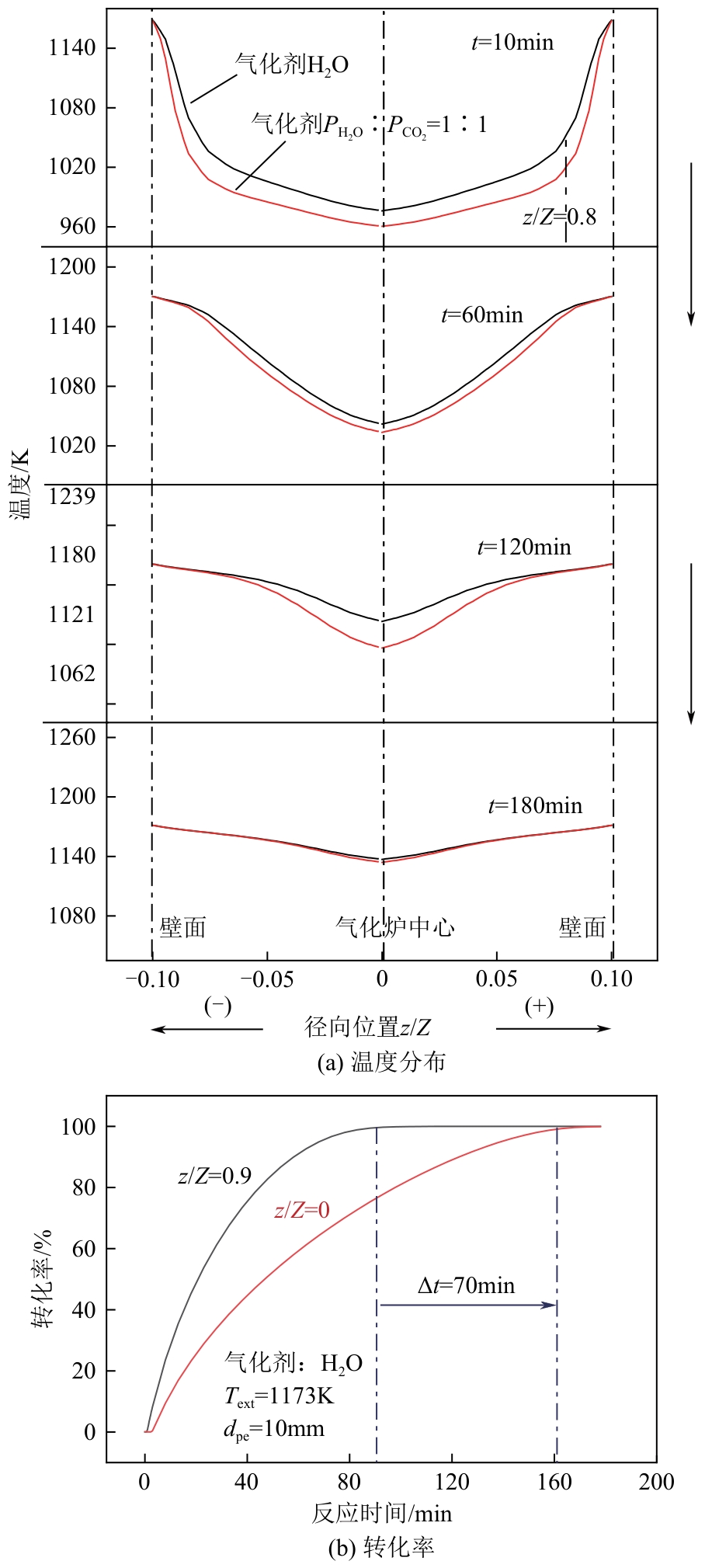
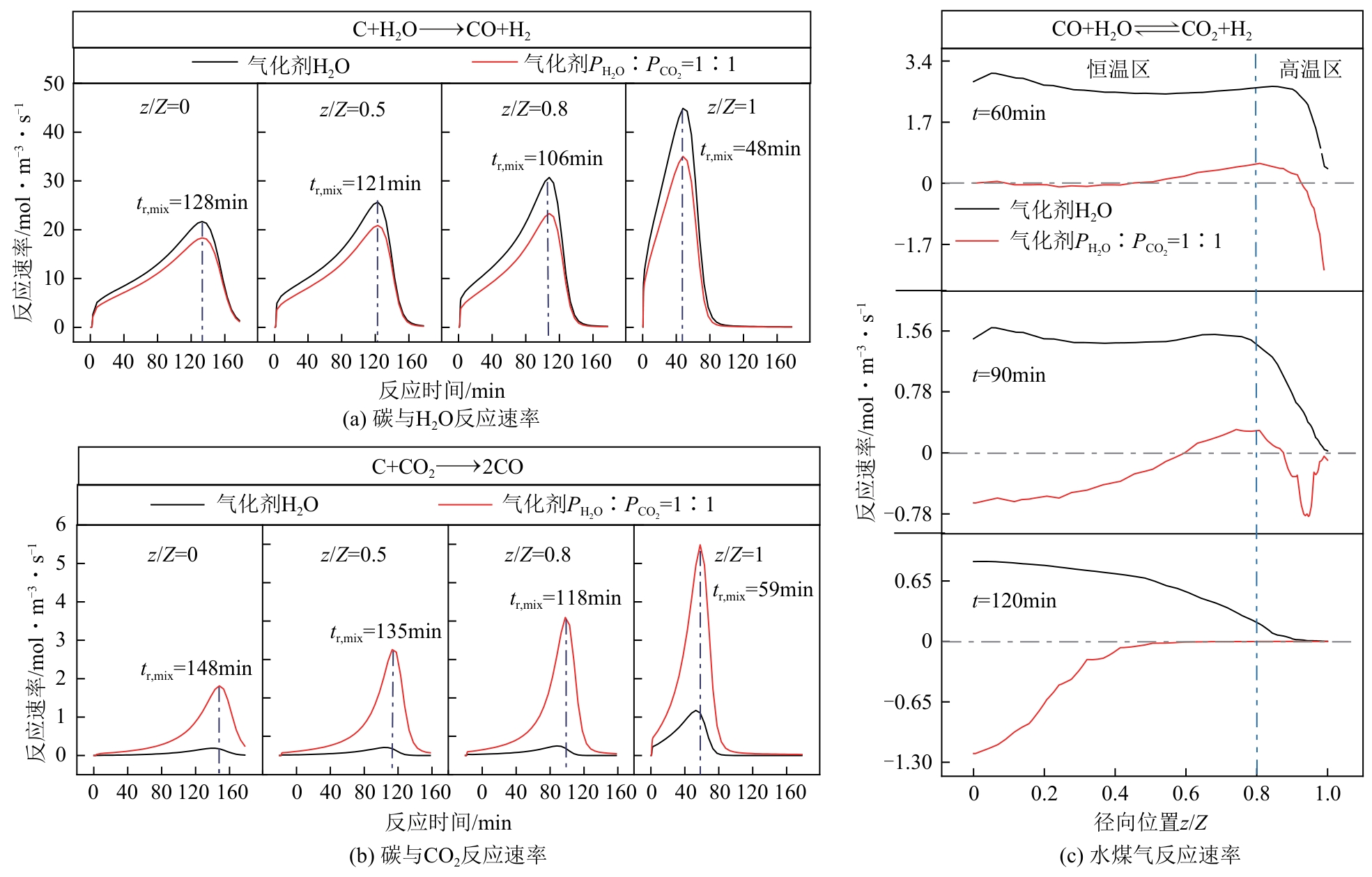
光热热源可解决中低价煤气化过程中元素利用率低等问题,但由于热源属性和传热方式改变,反应特性与传统煤气化过程不同,为消纳CO2引入的混合气化剂会对反应器内的温度场产生影响。为研究上述问题,本文利用多场耦合软件Comsol Multiphysics,构建了光热热源的褐煤固定床气化反应器,研究了不同导热特性的气化介质水蒸气、二氧化碳、不同比例混合气化剂下反应器内温度分布和产物组成以及反应器内热质传递行为变化。结果表明,随着气化剂中CO2添加比例由0增大至100%,由于CO2加入带来了流体黏度、密度不断增大,使得流体间黏滞力增强,动量传递减少;气/固相间对流换热系数从26.3W/(m2·K)降至22.7W/(m2·K)。相较于水蒸气气化,CO2的加入使得颗粒气化温度有所降低,煤气化反应速率下降,但CO2浓度的增加又提高了半焦CO2还原反应的速率,煤焦颗粒完全气化的综合时间没有明显改变。此外,光热储蓄热介质加热的方式使得反应器床层温度曲线整体呈“漏斗状”,导致炉内中心位置颗粒气化时间延长了70min,且在掺混CO2后,传热效率的降低使得温升曲线出现明显的“滞后”效应。通过改变CO2在气化剂中的添加比例发现,当CO2添加比例增至40%(体积分数)时,气化炉表现出消纳外源CO2的行为,其CO2消纳量达0.013g/g;且随着气化温度的增加,CO2气化反应速率升高, CO2的消纳量增加,有效合成气产量增加,但H2/CO下降。
中图分类号:
引用本文
袁梦丽, 宋云彩, 李文英, 冯杰. 光热驱动褐煤固定床气化过程热质传递规律[J]. 化工进展, 2025, 44(4): 2008-2019.
YUAN Mengli, SONG Yuncai, LI Wenying, FENG Jie. Heat and mass transfer law of photothermal-driven lignite fixed-bed gasification process[J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2008-2019.
| 反应 | 动力学表达式 | 动力学参数 | ΔH/kJ·mol-1 |
|---|---|---|---|
| C+H2O | 131.3 | ||
| C+CO2 | 172.5 | ||
| C+2H2 | -74.8 | ||
| CO+H2O | -41.2 | ||
| CH4+H2O | 206.1 |
表1 化学反应及动力学参数[23-25]
| 反应 | 动力学表达式 | 动力学参数 | ΔH/kJ·mol-1 |
|---|---|---|---|
| C+H2O | 131.3 | ||
| C+CO2 | 172.5 | ||
| C+2H2 | -74.8 | ||
| CO+H2O | -41.2 | ||
| CH4+H2O | 206.1 |
| 物理量 | 初始值 |
|---|---|
| 壁面温度/K | 1173.15 |
| 压力/Pa | 101325 |
| 气化剂入炉速度/m·s-1 | 0.023 |
| 气化剂入炉温度/K | 500 |
| 床层孔隙率 | 0.43 |
| 颗粒孔隙率 | 0.27 |
| 颗粒直径/m | 10-2 |
| 特征孔径/m | 10-7 |
| 颗粒密度/kg·m-3 | 1100 |
表2 气化炉初始条件
| 物理量 | 初始值 |
|---|---|
| 壁面温度/K | 1173.15 |
| 压力/Pa | 101325 |
| 气化剂入炉速度/m·s-1 | 0.023 |
| 气化剂入炉温度/K | 500 |
| 床层孔隙率 | 0.43 |
| 颗粒孔隙率 | 0.27 |
| 颗粒直径/m | 10-2 |
| 特征孔径/m | 10-7 |
| 颗粒密度/kg·m-3 | 1100 |
| 工业分析/% | 元素分析/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCdaf | C | H | N | S | O | |
| 0.55 | 28.36 | 4.63 | 95.37 | 97.75 | 0.99 | 0.66 | 0.53 | 0.07 | |
表3 半焦样的工业分析与元素分析[34]
| 工业分析/% | 元素分析/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCdaf | C | H | N | S | O | |
| 0.55 | 28.36 | 4.63 | 95.37 | 97.75 | 0.99 | 0.66 | 0.53 | 0.07 | |
| 参数 | [CO2/(CO2+H2O)] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | |
| 53.962 | 29.560 | 16.506 | 12.038 | 9.330 | 9.615 | 8.100 | 5.390 | 4.673 | |
| VCO/% | 38.115 | 23.064 | 14.218 | 11.204 | 9.356 | 10.132 | 9.060 | 6.499 | 5.941 |
| H2/CO | 1.415 | 1.281 | 1.161 | 1.074 | 0.997 | 0.949 | 0.894 | 0.829 | 0.787 |
| ξ/g·g-1 | — | — | — | — | 0.013 | 0.057 | 0.228 | 0.380 | 0.507 |
表4 不同CO2添加比例对气体组成的影响
| 参数 | [CO2/(CO2+H2O)] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | |
| 53.962 | 29.560 | 16.506 | 12.038 | 9.330 | 9.615 | 8.100 | 5.390 | 4.673 | |
| VCO/% | 38.115 | 23.064 | 14.218 | 11.204 | 9.356 | 10.132 | 9.060 | 6.499 | 5.941 |
| H2/CO | 1.415 | 1.281 | 1.161 | 1.074 | 0.997 | 0.949 | 0.894 | 0.829 | 0.787 |
| ξ/g·g-1 | — | — | — | — | 0.013 | 0.057 | 0.228 | 0.380 | 0.507 |
| 参数 | 温度 | ||||
|---|---|---|---|---|---|
| 1023K | 1073K | 1123K | 1173K | 1223K | |
| 5.511 | 7.117 | 7.665 | 9.615 | 10.451 | |
| VCO/% | 5.242 | 7.020 | 8.017 | 10.132 | 11.561 |
| H2/CO | 1.051 | 1.013 | 0.956 | 0.949 | 0.904 |
| ξ/g·g-1 | — | — | — | 0.057 | 0.216 |
表5 温度对气体组成的影响
| 参数 | 温度 | ||||
|---|---|---|---|---|---|
| 1023K | 1073K | 1123K | 1173K | 1223K | |
| 5.511 | 7.117 | 7.665 | 9.615 | 10.451 | |
| VCO/% | 5.242 | 7.020 | 8.017 | 10.132 | 11.561 |
| H2/CO | 1.051 | 1.013 | 0.956 | 0.949 | 0.904 |
| ξ/g·g-1 | — | — | — | 0.057 | 0.216 |
| 1 | BAI Zhang, GU Yucheng, WANG Shuoshuo, et al. Applying the solar solid particles as heat carrier to enhance the solar-driven biomass gasification with dynamic operation power generation performance analysis[J]. Applied Energy, 2023, 351: 121798. |
| 2 | SUÁREZ-ALMEIDA M, GÓMEZ-BAREA A, GHONIEM A F, et al. Solar gasification of biomass in a dual fluidized bed[J]. Chemical Engineering Journal, 2021, 406: 126665. |
| 3 | LI Xian, SHEN Ye, WEI Liping, et al. Hydrogen production of solar-driven steam gasification of sewage sludge in an indirectly irradiated fluidized-bed reactor[J]. Applied Energy, 2020, 261: 114229. |
| 4 | CHUEH William C, FALTER Christoph, ABBOTT Mandy, et al. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria[J]. Science, 2010, 330(6012): 1797-1801. |
| 5 | PADULA Stefano, TROIANO Maurizio, TREGAMBI Claudio, et al. Directly irradiated fluidized bed autothermal reactor (DIFBAR): Hydrodynamics, thermal behaviour and preliminary reactive tests[J]. Fuel, 2023, 346: 128222. |
| 6 | XU Dequan, WANG Bo, LI Xian, et al. Solar-driven biomass chemical looping gasification using Fe3O4 for syngas and high-purity hydrogen production[J]. Chemical Engineering Journal, 2024, 479: 147901. |
| 7 | ADINBERG Roman, EPSTEIN Michael, KARNI Jacob. Solar gasification of biomass: A molten salt pyrolysis study[J]. Journal of Solar Energy Engineering, 2004, 126(3): 850-857. |
| 8 | XIE Yingpu, YANG Haiping, ZENG Kuo, et al. Study on CO2 gasification of biochar in molten salts: Reactivity and structure evolution[J]. Fuel, 2019, 254: 115614. |
| 9 | 曾阔, 左宏杨, 陈汉平, 等. 一种太阳能蓄热热解气化生物质系统: CN115340885A[P]. 2022-11-15. |
| ZENG Kuo, ZUO Hongyang, CHEN Hanping, et al. A kind of solar energy regenerative pyrolysis gasification biomass system: CN115340885A[P]. 2022-11-15. | |
| 10 | LU Yongwen, WU Mingyang, ZUO Hongyang, et al. Melting enhancement in a shell-and-tube latent heat storage unit with staggered fin-foam synergistic configuration[J]. Journal of Energy Storage, 2024, 82: 110505. |
| 11 | KONG Jiayue, ZUO Hongyang, ZENG Kuo, et al. Parameter analysis and rapid design of porosity gradient distribution for open-cell metal foam in the latent thermal energy storage unit[J]. Journal of Energy Storage, 2024, 76: 109744. |
| 12 | PIATKOWSKI Nicolas, WIECKERT Christian, STEINFELD Aldo. Experimental investigation of a packed-bed solar reactor for the steam-gasification of carbonaceous feedstocks[J]. Fuel Processing Technology, 2009, 90(3): 360-366. |
| 13 | ZHANG Qi, SHAN Shiquan, YU Jinhong, et al. Coal gasification process driven by concentrated solar radiation for carbon neutralization: Reaction and energy characteristics[J]. Chemical Engineering Journal, 2022, 450: 138286. |
| 14 | BELLAN Selvan, GOKON Nobuyuki, MATSUBARA Koji, et al. Heat transfer analysis of 5kWth circulating fluidized bed reactor for solar gasification using concentrated Xe light radiation[J]. Energy, 2018, 160: 245-256. |
| 15 | CURCIO Axel, RODAT Sylvain, VUILLERME Valéry, et al. Dynamic simulation of a solar-autothermal hybridized gasifier: Model principle, experimental validation and parametric study[J]. Chemical Engineering Journal, 2023, 460: 141682. |
| 16 | WATANABE Hiroaki, TANNO Kenji, UMETSU Hiroki, et al. Modeling and simulation of coal gasification on an entrained flow coal gasifier with a recycled CO2 injection[J]. Fuel, 2015, 142: 250-259. |
| 17 | CHUAYBOON Srirat, ABANADES Stéphane. Carbon-neutral synfuel production via continuous solar H2O and CO2 gasification of oil palm empty fruit bunch[J]. Energy, 2023, 281: 128212. |
| 18 | PRABHAKAR Ashok, SADHUKHAN Anup Kumar, BHUNIA Shyamal, et al. Modelling and experimental investigations on gasification of coarse sized coal char particle with steam[J]. Journal of the Energy Institute, 2019, 92(5): 1502-1518. |
| 19 | FENG Bo, BHATIA Suresh K. On the validity of thermogravimetric determination of carbon gasification kinetics[J]. Chemical Engineering Science, 2002, 57(15): 2907-2920. |
| 20 | KWON Tae-Wahn, KIM Sang D, FUNG David P C. Reaction kinetics of char-CO2 gasification[J]. Fuel, 1988, 67(4): 530-535. |
| 21 | ROBERTS Daniel G, HARRIS David J. Char gasification kinetics in mixtures of CO2 and H2O: The role of partial pressure in determining the extent of competitive inhibition[J]. Energy & Fuels, 2014, 28(12): 7643-7648. |
| 22 | YANG Zhirong, GAO Meiqi, BAI Yonghui, et al. Model establishment for the kinetic evaluation of synergistic effect on the coal char gasification with H2O and CO2 mixtures[J]. Applied Thermal Engineering, 2017, 118: 682-690. |
| 23 | EVERSON Raymond C, NEOMAGUS Hein W J P, KASAINI Henry, et al. Reaction kinetics of pulverized coal-chars derived from inertinite-rich coal discards: Gasification with carbon dioxide and steam[J]. Fuel, 2006, 85(7/8): 1076-1082. |
| 24 | HUANG Zhimin, ZHANG Jiansheng, ZHAO Yong, et al. Kinetic studies of char gasification by steam and CO2 in the presence of H2 and CO[J]. Fuel Processing Technology, 2010, 91(8): 843-847. |
| 25 | LOHA Chanchal, CHATTOPADHYAY Himadri, CHATTERJEE Pradip K. Three dimensional kinetic modeling of fluidized bed biomass gasification[J]. Chemical Engineering Science, 2014, 109: 53-64. |
| 26 | GUNN D J. Transfer of heat or mass to particles in fixed and fluidised beds[J]. International Journal of Heat and Mass Transfer, 1978, 21(4): 467-476. |
| 27 | KRISHNA Rajamani, VAN BATEN Jasper M. Investigating the validity of the Bosanquet formula for estimation of diffusivities in mesopores[J]. Chemical Engineering Science, 2012, 69(1): 684-688. |
| 28 | 李位位, 黄戒介, 王志青, 等. 煤焦CO2气化反应动力学及内扩散对气化过程的影响分析[J]. 燃料化学学报, 2016, 44(12): 1416-1421. |
| LI Weiwei, HUANG Jiejie, WANG Zhiqing, et al. Reaction kinetics of coal char gasification with CO2 and the effect of internal diffusion on the gasification[J]. Journal of Fuel Chemistry and Technology, 2016, 44(12): 1416-1421. | |
| 29 | FULLER Edward N, SCHETTLER Paul D, Calvin GIDDINGS J. New method for prediction of binary gas-phase diffusion coefficients[J]. Industrial & Engineering Chemistry, 1996, 58(5): 18-27. |
| 30 | VELDSINK J W, VAN DAMME R M J, VERSTEEG G F, et al. The use of the dusty-gas model for the description of mass transport with chemical reaction in porous media[J]. The Chemical Engineering Journal and the Biochemical Engineering Journal, 1995, 57(2): 115-125. |
| 31 | NUGRAHA Maulana G, ANDERSSON Ronnie, ANDERSSON Bengt. On the Sherwood number correction due to Stefan flow[J]. Chemical Engineering Science, 2022, 249: 117292. |
| 32 | DALLAVALLE J M. Book reviews: Flow of gases through porous media[J]. Science, 1956, 124(3234): 1254-1255. |
| 33 | 徐鹏, 邱淑霞, 姜舟婷, 等. 各向同性多孔介质中Kozeny-Carman常数的分形分析[J]. 重庆大学学报, 2011, 34(4): 78-82. |
| XU Peng, QIU Shuxia, JIANG Zhouting, et al. Fractal analysis of Kozeny-Carman constant in the homogenous porous media[J]. Journal of Chongqing University, 2011, 34(4): 78-82. | |
| 34 | 徐春霞, 徐振刚, 董卫果, 等. CO2及水蒸气与煤焦共气化煤气组成分析[J]. 煤气与热力, 2010, 30(9): 6-10. |
| XU Chunxia, XU Zhengang, DONG Weiguo, et al. Analysis on gas composition from co-gasification of CO2 and steam with coal char[J]. Gas & Heat, 2010, 30(9): 6-10. | |
| 35 | XU Qixiang, PANG Shusheng, LEVI Tana. Reaction kinetics and producer gas compositions of steam gasification of coal and biomass blend chars, part 1: Experimental investigation[J]. Chemical Engineering Science, 2011, 66(10): 2141-2148. |
| 36 | 刘梦杰. 焦炭与水蒸气的气化特性研究[D]. 鞍山: 辽宁科技大学, 2017. |
| LIU Mengjie. Study on characteristics of coke and steam gasification[D]. Anshan: University of Science and Technology Liaoning, 2017. | |
| 37 | CHE Hanqiao, YUE Yuanhe, JIANG Zhaohua, et al. Numerical investigation of transient gas-solid heat transfer in a packed bed: Impact of intra-particle thermal diffusion[J]. Particuology, 2024, 90: 404-411. |
| 38 | 邬田华, 王晓墨, 许国良. 工程传热学[M]. 2版. 武汉: 华中科技大学出版社, 2020: 42-44. |
| WU Tianhua, WANG Xiaomo, XU Guoliang. Engineering heat transfer[M]. 2nd ed. Wuhan: Huazhong University of Science and Technology Press, 2020: 42-44. | |
| 39 | GUO Feiqiang, DONG Yuping, DONG Lei, et al. Effect of design and operating parameters on the gasification process of biomass in a downdraft fixed bed: An experimental study[J]. International Journal of Hydrogen Energy, 2014, 39(11): 5625-5633. |
| [1] | 王磊, 王艳, 甘玉凤, 罗凯, 费华, 栾俨丁. 水平流向不同小流道加热管内超临界CO2的传热特性[J]. 化工进展, 2025, 44(4): 1945-1956. |
| [2] | 王佳琪, 刘佳兴, 魏皓琦, 周昕霖, 程传晓, 葛坤. 鼠李糖脂强化CO2水合物生成[J]. 化工进展, 2025, 44(4): 1998-2007. |
| [3] | 王奇, 张乾, 杨凯, 高晨明, 孙岳鹏, 黄伟. 煤气化渣提炭分质用于橡胶补强填充料[J]. 化工进展, 2025, 44(3): 1749-1757. |
| [4] | 孙雅娟, 段思宇, 张宏, 周冬冬, 路广军, 马志斌. 化学外加剂对固废基胶凝材料性能及水化行为的影响[J]. 化工进展, 2025, 44(3): 1739-1748. |
| [5] | 王美杰, 韦刘轲, 贾保印, 蓝兴英, 高金森, 石孝刚. LNG开架式气化器传热强化的研究进展[J]. 化工进展, 2025, 44(3): 1206-1217. |
| [6] | 张喆, 纪献兵, 杨聿昊, 刘家璇, 姚泊丞. 多尺度结构烧结沟槽表面沸腾传热性能[J]. 化工进展, 2025, 44(2): 669-676. |
| [7] | 王思懿, 许建良, 代正华, 武国义, 王辅臣. 多晶硅还原炉气相沉积反应数值模拟[J]. 化工进展, 2025, 44(2): 706-716. |
| [8] | 李昊阳, 李洪伟, 谭建宇. 瞬态振荡加热条件下沸腾气泡运动特性[J]. 化工进展, 2025, 44(2): 735-742. |
| [9] | 白依冉, 翟玉玲, 戴晶慧, 李舟航. 微纳尺度池沸腾表面润湿性的气泡成核及强化传热机制[J]. 化工进展, 2025, 44(2): 743-751. |
| [10] | 蔡楷楠, 陈健勇, 陈颖, 罗向龙, 梁颖宗, 何嘉诚. 非共沸工质在分液板式冷凝器中的热力性能[J]. 化工进展, 2025, 44(1): 48-56. |
| [11] | 孙建辰, 杨捷, 李军, 孙会东, 牛俊敏, 廖逸飞, 任俊颖, 商辉. 催化剂颗粒排列方式对微波加热效果的影响[J]. 化工进展, 2025, 44(1): 57-65. |
| [12] | 张天昊, 李双喜, 贾祥际, 胡鼎国, 崔瑞焯, 李世聪. 干摩擦釜用机械密封DLC涂层-石墨配副摩擦磨损与温度变形场分析[J]. 化工进展, 2024, 43(S1): 121-133. |
| [13] | 张青, 黄理浩, 陶乐仁, 朱天意, 金云飞. R513A在不同肋结构水平管内的流动沸腾换热性能[J]. 化工进展, 2024, 43(S1): 134-143. |
| [14] | 苏瑶, 陈占秀, 杨历, 邢赫威, 呼和仓, 李源华. 热源温度对非对称纳米通道流动换热的影响[J]. 化工进展, 2024, 43(S1): 144-153. |
| [15] | 赵梦磊, 赵军, 鲁鸿滨, 陶少辉, 赵文英, 项曙光. 基于吉布斯自由能最小化法煤气化反应器模型开发及应用[J]. 化工进展, 2024, 43(9): 4793-4799. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||