化工进展 ›› 2022, Vol. 41 ›› Issue (8): 4077-4085.DOI: 10.16085/j.issn.1000-6613.2021-1943
换热壁面碳酸钙吸附与脱水行为的分子动力学
- 东北电力大学能源与动力工程学院,吉林 吉林 132012
-
收稿日期:2021-09-09修回日期:2021-10-31出版日期:2022-08-25发布日期:2022-08-22 -
通讯作者:王兵兵 -
作者简介:肖毅(1997—),男,硕士研究生,研究方向为换热表面污垢形成机理与抑制方法。E-mail:975152973@qq.com 。 -
基金资助:国家自然科学基金(51706038);吉林省科技厅优秀青年人才基金(20190103059JH)
Molecular dynamics simulation on adsorption and dehydration behavior of calcium carbonate on heat exchange surface
XIAO Yi( ), WANG Bingbing(
), WANG Bingbing( ), YU Xuliang, WANG Xin, CAI Hanyou
), YU Xuliang, WANG Xin, CAI Hanyou
- School of Energy and Power Engineering, Northeast Electric Power University, Jilin 132012, Jilin, China
-
Received:2021-09-09Revised:2021-10-31Online:2022-08-25Published:2022-08-22 -
Contact:WANG Bingbing
摘要:
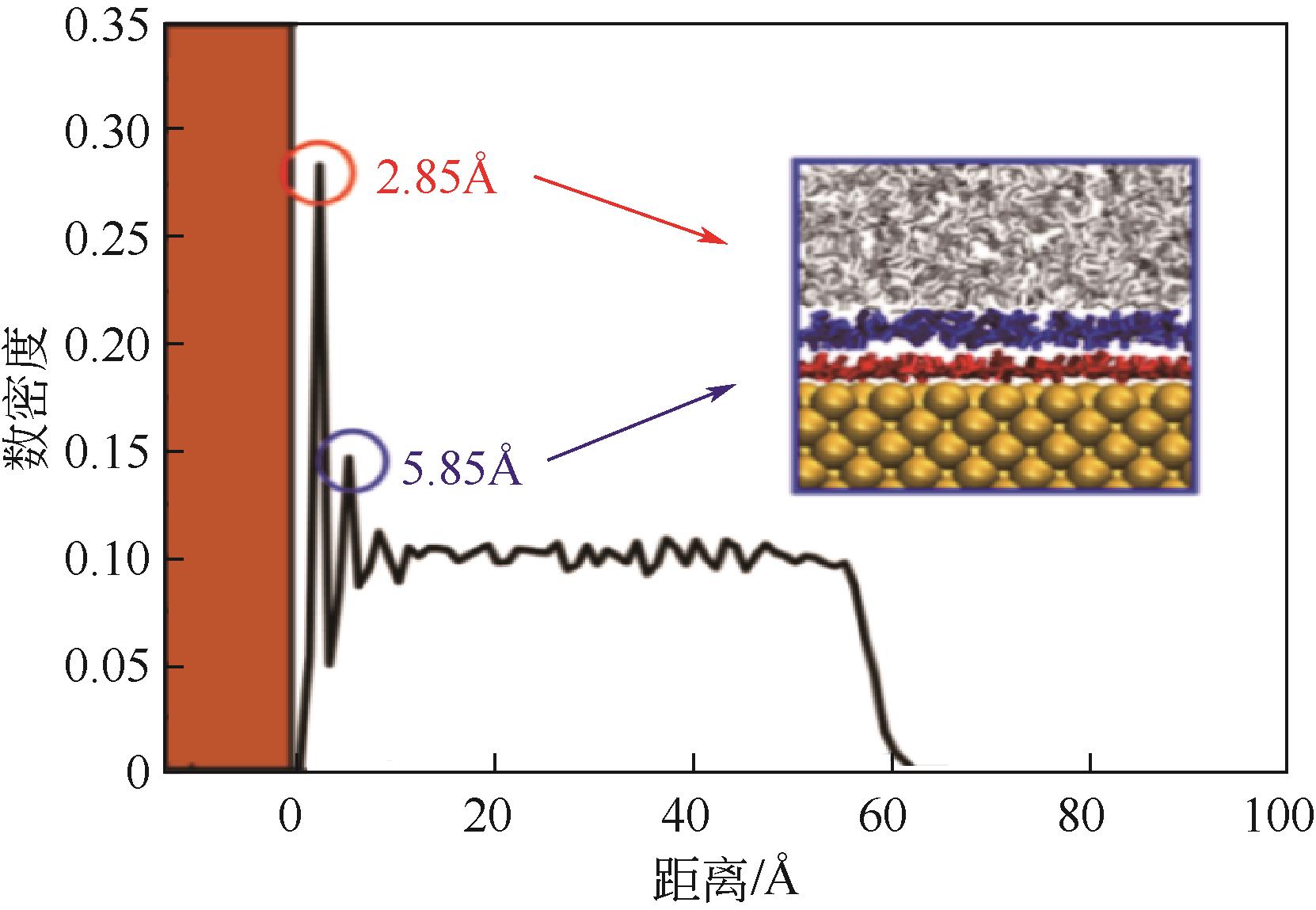
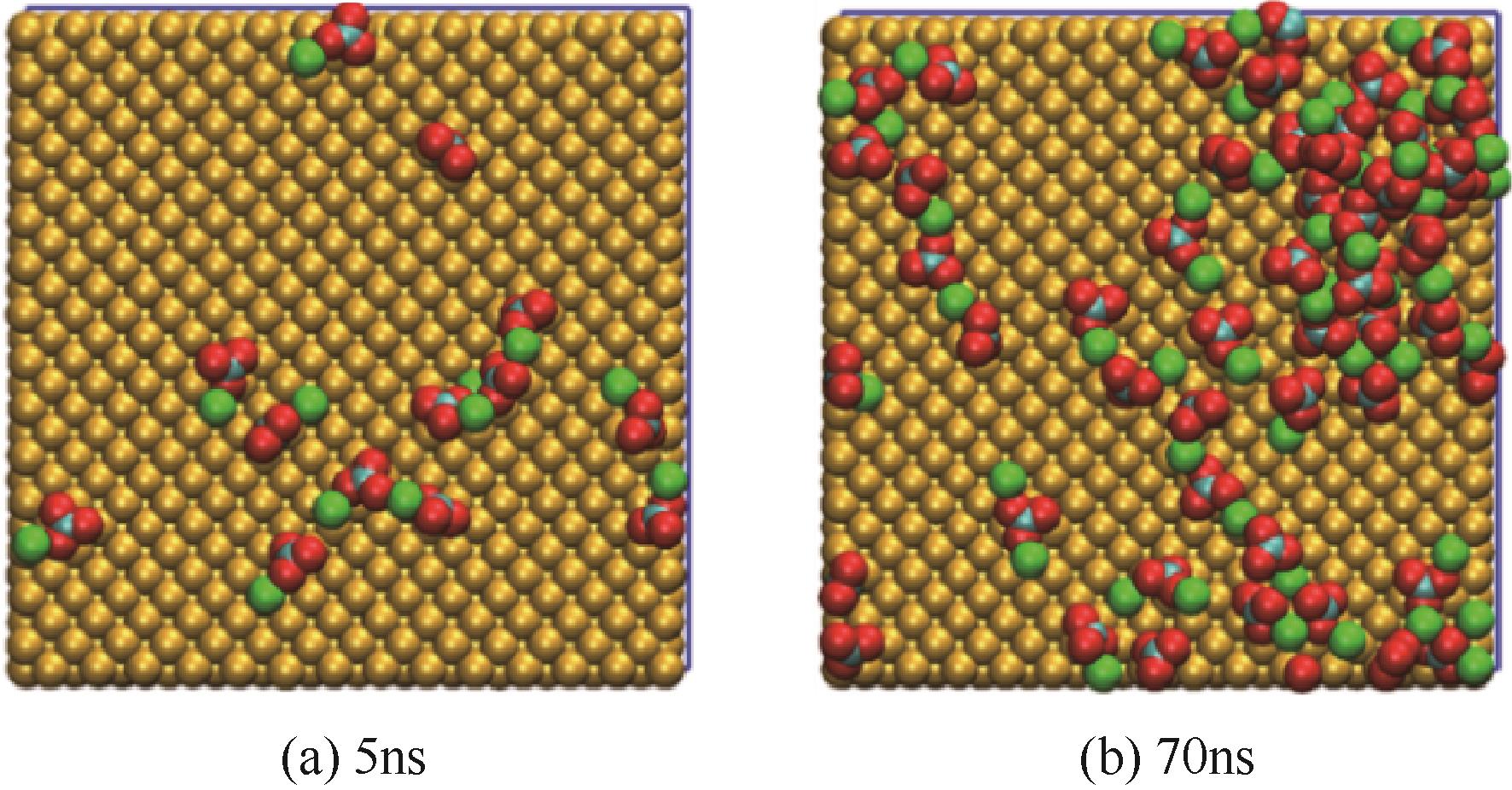
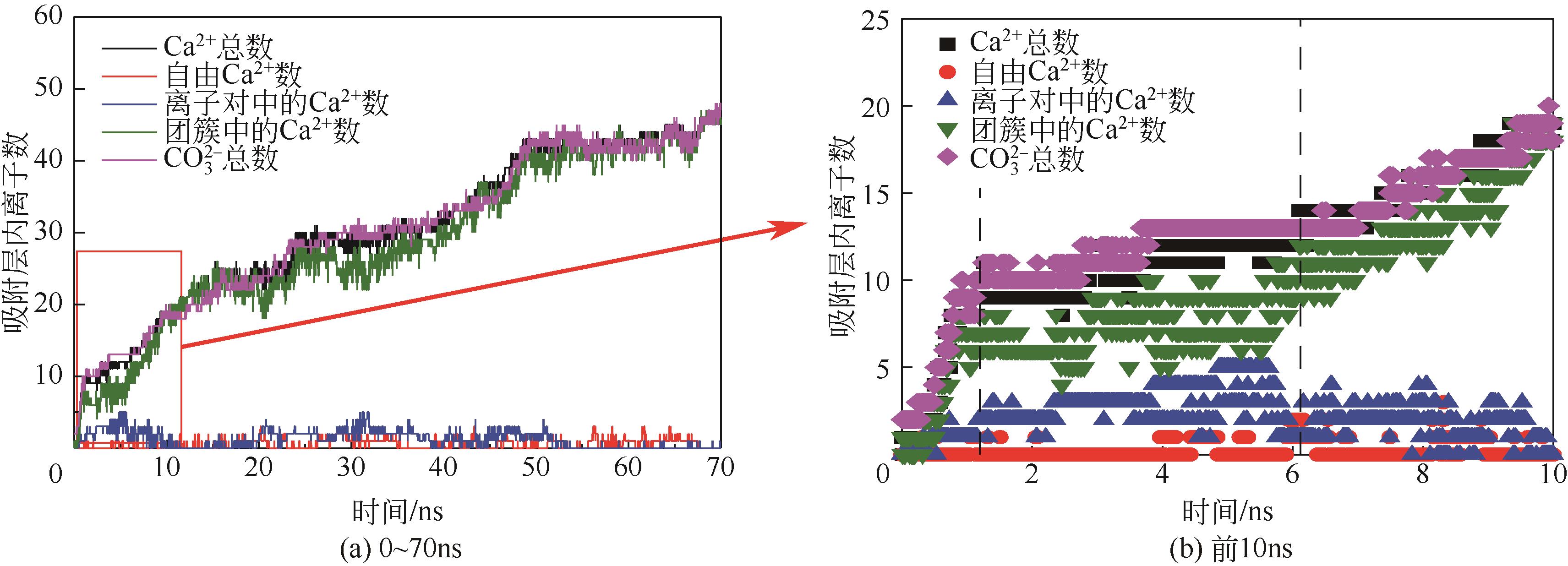
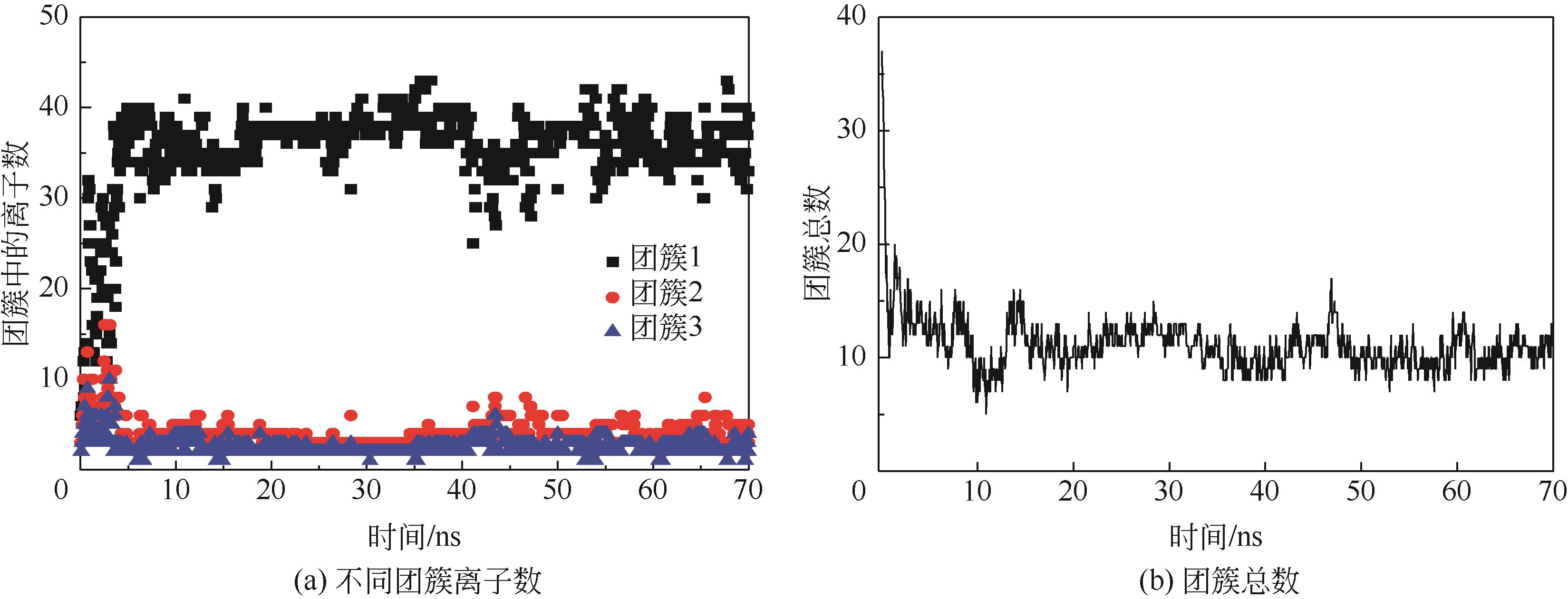
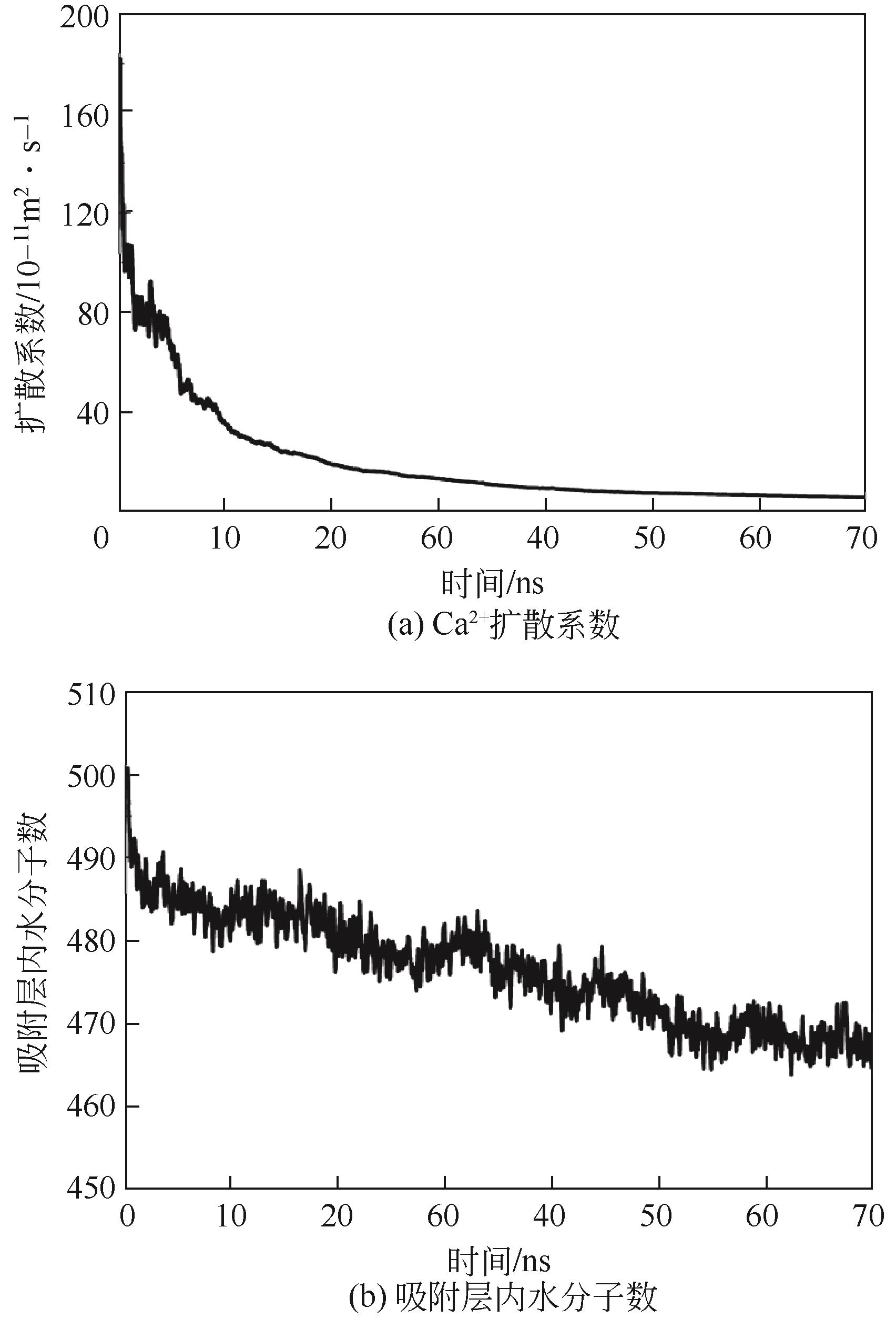
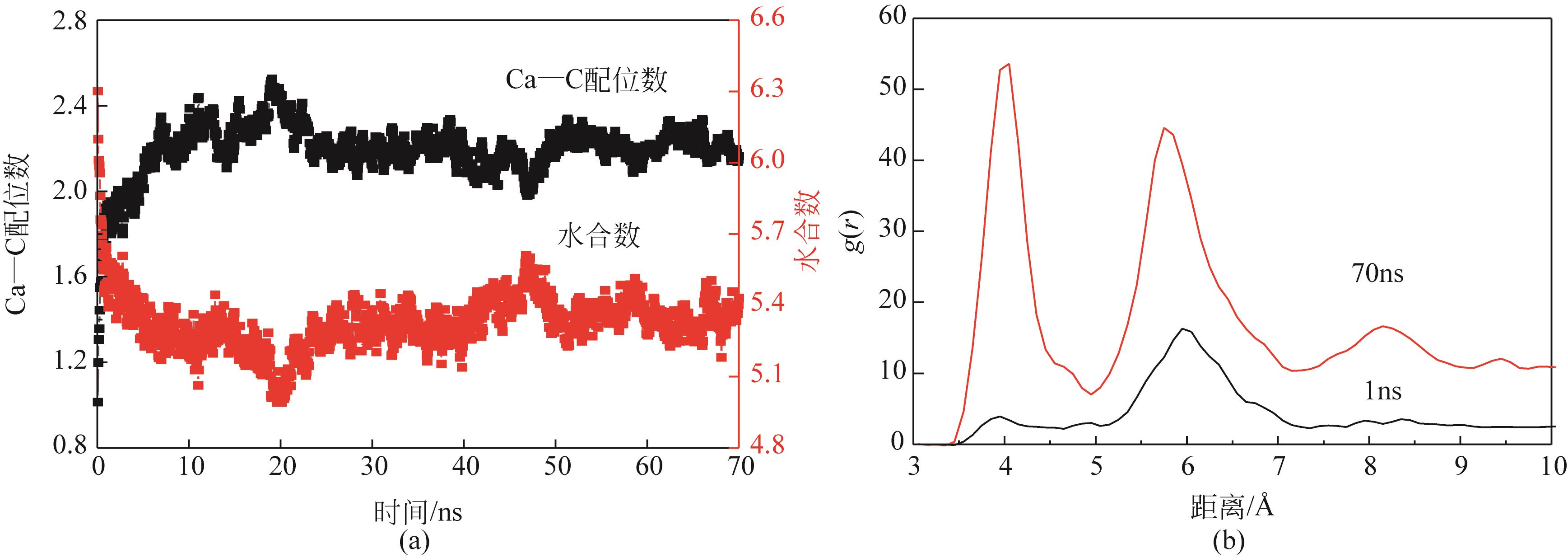
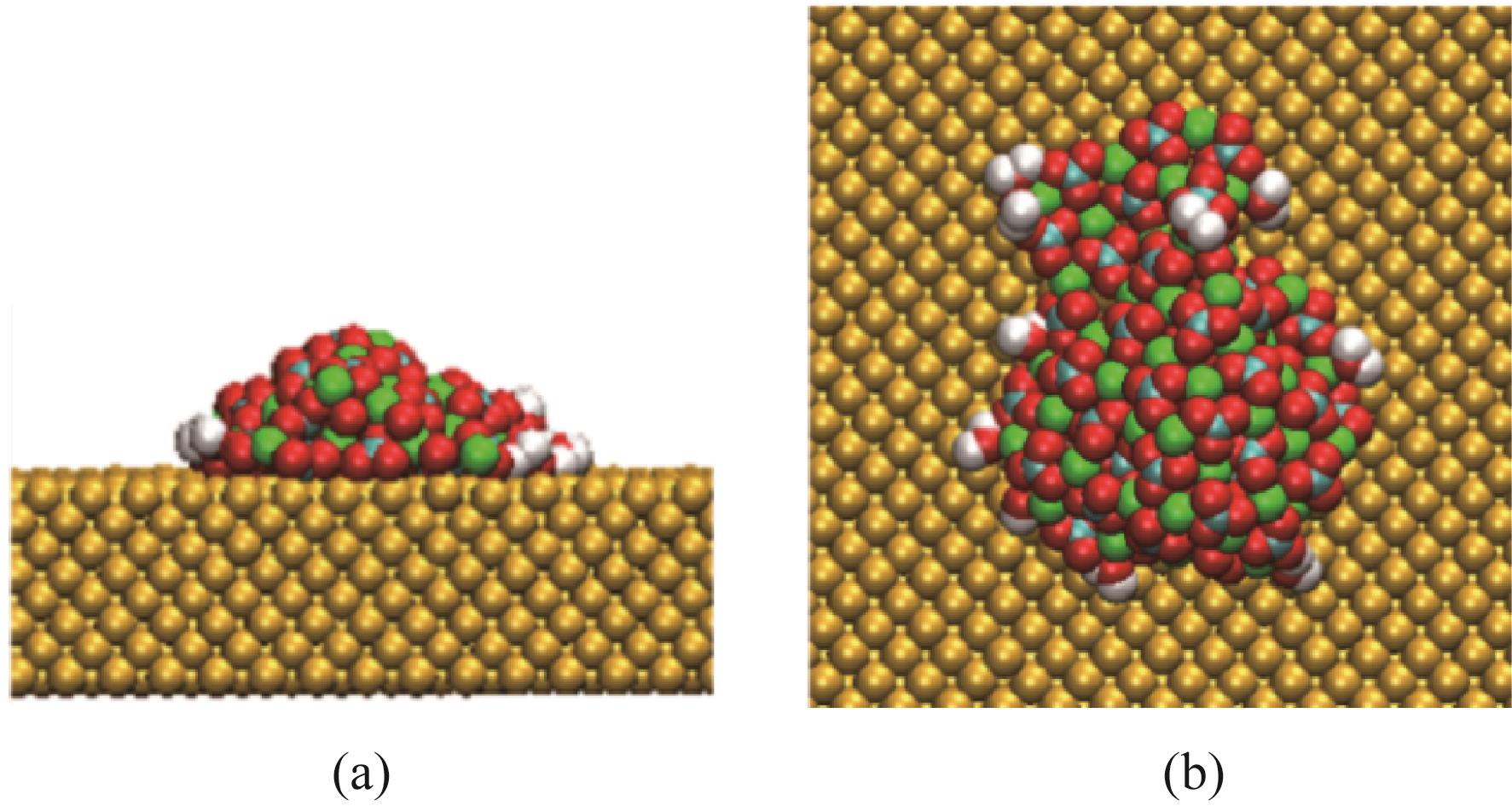
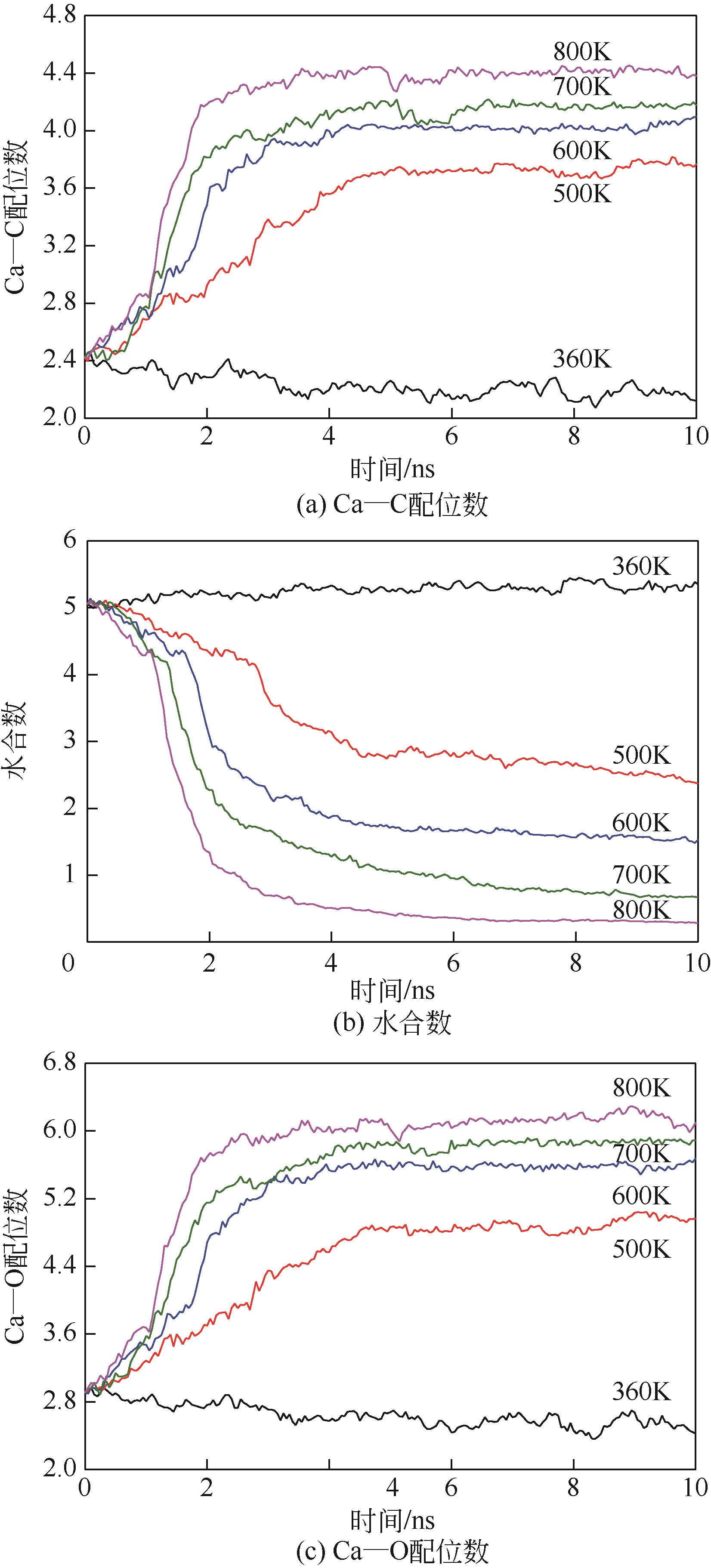
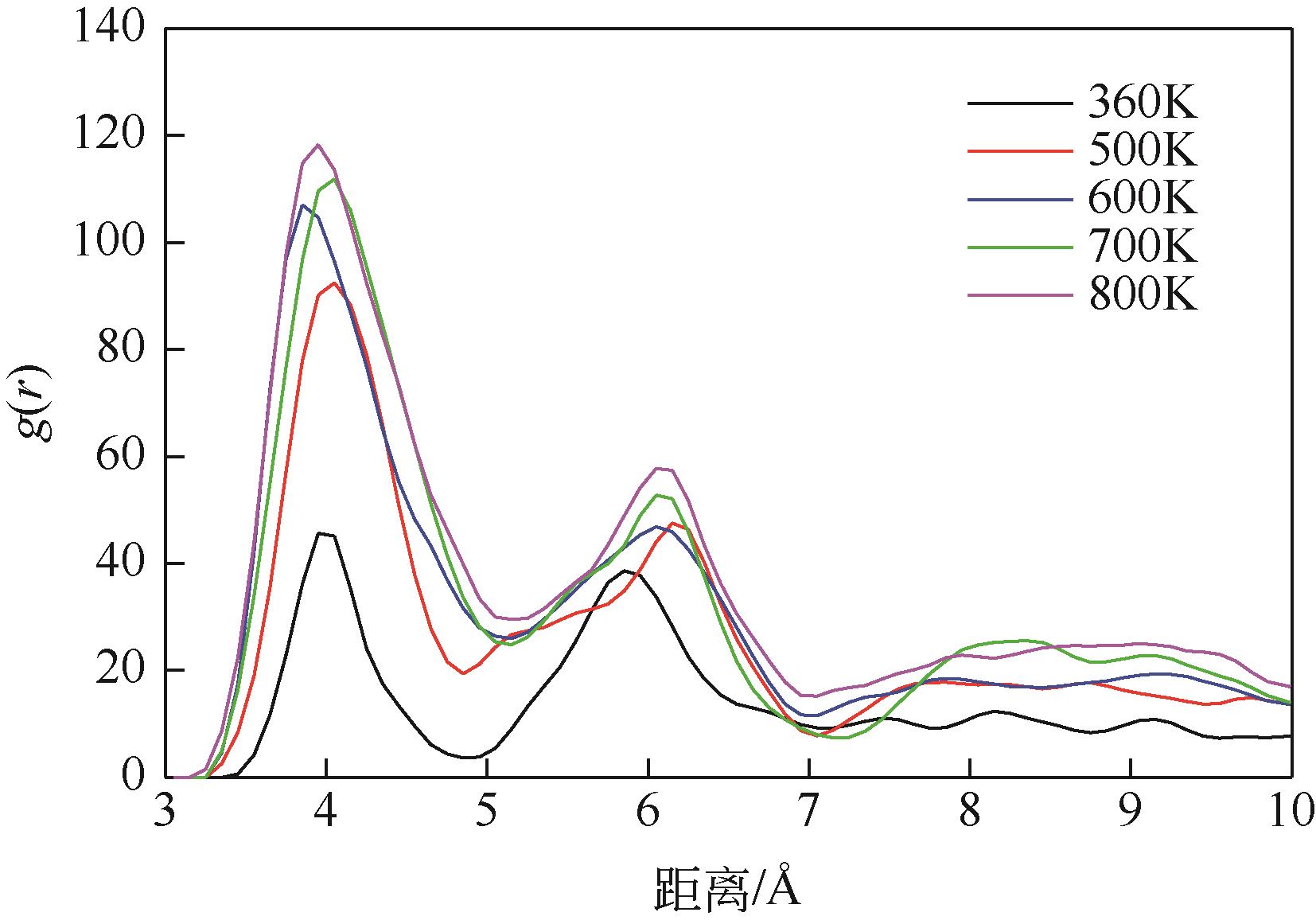
碳酸钙污垢具有较高的热阻,换热壁面碳酸钙的沉积会导致换热器效率显著下降,因此,换热壁面碳酸钙的形成机理与抑制是换热器设计研究的重点。本文采用分子动力学方法模拟分析过饱和溶液中碳酸钙在高温铜金属壁面上的吸附与脱水行为。模拟结果表明,在碳酸钙向壁面吸附过程中Ca—C配位数先增加后趋近于定值,离子的水合数先减少后趋近于定值。吸附在金属壁面的碳酸钙内部结构未发生明显变化,碳酸钙为具有一定水合数的非晶体结构。壁面温度越高,吸附的碳酸钙脱水越完全,Ca—C和Ca—O的配位数越高,高温壁面吸附的碳酸钙从水合结构向无水晶体转化。当壁面温度提高到800K,离子水合数接近于0,Ca—O配位数约为6,与宏观尺度下无水碳酸钙晶体的Ca—O配位数相接近。
中图分类号:
引用本文
肖毅, 王兵兵, 于旭亮, 王鑫, 蔡汉友. 换热壁面碳酸钙吸附与脱水行为的分子动力学[J]. 化工进展, 2022, 41(8): 4077-4085.
XIAO Yi, WANG Bingbing, YU Xuliang, WANG Xin, CAI Hanyou. Molecular dynamics simulation on adsorption and dehydration behavior of calcium carbonate on heat exchange surface[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4077-4085.
| 项目 | 类型 | k/eV·?-2 | r0/? | kθ/eV·rad-2 | θ0/(°) |
|---|---|---|---|---|---|
| Hw—Ow | Harmonic | 45.93 | 1.012 | ||
| Hw—Ow—Hw | Harmonic | 3.291 | 113.24 |
表1 水分子内原子相互作用势能参数
| 项目 | 类型 | k/eV·?-2 | r0/? | kθ/eV·rad-2 | θ0/(°) |
|---|---|---|---|---|---|
| Hw—Ow | Harmonic | 45.93 | 1.012 | ||
| Hw—Ow—Hw | Harmonic | 3.291 | 113.24 |
| 项目 | 类型 | k/eV·?-2 | r0/? | ka/eV·rad-2 | kbb/eV·?-2 | kba/eV·?-1·rad-1 | k2/eV·?-2 | k4/eV·?-4 |
|---|---|---|---|---|---|---|---|---|
| C—O | Harmonic | 40.8493 | 1.3042 | |||||
| O—C—O | class2 | 13.234 | 12.818 | 1.53319 | ||||
| C—O/O/O | Distance | 13.647 | 360.0 |
表2 碳酸根分子内相互作用势能参数
| 项目 | 类型 | k/eV·?-2 | r0/? | ka/eV·rad-2 | kbb/eV·?-2 | kba/eV·?-1·rad-1 | k2/eV·?-2 | k4/eV·?-4 |
|---|---|---|---|---|---|---|---|---|
| C—O | Harmonic | 40.8493 | 1.3042 | |||||
| O—C—O | class2 | 13.234 | 12.818 | 1.53319 | ||||
| C—O/O/O | Distance | 13.647 | 360.0 |
| 原子 | 电荷量/e | 原子量 |
|---|---|---|
| Hw | 0.41 | 1.008 |
| Ow | -0.82 | 16.000 |
| Ca | 2 | 40.080 |
| C | 1.123285 | 12.010 |
| O | -1.041095 | 16.000 |
| Cu | 0 | 63.550 |
表3 各原子电荷量与原子量
| 原子 | 电荷量/e | 原子量 |
|---|---|---|
| Hw | 0.41 | 1.008 |
| Ow | -0.82 | 16.000 |
| Ca | 2 | 40.080 |
| C | 1.123285 | 12.010 |
| O | -1.041095 | 16.000 |
| Cu | 0 | 63.550 |
| Lennard-Jones | ε/eV | σ/? | |
|---|---|---|---|
| Ow—Ow(lj/cut) | 0.00674 | 3.16549 | |
| Ow—Ca(lj/mdf) | 0.00095 | 3.35 | |
| Ow—Cu(lj/cut) | 0.05252 | 2.72029 | |
| Ca—Cu(lj/cut) | 0.09211 | 2.35409 | |
| C—Cu(lj/cut) | 0.03956 | 2.98960 | |
| O—Cu(lj/cut) | 0.04969 | 2.68805 | |
| Cu—Cu(lj/cut) | 0.4093 | 2.3377 | |
| Buckingham | A/eV | ρ/? | C/eV·?6 |
| Ca—O(buck/mdf) | 3161.63 | 0.27151 | 0 |
| Ca—C(buck/mdf) | 120000000 | 0.12 | 0 |
| O—O(buck/mdf) | 63840.20 | 0.19891 | 27.899 |
| O—Ow(buck/mdf) | 12534.46 | 0.2020 | 12.090 |
| O—Hw(buck/mdf) | 396.30 | 0.2170 | 0 |
表4 分子间势能参数
| Lennard-Jones | ε/eV | σ/? | |
|---|---|---|---|
| Ow—Ow(lj/cut) | 0.00674 | 3.16549 | |
| Ow—Ca(lj/mdf) | 0.00095 | 3.35 | |
| Ow—Cu(lj/cut) | 0.05252 | 2.72029 | |
| Ca—Cu(lj/cut) | 0.09211 | 2.35409 | |
| C—Cu(lj/cut) | 0.03956 | 2.98960 | |
| O—Cu(lj/cut) | 0.04969 | 2.68805 | |
| Cu—Cu(lj/cut) | 0.4093 | 2.3377 | |
| Buckingham | A/eV | ρ/? | C/eV·?6 |
| Ca—O(buck/mdf) | 3161.63 | 0.27151 | 0 |
| Ca—C(buck/mdf) | 120000000 | 0.12 | 0 |
| O—O(buck/mdf) | 63840.20 | 0.19891 | 27.899 |
| O—Ow(buck/mdf) | 12534.46 | 0.2020 | 12.090 |
| O—Hw(buck/mdf) | 396.30 | 0.2170 | 0 |
| 1 | PÄÄKKÖNEN T M, RIIHIMÄKI M, SIMONSON C J, et al. Crystallization fouling of CaCO3-analysis of experimental thermal resistance and its uncertainty[J]. International Journal of Heat & Mass Transfer, 2012, 55(23-24): 6927-6937. |
| 2 | CHAUSSEMIER M, POURMOHTASHAM E, GELUS D, et al. State of art of natural inhibitors of calcium carbonate scaling[J]. Desalination, 2015, 356: 47-55. |
| 3 | 贺姗姗. 圆管内三角翼涡流发生器CaCO3污垢特性的数值模拟[D]. 吉林: 东北电力大学, 2018. |
| HE S S. Numerical simulation of CaCO3 fouling characteristics in tube with delta wing vortex generator[D]. Jilin: Northeast Electric Power University, 2018. | |
| 4 | 罗志强, 杨庆峰. 旋转磁场与水量耦合对CaCO3结晶的影响[J]. 化工学报, 2018, 69(7): 3029-3037. |
| LUO Z Q, YANG Q F. Effect of rotating magnetic field coupled with water volume on CaCO3 crystallization[J]. CIESC Journal, 2018, 69(7): 3029-3037. | |
| 5 | WANG J G, LIANG Y D. Anti-fouling effect of axial alternating electromagnetic field on calcium carbonate fouling in U-shaped circulating cooling water heat exchange tube[J]. International Journal of Heat & Mass Transfer, 2017, 115: 774-781. |
| 6 | 李海花, 刘振法, 高玉华, 等. 静电场对CaCO3结晶过程的影响及与绿色阻垢剂的协同阻垢性能[J]. 化工学报, 2013, 64(5): 1736-1742. |
| LI H H, LIU Z F, GAO Y H, et al. Influence of electrostatic water treatment on crystallization behavior of CaCO3 and synergistic scale inhibition with a green scale inhibitor[J]. CIESC Journal, 2013, 64(5): 1736-1742. | |
| 7 | ALIMI F, TLILI M, AMOR M B, et al. Influence of magnetic field on calcium carbonate precipitation[J]. Desalination, 2007, 206(1/2/3): 163-168. |
| 8 | TIJING L D, LEE D H, KIM D W, et al. Effect of high-frequency electric fields on calcium carbonate scaling[J]. Desalination, 2011, 279(1/2/3): 47-53. |
| 9 | 徐志明, 常宏亮, 王兵兵, 等. 电场作用下CaCO3污垢特性的实验研究[J]. 中国电机工程学报, 2018, 38(21): 6346-6352. |
| XU Z M, CHANG H L, WANG B B, et al. Experimental study on CaCO3 fouling characteristics under electric field[J]. Proceedings of the CSEE, 2018, 38(21): 6346-6352. | |
| 10 | SMEETS P, KANG R C, KEMPEN R, et al. Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy[J]. Nature Materials, 2015, 14(4): 394-399. |
| 11 | GEBAUER D, CÖLFEN H. Prenucleation clusters and non-classical nucleation[J]. Nano Today, 2011, 6(6): 564-584. |
| 12 | GEBAUER D, VÖLKEL A, CÖLFEN H. Stable prenucleation calcium carbonate clusters[J]. Science, 2008, 322(5909): 1819-1822. |
| 13 | POUGET E M, BOMANS P H H, GOOS J A C M, et al. The initial stages of template-controlled CaCO3 formation revealed by Cryo-TEM[J]. Science, 2009, 323(5920): 1455-1458. |
| 14 | GOODWIN A L, MICHEL F M, PHILLIPS B L, et al. Nanoporous structure and medium-range order in synthetic amorphous calcium carbonate[J]. Chemistry of Materials, 2010, 22(10): 3197-3205. |
| 15 | RAITERI P, GALE J D. Water is the key to nonclassical nucleation of amorphous calcium carbonate[J]. Journal of the American Chemical Society, 2010, 132(49): 17623–17634. |
| 16 | RODRIGUEZ-BLANCO J D, SHAW S, BENNING L G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite[J]. Nanoscale, 2010, 3(1): 265-271. |
| 17 | NIELSEN M H, ALONI S, YOREO J J D. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways[J]. Science, 2014, 345(6201): 1158-1162. |
| 18 | SAHARAY M, YAZAYDIN A O, KIRKPATRICK R J. Dehydration-induced amorphous phases of calcium carbonate[J]. Journal of Physical Chemistry B, 2013, 117(12): 3328-3336. |
| 19 | SAHARAY M, KIRKPATRICK R J. Water dynamics in hydrated amorphous materials: a molecular dynamics study of the effects of dehydration in amorphous calcium carbonate[J]. Physical Chemistry Chemical Physics, 2017, 19(43): 29594-29600. |
| 20 | PLIMPTON S. Fast parallel algorithms for short-range molecular dynamics[J]. Journal of Computational Physics, 1995, 117(1): 1-19. |
| 21 | MA M, WANG Y H, CAO X F, et al. Temperature and supersaturation as key parameters controlling the spontaneous precipitation of calcium carbonate with distinct physicochemical properties from pure aqueous solutions[J]. Crystal Growth and Design, 2019, 19(12): 6972-6988. |
| 22 | WU Y J, TEPPER H L, VOTH G A. Flexible simple point-charge water model with improved liquid-state properties[J]. The Journal of Chemical Physics, 2006, 124(2): 24503-24503. |
| 23 | DEMICHELIS R, RAITERI P, GALE J D, et al. Stable prenucleation mineral clusters are liquid-like ionic polymers[J]. Nature Communications, 2011, 2(6): 590. |
| 24 | XIAO S J, EDWARDS S A, GRÄTER F. A new transferable forcefield for simulating the mechanics of CaCO3 crystals[J]. Journal of Physical Chemistry C, 2011, 115(41): 20067-20075. |
| 25 | SWOPE W C, ANDERSEN H C, BERENS P H, et al. A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: application to small water clusters[J]. Journal of Chemical Physics, 1982, 76(1): 637-649. |
| 26 | BECKERS J V L, LOWE C P, LEEUW S W D. An iterative PPPM method for simulating coulombic systems on distributed memory parallel computers[J]. Molecular Simulation, 1998, 20(6): 369-383. |
| 27 | GEBAUER D, GUNAWIDJAJA P N, KO J Y P. Proto-calcite and proto-vaterite in amorphous calcium carbonates[J]. Angewandte Chemie International Edition, 2010, 49(47): 8889-8891. |
| 28 | SURFACEACE A F, HEDGES L O, FERNANDEZ-MARTINEZ A, et al. Microscopic evidence for liquid-liquid separation in supersaturated CaCO3 solutions[J]. Science, 2013, 341(6148): 885-889. |
| 29 | LHLI J, WONG W C, NOEL E H, et al. Dehydration and crystallization of amorphous calcium carbonate in solution and in air[J]. Nature Communications, 2014, 5(1): 3169. |
| 30 | ZOU Z Y, BERTINETTI L, POLITI Y, et al. Opposite particle size effect on amorphous calcium carbonate crystallization in water and during heating in air[J]. Chemistry of Materials, 2015, 27(12): 4237-4246. |
| [1] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 徐若思, 谭蔚. C形管池沸腾两相流流场模拟与流固耦合分析[J]. 化工进展, 2023, 42(S1): 47-55. |
| [6] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [7] | 张凤岐, 崔成东, 鲍学伟, 朱炜玄, 董宏光. 胺液吸收-分步解吸脱硫工艺的设计与评价[J]. 化工进展, 2023, 42(S1): 518-528. |
| [8] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [9] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [10] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [11] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [12] | 张瑞杰, 刘志林, 王俊文, 张玮, 韩德求, 李婷, 邹雄. 水冷式复叠制冷系统的在线动态模拟与优化[J]. 化工进展, 2023, 42(S1): 124-132. |
| [13] | 王太, 苏硕, 李晟瑞, 马小龙, 刘春涛. 交流电场中贴壁气泡的动力学行为[J]. 化工进展, 2023, 42(S1): 133-141. |
| [14] | 孙继鹏, 韩靖, 唐杨超, 闫汉博, 张杰瑶, 肖苹, 吴峰. 硫黄湿法成型过程数值模拟与操作参数优化[J]. 化工进展, 2023, 42(S1): 189-196. |
| [15] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||