化工进展 ›› 2024, Vol. 43 ›› Issue (11): 6206-6214.DOI: 10.16085/j.issn.1000-6613.2023-1919
• 工业催化 • 上一篇
锆修饰载体对锰铈复合催化剂NH3-SCR性能的影响
高梓寒1( ), 杨润农1, 王钊颖1, 宋燕海2, 覃斌3, 余林1(
), 杨润农1, 王钊颖1, 宋燕海2, 覃斌3, 余林1( )
)
- 1.广东工业大学轻工化工学院,广东省现代精细化工研究开发中心,广东 广州 510006
2.河北华特汽车部件 有限公司,河北 衡水 053000
3.江门气派摩托车有限公司,广东 江门 529000
-
收稿日期:2023-11-01修回日期:2024-03-12出版日期:2024-11-15发布日期:2024-12-07 -
通讯作者:余林 -
作者简介:高梓寒(1996—),男,硕士研究生,研究方向为SCR脱硝催化剂。E-mail:706479968@qq.com。 -
基金资助:国家自然科学基金(22278086)
Influence of zirconium modified carriers on the NH3-SCR performance of manganese-cerium composite catalysts
GAO Zihan1( ), YANG Runnong1, WANG Zhaoying1, SONG Yanhai2, QIN Bin3, YU Lin1(
), YANG Runnong1, WANG Zhaoying1, SONG Yanhai2, QIN Bin3, YU Lin1( )
)
- 1.Guangdong Engineering Technology Research Center of Modern Fine Chemical Engineering, School of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou 510006, Guangdong, China
2.Hebei HWAT Auto Parts Co. , Ltd. , Hengshui 053000, Hebei, China
3.Jiangmen Qipai Motorcycle Co. , Ltd. , Jiangmen 529000, Guangdong, China
-
Received:2023-11-01Revised:2024-03-12Online:2024-11-15Published:2024-12-07 -
Contact:YU Lin
摘要:
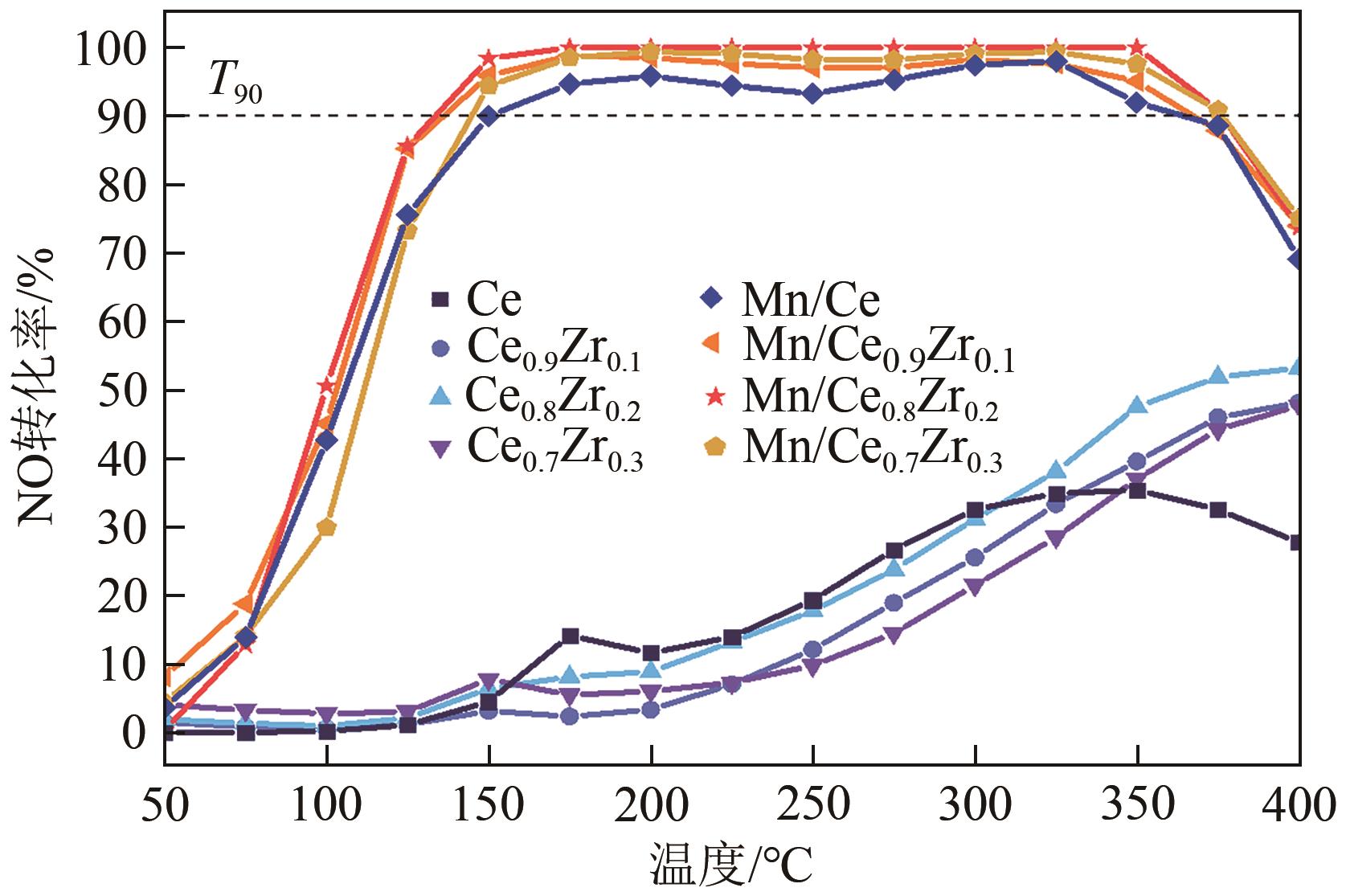
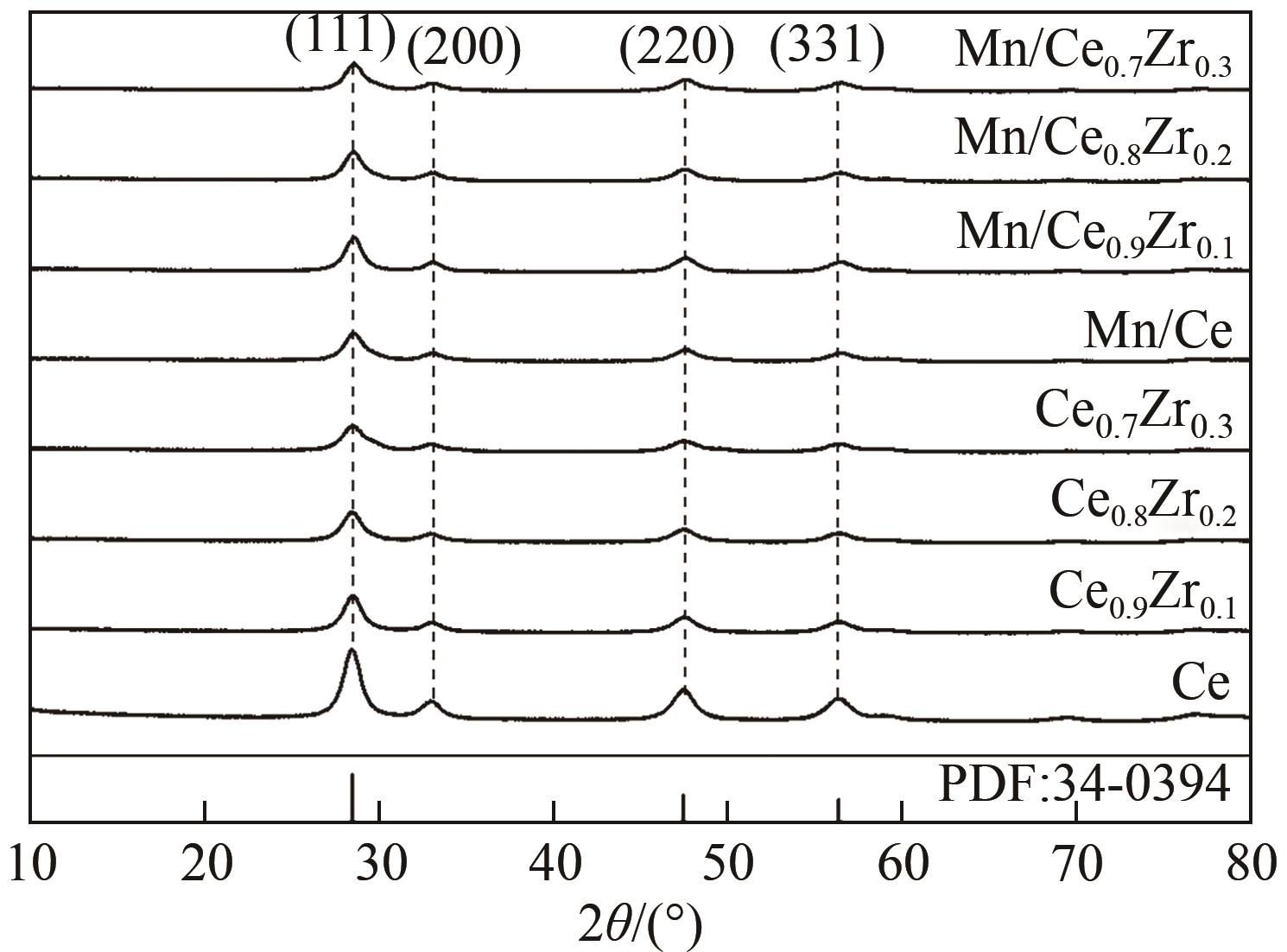
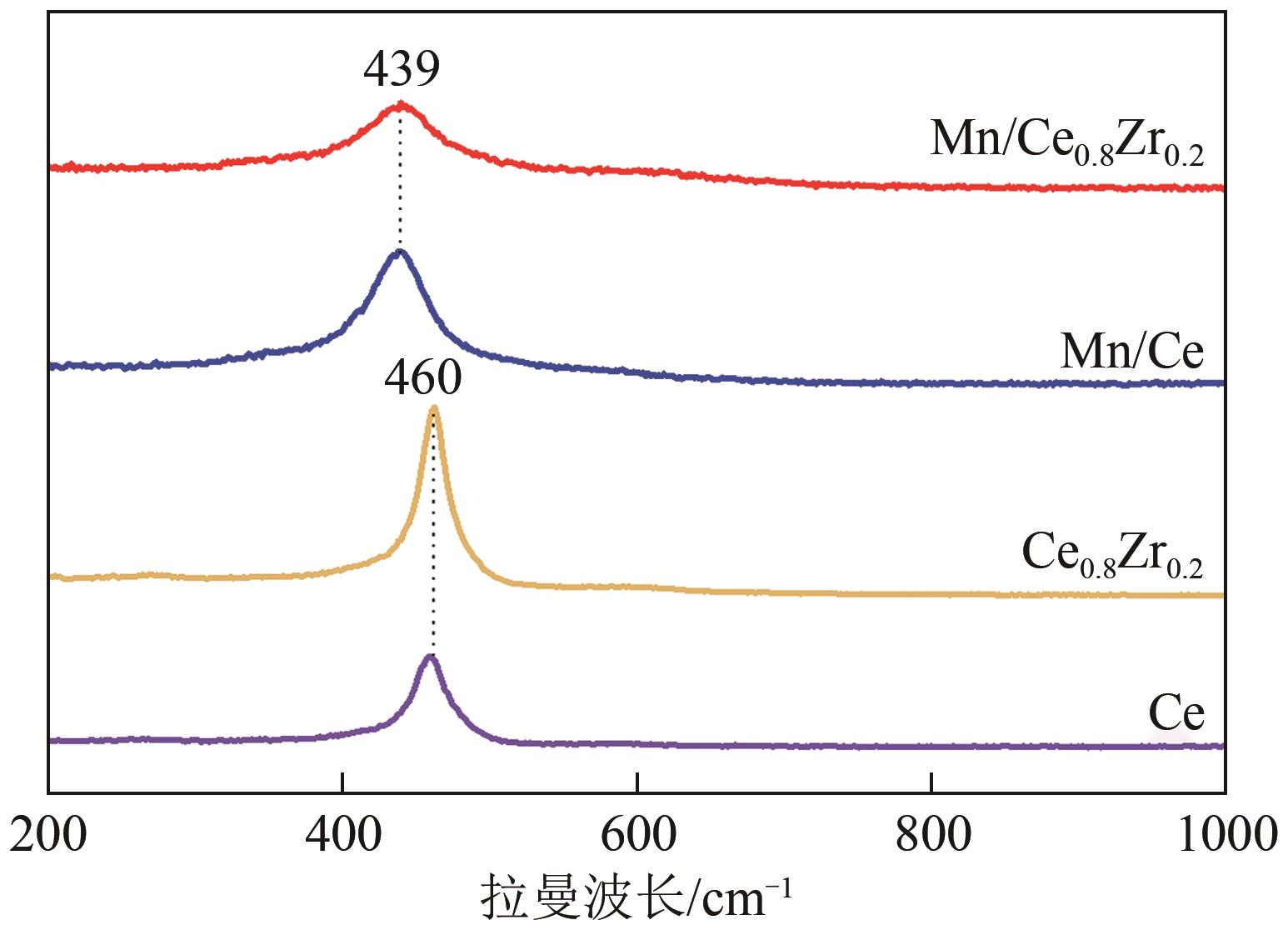
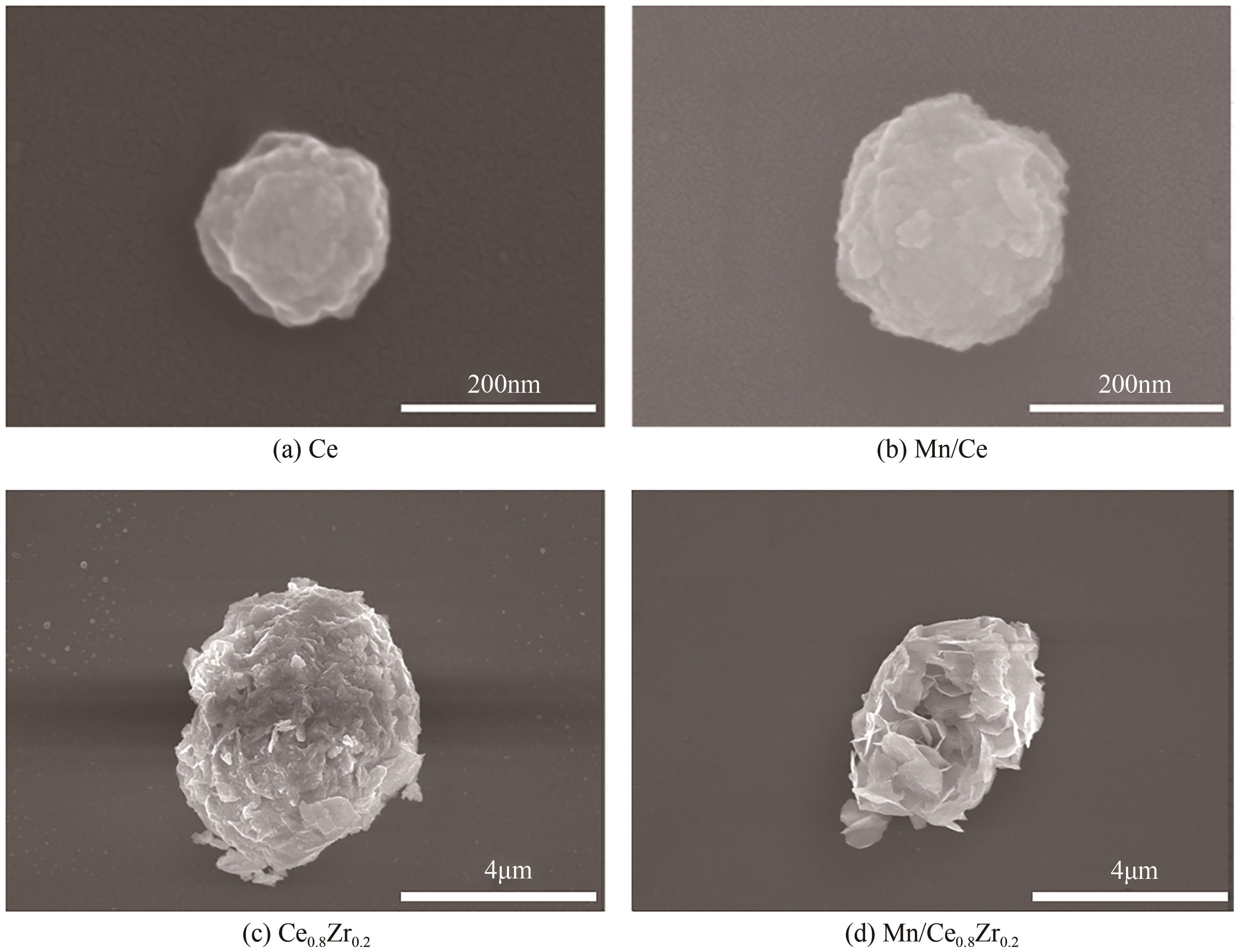
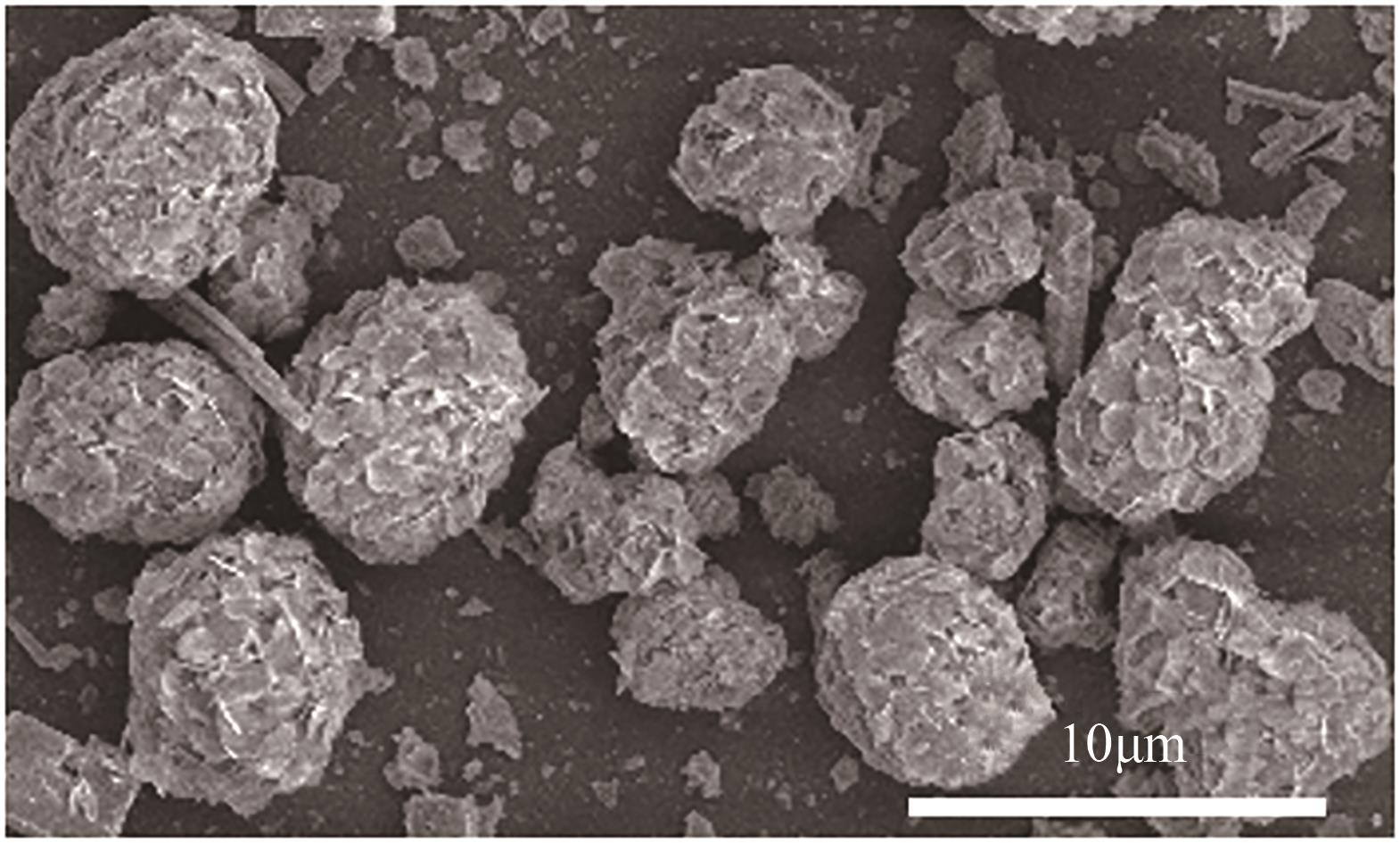
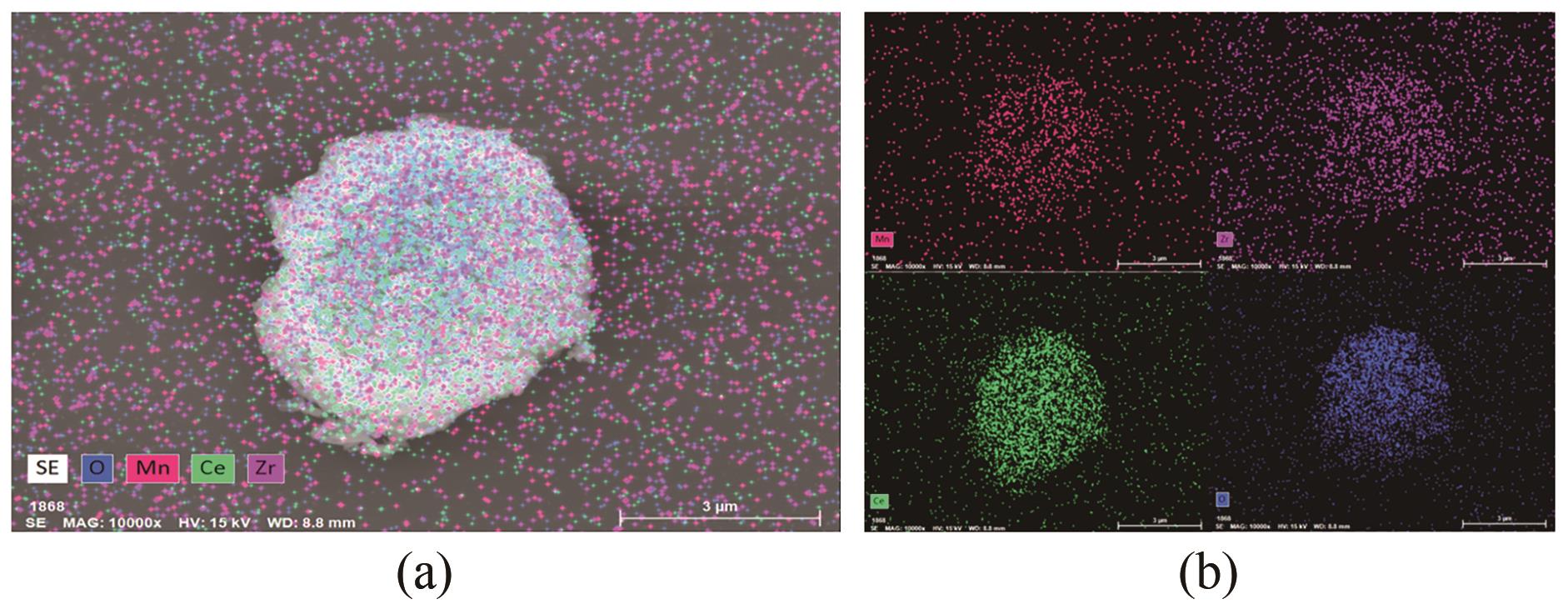
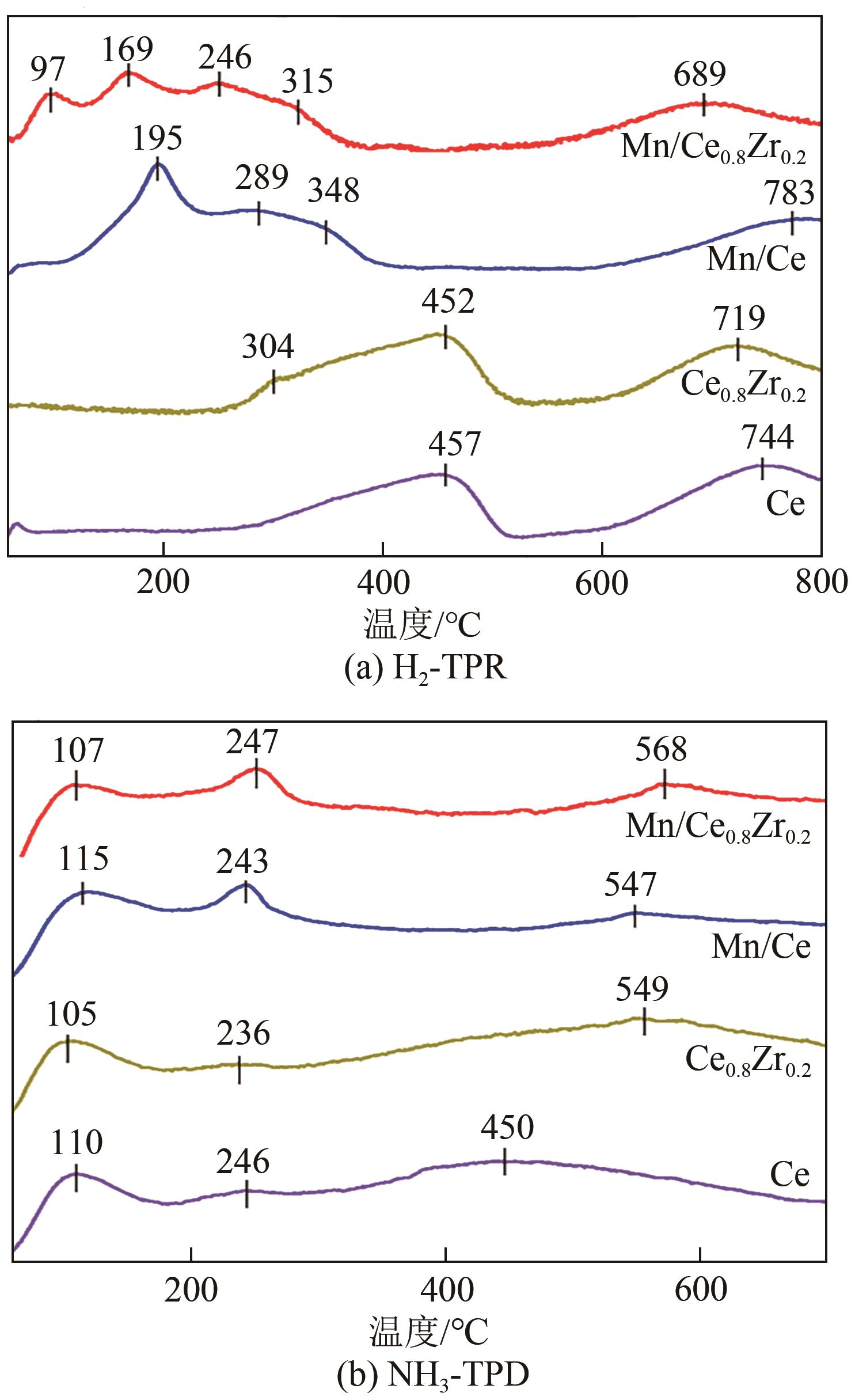
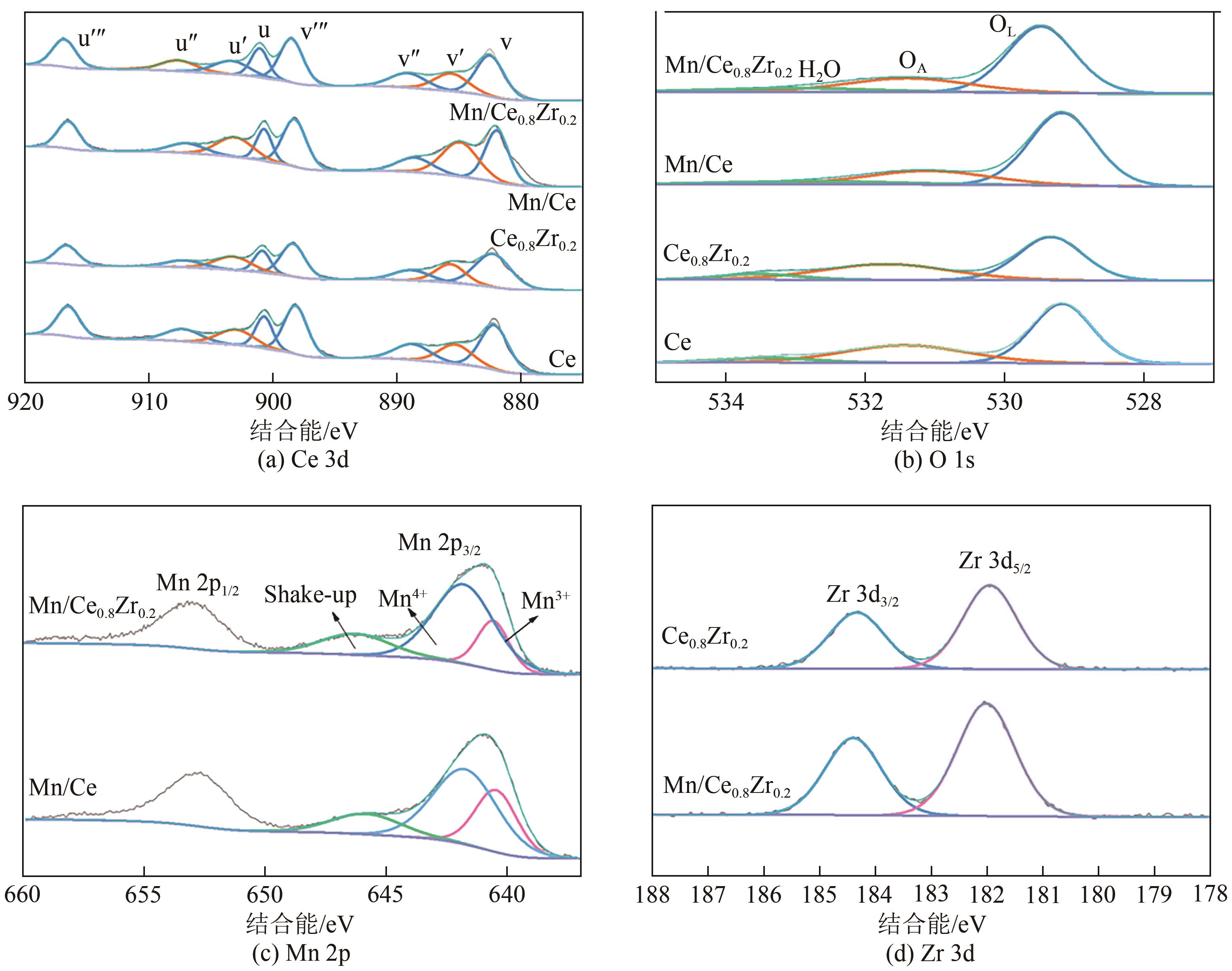
采用水热法合成不同比例的铈锆载体,通过浸渍法将MnO x 作为活性组分制备一系列复合催化剂,并考察其在以NH3为还原剂的选择性催化还原NO x 反应(NH3-SCR)中的脱硝活性。通过XRD、Raman、SEM、N2吸脱附、H2-TPR、NH3-TPD、XPS等技术手段对催化剂的结构、形貌、表面酸性、表面氧化价态等进行了表征。结果表明,水热法制备的Ce n Zr1-n 较CeO2载体具有更好的NH3-SCR催化活性,8Mn/Ce n Zr1-n 催化剂均有性能提升,其中8Mn/Ce0.8Zr0.2催化剂具有最佳的催化活性,其T50和T90分别为100.2℃和130.2℃,工作温度窗口为245.5℃(130.2~377.2℃),并且159.6~349.0℃范围内保持100%的NO转化率,具有更好的低温还原性能和更多的酸性位点,有利于反应物在更低的温度被吸附还原;其表面高价的Mn4+物种相对含量更高,有利于提升NH3-SCR催化活性。
中图分类号:
引用本文
高梓寒, 杨润农, 王钊颖, 宋燕海, 覃斌, 余林. 锆修饰载体对锰铈复合催化剂NH3-SCR性能的影响[J]. 化工进展, 2024, 43(11): 6206-6214.
GAO Zihan, YANG Runnong, WANG Zhaoying, SONG Yanhai, QIN Bin, YU Lin. Influence of zirconium modified carriers on the NH3-SCR performance of manganese-cerium composite catalysts[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6206-6214.
| 催化剂名称 | T50/℃ | T90/℃ | 最高NO转化率/% | 工作温度窗口/℃ |
|---|---|---|---|---|
| Ce | — | — | 35.7 | — |
| Ce0.9Zr0.1 | — | — | 48.3 | — |
| Ce0.8Zr0.2 | 364.3 | — | 53.4 | — |
| Ce0.7Zr0.3 | — | — | 48.8 | — |
| Mn/Ce | 104.0 | 150.4 | 98.0 | 150.4~353.5 |
| Mn/Ce0.9Zr0.1 | 102.7 | 132.3 | 99.1 | 132.3~368.1 |
| Mn/Ce0.8Zr0.2 | 100.2 | 130.2 | 100.0 | 130.2~375.7 |
| Mn/Ce0.7Zr0.3 | 112 | 141.2 | 99.8 | 141.2~377.2 |
表1 不同铈锆比载体及负载锰催化剂的起燃温度、完全转化温度、最高NO转化率和温度窗口
| 催化剂名称 | T50/℃ | T90/℃ | 最高NO转化率/% | 工作温度窗口/℃ |
|---|---|---|---|---|
| Ce | — | — | 35.7 | — |
| Ce0.9Zr0.1 | — | — | 48.3 | — |
| Ce0.8Zr0.2 | 364.3 | — | 53.4 | — |
| Ce0.7Zr0.3 | — | — | 48.8 | — |
| Mn/Ce | 104.0 | 150.4 | 98.0 | 150.4~353.5 |
| Mn/Ce0.9Zr0.1 | 102.7 | 132.3 | 99.1 | 132.3~368.1 |
| Mn/Ce0.8Zr0.2 | 100.2 | 130.2 | 100.0 | 130.2~375.7 |
| Mn/Ce0.7Zr0.3 | 112 | 141.2 | 99.8 | 141.2~377.2 |
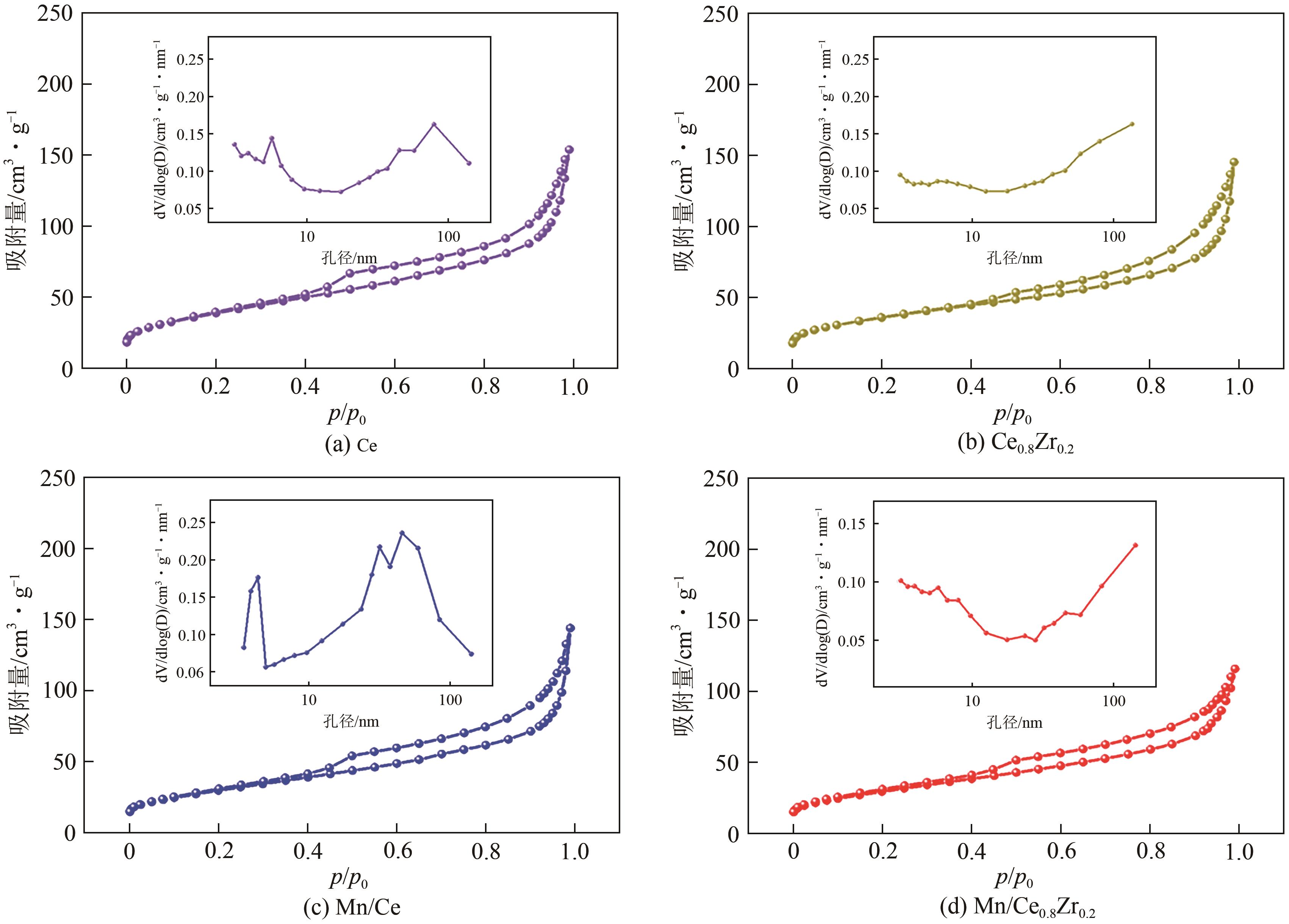
| 催化剂 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| Ce | 117.6 | 0.196 | 3.057 |
| Ce0.8Zr0.2 | 125.7 | 0.183 | 3.057 |
| Mn/Ce | 106.1 | 0.192 | 3.063 |
| Mn/Ce0.8Zr0.2 | 104.7 | 0.147 | 3.063 |
表2 不同铈锆比载体及负载锰催化剂的比表面积、孔容和平均孔径
| 催化剂 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| Ce | 117.6 | 0.196 | 3.057 |
| Ce0.8Zr0.2 | 125.7 | 0.183 | 3.057 |
| Mn/Ce | 106.1 | 0.192 | 3.063 |
| Mn/Ce0.8Zr0.2 | 104.7 | 0.147 | 3.063 |
| 催化剂 | H总/mmol·g-1 | H低温/mmol·g-1 | 相对酸性位点总量 |
|---|---|---|---|
| Ce | 1.202 | 0.639 | 4.48 |
| Ce0.8Zr0.2 | 1.447 | 0.848 | 4.97 |
| Mn/Ce | 1.564 | 1.095 | 4.94 |
| Mn/Ce0.8Zr0.2 | 1.776 | 1.211 | 5.12 |
表3 不同铈锆比载体及负载锰催化剂H2消耗量和NH3脱附量
| 催化剂 | H总/mmol·g-1 | H低温/mmol·g-1 | 相对酸性位点总量 |
|---|---|---|---|
| Ce | 1.202 | 0.639 | 4.48 |
| Ce0.8Zr0.2 | 1.447 | 0.848 | 4.97 |
| Mn/Ce | 1.564 | 1.095 | 4.94 |
| Mn/Ce0.8Zr0.2 | 1.776 | 1.211 | 5.12 |
| 催化剂 | Ce3+/(Ce3++Ce4+) | Mn4+/(Mn3++Mn4+) | OA/(OA+OL) |
|---|---|---|---|
| Ce | 0.179 | — | 0.225 |
| Ce0.8Zr0.2 | 0.204 | — | 0.264 |
| Mn/Ce | 0.224 | 0.602 | 0.167 |
| Mn/ Ce0.8Zr0.2 | 0.184 | 0.658 | 0.174 |
表4 不同铈锆比载体及负载锰催化剂的表面元素组成
| 催化剂 | Ce3+/(Ce3++Ce4+) | Mn4+/(Mn3++Mn4+) | OA/(OA+OL) |
|---|---|---|---|
| Ce | 0.179 | — | 0.225 |
| Ce0.8Zr0.2 | 0.204 | — | 0.264 |
| Mn/Ce | 0.224 | 0.602 | 0.167 |
| Mn/ Ce0.8Zr0.2 | 0.184 | 0.658 | 0.174 |
| 1 | BOHON Myles D, RASHIDI Mariam J AL, Mani SARATHY S, et al. Experiments and simulations of NO x formation in the combustion of hydroxylated fuels[J]. Combustion and Flame, 2015, 162(6): 2322-2336. |
| 2 | LIU Zhiming, LI Junhua, Seong Ihl WOO. Recent advances in the selective catalytic reduction of NO x by hydrogen in the presence of oxygen[J]. Energy & Environmental Science, 2012, 5(10): 8799-8814. |
| 3 | 王艳, 李兆强, 张丞, 等. CeO2含量对柴油机商用稀土SCR催化剂脱硝性能的影响[J]. 化工进展, 2020, 39(7): 2662-2669. |
| WANG Yan, LI Zhaoqiang, ZHANG Cheng, et al. Influence of CeO2 contents on the SCR performance of commercial rare earth catalysts[J]. Chemical Industry and Engineering Progress, 2020, 39(7): 2662-2669. | |
| 4 | DEKA Upakul, Ines LEZCANO-GONZALEZ, WECKHUYSEN Bert M, et al. Local environment and nature of Cu active sites in zeolite-based catalysts for the selective catalytic reduction of NO x [J]. ACS Catalysis, 2013, 3(3): 413-427. |
| 5 | GRANGER Pascal, PARVULESCU Vasile I. Catalytic NO x abatement systems for mobile sources: From three-way to lean burn after-treatment technologies[J]. Chemical Reviews, 2011, 111(5): 3155-3207. |
| 6 | BUSCA Guido, LIETTI Luca, RAMIS Gianguido, et al. Chemical and mechanistic aspects of the selective catalytic reduction of NO by ammonia over oxide catalysts: A review[J]. Applied Catalysis B: Environmental, 1998, 18(1/2): 1-36. |
| 7 | KOMPIO Patrick G W A, Angelika BRÜCKNER, HIPLER Frank, et al. A new view on the relations between tungsten and vanadium in V2O5-WO3/TiO2 catalysts for the selective reduction of NO with NH3 [J]. Journal of Catalysis, 2012, 286: 237-247. |
| 8 | ZHANG Jie, HUANG Zhiwei, DU Yueyao, et al. Atomic-scale insights into the nature of active sites in Fe2O3-supported submonolayer WO3 catalysts for selective catalytic reduction of NO with NH3 [J]. Chemical Engineering Journal, 2020, 381: 122668. |
| 9 | BRANDENBERGER Sandro, Oliver KRÖCHER, TISSLER Arno, et al. The state of the art in selective catalytic reduction of NO x by ammonia using metal-exchanged zeolite catalysts[J]. Catalysis Reviews, 2008, 50(4): 492-531. |
| 10 | ROY Sounak, BAIKER Alfons. NO x storage-reduction catalysis: From mechanism and materials properties to storage-reduction performance[J]. Chemical Reviews, 2009, 109(9): 4054-4091. |
| 11 | FANG Xue, LIU Yongjun, CEN Wanglai, et al. Birnessite as a highly efficient catalyst for low-temperature NH3-SCR: The vital role of surface oxygen vacancies[J]. Industrial & Engineering Chemistry Research, 2020, 59(33): 14606-14615. |
| 12 | MENG Dongmei, ZHAN Wangcheng, GUO Yun, et al. A highly effective catalyst of Sm-MnO x for the NH3-SCR of NO x at low temperature: Promotional role of Sm and its catalytic performance[J]. ACS Catalysis, 2015, 5(10): 5973-5983. |
| 13 | TANG Xingfu, LI Junhua, SUN Liang, et al. Origination of N2O from NO reduction by NH3 over β-MnO2 and α-Mn2O3 [J]. Applied Catalysis B: Environmental, 2010, 99(1/2): 156-162. |
| 14 | GAO Ge, SHI Jianwen, LIU Chang, et al. Mn/CeO2 catalysts for SCR of NO x with NH3: Comparative study on the effect of supports on low-temperature catalytic activity[J]. Applied Surface Science, 2017, 411: 338-346. |
| 15 | SHEN Boxiong, WANG Yinyin, WANG Fumei, et al. The effect of Ce-Zr on NH3-SCR activity over MnO x (0.6)/Ce0.5Zr0.5O2 at low temperature[J]. Chemical Engineering Journal, 2014, 236: 171-180. |
| 16 | DING Shipeng, LIU Fudong, SHI Xiaoyan, et al. Significant promotion effect of Mo additive on a novel Ce-Zr mixed oxide catalyst for the selective catalytic reduction of NO x with NH3 [J]. ACS Applied Materials & Interfaces, 2015, 7(18): 9497-9506. |
| 17 | Fabien CAN, BERLAND Sébastien, ROYER Sébastien, et al. Composition-dependent performance of Ce x Zr1– x O2 mixed-oxide-supported WO3 catalysts for the NO x storage reduction-selective catalytic reduction coupled process[J]. ACS Catalysis, 2013, 3(6): 1120-1132. |
| 18 | MA Lei, WANG Dingsheng, LI Junhua, et al. Ag/CeO2 nanospheres: Efficient catalysts for formaldehyde oxidation[J]. Applied Catalysis B: Environmental, 2014, 148/149: 36-43. |
| 19 | LIU Zhiming, YI Yang, ZHANG Shaoxuan, et al. Selective catalytic reduction of NO x with NH3 over Mn-Ce mixed oxide catalyst at low temperatures[J]. Catalysis Today, 2013, 216: 76-81. |
| 20 | DANIEL Marlène, LORIDANT Stéphane. Probing reoxidation sites by in situ Raman spectroscopy: Differences between reduced CeO2 and Pt/CeO2 [J]. Journal of Raman Spectroscopy, 2012, 43(9): 1312-1319. |
| 21 | SHANG Danhong, CAI Wei, ZHAO Wei, et al. Catalytic oxidation of NO to NO2 over Co-Ce-Zr solid solutions: Enhanced performance of Ce-Zr solid solution by Co[J]. Catalysis Letters, 2014, 144(3): 538-544. |
| 22 | SING K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984)[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 23 | WU Xiaomin, YU Xiaolong, HUANG Zhiwei, et al. MnOx-decorated VO x /CeO2 catalysts with preferentially exposed {110} facets for selective catalytic reduction of NO x by NH3 [J]. Applied Catalysis B: Environmental, 2020, 268: 118419. |
| 24 | SHEN Boxiong, YAO Yan, CHEN Jianhong, et al. Alkali metal deactivation of Mn-CeO x /Zr-delaminated-clay for the low-temperature selective catalytic reduction of NO x with NH3 [J]. Microporous and Mesoporous Materials, 2013, 180: 262-269. |
| 25 | DELIMARIS Dimitrios, IOANNIDES Theophilos. VOC oxidation over MnO x -CeO2 catalysts prepared by a combustion method[J]. Applied Catalysis B: Environmental, 2008, 84(1/2): 303-312. |
| 26 | LI Hongfeng, LU Guanzhong, DAI Qiguang, et al. Efficient low-temperature catalytic combustion of trichloroethylene over flower-like mesoporous Mn-doped CeO2 microspheres[J]. Applied Catalysis B: Environmental, 2011, 102(3/4): 475-483. |
| 27 | ZHANG Dengsong, ZHANG Lei, SHI Liyi, et al. In situ supported MnO x -CeO x on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3 [J]. Nanoscale, 2013, 5(3): 1127-1136. |
| 28 | TAN Hongyi, WANG Jin, YU Shuzhen, et al. Support morphology-dependent catalytic activity of Pd/CeO₂ for formaldehyde oxidation[J]. Environmental Science & Technology, 2015, 49(14): 8675-8682. |
| 29 | YOU Xiaochen, SHENG Zhongyi, YU Danqing, et al. Influence of Mn/Ce ratio on the physicochemical properties and catalytic performance of graphene supported MnO x -CeO2 oxides for NH3-SCR at low temperature[J]. Applied Surface Science, 2017, 423: 845-854. |
| 30 | PENG Yue, WANG Dong, LI Bing, et al. Impacts of Pb and SO2 poisoning on CeO2-WO3/TiO2-SiO2 SCR catalyst[J]. Environmental Science & Technology, 2017, 51(20): 11943-11949. |
| 31 | KONG Jiejing, XIANG Ziwei, LI Guiying, et al. Introduce oxygen vacancies into CeO2 catalyst for enhanced coke resistance during photothermocatalytic oxidation of typical VOCs[J]. Applied Catalysis B: Environmental, 2020, 269: 118755. |
| 32 | ZHANG Guodong, HAN Weiliang, DONG Fang, et al. One pot synthesis of a highly efficient mesoporous ceria-titanium catalyst for selective catalytic reduction of NO[J]. RSC Advances, 2016, 6(80): 76556-76567. |
| 33 | YU Xiaolong, WU Xiaomin, CHEN Ziyi, et al. Oxygen vacancy defect engineering in Mn-doped CeO2 nanostructures for nitrogen oxides emission abatement[J]. Molecular Catalysis, 2019, 476: 110512. |
| 34 | LI Guangfeng, WANG Qiuyan, ZHAO Bo, et al. Effect of iron doping into CeO2-ZrO2 on the properties and catalytic behaviour of Pd-only three-way catalyst for automotive emission control[J]. Journal of Hazardous Materials, 2011, 186(1): 911-920. |
| 35 | LIU Xiangwen, ZHOU Kebin, WANG Lei, et al. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods[J]. Journal of the American Chemical Society, 2009, 131(9): 3140-3141. |
| 36 | ZHOU Aiyi, YU Danqing, YANG Liu, et al. Combined effects Na and SO2 in flue gas on Mn-Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO by NH3 simulated by Na2SO4 doping[J]. Applied Surface Science, 2016, 378: 167-173. |
| 37 | GENUINO Homer C, MENG Yongtao, HORVATH Dayton T, et al. Enhancement of catalytic activities of octahedral molecular sieve manganese oxide for total and preferential CO oxidation through vanadium ion framework substitution[J]. ChemCatChem, 2013, 5(8): 2306-2317. |
| 38 | SUN Liang, CAO Qingqing, HU Bingqing, et al. Synthesis, characterization and catalytic activities of vanadium-cryptomelane manganese oxides in low-temperature NO reduction with NH3 [J]. Applied Catalysis A: General, 2011, 393(1/2): 323-330. |
| 39 | DU Haiwei, WANG Yuan, ARANDIYAN Hamidreza, et al. Design and synthesis of CeO2 nanowire/MnO2 nanosheet heterogeneous structure for enhanced catalytic properties[J]. Materials Today Communications, 2017, 11: 103-111. |
| 40 | TANG Xingfu, LI Yonggang, CHEN Junli, et al. Synthesis, characterization, and catalytic application of titanium-cryptomelane nanorods/fibers[J]. Microporous and Mesoporous Materials, 2007, 103(1/2/3): 250-256. |
| 41 | LI Weiman, LIU Haidi, CHEN Yunfa. Promotion of transition metal oxides on the NH3-SCR performance of ZrO2-CeO2 catalyst[J]. Frontiers of Environmental Science & Engineering, 2017, 11(2): 6. |
| 42 | ZUO Jianliang, CHEN Zhihang, WANG Furong, et al. Low-temperature selective catalytic reduction of NO x with NH3 over novel Mn-Zr mixed oxide catalysts[J]. Industrial & Engineering Chemistry Research, 2014, 53(7): 2647-2655. |
| [1] | 付维, 宁淑英, 蔡晨, 陈佳音, 周皞, 苏亚欣. Cu改性MIL-100(Fe)催化剂的SCR-C3H6脱硝特性[J]. 化工进展, 2024, 43(9): 4951-4960. |
| [2] | 龙涛, 周锋, 张伟, 吴泓, 王建, 陈霖. CO-CO2体系制备氘代甲醇催化剂的合成与改性[J]. 化工进展, 2024, 43(8): 4411-4420. |
| [3] | 周皞, 王旭瑞, 赵辉爽, 温妮妮, 苏亚欣. CuCe-SAPO-34选择性催化丙烯还原柴油车尾气氮氧化物[J]. 化工进展, 2024, 43(6): 3093-3099. |
| [4] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [5] | 李佳, 樊星, 陈莉, 李坚. 硝酸生产尾气中NO x 和N2O联合脱除技术研究进展[J]. 化工进展, 2023, 42(7): 3770-3779. |
| [6] | 马敬文, 牛佳钰, 李秀芬. 好氧堆肥腐熟的促进技术[J]. 化工进展, 2023, 42(5): 2744-2750. |
| [7] | 赵巧男, 刘雪敏, 刘峰, 徐洪涛, 刘兆海, 廖晓炜. 燃气锅炉掺氢燃烧氮氧化物排放机理数值模拟[J]. 化工进展, 2023, 42(11): 5637-5647. |
| [8] | 龙红明, 丁龙, 钱立新, 春铁军, 张洪亮, 余正伟. 烧结烟气中NO x 和二 英的减排现状及发展趋势[J]. 化工进展, 2022, 41(7): 3865-3876. 英的减排现状及发展趋势[J]. 化工进展, 2022, 41(7): 3865-3876. |
| [9] | 杨希刚, 陈国庆, 黄林滨, 古世军, 李昌松, 张勇, 金保昇. 尿素法SNCR对大型电站煤粉锅炉运行影响的工业试验[J]. 化工进展, 2022, 41(7): 3573-3581. |
| [10] | 韩徳琳, 李丹, 王天天, 张海, 张扬, 王随林. 位移钝体稳燃的旋流预混燃烧污染物生成特性[J]. 化工进展, 2022, 41(6): 2915-2923. |
| [11] | 王新宇, 黄亚继, 徐力刚, 李志远, 李偲, 刘晓东. 调节同层二次风以缓解双切圆锅炉高温腐蚀的数值模拟[J]. 化工进展, 2022, 41(5): 2292-2300. |
| [12] | 谭潇, 齐随涛, 周一鸣, 石利斌, 程光旭, 伊春海, 杨伯伦. 非热等离子体协同锰铁双金属催化剂直接催化还原NO[J]. 化工进展, 2022, 41(11): 5850-5857. |
| [13] | 杨景瑞, 王莹, 陈虎, 吕永康. 氧浓度对调节NO x 氧化度协同CABR法脱硝性能及菌群结构的影响[J]. 化工进展, 2022, 41(11): 6139-6148. |
| [14] | 尹子骏, 苏胜, 王中辉, 王乐乐, 安晓雪, 赵志刚, 陈逸峰, 刘涛, 汪一, 胡松, 向军. 燃煤烟气中SO3与NH4HSO4生成特性及其控制方法研究进展[J]. 化工进展, 2021, 40(4): 2328-2337. |
| [15] | 刘月华, 上官炬, 刘守军, 杨颂, 杜文广. 铁镍复合助剂对煤热解过程中氮迁移规律的影响[J]. 化工进展, 2021, 40(1): 164-172. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||